Abstract
As an important part of global carbon neutrality strategies, carbon dioxide (CO2) capture, utilization, and storage technologies have emerged as critical solutions for reducing carbon emissions. However, conventional CO2 applications, including food preservation, industrial synthesis, and enhanced oil recovery, face inherent limitations such as suboptimal gas–liquid mass transfer efficiency and inadequate long-term stability. Recent advancements in CO2 micro-nanobubbles (CO2 MNBs) have demonstrated remarkable potential across multidisciplinary domains, owing to their distinctive physicochemical characteristics encompassing elevated internal pressure, augmented specific surface area, exceptional stability, etc. In this review, we try to comprehensively explore the unique physicochemical properties of CO2 MNBs and their emerging applications, including industrial, agricultural, environmental, and energy fields. Furthermore, we provide a prospective analysis of how these minuscule bubbles can emerge as pivotal in future technological innovations. We also offer novel insights and directions for research and applications across related fields. Finally, we engage in predicting their future development trends as a promising technological pathway for advancing carbon neutrality objectives.
1. Introduction
With the rapid advancement of global industrialization and urbanization, anthropogenic impacts on the natural environment have escalated significantly. The massive combustion of fossil fuels and exploitation of non-renewable resources have established carbon dioxide (CO2) as the dominant greenhouse gas emission, driving critical issues, including global climate warming, increased frequency of extreme weather events, ecosystem degradation, and biodiversity loss [1,2]. To address these pressing concerns, the international community has advocated for low-carbon initiatives to mitigate greenhouse gas emissions, optimize energy efficiency, develop renewable energy systems, and facilitate green economic transitions. Under this background, atmospheric CO2 reduction has become a focal point of environmental governance. Existing technologies such as carbon capture and storage (CCS), carbon capture, utilization, and storage (CCUS), CO2-enhanced oil recovery (CO2-EOR), and CO2 geological sequestration (CGS) face significant limitations [3]. These approaches not only fail to align with low-carbon principles but may exacerbate environmental burdens through groundwater contamination risks from CO2 leakage, persistent chemical residues in reservoirs, and induced micro-seismic activity. Furthermore, their implementation incurs substantial energy penalties and economic costs. For instance, compressing 80% of CO2 emissions in coal-fired power plants increases energy consumption by 24–40% [4]. In China, CCS adoption escalates electricity generation costs by 30–50% due to extra power and steam consumption, with post-combustion capture costs reaching USD 24.3 per ton at the Huaneng Beijing plant, achieving merely 80–85% capture efficiency [5]. These energy-intensive, cost-prohibitive, and low-return characteristics hinder industrial adoption and national-scale implementation.
Recent advancements in micro-nanobubbles (MNBs) have revealed unique physicochemical properties distinct from conventional macro-bubbles and may provide significant contributions in decreasing carbon pollution and efficient utilization of CO2. MNBs are generally defined as gas bubbles with diameters ranging from micrometers (μm) down to nanometers (nm). MNBs are classified into two primary types: surface MNBs attached to solid interfaces and bulk MNBs suspended within liquid media [6,7,8]. This study focuses specifically on bulk MNBs. A key characteristic of MNBs is their exceptional stability: contrary to the rapid rupture predicted for bubbles within microseconds by the classical Epstein–Plesset equation, numerous experiments demonstrate that MNBs can maintain their structure in aqueous solutions for hours or even days without readily coalescing or rupturing [9,10,11]. This long-lasting stability, far exceeding theoretical expectations, represents a core advantage. MNBs also typically sustain high internal pressure, forming the basis for their distinct physical and chemical behaviors [12]. Furthermore, they possess a significantly increased specific surface area, which greatly enhances their contact efficiency with surrounding substances such as pollutants or reactants [13]. Given the direct relationship between gas mass transfer efficiency and gas–liquid interfacial contact area, this high specific surface area significantly boosts the gas–liquid mass transfer efficiency of MNBs, enabling them to efficiently promote gas dissolution and transfer while prolonging gas residence time in liquids [14]. For instance, experiments by Matsumoto confirmed that O3 MNBs improve ozone mass transfer efficiency and extend its half-life [15]. Additionally, OH− ions are typically adsorbed onto the gas–liquid interface of MNBs, resulting in a negative surface charge[16]. At near-neutral pH conditions, the Zeta potential of Air MNBs, O2 MNBs, and N2 MNBs typically ranges from approximately −27 to −45 mV [17]. These properties have prompted MNB applications in diverse fields, including biomedicine, wastewater treatment, crop enhancement in agriculture, surface cleaning, mineral flotation, food production, etc. [18,19,20,21,22,23,24]. Notably, the advantages of using MNBs include simple material preparation, low cost, and environmental friendliness. This may provide solutions to environmental pollution challenges brought about by various fields, and is in line with the current low-carbon and environmental protection concept. For instance, in treating black and odorous river water, Hong et al. found that under identical aeration intensity, MNB aeration produced significantly higher dissolved oxygen levels (reaching 9.87 mg/L at 60 min) compared to ordinary aeration (6.54 mg/L). Furthermore, the maximum removal rates for COD, NH3-N, geosmin, and 2-methylisoborneol were 12%, 10%, 16%, and 12% higher, respectively [25]. Similarly, when treating simulated wastewater containing azo dyes, Chu et al. observed that O3 MNBs increased the total mass transfer coefficient and TOC removal rate by 80% and 30%, respectively [26]. Liu et al.’s experiments on printing and dyeing wastewater treatment revealed that MNB air flotation technology not only enhanced pretreatment efficiency and reduced flocculant dosage but also increased the removal rates of oil, COD, and chromaticity by 40%, 30%, and 110%, respectively, compared to traditional air flotation processes [27].
Herein, we systematically review the unique physicochemical properties of CO2 MNBs and their potential applications in various fields. Figure 1 presents all the content involved in this review. The characteristics of CO2 MNBs were first deeply analyzed in terms of stability, mass transfer efficiency, surface charge, and elimination of hydroxyl radical. Then, the specific applications in seven major fields were mainly discussed: agricultural planting, food processing, oil and gas extraction, construction mining, anaerobic digestion, microalgae cultivation, and industrial crystallization. It demonstrates CO2 MNB technology’s triple advantages: process efficiency amplification, energy demand reduction, and operational cost minimization. These studies underline CO2 MNBs’ transformative potential in green manufacturing, environmental engineering, and biotechnological applications, positioning them as key enablers for achieving carbon neutrality and circular economy objectives.
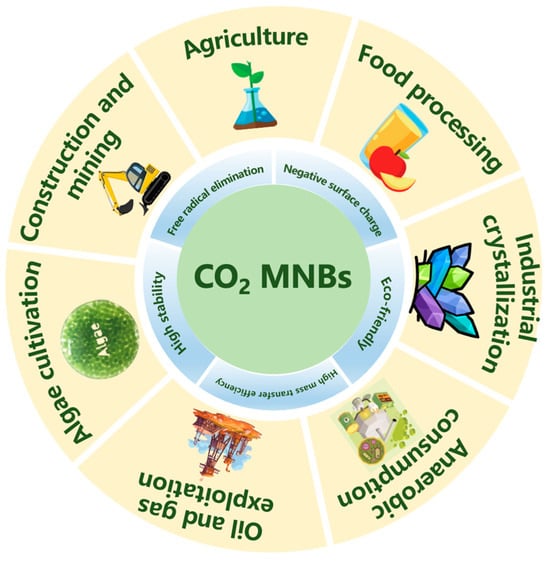
Figure 1.
Properties of CO2 MNBs and their significant applications.
2. Properties of CO2 MNBs
2.1. High Stability
MNBs are distinguished from macroscopic bubbles due to their unique physicochemical properties, and their submicron size confers extraordinary stability to the system, with their survival time in aqueous media reported in the literature to be up to tens of days or even weeks [28,29]. However, significant differences lie in the stability performance of CO2 MNBs. Table 1 summarizes the lifespan, pH, zeta potential, and key findings of CO2MNBs generated using different methods. When prepared via the nanobubble film method, CO2 MNBs with ~60% population collapse within 72 h post-formation and near-complete dissolution by 5 days [30]. Studies using the periodic pressure change method revealed that CO2 MNBs exhibited markedly reduced stability compared to N2 and O2 MNBs, with a lifetime of less than 48 h [9]. Contrasting stability profiles emerged in hydrodynamic cavitation-synthesized MNBs (N2, O2, Ar + 8% H2, air, and CO2) across aqueous matrices. In deionized water, CO2 MNBs persisted for 5 days, whereas N2/O2-based MNBs maintained stability for 60 days. Remarkably, this trend reversed in ionic environments (NaCl, CaCl2, and AlCl3): CO2 MNBs achieved 14-day stability, while other MNBs underwent complete dissolution within 2 weeks [31]. Although different preparation methods may lead to differences in the lifetimes of CO2 MNBs in deionized water, all studies consistently showed that the lifetimes of CO2 MNBs in deionized water were significantly shorter than those of other MNBs. Notably, researchers attribute the shorter stability of CO2 MNBs relative to other gas species to their chemical reactivity with water, as illustrated by the following equations [30,31]:
This dissolution process generates carbonic acid, thereby acidifying the solution. Cerrón-Calle documented that CO2 MNB generation in an initial pH 5.8 solution caused rapid acidification, with pH dropping to 4.5 within 5 min and stabilizing at 4.0 [30]. These observations align with findings from others, all confirming that the CO2 MNBs system has a significant acidification effect [22,31,32,33,34].
Cerrón-Calle further investigated the pH effect on CO2 MNBs size by titrating NaOH into a pH 4.2 CO2 MNB solution to systematically raise pH to 7.5 and 9.0 [30]. This manipulation induced a distinct MNBs’ size (Figure 2):

Figure 2.
