Study on Flotation Behavior of Fine Flake Graphite Enhanced by Nanobubbles
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Mineral Composition
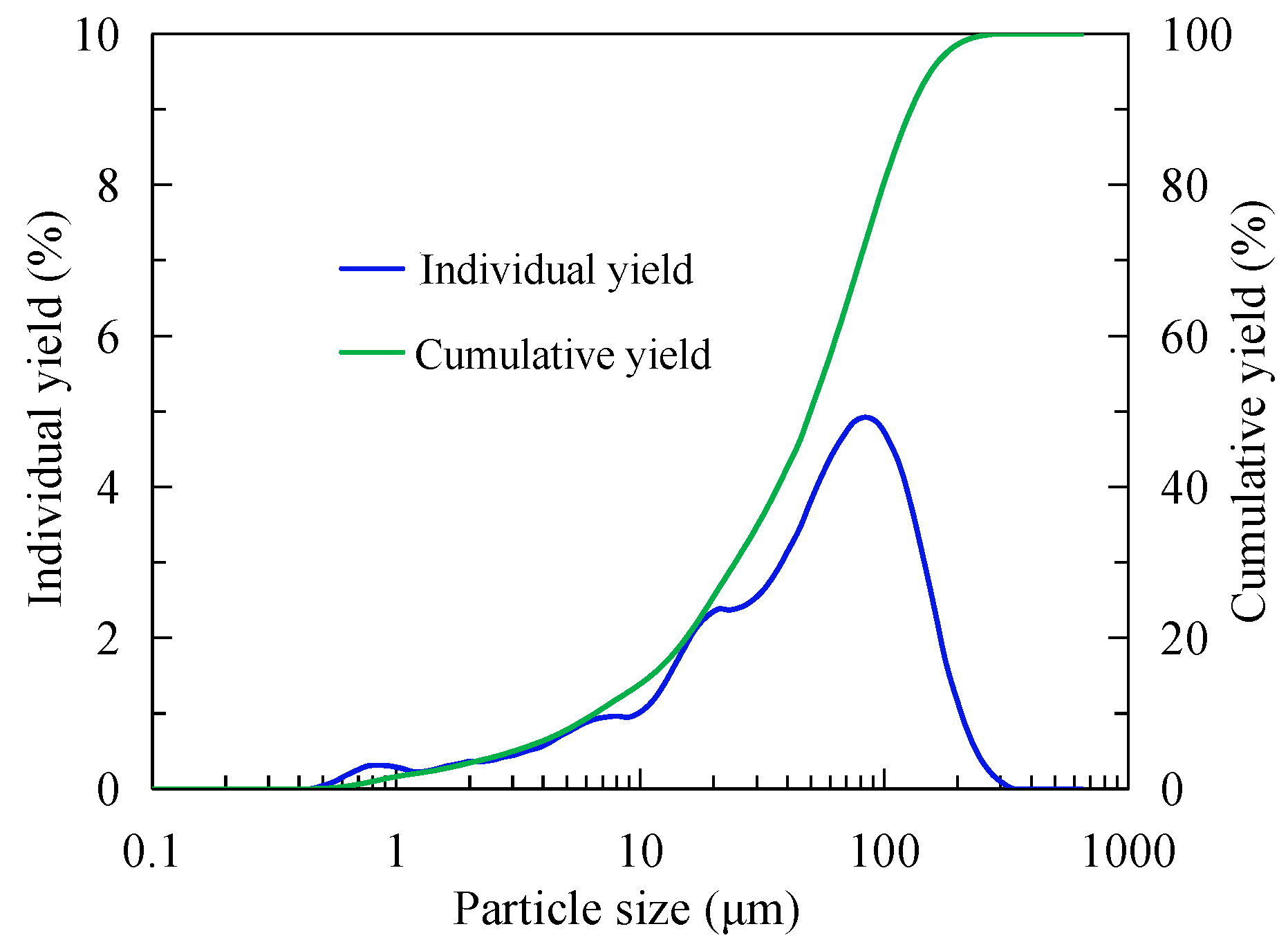
2.1.2. Particle Size Composition
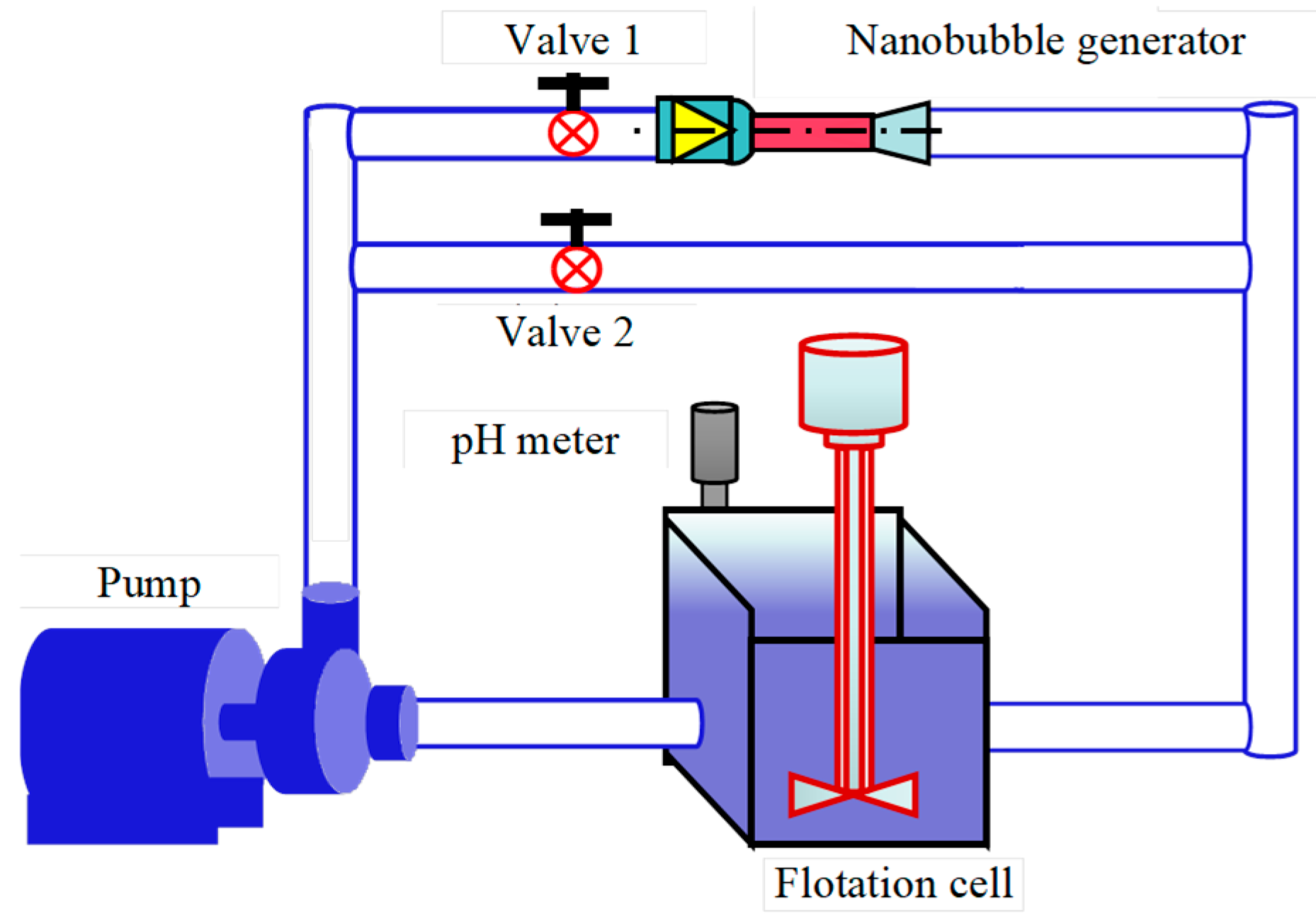
2.2. Nanobubble Generation Methods
