Abstract
To overcome the limitations of template-dependent and anion-assisted methods, this work presents a solvent-controlled strategy for the one-step solvothermal synthesis of octahedral CeO2. Using only Ce(NO3)3·6H2O in methanol/water (MeOH/H2O) mixtures without the addition of auxiliary templates or surfactants, phase-pure cubic CeO2 was obtained. Well-defined octahedra were exclusively formed in a 15 mL MeOH/5 mL H2O system at 180 °C for 12 h, whereas other alcohols (including ethanol (EtOH), n-propanol (n-PrOH), and iso-propanol (i-PrOH)) yielded irregular aggregates. Time-dependent evolution revealed continuous crystallinity optimization between 3 and 24 h, beyond which surface dissolution occurred. The solvothermal mother liquor could be recycled four times without compromising phase purity or octahedral morphology, as confirmed by XRD and SEM. This work provides a green and practical route for morphology-controlled oxide synthesis while significantly reducing solvent consumption.
1. Introduction
Ceria (CeO2) with a cubic fluorite-type structure is the most stable oxide of the rare earth element cerium (Ce), as well as one of the most reactive rare earth metal oxides. CeO2 has been extensively researched due to its wide applications as promoters in the three-way catalysts (TWCs) to eliminate volatile organic compounds (VOCs) [1], as oxygen sensors or solid electrolytes in the solid oxide fuel cells (SOFCs) based on their oxygen storage capacity (OSC) [2], as adsorbents for bonding In3+, Ga3+ and As5+ [3] ions or anionic organic dyes [4], and as barriers against ultraviolet radiation [5].
CeO2 with a specific size and morphology is preferred due to its remarkable chemical and physical properties. Consequently, increasing efforts have been focused on controlling the morphological characteristics of CeO2 micro/nanocrystals, including fine sphere, rod, wire, tube, dendrite, spindle and 3D-flower shapes. Generally, CeO2 with specific morphologies can be synthesized using “hard” or “soft” templates. The utilization of “hard” templates primarily involves metal oxide (Al2O3 [6] and Cu2O [7]), mesoporous silica (MCM-48 [8], MCM-41 [9], KIT-6 [10] and SBA-15 [11]), carbon/polymer-based templates (mesoporous carbon [12] and active carbon [13], carbon nanotubes [14] and chitosan film [15]), while the “soft” templates predominantly consist of surfactants (cetyltrimethylammonium bromide, CTAB [16]), biomolecules (amino acids [17], powdered cellulose [18], egg-shell membrane [19]) and polymers (polymethylmethacrylate, PMMA [20]; polyvinyl alcohol/polyvinylpyrrolidone, PVA/PVP [21]; polystyrene spheres [22]). In this way, tedious procedures were required to remove these. In this manner, laborious procedures were necessary to eliminate these “hard” or “soft” templates. This has spurred the development of alternative green synthesis routes, such as water-based systems that enable morphological control without the use of surfactants [23].
Recently, there has been a growing interest in the template- and surfactant-free synthesis of CeO2 [24,25,26,27,28,29]. However, it is essential to control the growth of CeO2 crystals by adding specific anions that act as capping reagents, such as OH− from NaOH [30] or NH4OH [31], NO3− from NaNO3 [32], S2O82− from (NH4)2S2O8 [33], or PO43− from Na3PO4 [34]. Specifically, urea has been commonly used in the hydrothermal synthesis of CeO2 [35,36,37]. While effective, such anion-assisted strategies introduce practical and sustainability concerns. The post-synthesis removal of these ions adds operational complexity, increases energy and time costs, and raises the risk of residual anions adversely affecting the catalytic or functional properties of the final CeO2 product. Furthermore, the consumption of auxiliary chemicals and the generation of associated wastewater contradict the principles of green chemistry and scalable, sustainable manufacturing.
Hence, we had developed a cost-effective and environmentally friendly one-step solvothermal method for the synthesis of unique octahedral CeO2 that eliminates the need for any external templates, surfactants, or controlling anions. The entire reaction system consisted solely of a cerium source (Ce(NO3)3∙6H2O) and a mixed solvent (MeOH/H2O). This approach, which uses only the binary solvent without additional shape-directing agents, not only simplifies the process but also inherently avoids the contamination and purification challenges associated with auxiliary agents. Moreover, we integrate a closed-loop solvent recycling strategy, significantly reducing waste generation and enhancing the overall sustainability profile of the synthesis.
2. Materials and Methods
2.1. Materials
Ce(NO3)3∙6H2O was obtained from Aladdin Co. Ltd. (Shanghai, China). Methanol (MeOH), ethanol (EtOH), n-propanol (n-PrOH) and iso-propanol (i-PrOH) were supplied by Chengdu Kelong Chemical Co., Ltd. (Chengdu, China). All reagents were utilized without further purification. Purified water (H2O), obtained from an HRO-402 ultrapure water system (Chengdu Zhonghan Water Treatment Equipment Co., Ltd., Chengdu, China), was employed in all experiments conducted throughout the study.
