Abstract
Semiconducting clathrates are a distinct class of inclusion compounds with considerable interest for thermoelectric applications. We report here the synthesis, crystal structure and thermoelectric properties of Sn38Sb8I8. The compound was synthesized via planetary ball milling of the corresponding elements for 6 h and then sintering of amorphous mixture at 620 K for 3 days. The crystal structure of the polycrystalline product was determined via X-ray powder diffraction and Rietveld refinement as a type-I clathrate (a = 12.0390(2), space group Pm-3n, No. 223) with mixed-occupied Sn/Sb framework sites and fully occupied I guest sites. Further analysis on the chemical composition, nanomorphology and vibrational modes of the material was carried out via Induced-Coupled-Plasma–Mass Spectrometry, SEM/EDX microscopy and Raman spectroscopy, respectively. Thermoelectric measurements were performed on hot-pressed samples with ca. 98% of the crystallographic density. The clathrate compound behaves as an n-type semiconductor with a band gap of 0.737 eV and exhibits a maximum ZT of 0.0016 at 473 K. Theoretical calculations on the formation enthalpy, electron density of states and transport properties provide insights into the experimentally observed physical behavior.
1. Introduction
Clathrate compounds are a class of inclusion materials in which guest atoms or molecules are completely and irreversibly encapsulated within the cavities of a host framework [1]. Depending on the chemical composition of this framework, they are broadly categorized into clathrate hydrates, where the framework is built of water molecules, and ‘intermetallic’ or ‘semiconducting’ clathrates, which consist primarily of four-bonded, group-fourteen elements (Si, Ge, Sn) [2]. Intermetallic clathrates can further be classified based on the framework’s charge, leading to polyanionic clathrates which incorporate electropositive guest atoms (e.g., alkali or alkaline-earth metals), and polycationic or inverse clathrates where the framework carries a positive charge and halogen atoms (I, Br, Cl) or Te atoms serve as guests [3,4]. Their exact composition is often described via the electron-counting rules of the Zintl–Klemm concept [5].
Compared to their polyanionic counterparts, the polycationic clathrates are far less explored. They are usually synthesized from the corresponding elements either by ball milling and sintering or by hot pressing and contain indium, phosphorus, antimony and zinc for framework substitution [6,7,8,9,10,11,12,13,14,15]. In terms of physical properties, polycationic clathrates exhibit so far moderate thermoelectric efficiency [3]. With regard to Sn-based clathrates, Sn38Sb8I8 has been initially reported by Kishimoto et al. [6], which adopts the type-I clathrate structure, with nominal charges (4b-Sn0)38(4b-Sb+)8I−8 (Figure 1) and exhibits semiconducting behavior. We hereby perform an integrated study on the synthesis, chemical composition, vibrational spectroscopy, DFT calculations and thermoelectric properties of the type-I clathrate Sn38Sb8I8.
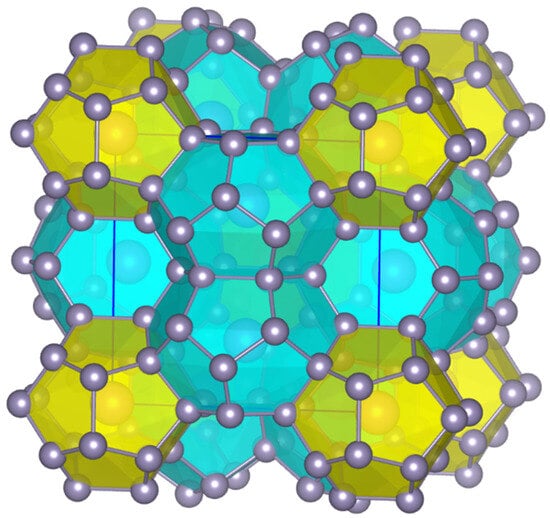
Figure 1.
Crystal structure of Sn38Sb8I8 clathrate with mixed-occupied Sn/Sb framework sites. I atoms reside in the center of the pentagonal dodecahedra (yellow) and the tetrakaidecahedra (light blue). The illustration of the crystal structure was performed using the VESTA software version 3.90.1 [16].
2. Materials and Methods
The reagents Sn (powder, 99.999%), Sb (powder, 99.999%) and I (grains, 99.999%) were obtained by Sigma-Aldrich (United States) and were initially ground together in an atomic ratio of 38:8:8, respectively, in an agate mortar. The mixture (ca. 3 g per batch) was then loaded into a Retsch PM100 planetary ball mill (Rastatt, Germany), inside a 25 mL stainless steel jar along with 5 mm diameter stainless steel balls and spun at 600 rpm for 6 h. The obtained dark gray powder was subsequently loaded in a pyrex tube that was sealed under vacuum (∼0.1 mbar) and heated to 623 K for 3 days. The resulting crystalline gray powder was air stable and was used for all thermoelectric measurements described below without any purification. Moreover, the same process was repeated for a Sn:Sb:I atomic ratio of 30:16:8 and it is discussed separately with regard to the homogeneity range in this ternary system.