2.3. Test Equipment
2.4. Experimental Condition
2.5. Particle Size Characterization
2.5.1. Pulp Particle Size Measurement
2.5.2. Particle Size Characterization of Flotation Concentrate
2.6. Zeta Potential Measurement
2.7. XPS Characterization
3. Results and Discussion
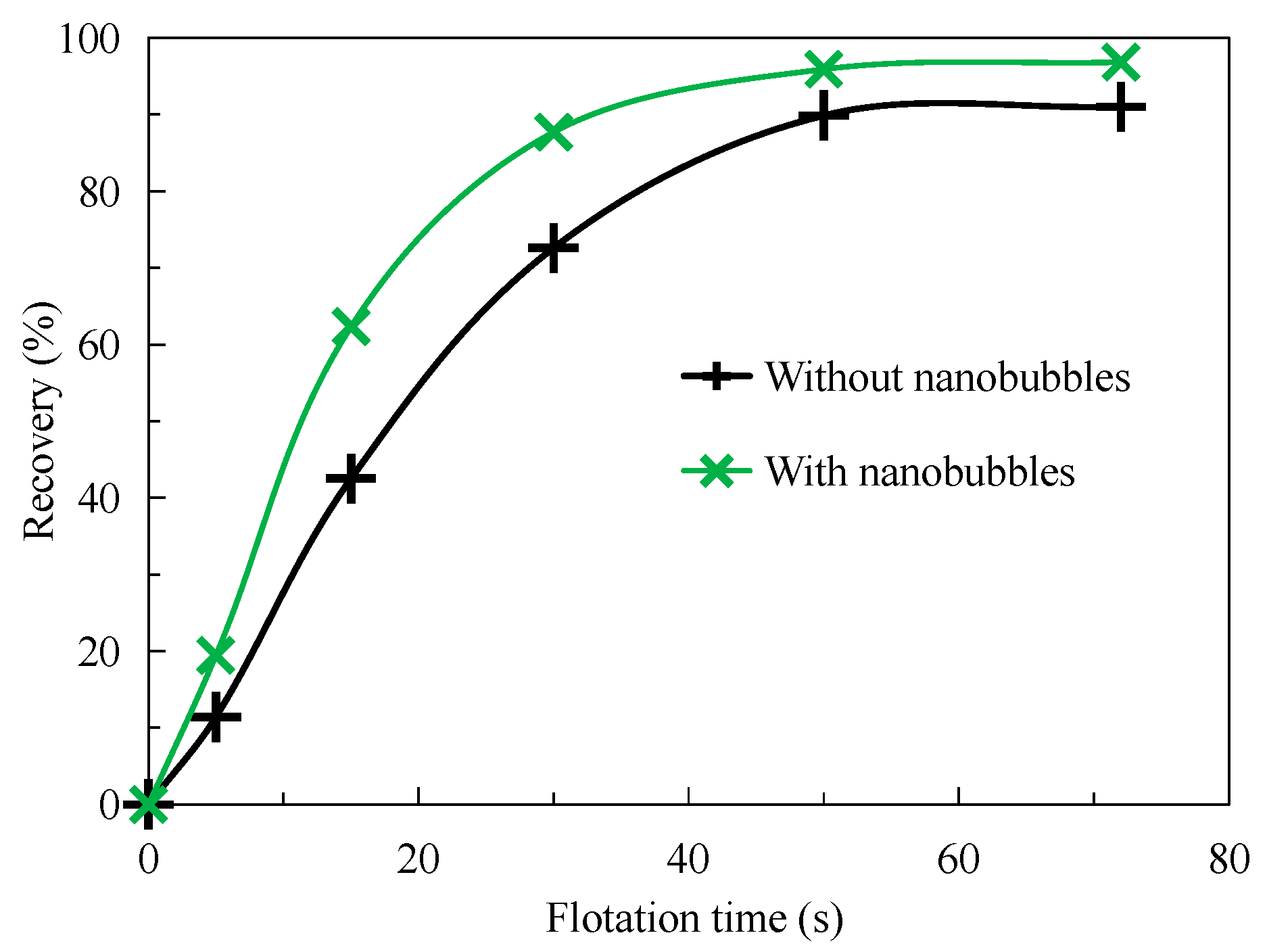
3.1. Flotation Rate
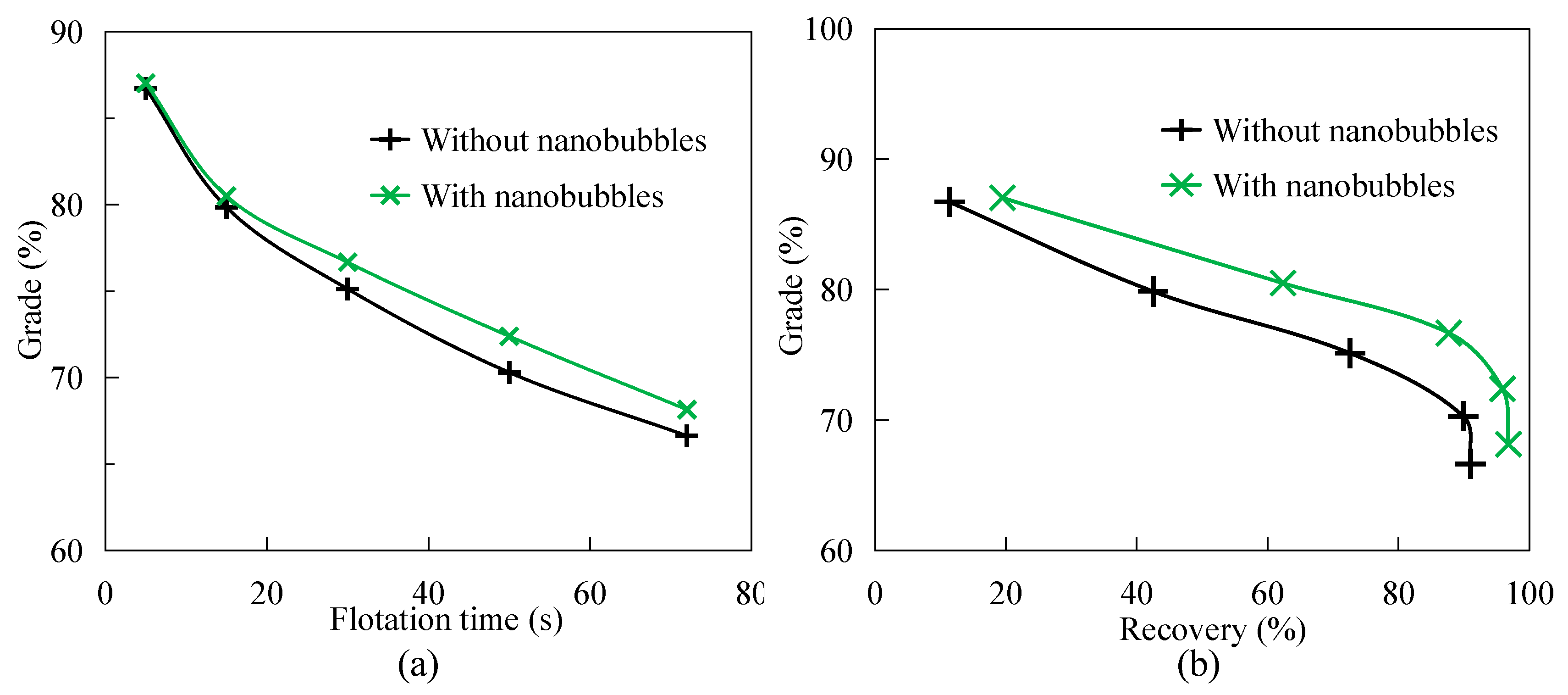
3.2. Flotation Selectivity
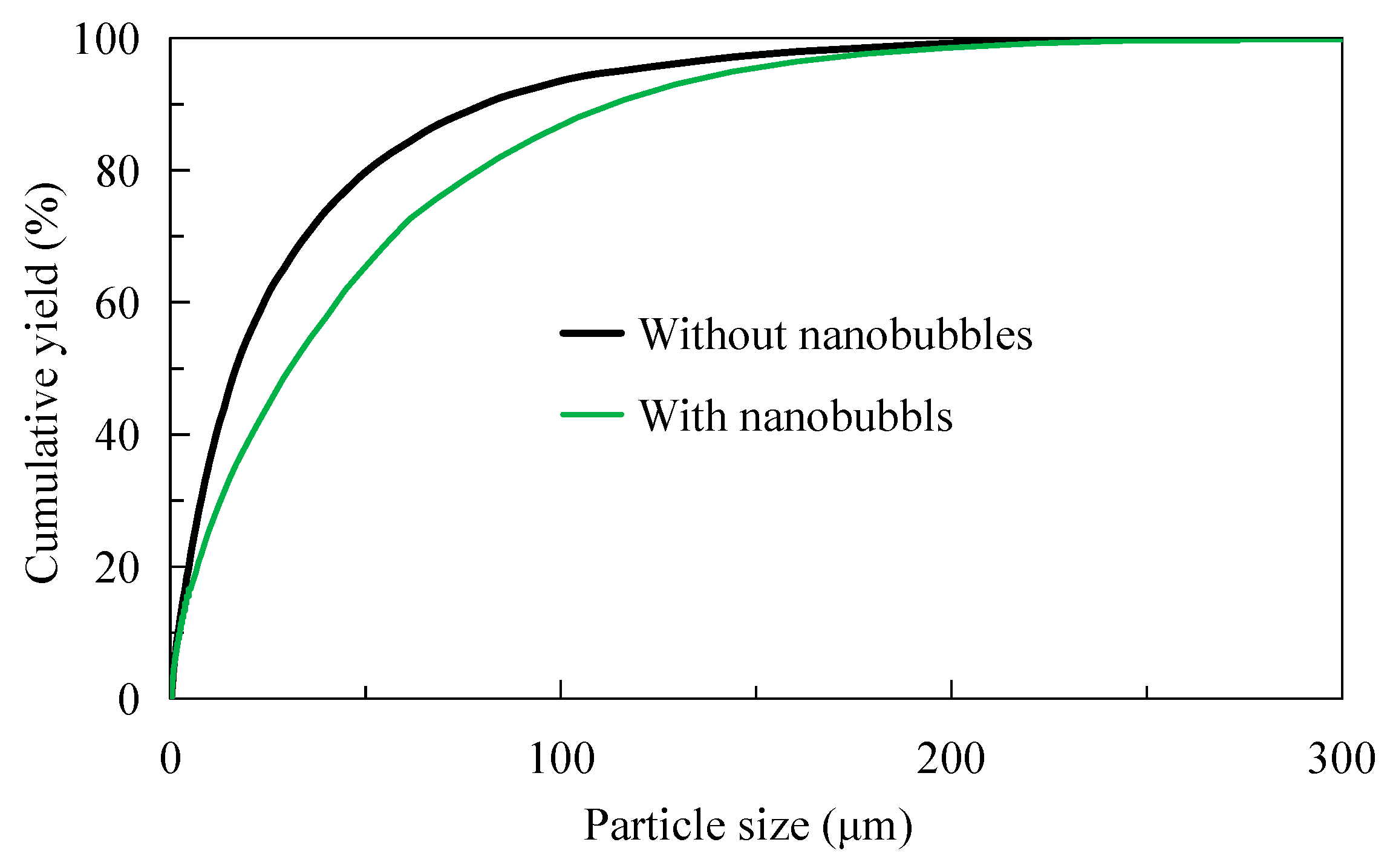
3.3. Pulp Particle Size
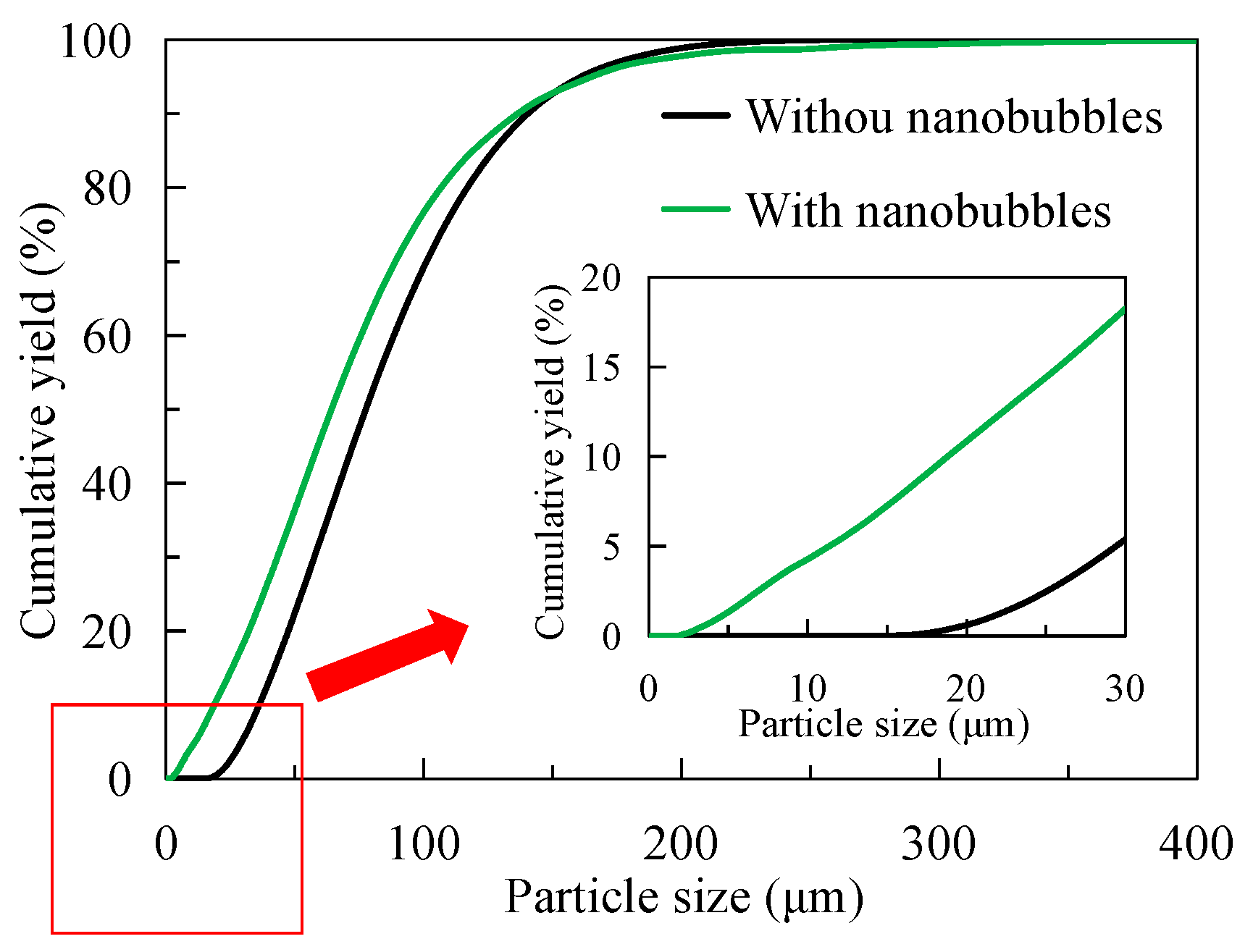
3.4. Concentrate Particle Size
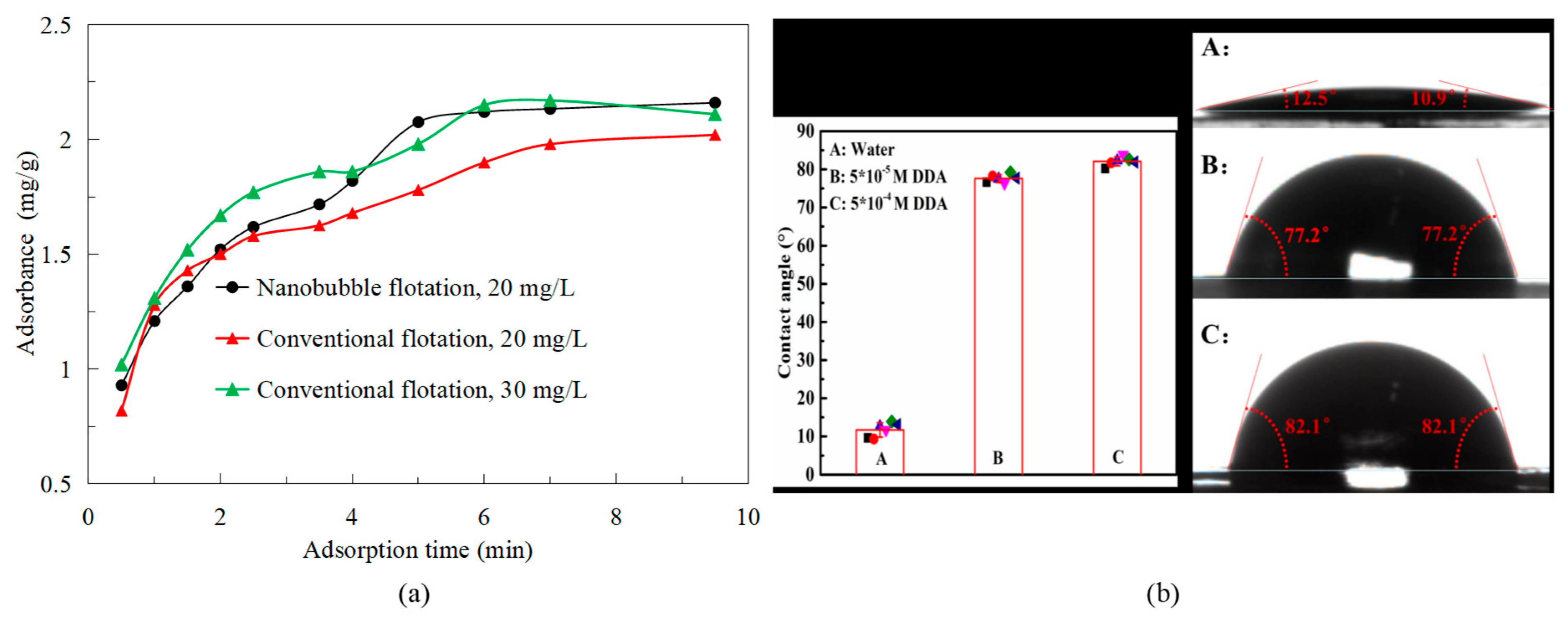
