Polyaspartamide-Based Nanoparticles Loaded with Fluticasone Propionate and the In Vitro Evaluation towards Cigarette Smoke Effects
Abstract
1. Introduction
2. Result and Discussion
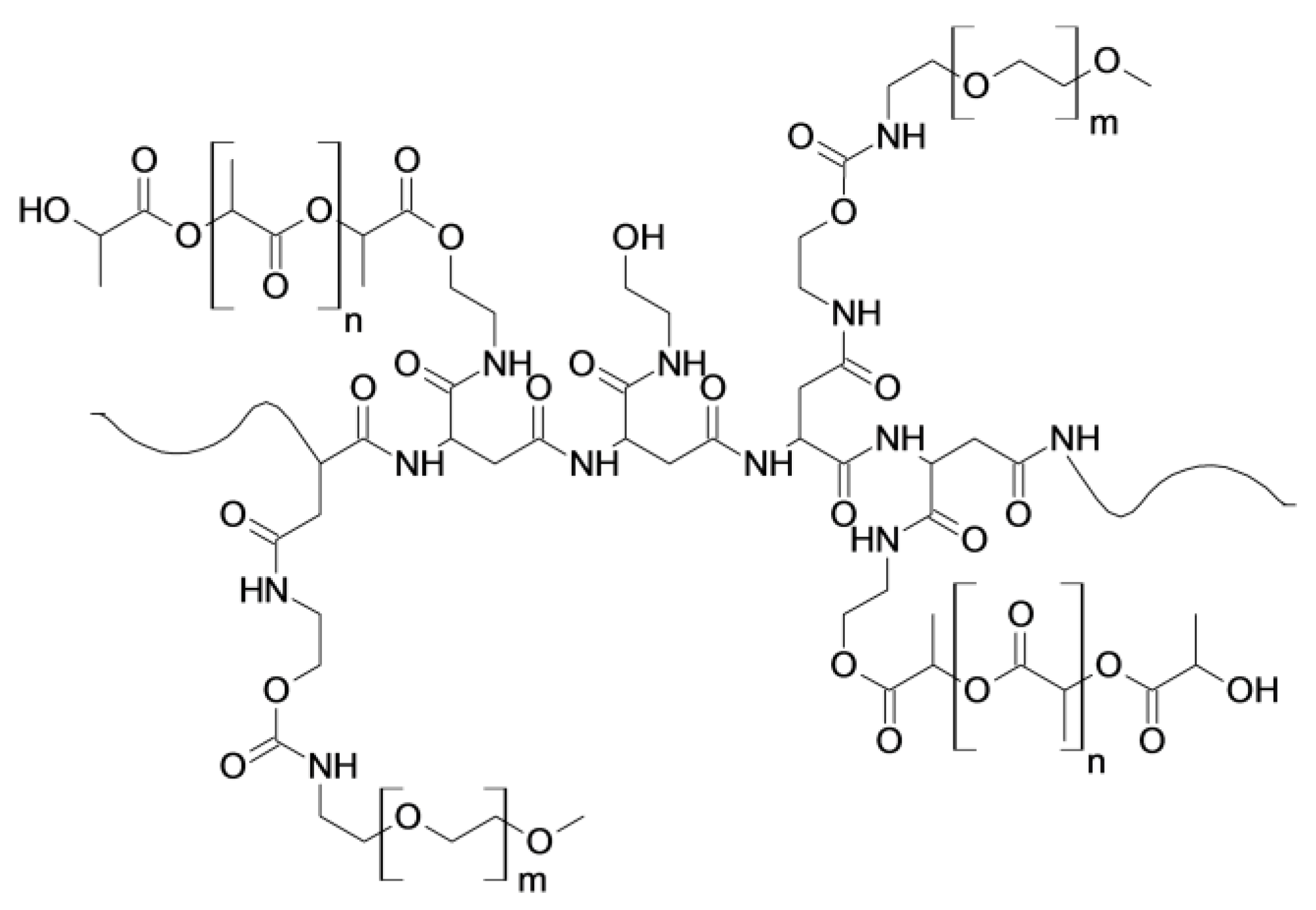
2.1. Preparation and Characterization of Polymeric Nanoparticles (NPs) Based on PHEA-PLA-PEG2000 Copolymer
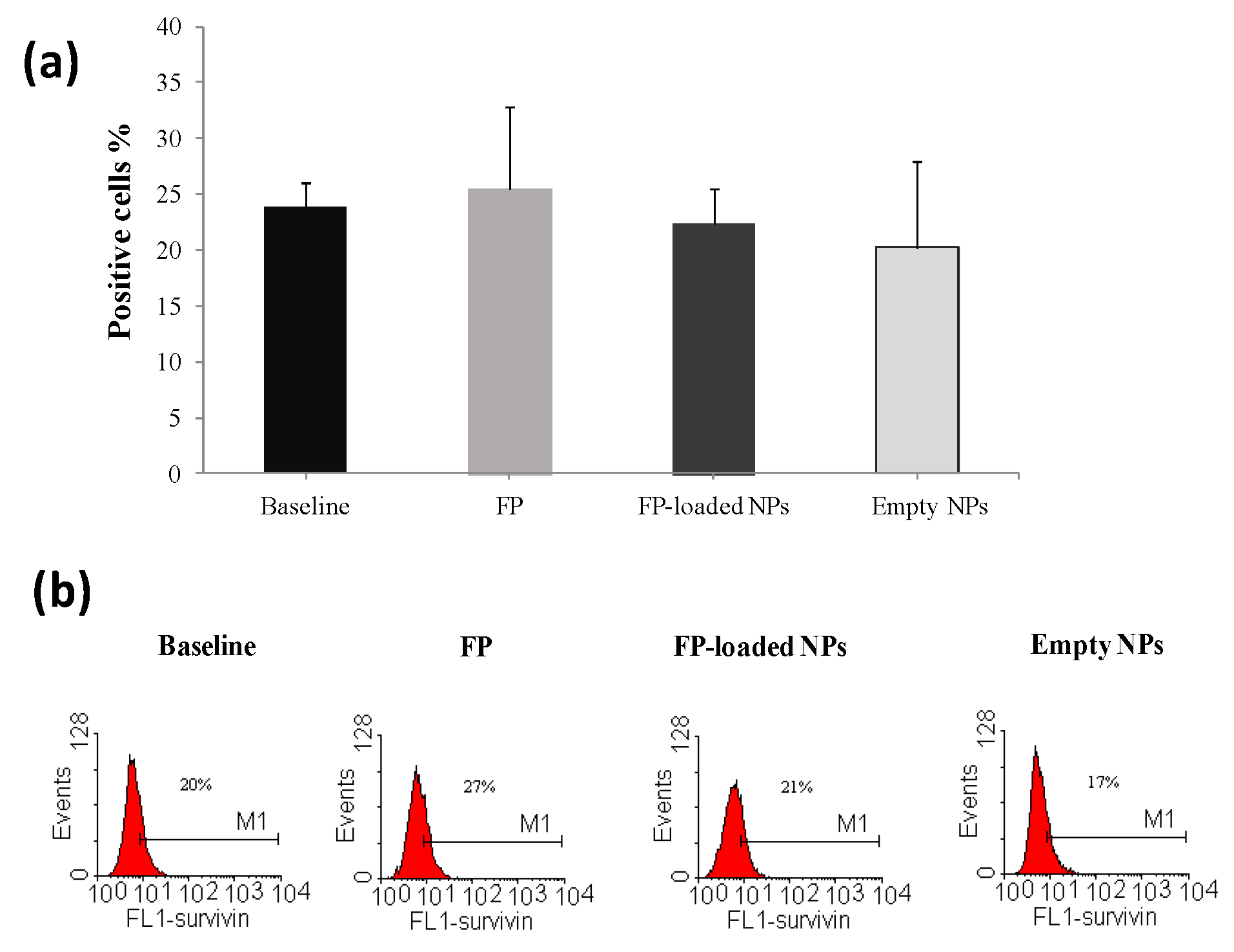
2.2. Biological Characterization
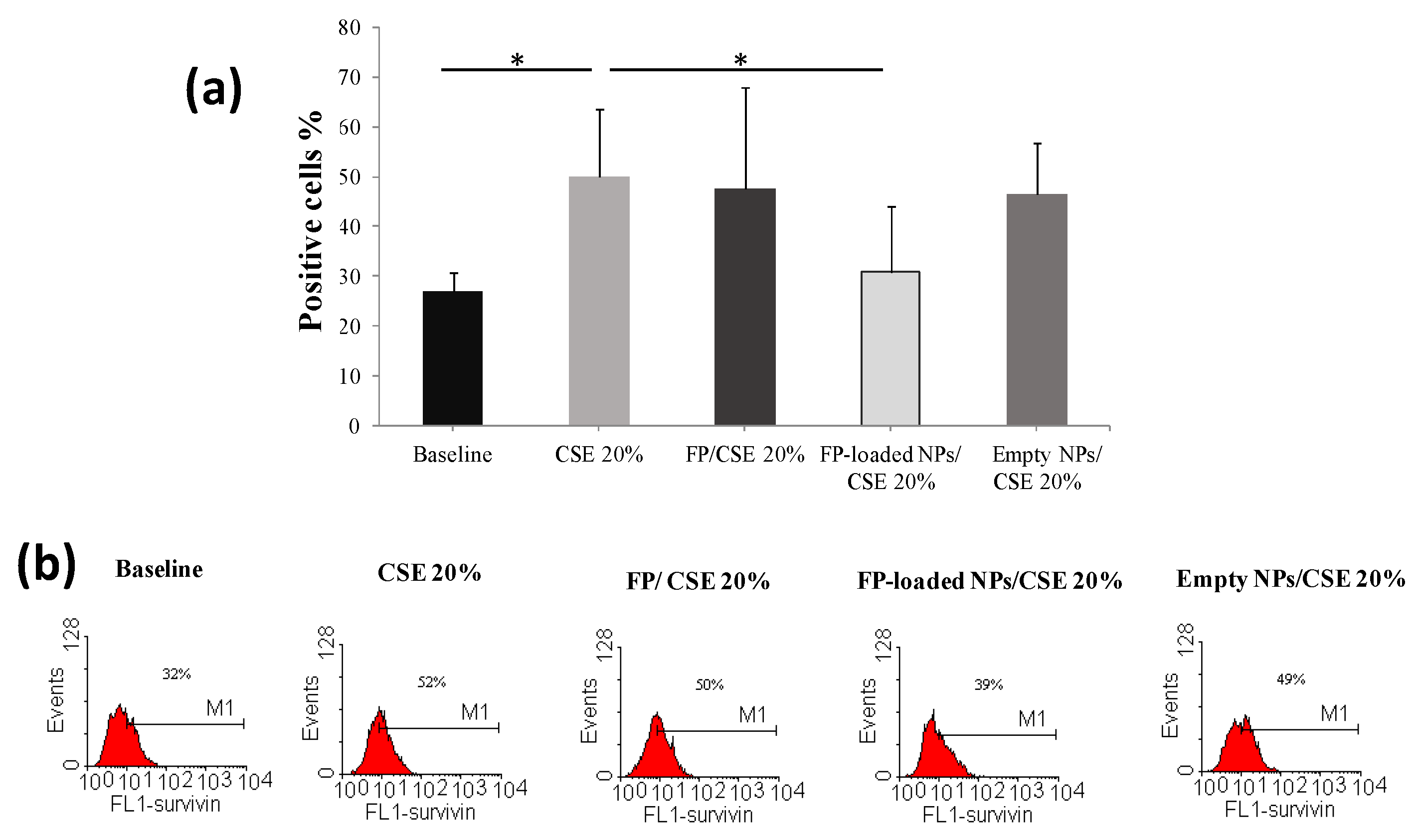
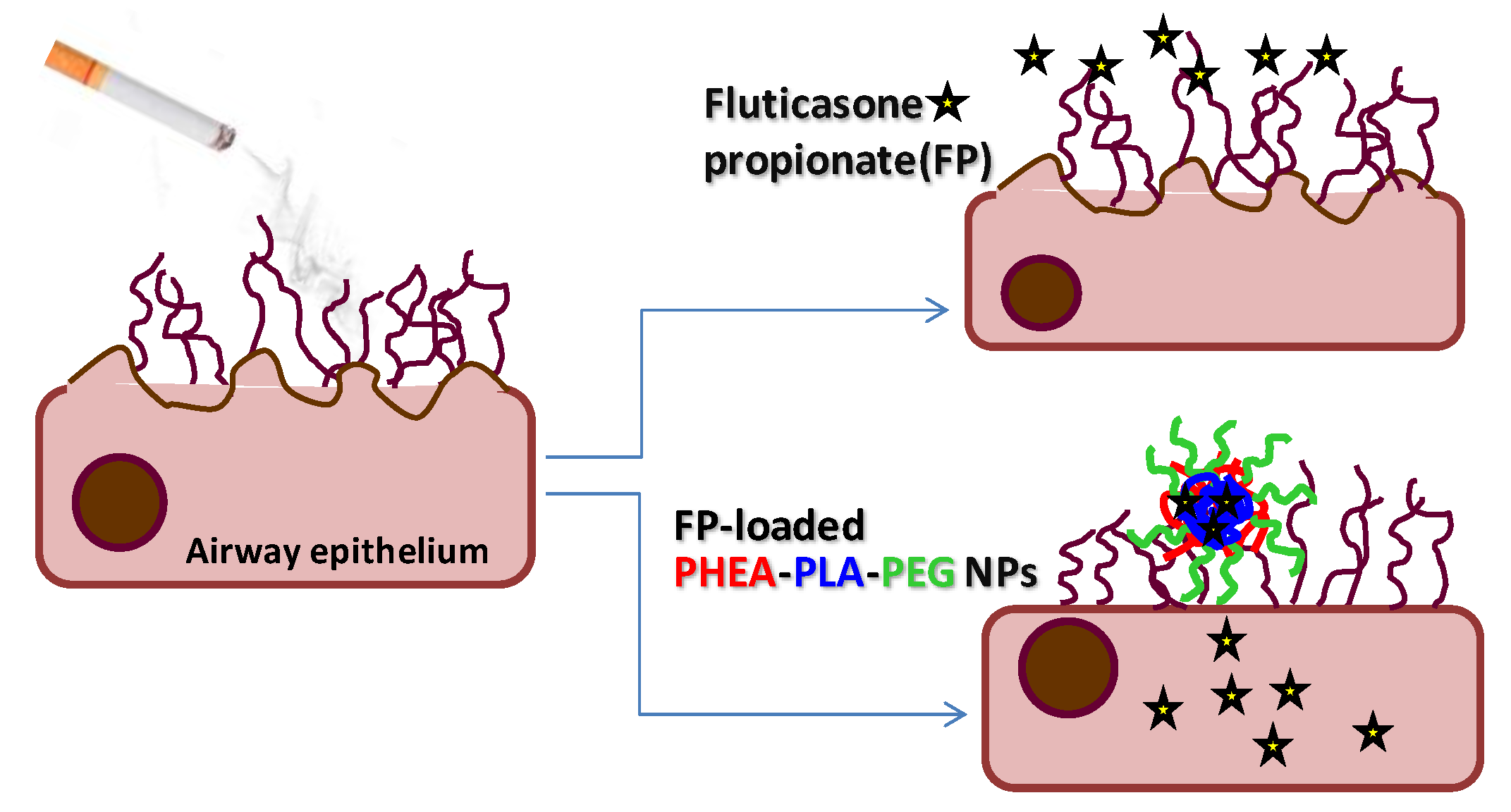
2.3. Biological Efficacy
3. Materials and Methods
3.1. General Methods
3.2. Synthetic Procedures and Nanoparticle Formation
3.2.1. Synthesis of PHEA-PLA Graft Copolymer and Characterization
3.2.2. Synthesis of PHEA-PLA-PEG2000 Graft Copolymer and Characterization
3.2.3. General Procedure for Nanoparticle Preparation
3.3. NPs Characterization
3.3.1. Mean Dimensions and ζ Potential
3.3.2. Drug Loading
3.3.3. Stability Studies
3.3.4. Drug Release in Simulated Lung Fluid (SLF)
3.4. Biological Characterization
3.4.1. Cell Culture
3.4.2. Cell Viability Assay
3.4.3. Cell Apoptosis
3.4.4. Preparation of Cigarette Smoke Extract (CSE)