NTA shows the size distribution and concentration of CO2 MNBs collected 50 min after generation in solutions with different pH values (4.2, 7.5, and 9.0) [30].
- At pH 4.2: Dominant size distribution 150–250 nm;
- At pH 7.5: Decrease in quantity, and the size distribution is concentrated between 100 and 150 nm;
- At pH 9.0: An increase in quantity, and the size distribution is concentrated between 70 and 100 nm.
The researchers interpreted this phenomenon as pH-driven gas transfer from bubbles to solution, ultimately leading to bubble dissolution. This phenomenon was amplified in low-CO2-concentration systems, where MNBs’ shrinkage not only reduced diameters but also supplied dissolved carbon for reactions.
Supporting this mechanism, Takemura demonstrated in shallow aquifers that CO2 MNB stability is governed by CO2 solubility rather than electrostatic repulsion [35]. This discovery reveals the essence of the anomalous stability of CO2 MNBs in salt solutions at the molecular scale—the increase in ionic strength affects the gas–liquid mass transfer kinetics by altering the CO2 dissolution equilibrium (CO2 (aq) ⇌ CO2 (g)), rather than through the compression of the double electric layer effect. The stability of CO2 MNBs prepared in the salt solution environment was significantly improved, which also confirmed this conclusion [31].
Therefore, their real lifetime is easily influenced by some factors such as production methods, different pH and ionic compositions, etc. Practical applications may require a continuous CO2 MNB generator to sustain their concentration.
2.2. High Mass Transfer Efficiency
The gas mass transfer rate depends on the interfacial contact area between the gaseous and liquid phases [16]. Notably, the gas–liquid interfacial area provided by CO2 MNBs has been demonstrated to exceed conventional macro-bubbles by several orders of magnitude. This enhanced interfacial characteristic directly translates to superior mass transfer performance. Song et al. observed that CO2 MNBs water exhibited a longer nuclear magnetic resonance relaxation time than deionized water, indicating enhanced CO2 MNBs water mobility [36]. When evaluating CO2 MNBs’ systems through the mass transfer coefficient, experimental measurements revealed an 11-fold enhancement compared to traditional bubbling systems under equivalent gas conditions [30]. Moreover, as the bubble size decreases, the CO2 MNBs continuously contract under the action of surface tension. The internal pressure of the bubbles is relatively high, and the mass transfer rate is higher.
2.3. Negative Surface Charge
CO2 MNBs exhibit surface negative charges, aligning with reported interfacial properties of other gaseous MNBs [17,30,37,38]. The elevated zeta potential magnitude (|ζ|) enhances MNBs’ stability through intensified electrostatic repulsion between adjacent MNBs. Notably, while all reported ζ-values for CO2 MNBs fall within the negative range (−2.68 mV to −27 mV), a distinct dichotomy emerges: some experimental data show that their zeta potential values are in the strongly negative charge range from −8 to −27 mV, while the negative charge range recorded by other studies is relatively weak (−2.68 to −5.91 mV) [30,32,34,36,39]. This magnitude difference may be closely related to the characteristics of the solution environment (such as ionic strength, pH value) and the specificity of the bubble generation method.
Time-dependent analyses reveal dynamic interfacial charge evolution. During generation (10–70 min), ζ-potential progressively shifts from −2.68 mV to −3.05 mV concomitant with pH reduction (4.43→4.23), suggesting acid-enhanced charge density and nucleation efficiency [30]. Similarly, during storage, |ζ| amplification peaks at −19 mV after 7 days, correlating with pH elevation via CO2 outgassing at the gas–liquid interface [39]. The pH-mediated charge modulation mechanism exhibits universality. Abdella et al. confirmed air/O2/N2 MNBs dependence for pH: lower pH reduces |ζ|, whereas alkaline conditions intensify surface electronegativity and stability [17]. This consistency underscores the generalized nature of pH-responsive interfacial charge regulation in MNB systems.
2.4. Inhibition and Synergistic Effect of CO2 MNBs on Hydroxyl Radical Generation
The longstanding controversy regarding whether MNBs can generate hydroxyl radicals remains unresolved: while some experiments have detected hydroxyl radicals in water containing high-concentration MNBs [40,41], others have failed to observe detectable signals [42,43]. For these contradictory findings, Liu et al. postulated that the observed hydroxyl radical signals might originate from H2O2 produced through hydrodynamic cavitation during MNB generation, rather than the direct free radical itself [44].
CO2 MNBs generate radicals through Brownian motion-induced collapse, attributed to their low absolute zeta potential [33]. Although limited radical production was observed in acidic ethanol solutions (pH = 5), electron spin resonance analysis of CO2 mixed with hydrogen-containing gases (8% H2/Ar) revealed no detectable peaks for either superoxide anion radicals or hydroxyl radicals. This observation aligns remarkably with this study: sequential introduction of H2 and CO2 MNBs synergistically maximized radical elimination, whereas reverse sequencing (CO2 followed by H2) paradoxically increased both radical and superoxide anion concentrations. This bidirectional regulatory effect was similarly observed in superoxide anion inhibition patterns [45]. Ultrasonic collapse experiments further highlight the unique behavior of CO2 MNBs: unlike air, O2, or N2 MNBs that substantially generate reactive oxygen species, CO2 MNBs exhibit negligible hydroxyl radical production under comparable conditions [22].
Collectively, these findings demonstrate that CO2 MNBs possess dual functionality–not only suppressing radical generation during collapse, but also actively scavenging free radicals through synergistic interactions with H2 MNBs. This dual-action mechanism provides critical support for their enhanced biocompatibility and environmental remediation potential.

Table 1.
Preparation methods and properties of CO2 MNB.
Table 1.
Preparation methods and properties of CO2 MNB.
| Preparation Method | Solvent | Characterization Method | Lifetime | pH Value | Average Bubble Size | Zeta Potential | Dissolved CO2 Concentration | Mass Transfer Performance | Main Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| nanobubble membrane | Deionized water (DW) | NTA | ≤5 days (The loss was over 60% after three days) | 4~4.5 | 75–250 nm | −3.05 mV~+2.68 mV | —— | CO2 MNBs are 11 times that of macroscopic large bubbles | CO2 MNBs optimize mass transfer and buffering simultaneously through their ultra-high specific surface area and interfacial reactivity [30]. |
| DW, Tap water (TW) | Zetasizer Nano ZS, ASTM D513-92 (1996) standard measurement | —— | 3.78 (TW); 2.13 (DW) | 18.17 nm~299.5 nm (TW); 1.63 nm~216.1 nm (DW) | −5.91 mV (TW); −3.23 mV (DW) | It rose from 2 ppm to 24 ppm | —— | CO2 MNBs and biochar work in synergy to achieve the triple goals of CO2 resource utilization, soil quality improvement, efficiency enhancement, and crop yield increase [32]. | |
| DW | DLS, Zetasizer, CO2 Sensor | ≥7 days | <4.0 | 200–500 nm | −8~−19 mV | 2000 ppm | —— | The high solubility and acidic environment of CO2 MNBs can provide a basis for food processing applications [39]. | |
| periodic transformer device | DW | DLS | <48 h | —— | 41 nm and 338 nm | —— | —— | —— | CO2 MNBs take advantage of their high solubility to achieve a small size advantage, but their chemical activity limits their long-term stability [9]. |
| high-pressure fluid dynamics | DW | Zetasizer, High-temperature NMR imaging analyzer | —— | —— | 255~712 nm | −3.68 mV | —— | A longer relaxation time table of CO2 MNBs than DW indicates an increase in the fluidity of water molecules | The addition of CO2 MNBs can significantly increase the methane yield of anaerobic digestion of corn stalks (up to 17%), and the mechanisms include enhancing microbial enzyme activity, improving pH buffering capacity, and promoting substrate degradation [36]. |
| the hydrodynamic cavitation method | DW, 1 mM salt water (NaCl, CaCl2, AlCl3) | DLS, the phase analysis light scattering method | ≤5 days (DW); ≥14 days (1 mM salt water) | 4~4.5 | 160 nm (DW); 200~300 nm (1 mM salt water) | +9 mV (DW); +10 mV (1 mM salt water) | —— | —— | The stability of CO2 MNBs in salt water is enhanced due to ion inhibition dissolution [31]. |
| mechanical high-speed cavitation equipment (self-made equipment) | Aqueous solution of ethanol (10%, 30%, 50% ethanol) | DLS, NTA, the phase analysis light scattering method | 20 days | 4~5 | 500 nm (10%); 3500 nm (50%); | −5 mV~0 mV; | —— | —— | The stability of CO2 MNBs is highly dependent on pH and ethanol concentration. When acidity collapses, hydroxyl radicals are produced, but a mixture of H2 can inhibit free radicals [33]. |
| mechanical shear combined with pressure drop nucleation method | DW | Zeta Potential Analyzer | —— | 4.0 | —— | −20~−27 mV | —— | —— | The type of gas affects the stability of MNBs by altering the zeta potential. Among them, O2 MNBs and N2 MNBs are the most stable due to their high negative charges, while the stability of CO2 MNBs is weakened by an acidic environment [34]. |
3. Applications of CO2 MNBs in Different Fields
While the formation mechanisms underlying CO2 MNBs’ unique properties remain under active investigation, CO2 MNBs have demonstrated extraordinary potential across diverse aspects. Notable applications span agriculture, food processing, enhanced oil recovery, green construction, and mineral flotation, each demonstrating outstanding improvements in process sustainability and economic viability. This section systematically discusses these frontier applications and analyzes how they bring innovation and improvement in their respective fields.