2.2. Synthesis of CeO2 via One-Step Solvothermal Method
CeO2 was synthesized based on a straightforward solvothermal process without any post-treatment calcination. In brief, 4 mmol of Ce(NO3)3·6H2O was dissolved into a 20 mL binary solvent mixture composed of an organic solvent (MeOH, EtOH, n-PrOH, or i-PrOH) and H2O. The mixture was then transferred into a sealed 50 mL Teflon-lined stainless-steel autoclave and heated at 180 °C for a specified duration. After the reaction, the precipitates were collected, washed repeatedly with H2O and EtOH, and finally, a series of CeO2 powders were obtained by following drying in air at 80 °C for 24 h.
To systematically investigate the effects of solvent composition, a series of experiments was designed as summarized in Table 1. This included: (1) syntheses using pure organic solvents for comparison; (2) syntheses using binary mixtures with a fixed organic solvent/H2O volume ratio of 15:5 mL; (3) a study on the MeOH/H2O volume ratio with a constant total volume; (4) a study on the total volume with a constant H2O amount; and (5) the recycling experiments of the mother liquor.

Table 1.
Summary of solvent compositions and reaction conditions for the synthesis of CeO2.
To assess the sustainability of the process, the solvothermal mother liquor was recycled over multiple cycles. After each synthesis conducted under standard conditions (4 mmol Ce(NO3)3·6H2O in 15 mL MeOH and 5 mL H2O, 180 °C for 12 h), the mother liquor was collected and allowed to stand undisturbed for 12 h. The supernatant was then carefully decanted and reused directly as the solvent for the subsequent cycle, without any purification or supplementation. In each new cycle, 4 mmol of fresh Ce(NO3)3·6H2O was dissolved into the recycled supernatant, and the mixture was subjected to the same solvothermal conditions. This recycling procedure was repeated successively for up to eight cycles. The 12 h settling step was implemented to minimize the carryover of residual nanocrystalline nuclei or particulate matter, which could otherwise act as unintended seeds and influence growth kinetics in subsequent runs. The phase and morphology of the CeO2 products from each cycle were characterized by XRD and SEM to evaluate the impact of recycling.
2.3. Characterization
The crystallographic phases of the samples were characterized by X-ray diffraction (XRD) on a DX-2700 diffractometer (Dandong Haoyuan Instrument Co., Ltd., Dandong, China) using Cu Kα radiation. The patterns were recorded in the 2θ range of 20–80° with a step size of 0.1°, operating at 30 kV and 25 mA. The morphology of the samples was examined by scanning electron microscopy (SEM) on a SEM5000 instrument (CIQTEK Co., Ltd., Hefei, China). Prior to observation, the samples were sputter-coated with a thin layer of platinum to enhance conductivity. Images were acquired in SE mode at an accelerating voltage of 5 kV.
3. Results and Discussion
3.1. Effect of Solvent Type and Binary Solvent System on Phase and Morphology
The XRD patterns in Figure 1 confirm the formation of pure-phase cubic CeO2 (JCPDS no. 43-1002) under all tested solvent conditions, whether using pure alcohols (20 mL of MeOH, EtOH, n-PrOH, or i-PrOH) or binary alcohol/H2O mixtures (15 mL alcohol + 5 mL H2O). The primary (111), (200), (220), and (311) diffraction peaks are prominent in every pattern (Figure 1a,b). Notably, the introduction of H2O significantly enhanced the diffraction intensity compared to the pure alcohol systems, indicating improved crystallinity (Table 2). Among the binary systems, MeOH/H2O yielded the highest peak intensity, followed by i-PrOH/H2O, whereas EtOH/H2O and n-PrOH/H2O produced lower intensities (Figure 1b). Although a lower alcohol boiling point (MeOH: 64.7 °C < EtOH: 78.3 °C < i-PrOH: 82.6 °C < n-PrOH: 97.1 °C) typically favors higher autogenous pressure and crystallization, the observed intensity trend does not follow this sequence. This suggests that solvent polarity and specific synergies with H2O collectively govern crystallization kinetics and final crystallinity.
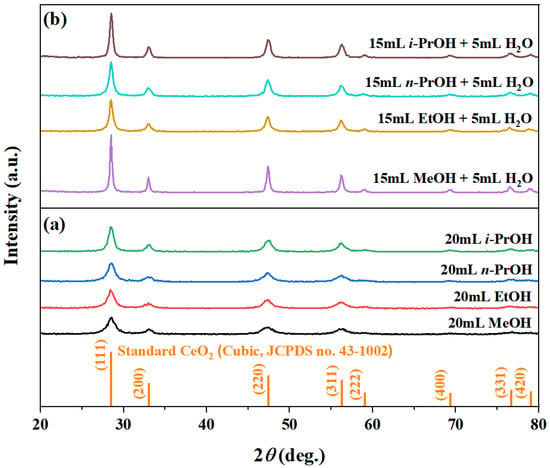
Figure 1.
XRD patterns of the samples synthesized solvothermally at 180 °C for 12 h using (a) pure alcohols (20 mL) and (b) binary aqueous-mixed solutions (15 mL alcohol + 5 mL H2O). Alcohols: MeOH, EtOH, n-PrOH, and i-PrOH.

Table 2.
Structural and morphological parameters of CeO2 synthesized in different solvent systems.
In binary solvent systems, additional well-defined peaks corresponding to the (222), (400), (331), and (420) planes became visible (Figure 1b), implying that H2O not only promotes complete crystallization but may also modulate the relative growth rates of different crystal facets. The absence of impurity peaks underscores the efficacy of this one-step, solvent-controlled strategy. The crystallite sizes, calculated from the (111) peak broadening, increased from ~6.7–9.0 nm in pure alcohols to ~9.5–12.8 nm in the binary system. The phase purity and controlled crystallinity are crucial for functional performance, as demonstrated in analogous material systems where composition and morphology dictate properties [38].