3.4.5. Stimulation of 16-HBE
3.4.6. Survivin Expression
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Umerska, A.; Mouzouvi, C.R.A.; Bigot, A.; Saulnier, P. Formulation and Nebulization of Fluticasone Propionate-Loaded Lipid Nanocarriers. Int. J. Pharm. 2015, 493, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.; McConville, J.T.; Lamprecht, A. Pulmonary Delivery of Anti-Inflammatory Agents. Expert Opin. Drug Deliv. 2015, 12, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Derendorf, H.; Nave, R.; Drollmann, A.; Cerasoli, F.; Wurst, W. Relevance of Pharmacokinetics and Pharmacodynamics of Inhaled Corticosteroids to Asthma. Eur. Respir. J. 2006, 28, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Schopf, L.; Bourassa, J.; Chen, H. Enhanced Pulmonary Delivery of Fluticasone Propionate in Rodents by Mucus-Penetrating Nanoparticles. Int. J. Pharm. 2016, 502, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Kim, A.J.; Trehan, K.; Schneider, C.S.; Cebotaru, L.; Woodward, O.M.; Boylan, N.J.; Boyle, M.P.; Lai, S.K.; Guggino, W.B.; et al. Lung Gene Therapy with Highly Compacted DNA Nanoparticles That Overcome the Mucus Barrier. J. Control. Release 2014, 178, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Boylan, N.J.; Suk, J.S.; Lai, S.K.; Jelinek, R.; Boyle, M.P.; Cooper, M.J.; Hanes, J. Highly Compacted DNA Nanoparticles with Low MW PEG Coatings: In Vitro, Ex Vivo and in Vivo Evaluation. J. Control. Release 2012, 157, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, V.C.F.; Legrand, P.; Morgat, J.L.; Vert, M.; Mysiakine, E.; Gref, R.; Devissaguet, J.P.; Barratt, G. Biodistribution of Long-Circulating PEG-Grafted Nanocapsules in Mice: Effects of PEG Chain Length and Density. Pharm. Res. 2001, 18, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Trapani, A.; Castellani, S.; Carbone, A.; Belgiovine, G.; Craparo, E.F.; Puglisi, G.; Cavallaro, G.; Trapani, G.; Conese, M. Nanocomplexes for Gene Therapy of Respiratory Diseases: Targeting and Overcoming the Mucus Barrier. Pulm. Pharmacol. Ther. 2015, 34, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Porsio, B.; Sardo, C.; Giammona, G.; Cavallaro, G. PegylatedPolyaspartamide—Polylactide-Based