3.1. Agriculture
As the primary substrate for plant photosynthesis, CO2 serves as the critical mediator for converting solar energy into biomass within agroecosystems, with its bioavailability directly governing crop growth efficiency and yield potential. Building upon the distinct physicochemical advantages of MNB technology, researchers began to explore innovative applications of CO2 MNBs in agriculture.
3.1.1. Enhanced Gas Transfer and Carbon Source Delivery
Due to their huge specific surface area and very low buoyancy, CO2 MNBs can remain stable in water for extended periods. This stability significantly enhances both the saturation concentration and retention time of dissolved CO2. Consequently, the system continuously supplies high concentrations of CO2 to plant roots. This not only directly increases the substrate availability for photosynthesis but also promotes root respiration and nutrient uptake.
In 2018, a study employed a nanoporous membrane to generate air, O2, N2, and CO2 MNBs solutions for comparative analysis of their effects on lettuce, carrot, fava bean, and tomato. Germination assays (6–10 days) revealed that all gas-infused MNB treatments enhanced germination rates relative to tap water controls, with N2 MNBs demonstrating maximum effect (100% germination after 6-day immersion), followed by CO2 MNBs (85%), both exceeding the tap water control (80%). Subsequent plant growth experiments showed that N2 MNBs significantly promoted the growth of all tested plants (fava bean, carrot and tomato) after continuous irrigation of MNB water every three days, while CO2 MNBs specifically enhanced the growth performance of fava bean and tomato, showing that the morphological indexes such as leaf number, stem length, stem diameter, and leaf length and width increased synchronously [22].
With the deepening of research, CO2 MNBs’ ability to enhance both germination rate and nutritional quality in amaranth becomes increasingly evident [46]. As shown in Figure 3 and Figure 4, treated plants exhibited optimized phenotypic indices (stem height, leaf area, root length) and increased accumulation of secondary metabolites (amino acids, vitamin C, soluble sugars, protein), showing positive correlations with MNB generator cycling time. The latest field trials have confirmed that the application of CO2 MNBs technology in the cultivation of tomatoes and kidney beans can increase the seed germination rate by 20% [32].
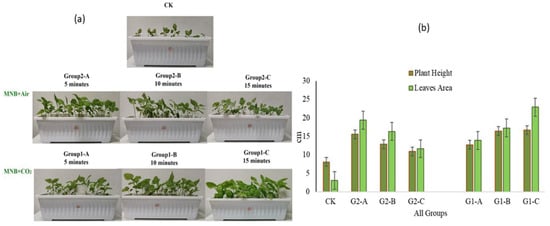
Figure 3.
(a) The plants treated with tap water served as the control group(CK) and MNBs-treated plants; (b) Effects of air MNBs and CO2 MNBs on the plant height and leaf area of amaranth; Groups 2-A, B, and C are, respectively, air MNBs circulating in the MNB generator for 5 min, 10 min, and 15 min; Groups 1-A, B, and C are, respectively, CO2 MNBs circulating in the MNB generator for 5 min, 10 min, and 15 min [46].

Figure 4.
The effects of air MNBs and CO2 MNBs on the nitrogen content (a) and protein (b) of amaranth (CK was the control group of tap water; G2-A, B, and C were, respectively, air MNBs that circulated in the MNB generator for 5 min, 10 min, and 15 min; G1-A, B, and C were, respectively, CO2 MNBs that circulated in the MNB generator for 5 min, 10 min, and 15 min [46].
3.1.2. Surface Negative Charge
The negative charge present on the surface of CO2 MNBs constitutes the core mechanism enabling their multifunctionality. Firstly, through electrostatic interactions, these surface charges effectively adsorb cationic nutrients (e.g., K+, Mg2+, NH4+) from the soil solution, significantly enhancing nutrient bioavailability [47]. The adsorbed cations migrate with the CO2 MNBs and accumulate within the plant rhizosphere, effectively mitigating the nutrient leaching loss common in traditional irrigation practices [32]. Secondly, the surface negative charges may also indirectly influence rhizosphere microbial community structure and function by affecting microbial cell membrane permeability. This effect was demonstrated in anaerobic fermentation, where CO2 MNBs increased microbial electron transport system activity by 23% and concurrently enhanced key enzyme activity. This ultimately improved corn stalk methane production efficiency by 4% to 17% while reducing secondary pollution risk [36]. In amaranth cultivation experiments, CO2 MNBs enhanced root cell membrane permeability, thereby improving the root’s capacity for water and nutrient uptake. This consequently promoted increases in stem height and leaf area expansion in amaranth [46].
3.1.3. Soil Acidification Mitigation and Nutrient Mobilization
CO2 MNBs significantly extend the dissolution time of CO2 in water, enabling the stable formation of carbonic acid. This process leads to a substantial decrease in water pH. Within the resulting acidic aqueous environment, insoluble soil nutrients (such as phosphate) and trace elements (e.g., Zn, Mn, Mg) dissolve and become efficiently activated [32,46]. Concurrently, the HCO3−/CO32− buffer system, formed synchronously upon CO2 MNB dissolution, plays a crucial role. This buffer effectively mitigates potential sharp pH drops during the acidification stage [36]. Consequently, it promotes nutrient dissolution and activation while maintaining a relatively stable microenvironment more favorable for nutrient availability.
3.2. Food Processing
Within the food industry, the functional applications of food-grade gases such as CO2, N2, O2, and their mixtures have expanded from the traditional processing, packaging, and storage to the nanoscale control field. Particularly, CO2 remains pivotal for texture modification and shelf-life extension in carbonated beverages and dairy products, attributed to its high aqueous solubility (1.45 g/L at 25 °C), chemical inertness, and purification feasibility. Leveraging their unique physicochemical characteristics, CO2 MNBs are revolutionizing food processing through breakthrough applications in component separation, rheology control, crystallization optimization, and interfacial engineering.
3.2.1. Efficient Nucleation Medium
Truong’s research group has pioneered systematic investigations into CO2-mediated targeted regulation of fat and sugar crystallization for food applications since 2017. Initial studies revealed CO2’s exceptional solubility in anhydrous milk fat (AMF), demonstrating its capacity to fundamentally alter crystallization kinetics: by promoting nucleation and accelerating α-type fat crystals formation, CO2 effectively modulated AMF’s macroscopic hardness through concurrent crystal size reduction and population enhancement [48,49]. These findings established critical physicochemical foundations for dairy product microstructure and rheological properties. Building upon this foundation, the team utilized the mechanical method (mechanical stirring or ultrasonication) to generate CO2 MNBs. In supersaturated lactose solutions, CO2 MNBs functioned as highly efficient nucleation sites, inducing morphological transition of α-lactose monohydrate crystals from tomahawk configurations to regular triangular or trapezoidal geometries. At the same time, the crystal yield is increased, and the crystal size is reduced without affecting the crystal density field [50]. This conclusion is consistent with the findings in similar systems [51]. In the follow-up work, the team verified the universality of CO2 MNBs technology in butter processing and ultrasonic-assisted AMF crystallization [52,53,54]. The latest research in 2024 shows that CO2 MNBs generated in palm oil by nanoporous membrane method can significantly optimize its crystallization characteristics: at 23 °C and 4 °C, CO2 MNBs all initiate crystallization in advance, forming finer and more uniform crystals, while lowering the melting end temperature and increasing the melting enthalpy, and finally obtaining the crystallized product with enhanced surface gloss and soft texture as shown in Figure 5, which is of great value for the development of grease products such as butter and shortening [55]. The team further found that the nucleation effect of CO2 MNBs can be extended to the freezing field: in sugar solution containing solute, CO2 MNBs induced by ultrasound can effectively increase the nucleation temperature of ice crystals and shorten the freezing time by providing a large number of nucleation sites, which is confirmed in the gelatin system [56,57]. Cross-system verification shows that CO2 MNBs can accelerate the freezing setting of ice cream, concentrated apple juice, and milk, and improve their texture softness and anti-melting, which highlights the multifunctional regulation potential of CO2 MNBs in food processing and freezing engineering [58,59]. A series of research by the Truong team revealed that CO2 MNBs had precisely controlled crystal morphology, size, and phase transition through a physical nucleation mechanism, which provided important theoretical support for green processing technology innovation.
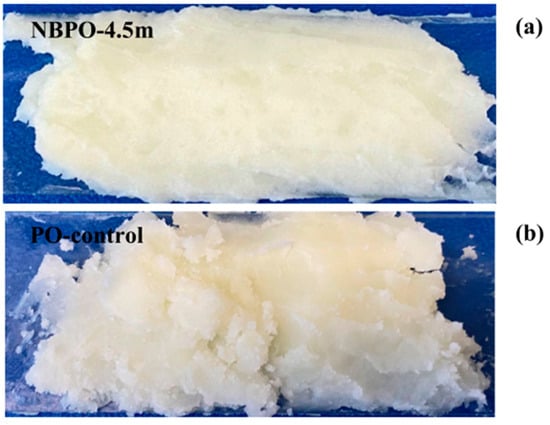
Figure 5.
Visual observation of palm oil with (a) and without (b) CO2 MNBs [55].