Figure 2 presents the morphological evolution of CeO2 synthesized under different solvent systems. In pure alcohols (20 mL), all samples formed irregular aggregates with poor definition (Figure 2a). Specifically, pure MeOH yielded incomplete large spheroids mixed with fine particles, while EtOH, n-PrOH, and i-PrOH produced featureless aggregates, indicating ineffective morphological control (Table 2). Replacing 5 mL of alcohol with H2O (15 mL alcohol + 5 mL H2O) induced a clear morphological transition (Figure 2b). Although EtOH/H2O, n-PrOH/H2O, and i-PrOH/H2O still gave irregular aggregates, the MeOH/H2O system uniquely generated well-defined, uniform octahedra with sharp edges, consistent with the highest crystallinity and largest crystallite size (11.8 nm) observed in XRD (Table 2).

Figure 2.
SEM images of the CeO2 samples synthesized solvothermally at 180 °C for 12 h using (a) pure alcohols (20 mL) and (b) binary aqueous-mixed solutions (15 mL alcohol + 5 mL H2O). Alcohols: MeOH, EtOH, n-PrOH, and i-PrOH.
Combined with the XRD results (Figure 1), H2O not only enhances crystallinity but also critically directs morphological evolution. The exclusive formation of octahedra in the MeOH/H2O system highlights the solvent-specific promotion of oriented growth, resulting in a distinct faceted morphology. This aligns with established principles where crystal morphology governs surface properties, as seen in analogous material systems [39]. The contrast between well-defined octahedra in MeOH/H2O and irregular aggregates in other systems underscores the decisive role of solvent selection in morphology-controlled oxide synthesis.
3.2. Effect of MeOH/H2O Ratio on Phase and Morphology
To elucidate how solvent composition governs CeO2 formation, we systematically varied the MeOH/H2O ratio following two experimental schemes. One maintained a fixed total solvent volume of 20 mL while varying the MeOH/H2O ratios at 5:15, 10:10, and 15:5 mL. Another kept the H2O volume constant at 5 mL and progressively increased the MeOH volume to 20 mL and 25 mL, corresponding to total volumes of 25 mL and 30 mL, respectively. All XRD patterns (Figure 3) confirm phase-pure cubic CeO2 (JCPDS no. 43-1002) without impurities. While variations in the MeOH/H2O ratio at a fixed 20 mL total volume caused only minor changes in peak intensity and width, increasing the total volume to 25 and 30 mL significantly reduced peak intensity and broadened the reflections (Table 3). This suggests that a higher solvent filling lowers the autogenous pressure and dilutes the precursor concentration, thereby impairing crystallinity and reducing crystallite size.
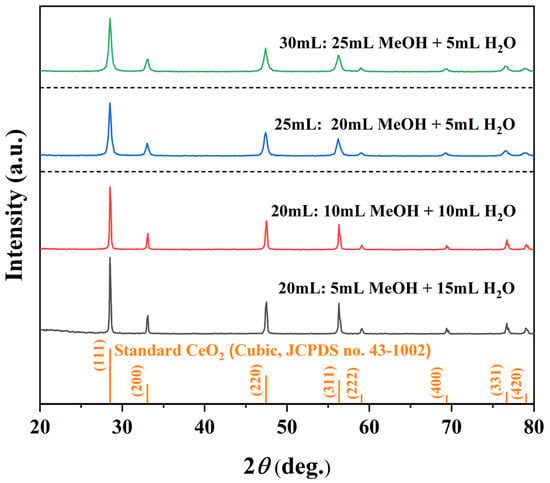
Figure 3.
XRD patterns of samples synthesized solvothermally at 180 °C for 12 h under different MeOH/H2O solvent compositions: 5 mL MeOH + 15 mL H2O, 10 mL MeOH + 10 mL H2O, 20 mL MeOH + 5 mL H2O, and 25 mL MeOH + 5 mL H2O.

Table 3.
Effect of MeOH/H2O volume ratio on the structural parameters of CeO2.
Corresponding SEM images (Figure 4) reveal the morphological sensitivity to solvent composition. At a fixed 20 mL system, with higher H2O content (5:15 and 10:10) produced aggregated nano-octahedra, whereas the 15:5 ratio yielded well-separated, faceted micro-octahedra. When the total volume was increased to 25 mL and 30 mL (with constant 5 mL H2O), the products exhibited mixed octahedral/spherical shapes with progressively degraded edges. These observations demonstrate that phase purity is maintained across all conditions, the MeOH/H2O ratio and total volume critically control particle dispersion, edge definition, and overall morphological uniformity.

Figure 4.
SEM images of CeO2 samples synthesized solvothermally at 180 °C for 12 h under different MeOH/H2O solvent compositions: 5 mL MeOH + 15 mL H2O, 10 mL MeOH + 10 mL H2O, 20 mL MeOH + 5 mL H2O, and 25 mL MeOH + 5 mL H2O.