The reaction products were structurally characterized by PXRD using a Bruker D8-Advance diffractometer (Karlsruhe, Germany) with Lynx-Eye XE-T silicon strip detector in Bragg−Brentano geometry with Cu-Kα1 (λ = 1.5406 Å) and Cu-Kα2 (λ = 1.5444 Å) radiation. Diffraction data were collected over the range 5° ≤ 2θ ≤ 80° counting for 1 s at each step of 0.02° in the detector position. Rietveld refinements were carried out using the FULLPROF software version 5.20 [17]. The parameters refined with the least-squares method are the sample displacement, the scale factor and the lattice parameters for each phase, the atomic coordinates (paired for every Sn/Sb position) for general positions, the isotropic displacement parameters (common for every Sn/Sb pair) for all atoms in the clathrate structure and some peak profile parameters. The background is fitted via linear interpolation for a set of refinable points.
SEM/EDX measurements were performed on a Zeiss EVO-MA10 (Oberkochen, Germany), equipped with an Oxford EDS analyzer, where the electron beam accelerating voltages used were between 5 and 12 kV with currents of 270 pA for imaging and up to 2 nA for EDX measurements. Charge accumulation was avoided by fast interlaced scanning and averaging techniques.
IPC-MS measurements were performed on an Agilent Technologies Model 7850 instrument (Agilent Technologies, Santa Clara, CA, USA) equipped with the ORS4 collision cell for the analysis of macro-elements and trace metals. The solid samples were dissolved in aqua regia and diluted in deionized water. Sampling was performed using an Agilent SPS 4 autosampler. The 7850 ICP-MS was configured with the standard ISIS 3 injection system. The IntelliQuant function in the ICPMS MassHunter 5.1 software provides the capability of a full mass-spectrum scan with only two seconds additional measurement time, though the samples were quantitated by internal standard seven-point calibration.
Raman spectroscopy was carried out under backscattering geometry on a Renishaw inVia Raman Microscope (Charfield, United Kingdom), equipped with a 2400 line/mm diffraction grating, a high sensitivity Peltier-cooled charged couple device (CCD), a motorized xyz microscope stage, a 50x magnification lens and a Rayleigh rejection notch filter, allowing for measurements down to 5 cm−1. All measurements were made at room temperature with a 2 cm−1 resolution using a 514.5 nm Ar ion laser for excitation employing ca. 0.10 mW μm−2 on the sample with no signs of laser-induced modifications observed. Each spectrum is an average of 35 scans in the range of 5–300 cm−1 accumulated for about 20 min to improve the signal-to-noise ratio.
Uniaxial hot press sintering was performed on the polycrystalline powders at 673 K and 80 MPa for 1 h under Ar atmosphere in a HP20, Thermal Technologies system. The thermal diffusivity (D) of the sample was measured by a Netzsch LFA 457 laser setup (Selb, Germany). Data were collected in 25 K increments on pellet coated with graphite over the temperature range 300–473 K. The determination of specific heat capacity (Cp) was performed based on the comparative method using a pyroceram sample as reference. The thermal conductivity was determined by using the formula:
κ = D ρ Cp
Electrical resistivity and Seebeck coefficient measurements were performed using a ZEM-3 ULVAC-RIKO (Japan) instrument over the temperature range 293–473 K. Estimated uncertainties for the measurements of electrical and thermal transport properties are ±5% and ±7%, respectively.
The DFT calculations were performed using the Vienna Ab initio Simulation Package (VASP, vs. 6) [18,19,20]. The exchange–correlation interactions were treated using the Perdew–Burke–Ernzerhof functional revised for solids (PBESOL), along with the soft pseudopotentials provided by VASP. Dispersion interactions were included by using the semi-empirical DFT-D2 method of Grimme [21], which has been shown to improve structural accuracy in van der Waals and guest–host systems. The plane-wave cutoff energy was set at 520 eV and the Brillouin zone was sampled with a 4 × 4 × 4 Monkhorst-Pack k-point grid. The self-consistent field (SCF) convergence criterion was set at 1.0 × 10−8 eV/atom, whereas the force tolerance for ionic relaxations was 0.01 eV/Å. A structural model was used without Sb- and Sn-mixed occupancies in the space group Pm-3 (No. 200), where the Sb atoms are fully occupied on the 8i Wyckoff site, located in pentagonal rings of the tetrakaidecahedra. This is a maximal translation-equivalent subgroup of the experimentally determined space group Pm-3n (No. 223). In all structural optimization attempts, both atomic coordinates and cell parameters were allowed to relax, with no symmetry constraints imposed. The conjugate gradient minimization scheme was used to optimize energies and forces. To assess the thermodynamic stability of the compound, the simplified formula for the formation enthalpy was used:
where Etot is the total energy and μi the chemical potential of each element at its most stable form. For the Density of States (DOS) calculations, a denser 8 × 8 × 8 k-point grid was employed and the resulting spectra were analyzed with the SUMO toolkit [22]. Transport coefficients were investigated using the semiclassical Boltzmann transport theory as implemented in BoltzTraP2 code (Wien, Austria) [23]. In addition, the influence of carrier doping was probed to simulate the impact of unintentional impurities or charge transfer at contacts.