3.2.2. Synergistic Effect of High Stability and Surface Negative Charge
In the field of regulating the rheological properties of food, it was discovered that introducing air, N2, O2, CO2, and their mixed gases into the dairy products system can significantly reduce the viscosity of liquid and maintain the stability effect for three days [60]. It is pointed out that this effect is not only due to the high stability of CO2 MNBs, but also related to the negative charge characteristics carried on their surfaces: CO2 MNBs, casein, and other particles with similar charges in the solution produce polarity synergy and form a buffer layer between nanoparticles through charge interaction, which promotes particle separation and inhibits aggregation behavior, thus reducing the viscosity of the system. As shown in Figure 6, generated CO2 MNBs in apple juice concentrate and canola oil via nanoporous membranes achieved 18% and 10% viscosity reduction, respectively, and persisted for seven days without altering pH, density, or soluble solids [61]. These findings collectively revealed the dual advantages of CO2 MNBs: on the one hand, it optimizes the fluidity of food processing fluid through the physical mechanism to improve production efficiency; on the other hand, it avoids the use of traditional chemical additives, which provides an innovative path for developing cleanable food and realizing environment-friendly production.

Figure 6.
Apparent viscosity of concentrated apple juice APJC (a) and rapeseed oil (CANO) (b) after treatment with CO2 MNBs and their controls. Time is the cycle time of the MNBs’ generator field [61].
Truong’s team recently pioneered a breakthrough using nanoporous membranes to generate CO2 MNBs in apple juice systems, demonstrating that CO2 MNBs can significantly reduce the surface tension of liquid and have no significant effect on the density of the matrix field [62]. This discovery provides an experimental basis for physical modification technology to replace chemical surfactants. Although the current research has been confirmed in the apple juice system, the universality of this technology to the surface tension of other food liquids still needs to be systematically verified. By using the interfacial activity characteristics of CO2 MNBs, the food processing performance can be optimized without introducing additives, which is of dual significance to food development and sustainable production mode construction in clean label.
3.2.3. Enhanced Interfacial Enrichment via Ultra-Low Buoyancy
In the field of food component separation technology, it is found that the hydrophobic region of hydrophobic can form a complex with CO2 molecules through intermolecular forces, and self-assembly generates MNBs (110–120 nm). Taking advantage of this feature, the research team used CO2 foam fractionation to extract protein after its fermentation, which successfully increased the concentration of hydrophobic compounds by four times. In this process, although nano-spray drying led to the partial decrease in protein activity, the inactivation phenomenon was effectively suppressed by pre-adding surfactants. Because of the low density of CO2 MNBs, the enrichment of CO2 MNBs at the interface of the two phases increased the purity of hydrophobic by 2.8 times. This method not only simplifies the chromatographic purification steps, reduces the cost, and maintains the activity of hydrophobic, but also shows the technical breakthrough of CO2 MNBs in the field of biomolecule separation [63].
3.3. CO2 EOR and CGS
With the continuous growth of global energy demand and the gradual depletion of conventional oil and gas resources, reservoir development faces serious challenges. Deep saline formations and oil reservoirs—considered ideal sites for geological CO2 storage—exhibit declining recovery rates and limited remaining recoverable reserves due to their development stage having entered the middle and late stages. Conventional CO2-EOR remains constrained by technical bottlenecks such as gas channeling, gravity override, and insufficient gas–reservoir contact time, further exacerbating energy supply limitations. Meanwhile, increasingly stringent environmental regulations have highlighted the carbon emissions of oil and gas development. In this part, CO2 MNBs effectively suppress viscous fingering and gravitational override phenomena, enhance sweep efficiency and volumetric displacement of CO2, and simultaneously improve CO2-EOR and CGS efficacy. This technology has recently gained traction as an innovative direction in oil and gas extraction.
3.3.1. Micro-Nano Size and Efficient Mass Transfer
The development history of CO2 MNBs in CO2-EOR and CGS has shown significant phased breakthroughs. Early basic research verified the permeation enhancement effect of CO2 MNBs in microporous media [64]. Their nanoscale size and reduced capillary force can effectively expand the contact area at the CO2-crude oil or water interface [65]. Subsequent simulations and experiments confirmed that supercritical CO2 MNBs can achieve a uniform distribution of high saturation in saline porous media, thereby improving sweep efficiency and mitigating gravitational override [66]. The pore-scale observation of Berea sandstone through X-ray computed tomography revealed the mechanism by which CO2 MNBs activate the low-permeability area by broadening the flow channel network [67]. Systematic studies have further clarified that CO2 MNBs offer dual advantages in enhancing crude CO2-EOR and improving CGS safety by strengthening mass transfer efficiency and dissolution kinetics [68,69,70,71,72]. In recent years, it was found that CO2 MNBs can not only improve the CO2-EOR efficiency by contacting the residual oil on the pore wall, but also dynamically regulate the seepage path by using the “Jamin effect”, with the help of a visual microfluidic platform [73].
3.3.2. High Stability
The latest experiments show that CO2 MNBs, by modifying nano-SiO2 particles, maintained the size stability by electrostatic adsorption and hydrogen bonding, and inhibited CO2 overflow. The constructed system has the advantages of temperature resistance, oil resistance, dimensional stability, and anti-wetting. The oil displacement experiment shows that the oil recovery ratio is increased by 17.64% compared with conventional CO2-EOR, and the risk of CO2 channeling is significantly reduced [74].
The high stability and low buoyancy of CO2 MNBs enable long-term discrete-phase storage in formation water, substantially lowering CGS leakage risks [70]. Field studies of Tokyo’s Setagaya-ku shallow aquifers revealed that injected CO2 MNBs persist for one day while gradually displacing groundwater(Figure 7) [35]. Notably, CO2 MNBs generation requires no chemical additives, aligning with green development principles. Although the understanding of CO2 MNBs’ stability and phase transitions under high-temperature and high-pressure conditions requires further investigation, their demonstrated recovery enhancement and storage safety have been extensively validated.

Figure 7.
Schematic diagram of a small-scale in situ injection test. The CO2 gas is conveyed to the MNB generator through the packer and forms bubbles in Well A [35].
3.3.3. Efficient Nucleation Medium
As a clean energy resource possessing reserves that far exceed traditional fossil fuels, natural gas hydrate extraction faces multifaceted challenges in exploration, technological implementation, and environmental risk management. Research reveals that CO2 MNBs accelerate hydrate formation by increasing nucleation frequency through shortened induction times [75].
3.3.4. Surface Negative Charge
Furthermore, CO2 MNBs demonstrate unique potential in microbial enhanced oil recovery: their surface charge characteristics enhance cation mass transfer efficiency in anaerobic environments. This mechanism enhances both the microbial activity and metabolite production [76,77].
Comprehensively, CO2 MNBs represent a breakthrough, addressing traditional technological limitations through multidimensional innovation. They demonstrate distinctive advantages in enhancing CO2-EOR, strengthening CGS, facilitating hydrate development, and advancing microbial processes. With ongoing refinements in MNB fundamental theories and engineering applications, this technology promises to catalyze sustainable development in petroleum extraction.
3.4. Construction and Mining
Extending the concrete structure has emerged as a pivotal strategy for reducing environmental impacts and optimizing resources in construction. While CO2-induced calcium ion carbonation generates pore-filling calcium carbonate, simultaneously densifying concrete surfaces and enabling crack self-healing, active regulation of atmospheric carbonation processes remains technically challenging.
3.4.1. Enhanced Gas Transfer and Carbon Source Delivery
Recent studies have shown that CO2 MNBs can significantly enhance the carbonization efficiency and optimize the material repair performance. Through experimental verification, concurrent calcium carbonate formation on both crack surfaces and interior regions significantly boosts self-healing capacity [78]. Further research has found that CO2 MNBs rapidly fill pores and accelerate precipitation reactions, achieving mass transfer efficiency far surpassing conventional CO2 gas (Figure 8) [79]. They innovatively combined calcium nitrite with CO2 MNBs water, revealing that structural densification via increased calcium carbonate production effectively reduces carbonation depth and porosity while enhancing mechanical properties [80].

Figure 8.
Schematic representation of carbonization of CO2 gas (left) and CO2 MNBs (right) [79].
In construction waste treatment, CO2 MNBs technology exhibits distinctive environmental benefits by utilizing its acidic properties to neutralize alkaline concrete wastewater, achieving a 1–2% increase in compressive strength of recycled cement paste [81]. Experimental studies show that CO2 MNBs enhance calcium carbonate deposition in recycled cement paste by 110%, dramatically reduce reaction duration, and boost CO2 utilization efficiency from 31.9% to 82.2%, with concurrent increases in specific surface area of porous calcium carbonate particles [82].
For mine filling materials, cementitious composites incorporating CO2 MNBs were developed to optimize pore structure. These materials exhibit improved compressive strength and hydration stability after 28-day curing, offering a novel low-carbon solution for sustainable mining [83,84].
3.4.2. Surface Negative Charge
CO2 MNBs exhibit multi-scale collaborative regulation capabilities in the mineral flotation system. For pyrite flotation, CO2 suppresses surface oxidation to maintain hydrophobicity while enhancing flotation recovery [85]. Subsequent work revealed rapid CO2 MNBs formation on pyrite surfaces in CO2-saturated solutions [86]. Atomic force microscopy and molecular dynamics simulations identified unique CO2 MNBs adhesion and diffusion mechanisms, facilitating millimeter-scale N2 bubble attachment through multimolecular structuring and synergistically reducing surface oxidation. Lian et al. pioneered a hybrid approach combining CO2 MNBs with microemulsion trapping: Firstly, microemulsion is used to improve the surface hydrophobicity of low-rank coal, and then, CO2 MNBs are induced into the flotation system, which can be selectively adsorbed on the surface of low-rank coal through its surface charge, thus weakening the electrostatic repulsion between coal particles, promoting the aggregation of coal particles and improving the collision probability between bubbles and particles, and finally, realizing the systematic optimization of flotation performance [87].
Comprehensive research shows that CO2 MNBs water forms a technical closed loop in enhancing the performance of building materials, reducing cement consumption, carbon sequestration, and emission reduction, and recycling waste through the synergistic effect of efficient mass transfer and chemical reaction, which opens up an innovative path for realizing the sustainable development of the construction industry and mining industry.