3.3. Effect of Solvothermal Duration on Crystallinity and Morphology
The crystallization pathway of octahedral CeO2 was investigated by varying the solvothermal time (1–36 h) while maintaining the optimal solvent composition (15 mL MeOH + 5 mL H2O) and temperature (180 °C). XRD patterns in Figure 5 display that all samples except the 1 h product exhibit the characteristic peaks of phase-pure cubic CeO2 (JCPDS no. 43-1002). The 1 h sample yielded insufficient material for reliable XRD, indicating early-stage nucleation. From 3 to 12 h, the diffraction peak intensified and sharpened, reflecting progressive crystallinity improvement and grain growth, with the highest crystallinity achieved at 12 h (Table 4). Peak profiles stabilized at 24 h, suggesting completion of primary crystallization. After 36 h, peaks broadening and a slight decrease in the (111) intensity indicate reduced crystallite size and the onset of surface dissolution or Ostwald ripening.
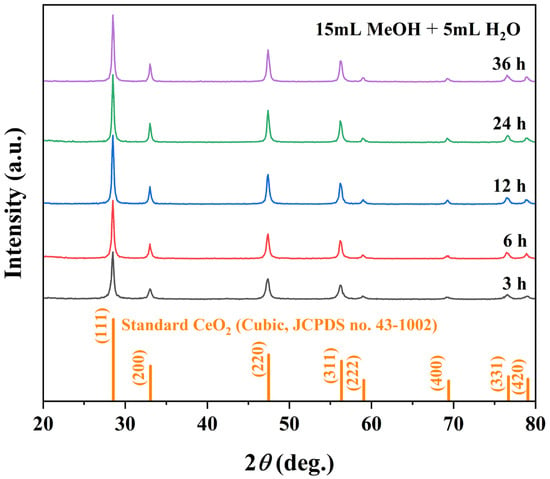
Figure 5.
Crystallographic evolution of samples with solvothermal duration: XRD patterns of samples synthesized at 180 °C using a binary solvent of 15 mL MeOH + 5 mL H2O at different reaction times (3, 6, 12, 24, and 36 h).

Table 4.
Evolution of structural parameters and morphology of CeO2 under optimal solvent composition (15 mL MeOH/5 mL H2O) with varying reaction time and upon mother liquor recycling.
The corresponding morphological evolution, as revealed by SEM in Figure 6, further supports this crystallization pathway. At 1 h, only undefined plate-like structures were observed. By 3 h, a mixture of nano- and micro-sized octahedra appeared. At 12 h, well-faceted micro-octahedra predominated, consistent with the optimal crystallinity seen in XRD. Crystal size continued to increase until 24 h. After 36 h, surface pitting and cavities became evident, confirming partial dissolution and aligning with the XRD peak broadening. Collectively, the results delineate a clear time-dependent trajectory: nucleation and crystallization (1–12 h), crystal growth and maturation (12–24 h), and surface restructuring or partial dissolution under extended conditions (>24 h). This understanding provides crucial guidance for selecting the optimal reaction window to obtain CeO2 octahedra with high crystallinity and well-defined morphology.
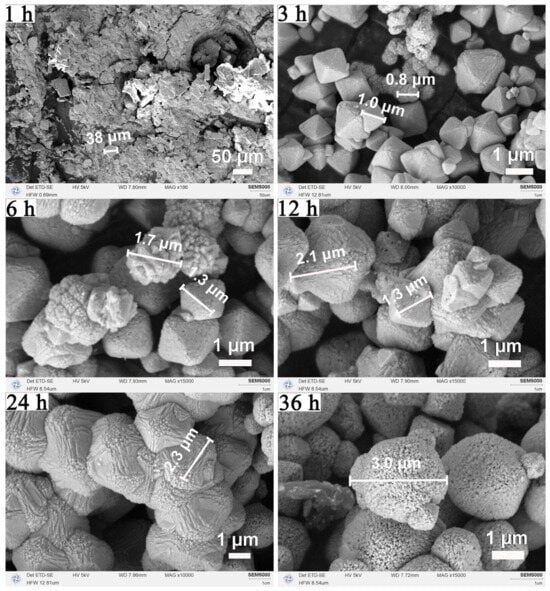
Figure 6.
Morphological evolution of CeO2 with solvothermal duration: SEM images of CeO2 samples synthesized at 180 °C using a binary solvent of 15 mL MeOH and 5 mL H2O at different reaction times (1, 3, 6, 12, 24, and 36 h).
3.4. Recycling and Reuse of Solvothermal Mother Liquor
To align with green chemistry principles and enhance the sustainability of the synthesis [40], the solvothermal mother liquor was recycled over multiple cycles under fixed conditions (180 °C, 12 h, initial solvent: 15 mL MeOH + 5 mL H2O). Figure 7 displays the XRD patterns of CeO2 synthesized over successive recycling rounds. During the first four cycles, all diffraction peaks correspond exclusively to the cubic fluorite structure of CeO2, with no detectable secondary phases. The consistency in peak position, intensity, and profile indicates excellent retention of crystallinity and phase purity throughout these initial reuses, which is corroborated by the stable crystallite size (12.4–15.7 nm) and high relative crystallinity (65.3–65.8%) recorded in Table 4. However, upon the fifth cycle, two weak impurity peaks emerged at 2θ = 51.3° and 70.4°. By the eighth cycle, additional impurity peaks became evident (marked by red arrows in Figure 7), accompanied by a discernible decrease in the intensity of the characteristic CeO2 reflections. These emerging peaks are attributed to orthorhombic CeCO3OH (JCPDS no. 41-0013) and hexagonal CeCO3OH (JCPDS no. 52-0352). The appearance of these impurities suggests the gradual accumulation of dissolved species or reaction byproducts in the recycled mother liquor, which ultimately interfere with the crystallization process and promote the formation of secondary phases, consistent with the observed loss of well-defined octahedral morphology and the onset of irregular aggregates noted in Table 4 for cycles 5–8.