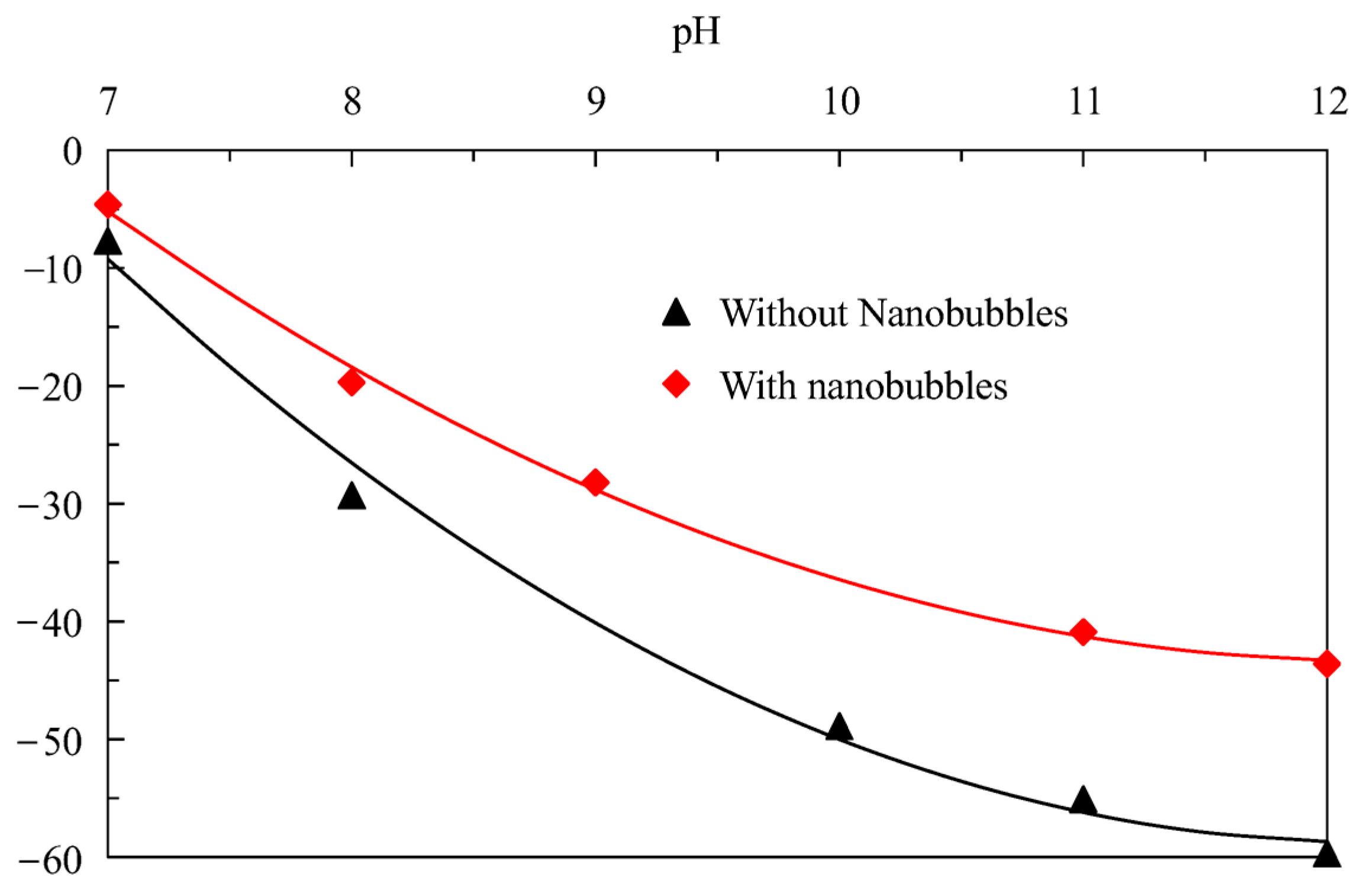
3.5. Zeta Potential
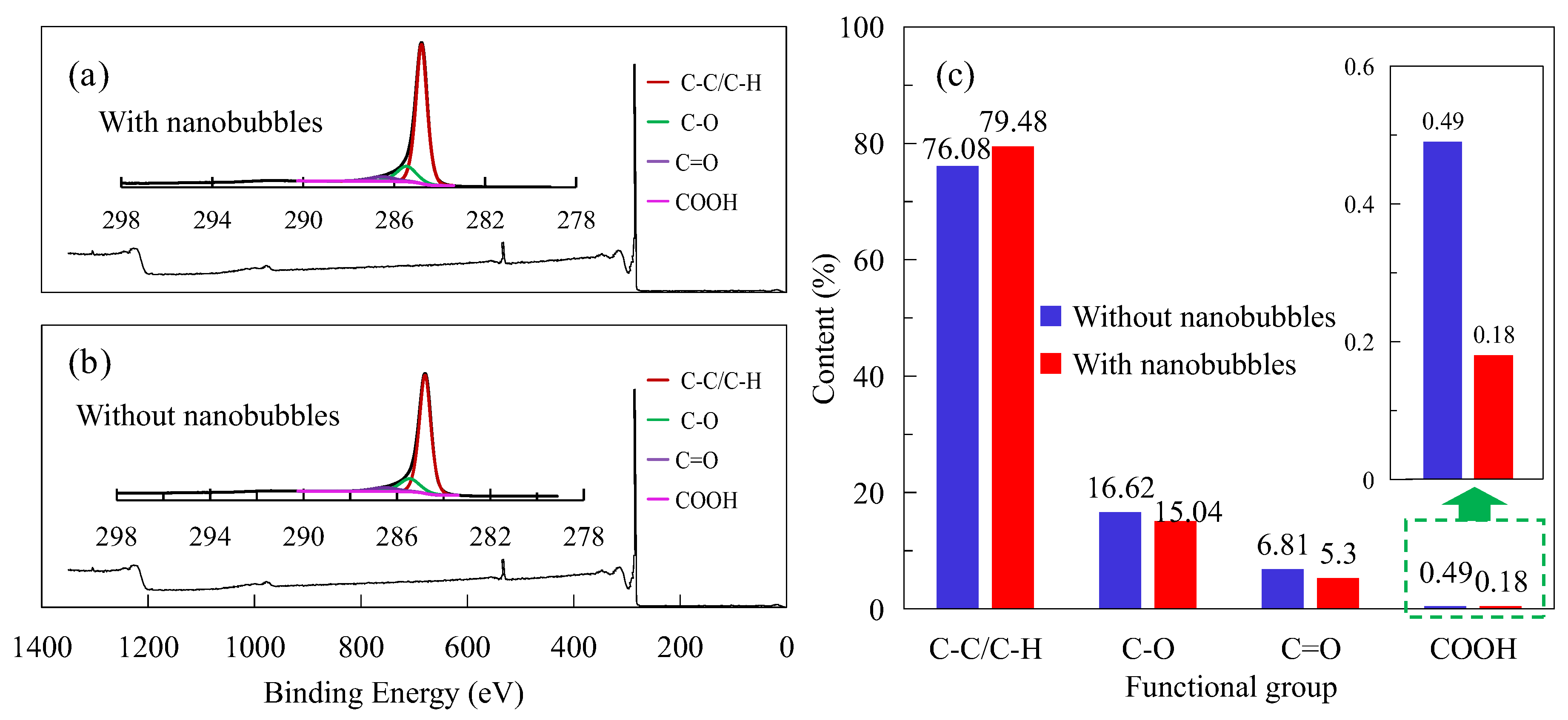
3.6. XPS
4. Conclusions
- (1)
- The nanobubble flotation rate is significantly higher than that of conventional flotation, especially within 15 s of flotation. In addition, the recovery of nanobubble flotation concentrate is significantly higher than that of traditional flotation concentrate, and the final concentrate recovery is about 5 percentage points higher than that of traditional flotation concentrate.
- (2)
- Nanobubble flotation selectivity is better than traditional flotation. Under the same flotation time, the grade of flotation concentrate is always higher than that of traditional flotation concentrate. In addition, nanobubble flotation concentrate has a higher grade than traditional flotation concentrate with the same graphite recovery.