3.5. Anaerobic Digestion
With the increasing global focus on renewable energy and environmental protection, anaerobic digestion has garnered significant attention for its dual advantages in simultaneous waste treatment and energy recovery. This technology utilizes microorganisms to decompose organic matter in an anoxic environment, producing renewable biogas (primarily methane) that can be used for power or heat generation. However, substrate degradation, microbial activity, and reaction conditions often constrain its efficiency, making improving digestion efficiency and gas production a critical research priority. Recent advancements in CO2 MNBs technology have introduced an innovative approach to address these limitations.
3.5.1. Enhanced Gas Transfer and Carbon Source Delivery
Early basic research first confirmed that injecting CO2 MNBs into waste-activated sludge significantly enhanced biogas and methane production. The mechanism of action stems from the enhanced nutrient transfer efficiency at the bubble interface, thereby accelerating substrate decomposition [88]. This discovery lays the foundation for subsequent technological expansion: In the anaerobic digestion system of pig manure, CO2 MNBs not only increase the gas production, but also accelerate the hydrolysis and acidification process and improve the biological activity of the system by enhancing the respiratory activity of microorganisms [89].
3.5.2. Buffering pH Fluctuation and Stabilizing Anaerobic Digestion
Further mechanism research revealed the steady-state regulatory function of CO2 MNBs: by dynamically buffering pH fluctuations and promoting the conversion of volatile fatty acids, thereby mitigating acid accumulation and methane inhibition. Molecular-scale analysis indicated that CO2 MNBs enhanced hydrolysis enzyme activity while optimizing water molecule mobility and nutrient transfer efficiency [90]. Expanding on these findings, verifying that CO2 MNBs reduced cellulose crystallinity through intensified microbial metabolic activity on high-loading cellulosic systems, concurrently improving hydrolysis-acidification efficiency and methane yield on high-loading cellulosic systems [91].
In summary, CO2 MNBs offer a critical optimization pathway for anaerobic digestion technology through three key mechanisms: pH regulation, enhancement of microbial metabolic environments, and improved substrate decomposition efficiency. The distinctive physicochemical characteristics of CO2 MNBs not only facilitate the efficient conversion of organic matter into biogas with reduced treatment costs and environmental risks but also establish a novel strategy for achieving resource recovery from organic waste. This dual functionality positions the technology as a promising solution with broad application prospects in both environmental protection and sustainable energy sectors.
3.6. Microalgae Cultivation
Microalgae utilize atmospheric CO2 through photosynthesis while assimilating nutrients such as carbon, nitrogen, and phosphorus from the water column. This dual functionality reduces atmospheric greenhouse gas concentrations and mitigates aquatic eutrophication. As a versatile feedstock for high-value-added products, including biofuels, vitamins, and biochar, microalgae demonstrate significant economic and environmental potential in climate change mitigation, driving growing global demand. However, ambient CO2 limitations and pH elevation induced by photosynthesis constrain natural carbon sequestration rates in microalgae. Elevated pH shifts inorganic carbon speciation toward HCO3− and CO32−, thereby reducing dissolved CO2 availability and ultimately impairing carbon fixation efficiency.
Enhanced Gas Transfer and Carbon Source Delivery
To address carbon utilization inefficiencies, researchers have investigated CO2 supplementation techniques to enhance the algal CO2 utilization ratio while lowering water pH [92,93,94]. At present, the most common supplement technology is the continuous bubbling method. Continuously bubbling air containing 10% CO2 into the algae membrane bioreactor improves the productivity and denitrification capacity of microalgae by 40% and reduces membrane pollutants in the algae membrane bioreactor [95]. However, the mass transfer efficiency of millimeter-to-centimeter bubbles generated by the traditional bubbling method is low, and the utilization rate of CO2 is limited. This limitation has spurred innovation in aerator design. The latest research boosted biomass productivity by 33.33% through bubble size reduction (27.97% smaller diameter, 46.88% lower rise velocity) by optimizing the aeration device [96]. Previous studies on optimizing aeration devices have jointly confirmed the promoting effect of smaller bubbles on growth, although these studies have all focused on millimeter-scale systems [97,98,99,100,101,102].
Early studies used MBs (air containing 20% CO2) of 300 μm and 500 μm for the first time to cultivate Dunaliella salina, and its mass transfer efficiency, dissolved oxygen removal ability, growth rate, and biomass density were significantly improved [103]. Later, a study uses CO2 MBs to obtain a similar conclusion [104]. However, research points out that the growth efficiency of microalgae is related to the radiation characteristics of bubbles. Experiments show that when the bubble volume fraction is 0.003 and the radius is 3.5 mm, microalgae growth and CO2 fixation are the best [105]. The latest technological breakthrough focuses on the application of MNBs. CO2 MNBs generated by ceramic nozzles under acidic conditions, with their high density and stability, retain more non-ionic CO2 in the culture medium, which promotes the cell yield and vitamin content of green algae to increase by 1.78 times and 1.5 times, respectively [106].
It is worth noting that all the CO2 MNBs studied above are MNBs produced by the gas mixed with CO2 and air or nitrogen. In the future, we can continue to explore the influence of mixing different concentrations of CO2 on algae growth in the field of microalgae culture. CO2 MNBs can not only enhance gas–liquid mass transfer but also increase the concentration of dissolved carbon in suspension. It is believed that CO2 MNBs can break through the traditional carbon source supply limitation in the field of microalgae culture, significantly improve the photosynthetic efficiency and biomass accumulation rate, and at the same time reduce the energy consumption cost by optimizing the carbon capture process, thus providing efficient and sustainable technical support for the large-scale production of high value-added microalgae products and the realization of carbon neutrality.
3.7. Industrial Crystallization
In addition to the application of optimizing crystallization in the food field, CO2 MNBs, as a new green additive that does not introduce external impurities and does not participate in the reaction, have also received extensive attention in the industrial field in recent years.
3.7.1. Efficient Nucleation Medium
Early basic research induced nucleation of CO2 bubbles through rapid pressure reduction at the gas–liquid interface, found that it can increase nucleation active sites, thereby accelerating ultrafine particle generation [107]. This principle was subsequently extended to polycrystalline systems: in complex systems such as curcumin, poultry pulp, piroxone, and cholesterol, CO2 MNBs demonstrated nucleation-promoting effects, verifying its technical applicability across substance categories [108,109,110,111]. An early method was developed to prepare Li2CO3 nanoparticles by microwave irradiation of CO2 MNBs’ solution. It was found that the crystallization rate of nanoparticles could be significantly improved by adjusting the bubble size and heating rate, in which the smaller the bubble size, the higher the heating rate and the stronger the crystallization rate, indicating that CO2 MNBs as a green additive can optimize the mass transfer process of cooling crystallization system, thus improving the nucleation rate and crystal growth [112]. Huang et al. identified a dual role of CO2 MNBs in high-temperature supersaturated solutions: while surface-adherent bubbles may physically disrupt crystal formation, their dominant effect enhances solution thermodynamics and kinetics, ultimately promoting crystallization efficiency [113,114].
3.7.2. Surface Negative Charge
In addition, the CO2 MNBs proposed by Kimura et al. can adsorb cations with negative charges on their surfaces, forming local supersaturated regions, thus inducing the formation of finer crystal particles [115]. The common surface of these studies is that CO2 MNBs can be used as efficient and environmentally friendly crystallization control means under different mechanisms.
4. Summary and Outlook
With unique physicochemical properties, CO2 MNBs have emerged as a transformative technology with cross-industrial applications. This comprehensive review systematically analyzes their innovative implementations in agriculture, food processing, oil/gas extraction, construction, mining, anaerobic digestion, microalgae cultivation, and industrial processes. CO2 MNBs reveal their excellent advantages of upgrading the traditional technology through high mass transfer efficiency, high stability, and surface negative charge. Among these properties, stability and surface negative charge play critical roles. Long-term stability is essential for ensuring CO2 MNBs maintain their function over extended periods, enabling them to sustain both a high-concentration CO2 microenvironment and high mass transfer efficiency. The surface negative charge drives specific interactions (such as electrostatic attraction or repulsion) between CO2 MNBs and other environmental components, thereby influencing related reaction processes and underlying mechanisms. It is particularly noteworthy that CO2 MNBs technology realizes the efficient utilization of gas resources through physical means, which provides a green technical path for the realization of carbon neutrality.
Although great progress has been made in the application research of CO2 MNBs, many characteristics and preparation aspects of CO2 MNBs are still relatively lacking. The following aspects can be further studied in the future: (1) To deepen the theoretical research, establishing the kinetic model of CO2 MNBs and the quantitative description of the interfacial reaction mechanism; (2) To develop a large-scale generator with high efficiency and low energy consumption to break through the technical bottleneck of engineering application; (3) To expand multi-technology collaborative application scenarios and expand the application scope of CO2 MNBs and explore the coupling innovation of CO2 MNBs with electrochemical catalysis, membrane separation, and other technologies; (4) To establish an industry standard system and formulate technical specifications and safety assessment standards for different application scenarios. With the collaborative breakthrough of basic research and engineering technology, CO2 MNBs technology is expected to develop into an important technical platform to support green manufacturing and sustainable development.