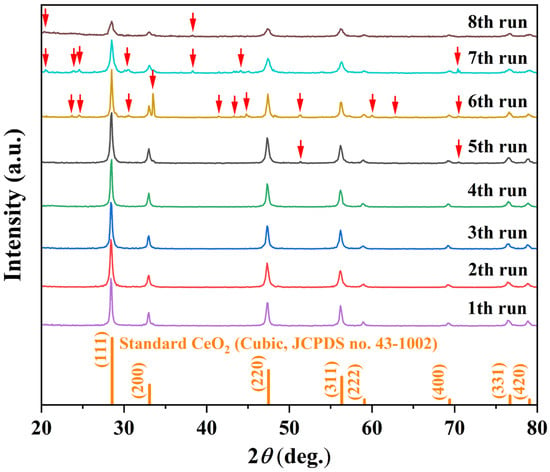
Figure 7.
Crystallographic evolution of the samples synthesized via recycled solvothermal mother liquor: XRD patterns of samples obtained after eight successive recycling cycles (180 °C, 12 h per cycle).
SEM images (Figure 8) reveal that uniform micron-scale octahedra are maintained through four cycles. From the fifth cycle onward, prismatic and nanoparticulate impurities appear, and by the eighth cycle, the product consists mainly of angular aggregates and near-spherical particles, consistent with the XRD detection of CeCO3OH. Together, XRD and SEM confirm that the mother liquor can be effectively recycled four times without degrading the phase purity or the octahedral morphology of CeO2. Beyond this point, accumulated byproducts lead to impurity formation and loss of morphological control. This closed-loop recycling strategy highlights the practical sustainability of the proposed synthesis route.
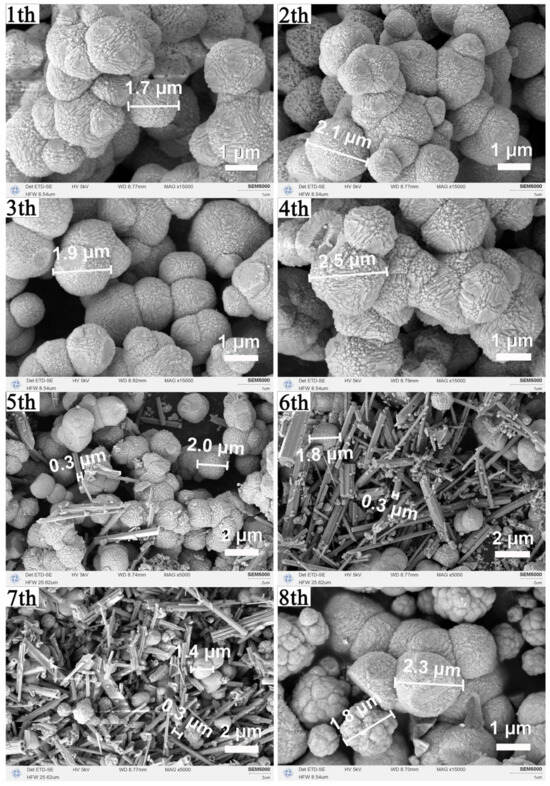
Figure 8.
Morphological evolution of the samples synthesized via recycled solvothermal mother liquor: SEM images of samples obtained after eight successive recycling cycles (180 °C, 12 h per cycle).
3.5. Mechanism Discussion
3.5.1. Formation Mechanism of CeO2
Based on the experimental results, the formation and evolution of CeO2 in the solvothermal reaction system can be described through the following mechanisms. In both pure alcohol and alcohol/H2O binary systems, CeO2 synthesis proceeds through a coordinated sequence of oxidation, catalysis, hydrolysis, and dissolution-recrystallization. Initially, Ce3+ ions from Ce(NO3)3·6H2O interact with monohydric alcohols (CnH2n+1OH) in the presence of dissolved molecular oxygen, initiating the oxidation and nucleation of CeO2. Continued oxygen participation facilitates the formation of intermediate cerium species, ultimately leading to CeO2 crystallization (Equations (1)–(3)). The oxygen originates from both trapped air in the reactor and oxygen dissolved in the solvent.
Once formed, CeO2 nanocrystals serve as catalysts for the subsequent oxidation of the alcohol solvent. The CnH2n+1OH can be oxidized to carboxylic acids (H2n−1CnOOH) (Equation (4)). H2O plays a crucial mediating role by promoting the hydrolysis and solvation of Ce3+ ions, thereby influencing the reaction environment (Equation (5)). Concurrently, nitrate ions (NO3−) from the cerium precursor (Ce(NO3)3·6H2O) serve as an intrinsic oxidant under solvothermal conditions, contributing to the oxidation of Ce3+ and the release of gaseous products (Equation (6)) [41].