- (3)
- Nanobubbles promote the agglomeration of fine graphite particles in the pulp, and the average particle size is 13 μm larger than that of the traditional flotation pulp. On the contrary, the particle size of nanobubble flotation concentrate is obviously finer than that of traditional flotation concentrate, and its average particle size is 13 μm smaller than that of traditional flotation concentrate. In addition, the minimum graphite size of nanobubble flotation is only 2 μm, 11 μm smaller than the minimum size of traditional flotation.
- (4)
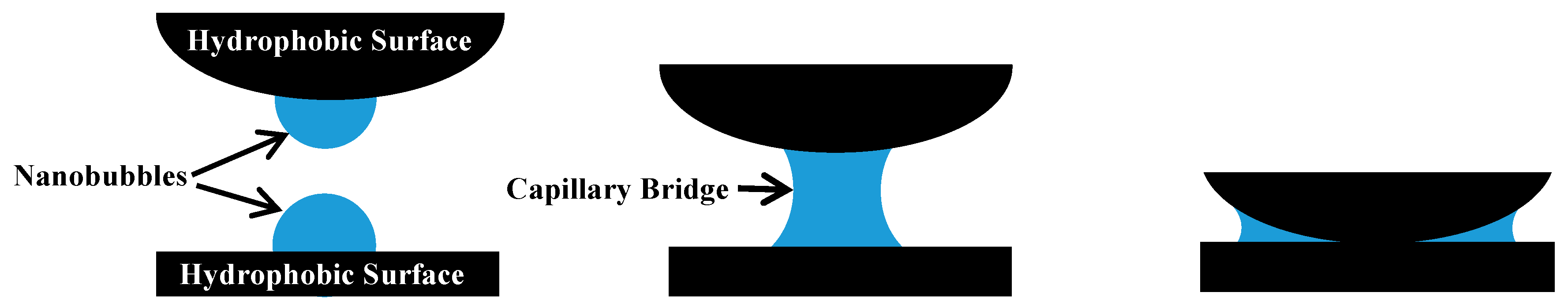
- Nanobubbles can reduce the electrostatic repulsion between fine particle graphite and enhance the hydrophobic attraction between graphite particles, which is conducive to maintaining the hydrophobic agglomeration structure of fine particle graphite and thereby strengthening the recovery of fine particle graphite. More importantly, nanobubbles can effectively cover the oxygen-containing groups on the surface of graphite, promoting the adsorption of collectors on the graphite surface and thereby enhancing the selectivity of fine particle graphite flotation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, F.Y.; Song, B.X.; Tao, Y.J. Study on mineralogical characteristics of fine flake graphite ore. Part. Sci. Technol. 2023, 41, 411–419. [Google Scholar] [CrossRef]
- Ma, F.Y. Study on Efficient Flotation of Graphite Ore by Nanobubble and Its Mechanism. Ph.D. Dissertation, China University of Mining and Technology, Xuzhou, China, 2021. (In Chinese). [Google Scholar]
- Kang, W.Z.; Li, H.J. Effect of ultrasonic pretreatment on cleaner flotation of graphite. J. China Univ. Min. Technol. 2020, 49, 1193–1198. [Google Scholar]
- Bu, X.; Zhang, T.; Chen, Y.; Peng, Y.; Xie, G.; Wu, E. Comparison of mechanical flotation cell and cyclonic microbubble flotation column in terms of separation performance for fine graphite. Physicochem. Probl. Miner. Process. 2018, 54, 732–740. [Google Scholar]
- Sun, K.; Qiu, Y.; Zhang, L.; Liu, Q.; Mao, Z.; Qian, Y. Enhanced fine flake graphite flotation and reduced carbon emission by a novel water-in-oil kerosene emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129603. [Google Scholar] [CrossRef]
- Ma, F.Y.; Tao, D.P.; Tao, Y.; Liu, S. An innovative flake graphite upgrading process based on HPGR, stirred grinding mill, and nanobubble column flotation. Int. J. Min. Sci. Technol. 2021, 31, 1063–1074. [Google Scholar] [CrossRef]
- Ma, F.Y.; Zhang, P.; Tao, D.P. Surface nanobubble characterization and its enhancement mechanisms for fine-particle flotation: A review. Int. J. Miner. Metall. Mater. 2022, 29, 727–738. [Google Scholar] [CrossRef]
- Li, C.W.; Zhang, H.J. A review of bulk nanobubbles and their roles in flotation of fine particles. Powder Technol. 2022, 395, 618–633. [Google Scholar] [CrossRef]
- Li, C.W.; Zhang, H.J. Surface nanobubbles and their roles in flotation of fine particles—A review. J. Ind. Eng. Chem. 2022, 106, 37–51. [Google Scholar] [CrossRef]
- Ma, F.Y.; Tao, D.P.; Tao, Y.J. Effects of nanobubbles in column flotation of Chinese sub-bituminous coal. Int. J. Coal Prep. Util. 2022, 42, 1126–1142. [Google Scholar] [CrossRef]
- Meng, L.X.; Zhao, T.L.; Fan, Z.L.; Ma, F.Y.; Liu, X.Y.; Zhang, D.; Zhang, M.Z.; Li, X.W.; Li, M.J. Research status of nanobubble enhanced flotation mechanism of ultrafine particles. Conserv. Util. Miner. Resour. 2023, 43, 162–168. (In Chinese) [Google Scholar]
- Oliveira, H.; Azevedo, A.; Rubio, J. Nanobubbles generation in a high-rate hydrodynamic cavitation tube. Miner. Eng. 2018, 116, 32–34. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Wu, Z.; Tao, D. An experimental study on size distribution and zeta potential of bulk cavitation nanobubbles. Int. J. Miner. Metall. Mater. 2020, 27, 152–161. [Google Scholar] [CrossRef]
- Sobhy, A.; Tao, D. Nanobubble column flotation of fine coal particles and associated fundamentals. Int. J. Miner. Process. 2013, 124, 109–116. [Google Scholar] [CrossRef]
- Sobhy, A.; Wu, Z.; Tao, D. Statistical analysis and optimization of reverse anionic hematite flotation integrated with nanobubbles. Miner. Eng. 2021, 163, 106799. [Google Scholar] [CrossRef]
- Zhang, J.S.; Que, X.L. Mineral Reagent; Metallurgical Industry Press: Beijing, China, 2008. [Google Scholar]