Author Contributions
Conceptualization by Z.Z. and L.Z.; original draft writing by Z.Z.; review and editing by X.W., T.T., J.H., X.Z. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 12274427, 22393934), the National Key R&D Program of China (2022YFA1603600), and the Young Scientists Fund of the National Natural Science Foundation of China (No. 12104469).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal Control Knob Governing Earth’s Temperature. Science 2010, 330, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the Greenhouse Effect and Global Warming: From the Pioneering Work of Arrhenius and Callendar to Today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary Advancements in Carbon Dioxide Valorization via Metal-Organic Framework-Based Strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Liu, H.J.; Were, P.; Li, Q.; Gou, Y.; Hou, Z. Worldwide Status of CCUS Technologies and Their Development and Challenges in China. Geofluids 2017, 2017, 6126505. [Google Scholar] [CrossRef]
- Huang, B.; Xu, S.; Gao, S.; Liu, L.; Tao, J.; Niu, H.; Cai, M.; Cheng, J. Industrial Test and Techno-Economic Analysis of CO2 Capture in Huaneng Beijing Coal-Fired Power Station. Appl. Energy 2010, 87, 3347–3354. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T. Preparation Method and Application of Nanobubbles: A Review. Coatings 2023, 13, 1510. [Google Scholar] [CrossRef]
- Lou, S.-T.; Ouyang, Z.-Q.; Zhang, Y.; Li, X.-J.; Hu, J.; Li, M.-Q.; Yang, F.-J. Nanobubbles on Solid Surface Imaged by Atomic Force Microscopy. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2000, 18, 2573–2575. [Google Scholar] [CrossRef]
- Tan, B.H.; An, H.; Ohl, C.-D. Stability of Surface and Bulk Nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 53, 101428. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Qi, N.; Qin, Y.; Zhang, X.; Li, Y. Generation and Stability of Size-Adjustable Bulk Nanobubbles Based on Periodic Pressure Change. Sci. Rep. 2019, 9, 1118. [Google Scholar] [CrossRef]
- Discher, B.M.; Bermudez, H.; Hammer, D.A.; Discher, D.E.; Won, Y.-Y.; Bates, F.S. Cross-Linked Polymersome Membranes: Vesicles with Broadly Adjustable Properties. J. Phys. Chem. B 2002, 106, 2848–2854. [Google Scholar] [CrossRef]
- Lohse, D.; Zhang, X. Surface Nanobubbles and Nanodroplets. Rev. Mod. Phys. 2015, 87, 981–1035. [Google Scholar] [CrossRef]
- Craig, V.S.J. Very Small Bubbles at Surfaces—The Nanobubble Puzzle. Soft Matter 2010, 7, 40–48. [Google Scholar] [CrossRef]
- Ji, X.; Liu, C.; Pan, G. Interfacial Oxygen Nanobubbles Reduce Methylmercury Production Ability of Sediments in Eutrophic Waters. Ecotoxicol. Environ. Saf. 2020, 188, 109888. [Google Scholar] [CrossRef]
- Haris, S.; Qiu, X.; Klammler, H.; Mohamed, M.M.A. The Use of Micro-Nano Bubbles in Groundwater Remediation: A Comprehensive Review. Groundw. Sustain. Dev. 2020, 11, 100463. [Google Scholar] [CrossRef]
- Matsumoto, M. Surface Tension and Stability of a Nanobubble in Water: Molecular Simulation. J. Fluid Sci. Technol. 2008, 3, 922–929. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, L.; Liu, G. Basic Characteristics and Application of Micro-Nano Bubbles in Water Treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 510, 042050. [Google Scholar] [CrossRef]
- Khaled Abdella Ahmed, A.; Sun, C.; Hua, L.; Zhang, Z.; Zhang, Y.; Marhaba, T.; Zhang, W. Colloidal Properties of Air, Oxygen, and Nitrogen Nanobubbles in Water: Effects of Ionic Strength, Natural Organic Matters, and Surfactants. Environ. Eng. Sci. 2018, 35, 720–727. [Google Scholar] [CrossRef]
- Singh, A.; Sekhon, A.S.; Unger, P.; Babb, M.; Yang, Y.; Michael, M. Impact of Gas Micro-nano-bubbles on the Efficacy of Commonly Used Antimicrobials in the Food Industry. J. Appl. Microbiol. 2021, 130, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiao, W.; Pu, W.; Hu, J.; Zhao, J.; Zhang, L. CH4 Nanobubbles on the Hydrophobic Solid–Water Interface Serving as the Nucleation Sites of Methane Hydrate. Langmuir 2018, 34, 10181–10186. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Ma, X.; He, W.; Liu, C.; Liu, Z. Functional Micro/Nanobubbles for Ultrasound Medicine and Visualizable Guidance. Sci. China Chem. 2021, 64, 899–914. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.; Park, H. Micro and Nanobubble Technologies as a New Horizon for Water-Treatment Techniques: A Review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124. [Google Scholar] [CrossRef]
- Jin, N.; Zhang, F.; Cui, Y.; Sun, L.; Gao, H.; Pu, Z.; Yang, W. Environment-Friendly Surface Cleaning Using Micro-Nano Bubbles. Particuology 2022, 66, 1–9. [Google Scholar] [CrossRef]
- Rosa, A.F.; Rubio, J. On the Role of Nanobubbles in Particle–Bubble Adhesion for the Flotation of Quartz and Apatitic Minerals. Miner. Eng. 2018, 127, 178–184. [Google Scholar] [CrossRef]
- Tao, H.; Chun, Y.E.; Chun-Hua, L.I.; Bao-Jun, Z.; Lei, Z. Treatment effect of microbubble aeration technology on black-odor river water. J. Environ. Eng. Technol. 2011, 1, 20–25. [Google Scholar] [CrossRef]
- Chu, L.-B.; Xing, X.-H.; Yu, A.-F.; Zhou, Y.-N.; Sun, X.-L.; Jurcik, B. Enhanced Ozonation of Simulated Dyestuff Wastewater by Microbubbles. Chemosphere 2007, 68, 1854–1860. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Ma, H.; Huang, P.; Li, J.; Kikuchi, T. Effect of Micro-Bubbles on Coagulation Flotation Process of Dyeing Wastewater. Sep. Purif. Technol. 2010, 71, 337–346. [Google Scholar] [CrossRef]
- Weijs, J.H.; Seddon, J.R.T.; Lohse, D. Diffusive Shielding Stabilizes Bulk Nanobubble Clusters. ChemPhysChem 2012, 13, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and Air Nanobubble Water Solution Promote the Growth of Plants, Fishes, and Mice. PLoS ONE 2013, 8, e65339. [Google Scholar] [CrossRef]
- Antonio Cerrón-Calle, G.; Luna Magdaleno, A.; Graf, J.C.; Apul, O.G.; Garcia-Segura, S. Elucidating CO2 Nanobubble Interfacial Reactivity and Impacts on Water Chemistry. J. Colloid Interface Sci. 2022, 607, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, Z.; He, C.; Feng, Q.; Wang, K.; Wang, Y.; Luo, N.; Dodbiba, G.; Wei, Y.; Otsuki, A.; et al. Long-Term Stability of Different Kinds of Gas Nanobubbles in Deionized and Salt Water. Materials 2021, 14, 1808. [Google Scholar] [CrossRef]
- Singh, E.; Kumar, A.; Lo, S.-L. Synergistic Roles of Carbon Dioxide Nanobubbles and Biochar for Promoting Direct CO2 Assimilation by Plants and Optimizing Nutrient Uptake Efficiency. Environ. Res. 2024, 244, 117918. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Kurokawa, H.; Matsui, H.; He, C.; Wang, K.; Wei, Y.; Dodbiba, G.; Otsuki, A.; Fujita, T. Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution. Nanomaterials 2022, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Ushikubo, F.Y.; Enari, M.; Furukawa, T.; Nakagawa, R.; Makino, Y.; Kawagoe, Y.; Oshita, S. Zeta-Potential of Micro- and/or Nano-Bubbles in Water Produced by Some Kinds of Gases. IFAC Proc. Vol. 2010, 43, 283–288. [Google Scholar] [CrossRef]
- Takemura, T.; Hamamoto, S.; Sato, M.; Suzuki, K.; Okuzawa, K. Migration Behavior and Lifetime of CO2 Micro-Nano Bubbles in Shallow Aquifer. Int. J. Greenh. Gas Control 2024, 137, 104207. [Google Scholar] [CrossRef]
- Song, H.; Hou, T.; Jiao, Y.; Liu, L.; Pan, X.; Li, G.; Zhang, Q.; Zeng, Y.; Cui, Z.; Li, P.; et al. Supplementation of CO2-Nanobubble Water to Enhance the Methane Production from Anaerobic Digestion of Corn Straw. Chemosphere 2023, 313, 137613. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. ζ Potential of Microbubbles in Aqueous Solutions: Electrical Properties of the Gas−water Interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, J.-M. Generation and Stability of Bulk Nanobubbles. Langmuir 2017, 33, 3818–3823. [Google Scholar] [CrossRef]
- Phan, K.K.T.; Truong, T.; Wang, Y.; Bhandari, B. Formation and Stability of Carbon Dioxide Nanobubbles for Potential Applications in Food Processing. Food Eng. Rev. 2021, 13, 3–14. [Google Scholar] [CrossRef]