3. Results and Discussion
3.1. Structural and Chemical Analysis
The X-ray powder diffraction data are shown in Figure 2. The formation of the clathrate phase takes place gradually and in low crystallinity upon ball milling for 6 h (Table 1). The sintering process leads to an almost pure phase sample, with minor impurities of β-Sn and a 1:1 Sn-Sb alloy (space group R-3m, No. 166, a = b = 4.3282(1) Å, c = 5.3500(1) Å) (Figure S1). No sign of underoccupied sites or superstructure reflections is observed through the structural refinements (Table S1), in contrast to other polycationic clathrates, e.g., Sn24P19.3I8 [14] and to polyanionic clathrates, e.g., Cs8Sn44 [24,25] which often have defect framework sites in order to fulfill the Zintl–Klemm concept, as well as lower symmetry due to defect ordering than the archetype structure. As expected from host–guest distances that range between 3.79 and 4.69 Å, the isotropic thermal displacement values of the I atoms are significantly larger (almost double in the case of the tetrakaidecahedron) compared to the framework atoms, due to their loose bonds.
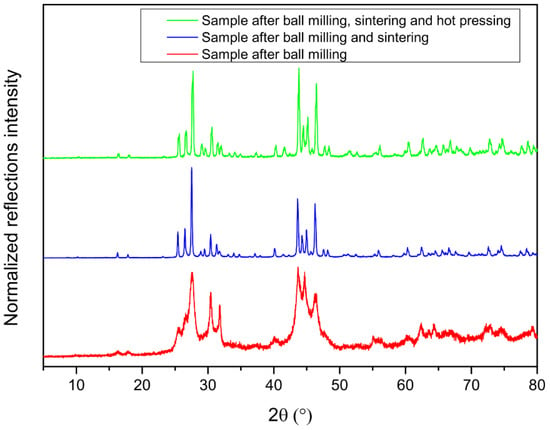
Figure 2.
PXRD patterns for different steps of the Sn38Sb8I8 clathrate synthesis.

Table 1.
Lattice parameters and quantitative analysis from XRPD and Rietveld refinement on various samples with the nominal composition Sn38Sb8I8 and Sn30Sb16I8.
The SEM/EDX analysis reveals that the sample after ball milling contains large amounts of Sn/Sb alloy in the form of small spherical particles (ca. 2 μm diameter) as well as a bulkier material with variable ratios of Sn, Sb and I atoms (Figure S2). After ball milling and sintering the sample becomes more homogeneous and the composition of the bulk phase is close to the anticipated 38:8:8 ratio for Sn, Sb and I atoms, respectively. On the other hand, the ICP-MS analysis has shown that the samples contained traces of Fe stemming from the ball mill jar and balls, during the synthesis. This amount is in ppm level and does not seem to have a significant effect on the thermoelectric properties of the sample. With regard to the homogeneity range in this system, the 30:16:8 reaction stoichiometry led to the PXRD pattern shown in Figure S3. Large amount of unreacted Sn–Sb alloy was detected via XRPD (Table 1). A significant reduction in the lattice parameters is found for the Sb-rich clathrates by ca. 0.1 Å, which shows that the electron count is lower than in the 38:8:8 stoichiometry. This leads to the conclusion that the actual Sb content in this clathrate is between the ratios 38:8:8 and 30:16:8. Due to the large concentration of metallic impurities, this material was not studied further for its thermoelectric properties.
3.2. Raman Spectroscopy
The room temperature Raman spectrum of Sn38Sb8I8 is shown in Figure 3. Similarly to previous studies [26,27], the low-energy peaks, characteristic of clathrate structures with restricted guest motions (known as “rattling modes”) are observed at ca. 35 cm−1. This is closely related to the large thermal displacement values determined for the iodine atoms via Rietveld refinement, as mentioned above. At higher frequencies, the peaks are attributed to the framework vibrations. In particular, the presence of Sb atoms leads to many distinct vibrational modes around 100–150 cm−1 due to its different mass and chemical bonding compared to Sn.
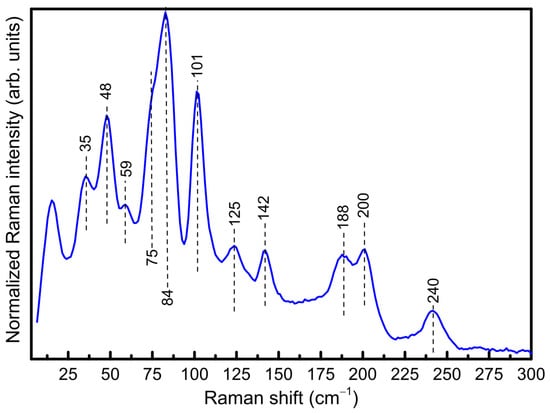
Figure 3.