The organic acids (H2n+1CnOOH) generated in situ, such as formic acid or acetic acid, participate in a reversible dissolution reaction with CeO2 (Equation (7)). This acid-mediated dissolution-recrystallization process is central to the morphological evolution of CeO2, promoting Ostwald ripening and enabling the transformation from irregular aggregates into well-defined octahedra in the MeOH/H2O system. This mechanism aligns with the observed time-dependent enhancement in crystallinity and particle uniformity between 3 and 24 h, as well as the surface restructuring observed beyond 24 h in Figure 6.
The dual role of MeOH in this system warrants further discussion, particularly in the context of morphological control. It is well-recognized that alcohol molecules, with their hydroxyl groups (−OH), can adsorb onto oxide surfaces and influence crystal growth kinetics. In our case, MeOH (and its in situ oxidation products like formaldehyde or formic acid) can indeed act as in situ-generated surface modifiers. They may preferentially adsorb onto specific crystallographic facets of CeO2, thereby modulating relative growth rates and contributing to the final octahedral morphology.
This mechanism, however, is fundamentally distinct from conventional templating or surfactant-assisted syntheses. In those methods, externally added agents (e.g., CTAB, polymers, or anionic capping agents) are introduced a priori with the primary intent of directing morphology, and their post-synthesis removal is often mandatory. In contrast, our strategy employs only the solvent itself as the reaction medium and the source of these modulating species. No auxiliary templates, surfactants, or anions are added. The surface-modifying species are generated in situ from the solvent during the reaction, are integral to the solvent matrix, and do not require a separate removal step. Therefore, while acknowledging the active role of MeOH-derived species in morphology control, we emphasize that our approach achieves shape control without the introduction of any foreign template or surfactant additives, relying solely on the intrinsic properties and reactivity of the binary solvent system.
3.5.2. Mechanism of Impurity Formation During Solvent Recycling
During the repeated recycling of the solvothermal mother liquor, the accumulation of carbonaceous species leads to the gradual formation of crystalline impurities, primarily cerium carbonate hydroxide (CeCO3OH), including orthorhombic and hexagonal CeCO3OH. With increasing cycles (≥5), formate or other organic anions accumulated in the mother liquor undergo decomposition or conversion to carbonate ions (Equation (8)). These carbonate ions then react with residual Ce3+ and hydroxide ions in the recycled solvent to precipitate as CeCO3OH (Equations (9) and (10)) [42]. Although CeCO3OH may partially decompose under prolonged heating, under the given recycling conditions, it persists as an impurity phase. The presence of these impurities coincides with the deterioration of octahedral morphology and the emergence of irregular aggregates, confirming that the solvent can be effectively recycled only up to four cycles without compromising product purity or morphology.
4. Conclusions
A facile one-step solvothermal strategy has been developed to synthesize octahedral CeO2 without the addition of external templates, surfactants, or post-calcination. Employing only Ce(NO3)3·6H2O in a binary MeOH/H2O solvent system under optimized conditions (15 mL MeOH + 5 mL H2O, 180 °C, 3–24 h) yields pure-phase cubic CeO2 with well-defined octahedral morphology. Solvent parameters critically govern morphological evolution: while variations in the MeOH/H2O ratio (5–25 mL) and total solvent volume (20–30 mL) preserve phase purity and crystallinity, they strongly influence particle aggregation and edge definition. Prolonged reaction beyond 24 h induces partial surface dissolution while maintaining phase integrity. The solvothermal mother liquor can be recycled four times without degrading phase purity or octahedral morphology, demonstrating a closed-loop sustainable process. This solvent-only approach, which requires no auxiliary additives, eliminates hazardous byproducts and complex purification, offering a green and practical route for the morphology-controlled CeO2 synthesis with minimal waste.
Author Contributions