- Takahashi, M.; Shirai, Y.; Sugawa, S. Free-Radical Generation from Bulk Nanobubbles in Aqueous Electrolyte Solutions: ESR Spin-Trap Observation of Microbubble-Treated Water. Langmuir 2021, 37, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Chiba, K.; Li, P. Free-Radical Generation from Collapsing Microbubbles in the Absence of a Dynamic Stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.H.; Kim, M.S.; Kim, J.-H.; Fortner, J.D. Nanobubble Reactivity: Evaluating Hydroxyl Radical Generation (or Lack Thereof) under Ambient Conditions. ACS EST Eng. 2023, 3, 1504–1510. [Google Scholar] [CrossRef]
- Tada, K.; Maeda, M.; Nishiuchi, Y.; Nagahara, J.; Hata, T.; Zhuowei, Z.; Yoshida, Y.; Watanabe, S.; Ohmori, M. ESR Measurement of Hydroxyl Radicals in Micro-Nanobubble Water. Chem. Lett. 2014, 43, 1907–1908. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS Produced by Nanobubbles and Their Positive and Negative Effects on Vegetable Seed Germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef]
- Fujita, T.; Kurokawa, H.; Han, Z.; Zhou, Y.; Matsui, H.; Ponou, J.; Dodbiba, G.; He, C.; Wei, Y. Free Radical Degradation in Aqueous Solution by Blowing Hydrogen and Carbon Dioxide Nanobubbles. Sci. Rep. 2021, 11, 3068. [Google Scholar] [CrossRef]
- Khan, P.; Wang, H.; Gao, W.; Huang, F.; Khan, N.A.; Shakoor, N. Effects of Micro-Nano Bubble with CO2 Treated Water on the Growth of Amaranth Green (Amaranthus Viridis). Environ. Sci. Pollut. Res. 2022, 29, 72033–72044. [Google Scholar] [CrossRef]
- Xue, S.; Gao, J.; Liu, C.; Marhaba, T.; Zhang, W. Unveiling the Potential of Nanobubbles in Water: Impacts on Tomato’s Early Growth and Soil Properties. Sci. Total Environ. 2023, 903, 166499. [Google Scholar] [CrossRef]
- Truong, T.; Palmer, M.; Bansal, N.; Bhandari, B. Effect of Solubilised Carbon Dioxide at Low Partial Pressure on Crystallisation Behaviour, Microstructure and Texture of Anhydrous Milk Fat. Food Res. Int. 2017, 95, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Palmer, M.; Bansal, N.; Bhandari, B. Investigation of Solubility of Carbon Dioxide in Anhydrous Milk Fat by Lab-Scale Manometric Method. Food Chem. 2017, 237, 667–676. [Google Scholar] [CrossRef]
- Xun, A.; Truong, T.; Bhandari, B. Effect of Carbonation of Supersaturated Lactose Solution on Crystallisation Behaviour of Alpha-Lactose Monohydrate. Food Biophys. 2017, 12, 52–59. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Truong, T.; Bansal, N.; Bhandari, B. Influence of Gas Addition on Crystallisation Behaviour of Lactose from Supersaturated Solution. Food Bioprod. Process. 2018, 109, 86–97. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Truong, T.; Bansal, N.; Bhandari, B. Effect of CO2 Bubbles on Crystallization Behavior of Anhydrous Milk Fat. J. Am. Oil Chem. Soc. 2020, 97, 363–375. [Google Scholar] [CrossRef]
- Truong, T.; Palmer, M.; Bansal, N.; Bhandari, B. Effect of Dissolved Carbon Dioxide on the Sonocrystallisation and Physical Properties of Anhydrous Milk Fat. Int. Dairy J. 2019, 93, 45–56. [Google Scholar] [CrossRef]
- Truong, T.; Palmer, M.; Bansal, N.; Bhandari, B. Effects of Dissolved Carbon Dioxide in Fat Phase of Cream on Manufacturing and Physical Properties of Butter. J. Food Eng. 2018, 226, 9–21. [Google Scholar] [CrossRef]
- Phan, K.; Truong, T.; Wang, Y.; Bhandari, B. Impact of Carbon Dioxide Nanobubbles on Crystallising Properties of Palm Oil. Food Bioprocess Technol. 2024, 17, 4290–4302. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, M.; Bhandari, B.; Sun, J.; Gao, Z. Infusion of CO2 in a Solid Food: A Novel Method to Enhance the Low-Frequency Ultrasound Effect on Immersion Freezing Process. Innov. Food Sci. Emerg. Technol. 2016, 35, 194–203. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Tung, V.P.; Truong, T.; Bansal, N.; Bhandari, B. Water Crystallisation of Model Sugar Solutions with Nanobubbles Produced from Dissolved Carbon Dioxide. Food Biophys. 2019, 14, 403–414. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Impact of Incorporation of CO2 on the Melting, Texture and Sensory Attributes of Soft-Serve Ice Cream. Int. Dairy J. 2020, 109, 104789. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Tung, V.P.; Truong, T.; Bansal, N.; Bhandari, B. Impact of In-Situ CO2 Nano-Bubbles Generation on Freezing Parameters of Selected Liquid Foods. Food Biophys. 2020, 15, 97–112. [Google Scholar] [CrossRef]
- Amamcharla, J.; Li, B.; Liu, Z. Use of Micro- and Nano-Bubbles in Liquid Processing. WO2017127636A1, 27 July 2021. [Google Scholar]
- Phan, K.; Truong, T.; Wang, Y.; Bhandari, B. Effect of CO2 Nanobubbles Incorporation on the Viscosity Reduction of Fruit Juice Concentrate and Vegetable Oil. Int. J. Food Sci. Technol. 2021, 56, 4278–4286. [Google Scholar] [CrossRef]
- Phan, K.; Truong, T.; Wang, Y.; Bhandari, B. Generation and Influence of Carbon Dioxide Nanobubbles on Physicochemical Properties Including the Surface Tension of Clarified Apple Juice. Food Biophys. 2024, 19, 131–142. [Google Scholar] [CrossRef]
- Khalesi, M.; Gebruers, K.; Riveros-Galan, D.; Deckers, S.; Moosavi-Movahedi, A.A.; Verachtert, H.; Derdelinckx, G. Hydrophobin Purification Based on the Theory of CO2-Nanobubbles. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 111–118. [Google Scholar] [CrossRef]
- Xue, Z.; Yamada, T.; Matsuoka, T.; Kameyama, H.; Nishio, S. Carbon Dioxide Microbubble Injection–Enhanced Dissolution in Geological Sequestration. Energy Procedia 2011, 4, 4307–4313. [Google Scholar] [CrossRef]
- Xue, Z.; Park, H.; Ueda, R.; Nakano, M.; Nishii, T.; Inagaki, S. Microbubble CO2 Injection for Enhanced Oil Recovery and Geological Sequestration in Heterogeneous and Low Permeability Reservoirs. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference, Melbourne, Australia, 21–26 October 2018; pp. 21–26. [Google Scholar]
- Patmonoaji, A.; Zhang, Y.; Xue, Z.; Park, H.; Suekane, T. Experimental and Numerical Simulation of Supercritical CO2 Microbubble Injection into a Brine-Saturated Porous Medium. Int. J. Greenh. Gas Control 2019, 91, 102830. [Google Scholar] [CrossRef]
- Zhai, H.; Xue, Z.; Park, H.; Aizawa, Y.; Baba, Y.; Zhang, Y. Migration Characteristics of Supercritical CO2 Microbubble Flow in the Berea Sandstone Revealed by Voxel-Based X-Ray Computed Tomography Imaging Analysis. J. Nat. Gas Sci. Eng. 2020, 77, 103233. [Google Scholar] [CrossRef]
- Seo, S.; Mastiani, M.; Hafez, M.; Kunkel, G.; Ghattas Asfour, C.; Garcia-Ocampo, K.I.; Linares, N.; Saldana, C.; Yang, K.; Kim, M. Injection of In-Situ Generated CO2 Microbubbles into Deep Saline Aquifers for Enhanced Carbon Sequestration. Int. J. Greenh. Gas Control 2019, 83, 256–264. [Google Scholar] [CrossRef]
- Aizawa, Y.; Baba, Y.; Xue, Z. Microbubble CO2 Injection for Geological Sequestration in Tight Sandstone Reservoirs. In Proceedings of the 15th Greenhouse Gas Control Technologies Conference, Abu Dhabi, United Arab Emirates, 15–18 March 2021. [Google Scholar]
- Xue, Z.; Nishio, S.; Hagiwara, N.; Matsuoka, T. Microbubble Carbon Dioxide Injection for Enhanced Dissolution in Geological Sequestration and Improved Oil Recovery. Energy Procedia 2014, 63, 7939–7946. [Google Scholar] [CrossRef]
- Jiang, L.; Xue, Z.; Park, H. Enhancement of CO2 Dissolution and Sweep Efficiency in Saline Aquifer by Micro Bubble CO2 Injection. Int. J. Heat Mass Transf. 2019, 138, 1211–1221. [Google Scholar] [CrossRef]
- Miyoshi, S.; Hitomi, T.; Miida, H.; Wada, H.; Inaba, K.; Yamaura, M. Numerical Study on Field-Scale Behavior of Carbon in CO2 Micro Bubble Storage (CMS). Energy Procedia 2013, 37, 5978–5985. [Google Scholar] [CrossRef]
- Jia, H.; Yu, H.; Xie, F.; Yuan, Z.; Xu, K.; Wang, Y. Research on CO2 microbubble dissolution kinetics and enhanced oil recovery mechanisms. Chin. J. Theor. Appl. Mech. 2023, 55, 755–764. [Google Scholar] [CrossRef]
- Cai, L.; Wu, J.; Zhang, M.; Wang, K.; Li, B.; Yu, X.; Hou, Y.; Zhao, Y. Investigating the Potential of CO2 Nanobubble Systems for Enhanced Oil Recovery in Extra-Low-Permeability Reservoirs. Nanomaterials 2024, 14, 1280. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Miyoshi, H.; Yamazaki, K.; Gohara, K. Promoting Effect of Ultra-Fine Bubbles on CO2 Hydrate Formation. Energies 2021, 14, 3386. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Wang, H.; Hu, B.; Lei, Z.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Zhang, Z. Effects of Nanobubble Water on the Growth of Lactobacillus Acidophilus 1028 and Its Lactic Acid Production. RSC Adv. 2019, 9, 30760–30767. [Google Scholar] [CrossRef]