Raman spectrum of the ball-milled and sintered Sn38Sb8I8 clathrate.
3.3. Thermoelectric Properties
The density of the hot-pressed pellet was ρ = 6.07 g cm−3, which corresponds to ca. 98% of the crystallographic value. The thermoelectric properties of Sn38Sb8I8 are given in Figure 4. The sample shows a semiconducting behavior with exponential electrical conductivity increase over temperature. According to the Arrhenius equation from the electrical conductivity measurements, the band gap is determined at 0.737 eV (inset in Figure 4). The Seebeck coefficient and thermal conductivity range between −245 to −270 μV K−1 and 0.87 to 1.03 W m−1 K−1, respectively. To our knowledge, the only previous experimental study [6] on this material shows electrical conductivity of 10−3 Ohm−1 cm−1 at RT and 0.1 10−3 Ohm−1 cm−1 at 500 K, Seebeck coefficient of −600 μV K−1 at 300 K and −500 μV K−1 at 550 K, an almost constant thermal conductivity of 0.7 W m−1 K−1 and a band gap of 0.8 eV. The main reason for the deviation with our results may be the partial decomposition of the sample during the hot-pressing process. As shown in Table 1 and Figure S4, the clathrate compound has decomposed by ca. 18% into β-Sn and Sn–Sb alloy which show more metallic behavior, which increases the electrical conductivity and decreases in the Seebeck coefficient in absolute value.
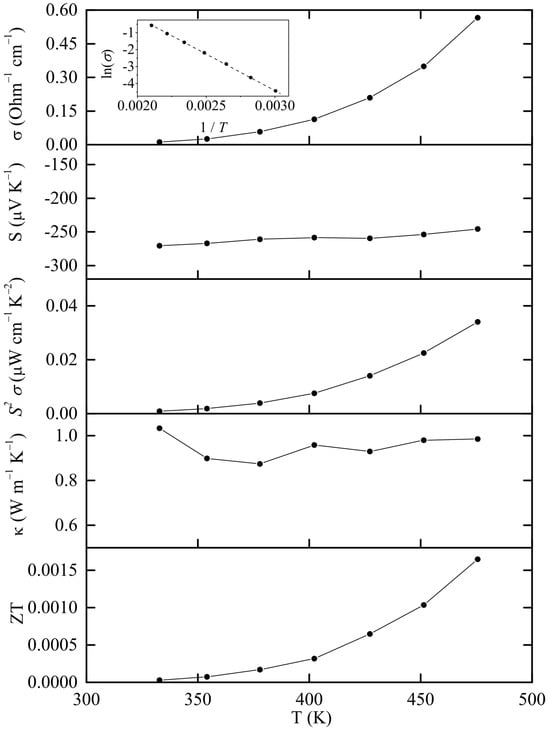
Figure 4.
Experimental thermoelectric properties of Sn38Sb8I8. The inset shows the Arrhenius fit of the electrical conductivity values as a function of temperature in order to determine the electronic band gap.
3.4. Computational Analysis
3.4.1. Structural Optimization and Thermodynamic Stability
The initial structural model was fully relaxed until both atomic positions and cell parameters reached equilibrium. Using the standard PBEsol functional, the optimized lattice constant was found to be 12.128 Å, which is in moderate agreement with our current experimental data (12.0390(2) Å) or the previous literature (e.g., 12.0447(3) Å [3]). When including the dispersion corrections using the PBEsol+D2 method, the lattice constant was calculated at 12.033 Å, essentially reproducing the experimental value with excellent accuracy. This confirms that long-range van der Waals interactions between the I guest atoms and the Sb/Sn framework are important in stabilizing the clathrate structure. The PBEsol+D2 geometry was therefore adopted for all subsequent electronic structure and transport calculations. Guest atoms were accommodated without off-centering in the dodecahedral and tetrakaidecahedral cages. To assess stability, the formation enthalpy was calculated at negative values of −15.98 eV per formula unit (which corresponds to −0.2854 eV per atom), which confirms the thermodynamic stability of the compound relative to its components (at their most stable form).
3.4.2. Electronic Structure: Density of States
The calculated electron band structure and the corresponding total and projected electronic density of states (DOS) of Sn38Sb8I8 are shown in Figure 5, where the Fermi level is set at 0 eV. The band structure indicates that the system exhibits a semiconducting behavior, with a direct energy band gap at the X-point in the Brillouin k-space. DOS calculations show a band gap of 0.71 eV for Sb8Sn38I8. This result is close to the experimental value of 0.737 eV but significantly larger than 0.24 eV reported by Eto et al. [13], likely reflecting improved structural relaxation and basis set convergence compared with less modern calculations. The projected DOS (Figure 5b) shows that the valence band is primarily composed of Sn(p) and I(p) states, with smaller contributions from Sb(p), while the conduction band is dominated by Sn s and p orbitals. The pronounced iodine p-character near the valence-band edge confirms strong guest–host hybridization, which produces the sharp DOS features also reported in [13]. This hybridization gives rise to mixed ionic–covalent bonding between the Sn/Sb framework and the iodine guests, stabilizing the structure and accords with the Zintl–Klemm concept.