Nanoparticles Penetrating Cystic Fibrosis Artificial Mucus. Biomacromolecules 2016, 17, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Giammona, G.; Carlisi, B.; Palazzo, S. Reaction of α,β-poly(N-Hydroxyethyl)-dl-Aspartamide with Derivatives of Carboxylic Acids. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2813–2818. [Google Scholar] [CrossRef]
- Mendichi, R.; Schieroni, A.G.; Cavallaro, G.; Licciardi, M.; Giammona, G. Molecular Characterization of α,β-poly(N-2-Hydroxyethyl)-dl-Aspartamide Derivatives as Potential Self-Assembling Copolymers Forming Polymeric Micelles. Polymer 2003, 44, 4871–4879. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cavallaro, G.; Bondi, M.L.; Mandracchia, D.; Giammona, G. PEGylated Nanoparticles Based on a Polyaspartamide. Preparation, Physico-Chemical Characterization, and Intracellular Uptake. Biomacromolecules 2006, 7, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Di Gioia, S.; Trapani, A.; Cellamare, S.; Belgiovine, G.; Mandracchia, D.; Giammona, G.; Cavallaro, G.; Conese, M. Realization of Polyaspartamide-Based Nanoparticles and in Vivo Lung Biodistribution Evaluation of a Loaded Glucocorticoid after Aerosolization in Mice. Int. J. Pharm. 