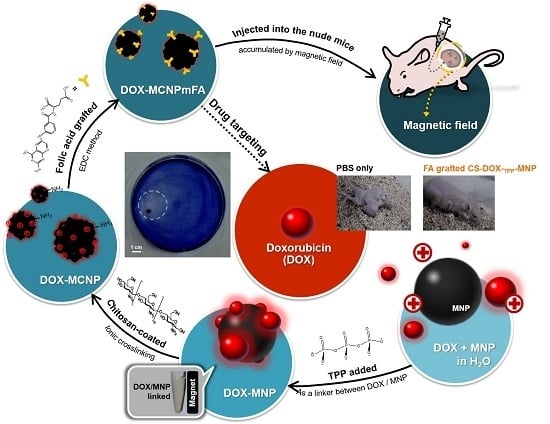
Release of Doxorubicin by a Folate-Grafted, Chitosan-Coated Magnetic Nanoparticle
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Synthesized Particles
2.2. Profile of DOX Release from DOX-TPP-MNP or CS-DOX-TPP-MNP
2.3. Effect of FA Molecules on Size Distribution of Synthesized Nanoparticles
2.4. In Vitro Micro-Chemotherapy and Cytotoxicity of Synthesized Nanoparticles
2.5. In Vivo Antitumor Effect
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Preparation of MNP, DOX-TPP-MNP, CS-DOX-TPP-MNP, or FA-Grafted CS-DOX-TPP-MNP
3.3. Characterizations
3.4. Encapsulation Efficiency and DOX Release of DOX-TPP-MNP, CS-DOX-TPP-MNP, or FA-Grafted CS-DOX-TPP-MNP
3.5. In Vitro Anti-Tumor Proliferation and Cytotoxicity of Synthesized Nanoparticles
3.6. Mouse Studies
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Braso-Maristany, F.; Filosto, S.; Catchpole, S.; Marlow, R.; Quist, J.; Francesch-Domenech, E.; Plumb, D.A.; Zakka, L.; Gazinska, P.; Liccardi, G.; et al. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat. Med. 2016, 22, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Cantelmo, A.R.; Conradi, L.C.; Brajic, A.; Goveia, J.; Kalucka, J.; Pircher, A.; Chaturvedi, P.; Hol, J.; Thienpont, B.; Teuwen, L.A.; et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 2016, 30, 968–985. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, A.; Scheinemann, K.; Zelcer, S.M.; Hukin, J.; Wilson, B.A.; Jabado, N.; Carret, A.S.; Lafay-Cousin, L.; Larouche, V.; Hawkins, C.E.; et al. Phase II weekly vinblastine for chemotherapy-naive children with progressive low-grade glioma: A canadian pediatric brain tumor consortium study. J. Clin. Oncol. 2016, 34, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Sellers, Z.P.; Ratajczak, M.Z. Induction of a tumor-metastasis-receptive microenvironment as an unwanted side effect after radio/chemotherapy and in vitro and in vivo assays to study this phenomenon. Methods Mol. Biol. 2016, 1516, 347–360. [Google Scholar] [PubMed]
- Zhang, M.Z.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.L.; Merlin, D. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.J.; Liao, Z.X.; Kao, S.H.; Zeng, Y.F.; Huang, K.Y.; Li, H.J.; Yang, C.L.; Deng, Y.F.; Huang, C.F.; Yang, S.C.; et al. Highly specific in vivo gene delivery for p53-mediated apoptosis and genetic photodynamic therapies of tumour. Nat. Commun. 2015, 6, 6456. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.J.; Kempson, I.M.; Peng, S.F.; Ke, B.H.; Chen, H.H.; Chen, P.F.; Hwu, Y. Environment acidity triggers release of recombinant adeno-associated virus serotype 2 from a tunable matrix. J. Control Release 2013, 170, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.J.; Zeng, Y.F.; Deng, Y.F.; Yang, P.C.; Liu, J.R.; Kempson, I.M. Switchable delivery of small interfering RNA using a negatively charged pH-responsive polyethylenimine-based polyelectrolyte complex. Chem. Commun. 2013, 49, 2670–2672. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.J.; Huang, K.Y.; Kempson, I.M.; Kao, S.H.; Liu, M.C.; Yang, S.C.; Liao, Z.X.; Yang, P.C. Remote control of light-triggered virotherapy. ACS Nano 2016, 10, 10339–10346. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Avti, P.K.; Pouliot, P.; Maafi, F.; Tardif, J.-C.; Rhéaume, É.; Lesage, F.; Kakkar, A. Fabricating Water Dispersible Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications through Ligand Exchange and Direct Conjugation. Nanomaterials 2016, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Tseng, S.J.; Kempson, I.M.; Yang, S.C.; Hong, T.M.; Yang, P.C. Extracellular delivery of modified oligonucleotide and superparamagnetic iron oxide nanoparticles from a degradable hydrogel triggered by tumor acidosis. Biomaterials 2013, 34, 4387–4393. [Google Scholar] [CrossRef] [PubMed]
- Al-Deen, F.M.N.; Xiang, S.D.; Ma, C.; Wilson, K.; Coppel, R.L.; Selomulya, C.; Plebanski, M. Magnetic Nanovectors for the Development of DNA Blood-Stage Malaria Vaccines. Nanomaterials 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Miest, T.S.; Cattaneo, R. New viruses for cancer therapy: Meeting clinical needs. Nat. Rev. Microbiol. 2014, 12, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Yousefpour, P.; Atyabi, F.; Vasheghani-Farahani, E.; Movahedi, A.A.; Dinarvand, R. Targeted delivery of doxorubicin-utilizing chitosan nanoparticles surface-functionalized with anti-Her2 trastuzumab. Int. J. Nanomed. 2011, 6, 1977–1990. [Google Scholar]
- Yan, T.; Zhang, H.; Huang, D.; Feng, S.; Fujita, M.; Gao, X.-D. Chitosan-functionalized graphene oxide as a potential immunoadjuvant. Nanomaterials 2017, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.X.; Ho, Y.C.; Chen, H.L.; Peng, S.F.; Hsiao, C.W.; Sung, H.W. Enhancement of efficiencies of the cellular uptake and gene silencing of chitosan/sirna complexes via the inclusion of a negatively charged poly(gamma-glutamic acid). Biomaterials 2010, 31, 8780–8788. [Google Scholar] [CrossRef] [PubMed]
- Mellati, A.; Kiamahalleh, M.V.; Madani, S.H.; Dai, S.; Bi, J.; Jin, B.; Zhang, H. Poly(N-isopropylacrylamide) hydrogel/chitosan scaffold hybrid for three-dimensional stem cell culture and cartilage tissue engineering. J. Biomed. Mater. Res. A 2016, 104, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Xu, R.; Darabi, M.A.; Zhong, W.; Luo, G.; Xing, M.M.; Wu, J. Fast and safe fabrication of a free-standing chitosan/alginate nanomembrane to promote stem cell delivery and wound healing. Int. J. Nanomed. 2016, 11, 2543–2555. [Google Scholar]
- Munteanu, B.S.; Dumitriu, R.P.; Profire, L.; Sacarescu, L.; Hitruc, G.E.; Stoleru, E.; Dobromir, M.; Matricala, A.L.; Vasile, C. Hybrid nanostructures containing sulfadiazine modified chitosan as antimicrobial drug carriers. Nanomaterials 2016, 6, 207. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.E.; Copp, J.A.; Karve, S.; Cummings, N.D.; Sukumar, R.; Li, C.; Napier, M.E.; Chen, R.C.; Cox, A.D.; Wang, A.Z. Folate-targeted polymeric nanoparticle formulation of docetaxel is an effective molecularly targeted radiosensitizer with efficacy dependent on the timing of radiotherapy. ACS Nano 2011, 5, 8990–8998. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Tehrani, Z.M.; Moghanaki, A.A. Folate-conjugated pH-responsive nanocarrier designed for active tumor targeting and controlled release of gemcitabine. Pharm. Res. 2016, 33, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Y.; Fang, C.Y.; Chang, C.C.; Chen, C.S.; Chen, Y.Y.; Chang, H.C. Receptor-mediated cellular uptake of folate-conjugated fluorescent nanodiamonds: A combined ensemble and single-particle study. Small 2009, 5, 2716–2721. [Google Scholar] [CrossRef] [PubMed]
- Sonvico, F.; Mornet, S.; Vasseur, S.; Dubernet, C.; Jaillard, D.; Degrouard, J.; Hoebeke, J.; Duguet, E.; Colombo, P.; Couvreur, P. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconjug. Chem. 2005, 16, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.P.; Patel, R.R.; Patel, J.K. Chitosan mediated targeted drug delivery system: A review. J. Pharm. Pharm. Sci. 2010, 13, 536–557. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Zhao, J.; Tang, B.; Chen, Y.; Hu, Y.; He, Z.; Wang, Y. Carboxymethyl chitosan-folic acid-conjugated Fe3O4@SiO2 as a safe and targeting antitumor nanovehicle in vitro. Nanoscale Res. Lett. 2014, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Sung, H.W.; Shyu, S.S.; Su, C.C.; Peng, C.K. Synthesis and characterization of biodegradable tpp/genipin co-crosslinked chitosan gel beads. Polymer 2003, 44, 6521–6530. [Google Scholar] [CrossRef]
- Mathew, M.E.; Mohan, J.C.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Folate conjugated carboxymethyl chitosan-manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohydr. Polym. 2010, 80, 442–448. [Google Scholar] [CrossRef]







| MNP | DOX-TPP-MNP | CS-DOX-TPP-MNP | FA-Grafted CS-DOX-TPP-MNP | |
|---|---|---|---|---|
| Zeta (mV) | +37.4 ±1.74 | +21.30 ± 2.69 | +31.93 ± 0.96 | −24.9 ± 0.66 |
| Size (nm) | 133.0 ± 8.9 | 180.2 ± 0.92 | 241.6 ± 1.82 | 246.8 ± 5.52 (D.I. water) 281 ± 10.31 (Culture medium) |
| Ms (emu·g−1) | 68.7 | 67.8 | 62.0 | 57.5 |
| Mr (emu·g−1) | 1.23 | 1.54 | 1.74 | 1.38 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-L.; Chen, J.-P.; Wei, K.-C.; Chen, J.-Y.; Huang, C.-W.; Liao, Z.-X. Release of Doxorubicin by a Folate-Grafted, Chitosan-Coated Magnetic Nanoparticle. Nanomaterials 2017, 7, 85. https://doi.org/10.3390/nano7040085
Yang C-L, Chen J-P, Wei K-C, Chen J-Y, Huang C-W, Liao Z-X. Release of Doxorubicin by a Folate-Grafted, Chitosan-Coated Magnetic Nanoparticle. Nanomaterials. 2017; 7(4):85. https://doi.org/10.3390/nano7040085
Chicago/Turabian StyleYang, Chung-Lin, Jyh-Ping Chen, Kuo-Chen Wei, Ju-Yu Chen, Chia-Wen Huang, and Zi-Xian Liao. 2017. "Release of Doxorubicin by a Folate-Grafted, Chitosan-Coated Magnetic Nanoparticle" Nanomaterials 7, no. 4: 85. https://doi.org/10.3390/nano7040085
APA StyleYang, C.-L., Chen, J.-P., Wei, K.-C., Chen, J.-Y., Huang, C.-W., & Liao, Z.-X. (2017). Release of Doxorubicin by a Folate-Grafted, Chitosan-Coated Magnetic Nanoparticle. Nanomaterials, 7(4), 85. https://doi.org/10.3390/nano7040085