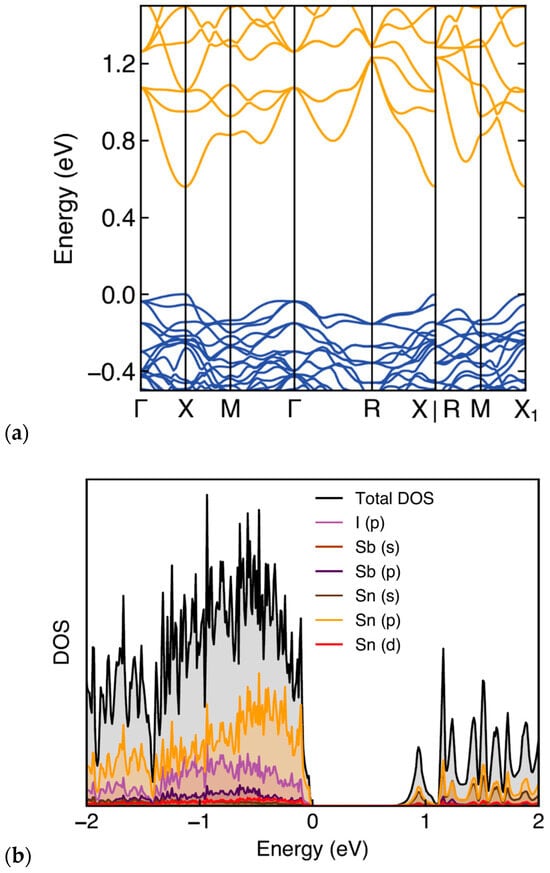
Figure 5.
(a) Electronic band structure of Sb8Sn38I8 along the high-symmetry directions in the Brillouin zone. (b) Total and projected density of states (DOS).
3.4.3. Transport Properties
We apply the Boltzmann transport equation to calculate the Seebeck coefficient S(T) and the conductivity prefactor σ(Τ)/τ, within the constant relaxation time approximation. Under this condition, the electron relaxation time is assumed to be independent of temperature, band index, and wave vector, simplifying the solution of the Boltzmann equation. Transport coefficients were computed using BoltzTraP2 code, based on the previously converged DFT band structure. The band interpolation was performed with a dense Fourier mesh and an energy window of ±0.5 eV around the Fermi level.
We calculated the electrical conductivity, Seebeck coefficient and power factor (S2σ), as functions of carrier concentration and temperature. The compound is intrinsically n-type, with the Fermi level located near the conduction-band minimum. Calculations were performed for electron concentrations between 1 × 1017 cm−3 and 1 × 1019 cm−3 under the constant relaxation time approximation, corresponding to different positions of the chemical potential within the conduction band. Typical values of tau (τ) in the literature are τ = 1 × 10−14 s or less [28,29]. Here, we apply the value of τ = 1 × 10−16 s to obtain calculations of electrical conductivity and power factor in the same order of magnitude with the experimental results, as shown in Figure 6.
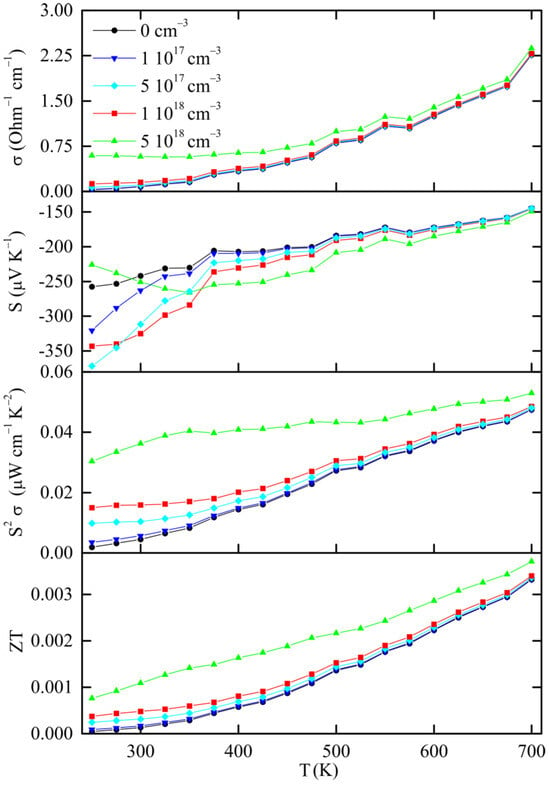
Figure 6.
Calculated thermoelectric properties of Sb8Sn38I8 after DFT relaxation.
The σ curves show two regimes characteristic of n-type semiconductors. At lower carrier concentrations, σ and σ/τ increase rapidly with temperature, reflecting a thermally activated transport mechanism. In contrast, for higher concentrations, it remains stable or decreases slightly for the high doping cases. It should be noted that the constant relaxation time model does not explicitly include temperature-dependent scattering mechanisms such as electron–phonon or impurity scattering. The observed mild decrease or U-shaped behavior of σ(T) at high carrier concentrations is therefore due to band-structure effects—mainly the downward shift in the chemical potential and the thermal broadening of the Fermi–Dirac distribution, which reduce the effective carrier velocity at elevated temperatures.