Conceptualization, Y.X. and Y.H.; validation, N.Z., H.W., Y.Z., Z.L. and X.C.; investigation, Y.X., N.Z., H.W., Y.Z., Z.L. and X.C.; resources, Y.X., Y.H. and Z.D.; data curation, Y.X., N.Z., H.W., Y.Z., Z.L. and X.C.; writing—original draft, Y.X.; writing—review and editing, Y.H. and Z.D.; supervision, Y.H. and Z.D.; project administration, Y.X., Y.H. and Z.D.; funding acquisition, Y.X., Y.H. and Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Innovative Experimental Project of Higher Education Institutions in Sichuan Province, China; College Student Innovation and Entrepreneurship Training Program, China (S202510649127, 202510649018). The APC was funded by Sichuan Province Science and Technology Plan Project, China (25JBGS0045).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bai, Y.; Yang, X.; Chen, J.; Shen, B. The removal of toluene by thermoscatalytic oxidation using CeO2-based catalysts: A review. Chemosphere 2024, 351, 141253. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Fan, L.; Akbar, M.; Gao, J.; Wang, B.; Xia, C.; Singh, M.; Chiu, T.W. Superionic conduction of self-assembled heterostructural LSCrF-CeO2 electrolyte for solid oxide fuel cell at 375–550 °C. Appl. Surf. Sci. 2024, 645, 158832. [Google Scholar] [CrossRef]
- Bi, X.; Westerhoff, P. Adsorption of III/V ions (In (III), Ga (III) and As (V)) onto SiO2, CeO2 and Al2O3 nanoparticles used in the semiconductor industry. Environ. Sci. Nano 2016, 3, 1014–1026. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; Rabea, M.F.; El-Sheikh, M.Y.; Okba, E.A. In situ polymerization of acrylamide on magnetic SnFe2O4/CeO2 nanocomposite: A novel adsorbent for removing various dyes. J. Mol. Struct. 2024, 1312, 138566. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.H.; Meng, X.Z.; Yan, H.J.; Hu, H.S.; Wu, L.K.; Cao, F.H. Active/passive protection and anti-UV waterborne epoxy coatings based on low defect functionalized PDA-GO-CeO2 material for excellent corrosion control. Chem. Eng. J. 2024, 479, 147859. [Google Scholar] [CrossRef]
- Wu, G.; Xie, T.; Yuan, X.; Cheng, B.; Zhang, L. An improved sol-gel template synthetic route to large-scale CeO2 nanowires. Mater. Res. Bull. 2004, 39, 1023–1028. [Google Scholar] [CrossRef]
- Fang, S.; Xin, Y.; Ge, L.; Han, C.; Qiu, P.; Wu, L. Facile synthesis of CeO2 hollow structures with controllable morphology by template-engaged etching of Cu2O and their visible light photocatalytic performance. Appl. Catal. B Environ. 2015, 179, 458–467. [Google Scholar] [CrossRef]
- Ying, F.; Wang, S.; Au, C.T.; Lai, S.Y. Highly active and stable mesoporous Au/CeO2 catalysts prepared from MCM-48 hard-template. Micropor. Mesopor. Mater. 2011, 142, 308–315. [Google Scholar] [CrossRef]
- Song, X.; Qu, P.; Jiang, N.; Yang, H.; Qiu, G. Synthesis and characterization of MCM-41 materials assembled with CeO2 nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 193–196. [Google Scholar] [CrossRef]
- Djinović, P.; Batista, J.; Pintar, A. WGS reaction over nanostructured CuO-CeO2 catalysts prepared by hard template method: Characterization, activity and deactivation. Catal. Today 2009, 147, S191–S197. [Google Scholar] [CrossRef]
- Lolli, A.; Amadori, R.; Lucarelli, C.; Cutrufello, M.G.; Rombi, E.; Cavani, F.; Albonetti, S. Hard-template preparation of Au/CeO2 mesostructured catalysts and their activity for the selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Micropor. Mesopor. Mater. 2016, 226, 466–475. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, X.; Li, W. Nanocast Ordered Mesoporous CeO2 as Support for Highly Active Gold Catalyst in CO Oxidation. Chin. J. Catal. 2009, 30, 1085–1090. [Google Scholar] [CrossRef]
- Cheng, T.; Fang, Z.; Hu, Q.; Han, K.; Zhang, Y. Preparation of CeO2 nanoparticles by using mesoporous active carbon as template. Asian J. Chem. 2007, 19, 2225–2228. [Google Scholar]
- Yang, Z.; Shen, Y.; Li, L.; Li, Z.; Wang, X.; Lu, X. In Situ Template-Synthesis of Hollow CeO2 Nanobeads in scCO2 with Improved Catalytic Activity Towards CO Oxidation. J. Nanosci. Nanotechnol. 2018, 18, 2068. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.; Narayan, A.; Bramhecha, I.; Sheikh, J. Development of multifunctional linen fabric using chitosan film as a template for immobilization of in-situ generated CeO2 nanoparticles. Int. J. Biol. Macromol. 2019, 121, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hu, Y.H.; Huang, J.G.; Liu, Z.G.; Wang, M.T. Study on the synthesis of CeO2 particles with spherical morphology by the control of CTAB. Chin. Rare Earths 2016, 37, 110–116. [Google Scholar] [CrossRef]
- Mitchell, S.L.; Guzman, J. Synthesis and characterization of nanocrystalline and mesostructured CeO2: Influence of the amino acid template. Mater. Chem. Phys. 2009, 114, 462–466. [Google Scholar] [CrossRef]
- Shishmakov, A.B.; Mikushina, Y.V.; Koryakova, O.V. Synthesis of CeO2 and CeO2/C Using Powdered Cellulose and Powdered Cellulose-Sucrose as a Template. Russ. J. Inorg. Chem. 2023, 68, 795–803. [Google Scholar] [CrossRef]
- Ruhaimi, A.H.; Aziz, M.A.A. Spherical CeO2 nanoparticles prepared using an egg-shell membrane as a bio-template for high CO2 adsorption. Chem. Phys. Lett. 2021, 779, 138842. [Google Scholar] [CrossRef]
- Schneider, J.J.; Naumann, M.; Schäfer, C.; Brandner, A.; Hofmann, H.J.; Claus, P. Template-assisted formation of microsized nanocrystalline CeO2 tubes and their catalytic performance in the carboxylation of methanol. Beilstein J. Nanotechnol. 2011, 2, 776–784. [Google Scholar] [CrossRef]