2016, 510, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Di Stefano, M.; Craparo, E.F.; Amato, G.; Fontana, G.; Cavallaro, G.; Giammona, G. PHEA-Graft-Polybutylmethacrylate Copolymer Microparticles for Delivery of Hydrophobic Drugs. Int. J. Pharm. 2012, 433, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Teresi, G.; Licciardi, M.; Bondí, M.L.; Cavallaro, G. Novel Composed Galactosylated Nanodevices Containing a Ribavirin Prodrug as Hepatic Cell-Targeted Carriers for HCV Treatment. J. Biomed. Nanotechnol. 2013, 9, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Pitarresi, G.; Palumbo, F.S.; Calabrese, R.; Craparo, E.F.; Giammona, G. CrosslinkedHyaluronan with a Protein-like Polymer: Novel Bioresorbable Films for Biomedical Applications. J. Biomed. Mater. Res. Part A 2008, 84, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Porsio, B.; Bondì, M.L.; Giammona, G.; Cavallaro, G. Evaluation of Biodegradability on Polyaspartamide-Polylactic Acid Based Nanoparticles by Chemical Hydrolysis Studies. Polym. Degrad. Stab. 2015, 119, 56–67. [Google Scholar] [CrossRef]
- Cavallaro, G.; Craparo, E.F.; Sardo, C.; Lamberti, G.; Barba, A.A.; Dalmoro, A. PHEA-PLA Biocompatible Nanoparticles by Technique of Solvent Evaporation from Multiple Emulsions. Int. J. Pharm. 2015, 495, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Teresi, G.; Ognibene, M.C.; Casaletto, M.P.; Bondì, M.L.; Cavallaro, G. Nanoparticles Based on Novel Amphiphilic Polyaspartamide Copolymers. J. Nanopart. Res. 2010, 12, 2629–2644. [Google Scholar] [CrossRef]
- Craparo, E.F.; Cavallaro, G.; Bondì, M.L.; Giammona, G. Preparation of Polymeric Nanoparticles by Photo-Crosslinking of an AcryloylatedPolyaspartamide in w/o Microemulsion. Macromol. Chem. Phys. 2004, 205, 1955–1964. [Google Scholar] [CrossRef]
- Stability Testing of New Drug Substances and Products Q1A (R2). Available online: https://urlsand.esvalabs.com/?u=http%3A%2F%2Facademy.gmp-compliance.org%2Fguidemgr%2Ffiles%2FQ1A%28R2%29STEP4.PDF&e=541e9c83&h=6551e918&f=y&p=y (accessed on 13 August 2017).