The calculated Seebeck coefficients are negative throughout the investigated range, confirming the n-type character of charge transport. At 300 K, |S| varies from approximately −360 µV K−1 at low electron concentrations (~5 × 1017 cm−3) to −200 µV K−1 at higher concentrations (~5 × 1018 cm−3). As temperature increases, |S| gradually decreases for highly doped cases but remains nearly constant or slightly increases for the lower carrier concentrations, indicating a crossover from degenerate to non-degenerate behavior at elevated temperatures.
The calculated power factor exhibits a maximum at high electron concentrations (~1018 cm−3) at temperatures near 700 K and reaches a maximum of approx. 6 µW m−1 K−2, where the opposing trends of decreasing Seebeck coefficient and increasing electrical conductivity are balanced. We note here that in our calculations we used a relaxation time value of τ = 10−16 s to approximately match the experimentally observed conductivity of iodine-filled clathrates. This is shorter than typical relaxation times (usually around 10−14–10−15 s) and is attributed to the strong scattering of charge carriers caused by the heavy iodine atoms and the mixed Sb/Sn framework. The high resistivity observed experimentally for Sb8Sn38I8 [13] supports this hypothesis. It is also noted that changing τ only scales the absolute values of σ but it does not affect the Seebeck coefficient or its temperature dependence, which are determined by the underlying band structure.
The overall behavior of σ(T) and S(T) in our results follows the typical trends observed in other semiconducting thermoelectrics. The moderate decrease in |S| with temperature and its dependence on carrier concentration are consistent with the n-type response predicted for doped SnSe and SnS by [29]. Likewise, the broad maximum in power factor around 500 K mirrors that seen in Sb-based clathrates such as Ba8Ga16Sn30 and in the iodine-doped Sn systems studied by [13]. The results indicate that charge transport in Sb8Sn38I8 is dominated by intrinsic band-structure features, particularly the weakly dispersive iodine-derived states near the band edges. This intrinsic semiconducting behavior leads to limited carrier mobility and moderate power factors.
The calculated ZT versus temperature for Sb8Sn38I8 is shown in Figure 6, where a simplified constant lattice thermal conductivity of κL = 1 W m−1 K−1 was presumed. It is calculated that higher carrier concentrations yield larger ZT values, primarily due to enhanced electrical conductivity. At 300 K, ZT values remain close or below 0.001 for all doping levels but increase steadily to reach approximately 0.003 at 700 K for the most highly doped case (n ≈ 5 × 1018 cm−3). At low and intermediate doping levels, we observe nearly parallel curves, consistent with the semiconducting nature of this compound. These computed ZT values match closely the experimental measurements (ZT ≈ 0.0016 at 473 K), confirming that the low performance arises mainly from limited carrier mobility and intrinsic scattering. Nevertheless, the present calculations are an estimation of the potential thermoelectric performance achievable in Sn38Sb8I8 upon controlled n-type doping. Optimization of the carrier concentration could significantly enhance its efficiency while retaining the low lattice thermal conductivity characteristic of clathrates [30]. In a broader context, many Sn-based materials have gained attention in the past years, such as SnO2 [31], SnTe [32] and type-VIII clathrate Ba8Ga16Sn30 [33], as they exhibit high thermoelectric efficiency combined with the inherent low cost of the row materials as well as ease of fabrication.
4. Conclusions
The title compound is synthesized in high purity in the scale of a few grams per batch. It behaves as an n-type semiconductor with maximum ZT of 0.0016 at 473 K. Despite the low figure of merit, this synthetic route allows for a reproducible synthesis of new polycationic clathrates with different types of framework doping. Moreover, the guest–host vibrational modes were investigated through Raman spectroscopy, revealing the characteristic low-energy rattling of the iodine atoms. The theoretical calculations predict an almost linear increase in the ZT with increasing temperatures up to 700 K. Future improvements in the thermoelectric performance may be achieved through controlled carrier doping or compositional tuning in order to reduce the electronic band gap and to enhance the carrier mobility.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano15221727/s1, Figure S1: Rietveld plot for Sn38Sb8I8 after ball milling and sintering; Table S1: Crystallographic parameters from the Rietveld refinement for Sn38Sb8I8 after ball milling and sintering; Figure S2: SEM images of Sn38Sb8I8 samples at different steps of the synthesis; Figure S3: Rietveld plot for Sn30Sb16I8 after ball milling and sintering; Figure S4: Rietveld plot for Sn38Sb8I8 after ball milling, sintering and hot pressing.