- Junais, P.M.; Athika, M.; Govindaraj, G.; Elumalai, P. Supercapattery performances of nanostructured cerium oxide synthesized using polymer soft-template. J. Energy Storage 2020, 28, 101241. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, D. Preparation and performance of CeO2 hollow spheres and nanoparticles. J. Rare Earth 2016, 34, 295–299. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Chen, X.; Liu, S.; Duan, C.; Xi, H. Water-based synthesis of nanoscale hierarchical metal-organic frameworks:boosting adsorption and catalytic performance. Nano Mater. Sci. 2023, 5, 361–368. [Google Scholar] [CrossRef]
- Xu, Y.; Li, R. Template-free synthesis of mesoporous CeO2 powders by integrating bottom-up and top-down routes for acid orange 7 adsorption. Rsc Adv. 2015, 5, 44828–44834. [Google Scholar] [CrossRef]
- Guan, Y.; Hensen, E.J.M.; Liu, Y.; Zhang, H.; Feng, Z.; Li, C. Template-free synthesis of sphere, rod and prism morphologies of CeO2 oxidation catalysts. Catal. Lett. 2010, 137, 28–34. [Google Scholar] [CrossRef]
- Yu, X.F.; Liu, J.W.; Cong, H.P.; Xue, L.; Arshad, M.N.; Albar, H.A.; Sobahi, T.R.; Gao, Q.; Yu, S.H. Template- and surfactant-free synthesis of ultrathin CeO2 nanowires in a mixed solvent and their superior adsorption capability for water treatment. Chem. Sci. 2015, 6, 2511–2515. [Google Scholar] [CrossRef]
- Tang, W.; Li, W.; Shan, X.; Wu, X.; Chen, Y. Template-free synthesis of hierarchical layer-stacking CeO2 nanostructure with enhanced catalytic oxidation activity. Mater. Lett. 2015, 140, 95–98. [Google Scholar] [CrossRef]
- Chen, J.F.; Yi, H. Self-assembly of flower-like CeO2 microspheres via a template-free synthetic approach and its use as support in enhanced co and benzene oxidation activity. Adv. Mater. Res. 2011, 308, 656–661. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Shi, L.; Fang, J. Template-Free Synthesis, Controlled Conversion, and CO Oxidation Properties of CeO2 Nanorods, Nanotubes, Nanowires, and Nanocubes. Eur. J. Inorg. Chem. 2010, 2008, 2429–2436. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.; Sun, X.; Peng, Q.; Li, Y. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J. Catal. 2005, 229, 206–212. [Google Scholar] [CrossRef]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, F.; Xiao, P.; Tao, H.; Wang, X.; Hu, Z.; Lu, Y. Great Influence of Anions for Controllable Synthesis of CeO2 Nanostructures: From Nanorods to Nanocubes. J. Phys. Chem. C 2008, 112, 17076–17080. [Google Scholar] [CrossRef]
- Xu, Y.; Li, R. Wet-chemical synthesis and characterization of nitrogen-doped CeO2 powders for oxygen storage capacity. Appl. Surf. Sci. 2018, 455, 997–1004. [Google Scholar] [CrossRef]
- Yan, L.; Yu, R.; Chen, J.; Xing, X. Template-Free Hydrothermal Synthesis of CeO2 Nano-octahedrons and Nanorods: Investigation of the Morphology Evolution. Cryst. Growth Des. 2008, 8, 1474–1477. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, L.; Jiu, H.; Sun, Y.; Liu, G.; Sun, Y.; Su, Q. Template-free hydrothermal synthesis and characterisation of single crystalline Ce(OH)CO3 and CeO2 with spindle-like structures. Micro Nano Lett. 2010, 5, 230–233. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, C.; Qin, H.; Wang, Y.; Li, Y.; Li, X.; Zuo, Y.; Kang, S.; Cui, L. A urea-assisted template method to synthesize mesoporous N-doped CeO2 for CO2 capture. Dalton Trans. 2015, 44, 18718–18722. [Google Scholar] [CrossRef]
- Anzorena, R.S.; Muñoz, F.F.; Bonelli, P.; Cukierman, A.L.; Larrondo, S.A. Hierarchical, template-free self-assembly morphologies IN CeO2 synthesized via urea-hydrothermal method. Ceram. Int. 2020, 46, 11776–11785. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gerasimov, G.N.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Phase composition, conductivity, and sensor properties of cerium-doped indium oxide. Nano Mater. Sci. 2024, 6, 193–200. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, W.; Yang, B.; Liao, C.; Kang, Q.; Chen, G.; Liu, M.; Liu, X.; Ma, R.; Zhang, N. Tuned d-band states over lanthanum doped nickel oxide for efficient oxygen evolution reaction. Nano Mater. Sci. 2023, 5, 228–236. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, X.; Chen, Y.; Shen, J.; Huang, J.; Chen, Y.; Duan, X.; Han, Y.; Chan, K.W.Y.; Lu, J. A hierarchical salt-rejection strategy for sustainable and high-efficiency solar-driven desalination. Nano Mater. Sci. 2024, 6, 38–43. [Google Scholar] [CrossRef]
- Mikheeva, N.N.; Zaikovskii, V.I.; Mamontov, G.V. The effect of Ag and CeO2 distribution on SBA-15 surface on the activity of Ag-CeO2/SBA-15 catalysts in CO and methanol oxidation. J. Sol Gel Sci. Technol. 2019, 92, 398–407. [Google Scholar] [CrossRef]
- He, L.; Li, J.; Feng, Z.; Sun, D.; Wang, T.; Li, R.; Xu, Y. Solvothermal synthesis and characterization of ceria with solid and hollow spherical and multilayered morphologies. Appl. Surf. Sci. 2014, 322, 147–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.