- Tao, D.P.; Wu, Z.X.; Sobhy, A. Investigation of nanobubble enhanced reverse anionic flotation of hematite and associated mechanisms. Powder Technol. 2021, 379, 12–15. [Google Scholar] [CrossRef]
- Zhang, X.Y. Characterization of Nanobubbles and Its Application in Reverse Flotation of Hematite. Master’s Dissertation, University of Science and Technology Liaoning, Anshan, China, 2020. (In Chinese). [Google Scholar]
- Meyer, C.J.; Deglon, D.A. Particle collision modeling—A review. Miner. Eng. 2011, 24, 719–730. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation using a multiphase pump, properties and features in flotation. Miner. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Ma, F.Y.; Tao, D.P. A Study of Mechanisms of Nanobubble-Enhanced Flotation of Graphite. Nanomaterials 2022, 12, 3361. [Google Scholar] [CrossRef]
- Tang, C.L.; Ma, F.Y.; Wu, T.Y.; Zhang, D.; Wang, Y.; Zhao, T.L.; Fan, Z.L.; Lui, X.U. Study on surface physical and chemical mechanism of nanobubble enhanced flotation of fine graphite. J. Ind. Eng. Chem. 2023, 122, 389–396. [Google Scholar] [CrossRef]
- Chen, G.H.; Ren, L.Y.; Zhang, Y.M.; Bao, S.X. Improvement of fine muscovite flotation through nanobubble pretreatment and its mechanism. Miner. Eng. 2022, 189, 107868. [Google Scholar] [CrossRef]
- Zhou, W.G.; Wu, C.N.; Lv, H.Z.; Zhao, B.L.; Lui, K.; Ou, L.M. Nanobubbles heterogeneous nucleation induced by temperature rise and its influence on minerals flotation. Appl. Surf. Sci. 2020, 508, 145282. [Google Scholar] [CrossRef]
- Ishida, N.; Inoue, T.; Miyahara, M.; Higashitani, K. Nano bubbles on a hydrophobic surface in water observed by tapping-mode atomic force microscopy. Langmuir 2000, 16, 6377–6380. [Google Scholar] [CrossRef]
- Song, B.; Walczyk, W.; Schonherr, H. Contact angles of surface nanobubbles on mixed self-assembled monolayers with systematically varied macroscopic wettability by atomic force microscopy. Langmuir 2011, 27, 8223–8232. [Google Scholar] [CrossRef]
- Calgaroto, S.; Azevedo, A.; Rubio, J. Flotation of quartz particles assisted by nanobubbles. Int. J. Miner. Process. 2015, 137, 64–70. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, A.V. Nanobubbles and the nanobubble bridging capillary force. Adv. Colloid Interface Sci. 2010, 154, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, S.; Liu, J.; Zhu, Y.; Han, Y. Nanobubble-enhanced flotation of ultrafine molybdenite and the associated mechanism. J. Mol. Liq. 2022, 346, 118312. [Google Scholar] [CrossRef]
- Wu, Z.X.; Tao, D.P.; Tao, Y.J.; Ma, G.X. New Insights into Mechanisms of Pyrite Flotation Enhancement by Hydrodynamic Cavitation Nanobubbles. Miner. Eng. 2023, 201, 108222. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Ren, L.Y.; Zhang, Y.M. Role of nanobubbles in the flotation of fine rutile particles. Miner. Eng. 2021, 172, 107140. [Google Scholar] [CrossRef]
- Yan, S.; Fang, X.; Zhao, G.; Qiu, T.; Ding, K. Nanobubbles Adsorption and Its Role in Enhancing Fine Argentite Flotation. Molecules 2025, 30, 79. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Z.; Wang, T.; Qiao, B.; Huang, H.; Ran, J.; Ma, G.; Tao, D. Mechanisms of nanobubble-enhanced flotation of galena from pyrite. Int. J. Miner. Metall. Mater. 2025, 32, 817–824. [Google Scholar] [CrossRef]



| Mineral | Graphite | Quartz | Pyrite | Calcite | Feldspar | Muscovite |
|---|---|---|---|---|---|---|
| Content (%) | 28.09 | 26.01 | 6.40 | 7.32 | 5.30 | 16.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Zhang, D.; Tao, D. Study on Flotation Behavior of Fine Flake Graphite Enhanced by Nanobubbles. Nanomaterials 2025, 15, 1542. https://doi.org/10.3390/nano15201542
Ma F, Zhang D, Tao D. Study on Flotation Behavior of Fine Flake Graphite Enhanced by Nanobubbles. Nanomaterials. 2025; 15(20):1542. https://doi.org/10.3390/nano15201542
Chicago/Turabian StyleMa, Fangyuan, Di Zhang, and Dongping Tao. 2025. "Study on Flotation Behavior of Fine Flake Graphite Enhanced by Nanobubbles" Nanomaterials 15, no. 20: 1542. https://doi.org/10.3390/nano15201542
APA StyleMa, F., Zhang, D., & Tao, D. (2025). Study on Flotation Behavior of Fine Flake Graphite Enhanced by Nanobubbles. Nanomaterials, 15(20), 1542. https://doi.org/10.3390/nano15201542