Author Contributions
Conceptualization, E.K. and A.K.; methodology, N.M., P.M., N.S.T., I.K., P.O. and T.S.; software, N.K. and E.K.; validation, E.K., T.K. and A.K.; formal analysis, P.M., E.K., T.K. and A.K.; investigation, N.M., P.M., N.K., N.S.T., I.K. and P.O.; resources, E.K., T.K. and A.K.; data curation, N.M., P.M., N.S.T., I.K., P.O., T.S. and A.K.; writing—original draft preparation, N.M., P.M., N.K., N.S.T., I.K., P.O., T.S. and A.K.; writing—review and editing, N.M., P.M., N.K., I.K., T.S. and A.K.; visualization, N.M., P.M., N.K., I.K., T.S. and A.K.; supervision, E.K., T.K. and A.K.; project administration, A.K.; funding acquisition, E.K. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research project was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the Call for “Basic research Financing (Horizontal support of all Sciences)” under the National Recovery and Resilience Plan “Greece 2.0” funded by the European Union–NextGenerationEU (H.F.R.I. Project Number: 14730).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are grateful to the NKUA X-ray Diffraction Core Facility for access to PXRD instrumentation. This work was also supported by computational time granted from the National Infrastructures for Research and Technology S.A. (GRNET S.A.) in the National HPC facility ARIS-under project ID pr017021.
Conflicts of Interest
Author Themistoklis Sfetsas was employed by the QLAB Private Company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PXRD | Powder X-ray Diffraction |
| ZT | Thermoelectric figure of merit |
| DFT | Density Functional Theory |
| DOS | Density of States |
| ICP-MS | Induced Coupled Plasma-Mass Spectrometry |
| SEM | Scanning Electron Microscopy |
| EDX | Energy-Dispersive X-ray analysis |
References
- Kovnir, K.; Shevelkov, A.V. Semiconducting Clathrates: Synthesis, Structure and Properties. Russ. Chem. Rev. 2004, 73, 923–938. [Google Scholar] [CrossRef]
- Park, Y.; Kim, D.; Kim, J.; Yoon, J.; Lee, S. Advances in Nanomaterials for Sustainable Gas Separation and Storage: Focus on Clathrate Hydrates. Acc. Chem. Res 2023, 56, 3111–3120. [Google Scholar] [CrossRef]
- Freer, R.; Ekren, D.; Ghosh, T.; Biswas, K.; Qiu, P.; Wan, S.; Chen, L.; Han, S.; Fu, C.; Zhu, T.; et al. Key properties of inorganic thermoelectric materials—Tables (version 1). J. Phys. Energy 2022, 4, 022002. [Google Scholar] [CrossRef]
- Menke, H.; von Schnering, H.G. Die Käfigverbindungen Ge38A8X8 mit A = P, As, Sb und X = Cl, Br, J. Z. Anorg. Allg. Chem. 1973, 395, 183–198. [Google Scholar] [CrossRef]
- Corbett, J. Polyatomic Zintl Anions of the Post-Transition Elements. Chem. Rev. 1985, 85, 383–397. [Google Scholar] [CrossRef]
- Kishimoto, K.; Arimura, S.; Koyanagi, T. Preparation and Thermoelectric Properties of Sintered Iodine-Containing Clathrate Compounds Ge38Sb8I8 and Sn38Sb8I8. Appl. Phys. Lett. 2006, 88, 222115. [Google Scholar] [CrossRef]
- Christensen, M.; Johnsen, S.; Iversen, B.B. Thermoelectric Clathrates of Type I. Dalton Trans. 2010, 39, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Dolyniuk, J.; Lee, K.; Kovnir, K.; Ovchinnikov, I.; Zaikina, J.V. Clathrate Thermoelectrics. Mater. Sci. Eng. R Rep. 2016, 108, 1–46. [Google Scholar] [CrossRef]
- Falmbigl, M.; Rogl, P.F.; Bauer, E.; Kriegisch, M.; Müeller, H.; Paschen, S. On the Thermoelectric Potential of Inverse Clathrates. MRS Online Proc. Libr. 2009, 1166, 603. [Google Scholar] [CrossRef]
- Shatruk, M.M.; Kovnir, K.A.; Lindsjo, M.; Presniakov, I.A.; Kloo, L.A.; Shevelkov, A.V. Novel Compounds Sn10In14P22I8 and Sn14In10P21.2I8 with Clathrate I Structure: Synthesis and Crystal and Electronic Structure. J. Solid State Chem. 2001, 161, 233–242. [Google Scholar] [CrossRef]
- Kovnir, K.A.; Shatruk, M.M.; Reshetova, L.N.; Presniakov, I.A.; Dikarev, E.V.; Baitinger, M.; Haarmann, F.; Schnelle, W.; Baenitz, M.; Grin, Y.; et al. Novel Compounds Sn20Zn4P22-vI8 (v = 1.2), Sn17Zn7P22I8, and Sn17Zn7P22Br8: Synthesis, Properties, and Special Features of Their Clathrate-like Crystal Structures. Solid State Sci. 2005, 7, 957–968. [Google Scholar] [CrossRef]