- Pace, E.; Ferraro, M.; Siena, L.; Melis, M.; Montalbano, A.M.; Johnson, M.; Bonsignore, M.R.; Bonsignore, G.; Gjomarkaj, M. Cigarette smoke increases Toll-like receptor 4 and modify lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 2008, 124, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Ferraro, M.; Di Vincenzo, S.; Cipollina, C.; Gerbino, S.; Cigna, D.; Caputo, V.; Balsamo, R.; Lanata, L.; Gjomarkaj, M. Comparative cytoprotective effects of carbocysteine and fluticasone propionate in cigarette smoke extract-stimulated bronchial epithelial cells. Cell Stress Chaperones 2013, 18, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Bondì, M.; Ferraro, M.; Di Vincenzo, S.; Gerbino, S.; Cavallaro, G.; Giammona, G.; Botto, C.; Gjomarkaj, M.; Pace, E. Effects in Cigarette Smoke Stimulated Bronchial Epithelial Cells of a Corticosteroid Entrapped into Nanostructured Lipid Carriers. J. Nanobiotechnol. 2014, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Cesselli, D.; Beltrami, C.A. At the Stem of Youth and Health. Pharmacol. Ther. 2011, 129, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Di Vincenzo, S.; Ferraro, M.; Bruno, A.; Dino, P.; Bonsignore, M.R.; Battaglia, S.; Saibene, F.; Lanata, L.; Gjomarkaj, M. Carbocysteine Counteracts the Effects of Cigarette Smoke on Cell Growth and on the SIRT1/FoxO3 Axis in Bronchial Epithelial Cells. Exp. Gerontol. 2016, 81, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biologic Fluids with Possible Application in Dissolution Testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Cozens, A.L.; Yezzi, M.J.; Yamaya, M.; Steiger, D.; Wagner, J.A.; Garber, S.S.; Chin, L.; Simon, E.M.; Cutting, G.R.; Gardner, P. A Transformed Human Epithelial Cell Line That Retains Tight Junctions Post Crisis. In Vitro. Cell. Dev. Biol. 1992, 28, 735–744. [Google Scholar] [CrossRef]
- Su, Y.; Han, W.; Giraldo, C.; De Li, Y.; Block, E.R. Effect of Cigarette Smoke Extract on Nitric Oxide Synthase in Pulmonary Artery Endothelial Cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 819–825. [Google Scholar] [CrossRef] [PubMed]



| Mean Size (nm) (±S.D.) | |||
| t0 | After freeze-drying | t12months, −20 °C | t12months, 5 °C |
| 147.4 ± 11.0 | 161.3 ± 14.0 | 204.7 ± 34.7 | 198.9 ± 22.6 |
| ζ Potential (mV) (±S.D.) | |||
| t0 | After freeze-drying | t12months, −20 °C | t12months, 5 °C |
| −6.9 ± 1.5 | −4.6 ± 2.3 | −3.8 ± 3.5 | −4.5 ± 3.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craparo, E.F.; Ferraro, M.; Pace, E.; Bondì, M.L.; Giammona, G.; Cavallaro, G. Polyaspartamide-Based Nanoparticles Loaded with Fluticasone Propionate and the In Vitro Evaluation towards Cigarette Smoke Effects. Nanomaterials 2017, 7, 222. https://doi.org/10.3390/nano7080222
Craparo EF, Ferraro M, Pace E, Bondì ML, Giammona G, Cavallaro G. Polyaspartamide-Based Nanoparticles Loaded with Fluticasone Propionate and the In Vitro Evaluation towards Cigarette Smoke Effects. Nanomaterials. 2017; 7(8):222. https://doi.org/10.3390/nano7080222
Chicago/Turabian StyleCraparo, Emanuela Fabiola, Maria Ferraro, Elisabetta Pace, Maria Luisa Bondì, Gaetano Giammona, and Gennara Cavallaro. 2017. "Polyaspartamide-Based Nanoparticles Loaded with Fluticasone Propionate and the In Vitro Evaluation towards Cigarette Smoke Effects" Nanomaterials 7, no. 8: 222. https://doi.org/10.3390/nano7080222
APA StyleCraparo, E. F., Ferraro, M., Pace, E., Bondì, M. L., Giammona, G., & Cavallaro, G. (2017). Polyaspartamide-Based Nanoparticles Loaded with Fluticasone Propionate and the In Vitro Evaluation towards Cigarette Smoke Effects. Nanomaterials, 7(8), 222. https://doi.org/10.3390/nano7080222