- Zaikina, J.V.; Schnelle, W.; Kovnir, K.A.; Olenev, A.V.; Grin, Y.; Shevelkov, A.V. Crystal Structure, Thermoelectric and Magnetic Properties of the Type-I Clathrate Solid Solutions Sn24P19.3(2)BrxI8-x (0 ≤ x ≤ 8) and Sn24P19.3(2)ClyI8-y (y ≤ 0.8). Solid State Sci. 2007, 9, 664–671. [Google Scholar] [CrossRef]
- Eto, T.; Koga, K.; Kamei, T.; Akai, K.; Matsuura, M. Electronic Structure and Thermoelectric Properties for Iodine-Doped Clathrate Compounds. Trans. Mater. Res. Soc. Jpn. 2006, 31, 315–318. [Google Scholar]
- Shatruk, M.M.; Kovnir, K.A.; Shevelkov, A.V.; Presniakov, I.A.; Popovkin, B.A. First Tin Pnictide Halides Sn24P19.3I8 and Sn24As19.3I8: Synthesis and the Clathrate-I Type of the Crystal Structure. Inorg. Chem. 1999, 38, 3455–3457. [Google Scholar] [CrossRef] [PubMed]
- Zaikina, J.V.; Mori, T.; Kovnir, K.; Teschner, D.; Senyshyn, A.; Schwarz, U.; Grin, Y.; Shevelkov, A.V. Bulk and Surface Structure and High-Temperature Thermoelectric Properties of Inverse Clathrate-III in the Si-P-Te System. Chem. Eur. J. 2010, 16, 12582–12589. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodriguez-Carvajal, J. Fullprof, Version September 2012; Institut Laue-Langevin: Grenoble, France, 2012. [Google Scholar]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Ganose, A.M.; Jackson, A.J.; Scanlon, D.O. sumo: Command-line tools for plotting and analysis of periodic ab initio calculations. J. Open Source Softw. 2018, 3, 717. [Google Scholar] [CrossRef]
- Madsen, G.K.H.; Carrete, J.; Verstraete, M.J. BoltzTraP2, a program for interpolating band structures and calculating semi-classical transport coefficients. Comp. Phys. Comm. 2018, 231, 140–145. [Google Scholar] [CrossRef]
- Kelaidis, N.; Klontzas, E.; Kaltzoglou, A. A DFT Computational Study of Type-I Clathrates A8Sn46−x (A = Cs or NH4, x = 0 or 2). Materials 2024, 17, 4595. [Google Scholar] [CrossRef]
- Shimizu, H.; Imai, T.; Kume, T.; Sasaki, S.; Kaltzoglou, A.; Fässler, T.F. Raman spectroscopy study of type-I clathrates A8Sn44□2 (A = Rb, Cs) and Rb8Hg4Sn42. Chem. Phys. Lett. 2008, 464, 54–57. [Google Scholar] [CrossRef]
- Shimizu, H.; Oe, R.; Ohno, S.; Kume, T.; Sasaki, S.; Kishimoto, K.; Koyanagi, T.; Ohishi, Y. Raman and x-ray diffraction studies of cationic type-I clathrate I8Sb8Ge38: Pressure-induced phase transitions and amorphization. J. Appl. Phys. 2009, 105, 043522. [Google Scholar] [CrossRef]
- Nolas, G.S.; Kendziora, C.A. Raman scattering study of Ge and Sn compounds with type-I clathrate hydrate crystal structure. Phys. Rev. B 2000, 62, 7157. [Google Scholar] [CrossRef]
- Møllnitz, L.; Blake, N.P.; Metiu, H. Effects of Morphology on the Electronic and Transport Properties of Sn-Based Clathrates. J. Chem. Phys. 2002, 117, 1302–1312. [Google Scholar] [CrossRef]
- Morales-Ferreiro, J.O.; Diaz-Droguett, D.E.; Celentano, D.; Luo, T. First-Principles Calculations of Thermoelectric Properties of IV–VI Chalcogenides 2D Materials. Front. Mech. Eng. 2017, 3, 15. [Google Scholar] [CrossRef]
- Jasrasaria, D.; Berkelbach, T.C. Strong anharmonicity dictates ultralow thermal conductivities of type-I clathrates. Phys. Rev. B 2005, 112, 014308. [Google Scholar] [CrossRef]
- Ishibe, T.; Tomeda, A.; Komatsubara, Y.; Kitaura, R.; Uenuma, M.; Uraoka, Y.; Yamashita, Y.; Nakamura, Y. Carrier and phonon transport control by domain engineering for high-performance transparent thin film thermoelectric generator. Appl. Phys. Lett. 2021, 118, 151601. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Shuai, J.; Tang, X.; Tan, G. Lead-free SnTe-based compounds as advanced thermoelectrics. Mater. Today Phys. 2021, 19, 100405. [Google Scholar] [CrossRef]
- Saiga, Y.; Suekuni, K.; Deng, S.K.; Yamamoto, T.; Kono, Y.; Ohya, N.; Takabatake, T. Optimization of thermoelectric properties of type-VIII clathrate Ba8Ga16Sn30 by carrier tuning. J. All. Comp. 2010, 507, 1–5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).