- Ito, M.; Sugai, Y. Nanobubbles Activate Anaerobic Growth and Metabolism of Pseudomonas Aeruginosa. Sci. Rep. 2021, 11, 16858. [Google Scholar] [CrossRef]
- Choi, H.; Inoue, M.; Kwon, S.; Choi, H.; Lim, M. Effective Crack Control of Concrete by Self-Healing of Cementitious Composites Using Synthetic Fiber. Materials 2016, 9, 248. [Google Scholar] [CrossRef]
- Kim, J.; Kitagaki, R.; Choi, H. Pore Filling Effect of Forced Carbonation Reactions Using Carbon Dioxide Nanobubbles. Materials 2020, 13, 4343. [Google Scholar] [CrossRef]
- Kim, H.; Choi, H.; Choi, H.; Lee, B.; Lee, D.; Lee, D.-E. Study on Physical Properties of Mortar for Section Restoration Using Calcium Nitrite and CO2 Nano-Bubble Water. Materials 2020, 13, 3897. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Park, D.; Doh, J.-H. Performance Improvement of Cement Materials by Mineral Carbonation Accelerated by CO2 Microbubble Water. Constr. Build. Mater. 2024, 447, 138210. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, Z.; Gu, Z.; Liu, F.; Shen, P.; Poon, C.S. A Novel Approach for Improving Aqueous Carbonation Kinetics with CO2 Micro- and Nano-Bubbles. Chem. Eng. J. 2024, 500, 157363. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, T.; Shimada, H.; Sasaoka, T.; Hamanaka, A. Influence of CO2 Nano-Bubble Water Concentration and Curing Time on the Macroscopic and Microscopic Mechanical Properties of Cemented Backfill Materials. J. Build. Eng. 2024, 98, 111099. [Google Scholar] [CrossRef]
- Cao, X.; Hamanaka, A.; Shimada, H.; Sasaoka, T. Effect of CO2 Nanobubble Water on the Fracture Properties of Cemented Backfill Materials under Different Aggregate Fractal Dimensions. Appl. Sci. 2024, 14, 7792. [Google Scholar] [CrossRef]
- Orlich, J.N.; Kappes, R.D.P.; Gathje, J.C. Method for Processing Mineral Material Containing Acid-Consuming Carbonate and Precious Metal in Sulfide Minerals. WO2014179134A1, 6 November 2014. [Google Scholar]
- Vaziri Hassas, B.; Jin, J.; Dang, L.X.; Wang, X.; Miller, J.D. Attachment, Coalescence, and Spreading of Carbon Dioxide Nanobubbles at Pyrite Surfaces. Langmuir 2018, 34, 14317–14327. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Deng, L.; Li, G.; Cao, Y.; Zhao, B.; Fan, K. Effective Purification of the Low-Rank Coal by the Collaboration of the Microemulsion Collector and the CO2 Nanobubbles. Fuel 2023, 339, 127370. [Google Scholar] [CrossRef]
- Wang, D.; Yang, X.; Tian, C.; Lei, Z.; Kobayashi, N.; Kobayashi, M.; Adachi, Y.; Shimizu, K.; Zhang, Z. Characteristics of Ultra-Fine Bubble Water and Its Trials on Enhanced Methane Production from Waste Activated Sludge. Bioresour. Technol. 2019, 273, 63–69. [Google Scholar] [CrossRef]
- Fan, Y.; Lei, Z.; Guo, Z.; Huang, W.; Wang, D.; Wang, X.; Zhang, Z.; Shimizu, K. Enhanced Solubilization of Solid Organics and Methane Production by Anaerobic Digestion of Swine Manure under Nano-Bubble Water Addition. Bioresour. Technol. 2020, 299, 122512. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lei, Z.; Yang, X.; Kobayashi, M.; Adachi, Y.; Zhang, Z.; Shimizu, K. Effect of Nano-Bubble Water on High Solid Anaerobic Digestion of Pig Manure: Focus on Digestion Stability, Methanogenesis Performance and Related Mechanisms. Bioresour. Technol. 2020, 315, 123793. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, T.; Guo, Z.; Han, H.; Lei, Z.; Shimizu, K.; Zhang, Z.; Lee, D.-J. Enhanced Hydrolysis and Acidification of Cellulose at High Loading for Methane Production via Anaerobic Digestion Supplemented with High Mobility Nanobubble Water. Bioresour. Technol. 2020, 297, 122499. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Perales, J.A. Capability of Different Microalgae Species for Phytoremediation Processes: Wastewater Tertiary Treatment, CO2 Bio-Fixation and Low Cost Biofuels Production. Water Res. 2014, 49, 465–474. [Google Scholar] [CrossRef]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 Capture, Wastewater Treatment and Biofuel Production by Microalgae Culturing—A Review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Arudchelvam, Y.; Nirmalakhandan, N. Energetic Optimization of Algal Lipid Production in Bubble Columns: Part II: Evaluation of CO2 Enrichment. Biomass Bioenergy 2012, 46, 765–772. [Google Scholar] [CrossRef]
- Tang, T.; Wan, P.; Hu, Z. CO2 Bubbling to Improve Algal Growth, Nutrient Removal, and Membrane Performance in an Algal Membrane Bioreactor. Water Environ. Res. 2018, 90, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Feng, W.; Li, J.; Zhang, X.; Liu, L.; Li, H. Effects of Bubble Cutting Dynamic Behaviors on Microalgal Growth in Bubble Column Photobioreactor with a Novel Aeration Device. Front. Bioeng. Biotechnol. 2023, 11, 1225187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Pei, H.; Han, F.; Wang, Y.; Hou, Q.; Chen, Y. Effects of Air Bubble Size on Algal Growth Rate and Lipid Accumulation Using Fine-Pore Diffuser Photobioreactors. Algal Res. 2018, 32, 293–299. [Google Scholar] [CrossRef]
- Cheng, J.; Song, Y.; Guo, W.; Miao, Y.; Chen, S.; Zhou, J. Developing Microporous Fibrous-Diaphragm Aerator to Decrease Bubble Generation Diameter for Improving Microalgal Growth with CO2 Fixation in a Raceway Pond. Bioresour. Technol. 2019, 276, 28–34. [Google Scholar] [CrossRef]
- Cheng, J.; Miao, Y.; Guo, W.; Song, Y.; Tian, J.; Zhou, J. Reduced Generation Time and Size of Carbon Dioxide Bubbles in a Volute Aerator for Improving Spirulina Sp. Growth. Bioresour. Technol. 2018, 270, 352–358. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, S.; Guo, W.; Song, Y.; Kumar, S.; Kubar, A.A.; Su, Y.; Li, Y. Developing Staggered Woven Mesh Aerator with Three Variable-Micropore Layers in Recycling Water Pipeline to Enhance CO2 Conversion for Improving Arthrospira Growth. Sci. Total Environ. 2021, 760, 143941. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, S.; Ding, Y.; Liao, Q.; Huang, Y.; Zhu, X. Optimizing the Gas Distributor Based on CO2 Bubble Dynamic Behaviors to Improve Microalgal Biomass Production in an Air-Lift Photo-Bioreactor. Bioresour. Technol. 2017, 233, 84–91. [Google Scholar] [CrossRef]
- Li, N.; Chen, C.; Zhong, F.; Zhang, S.; Xia, A.; Huang, Y.; Liao, Q.; Zhu, X. A Novel Magnet-Driven Rotary Mixing Aerator for Carbon Dioxide Fixation and Microalgae Cultivation: Focusing on Bubble Behavior and Cultivation Performance. J. Biotechnol. 2022, 352, 26–35. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Zandi, M.; Hemaka Bandulasena, H.C.; Tesař, V.; James Gilmour, D.; Ying, K. Design of an Airlift Loop Bioreactor and Pilot Scales Studies with Fluidic Oscillator Induced Microbubbles for Growth of a Microalgae Dunaliella Salina. Appl. Energy 2011, 88, 3357–3369. [Google Scholar] [CrossRef]
- Ying, K.; Al-Mashhadani, M.K.H.; Hanotu, J.O.; Gilmour, D.J.; Zimmerman, W.B. Enhanced Mass Transfer in Microbubble Driven Airlift Bioreactor for Microalgal Culture. Engineering 2013, 5, 735–743. [Google Scholar] [CrossRef]
- Fei, T.; Lin, L.; Li, X.; Yang, J.-Y.; Zhao, J.; Liu, L. Modeling Effect of Bubbles on Time-Dependent Radiation Transfer of Microalgae in a Photobioreactor for Carbon Dioxide Fixation. Photonics 2022, 9, 864. [Google Scholar] [CrossRef]
- Suzuki-Nagata, S.; Mase, N.; Kozuka, T.; Ng, J.C.; Suzuki, T. Effect of Ultrafine CO2 Bubbles on Euglena Gracilis Z Growth with CO2 Gas Bubble Size and Chlorophyll Content. Biosci. Biotechnol. Biochem. 2025, 89, 638–648. [Google Scholar] [CrossRef]
- Kumar, K.A.; Chattaraj, R.; Dhumal, U.; Mukhopadhyay, M.; Vinjamur, M.; Dalvi, S.V. Modeling of Precipitation of Ultra-Fine Particles by Pressure Reduction over CO2-Expanded Liquids. J. Supercrit. Fluids 2013, 79, 227–235. [Google Scholar] [CrossRef]
- Mondal, M.; Roy, S.; Mukhopadhyay, M. Process Intensification of Cooling Crystallization of Cholesterol from Acetone Solution Using CO2 Gas Bubbles: Experiments and Modeling. Chem. Eng. Process.-Process Intensif. 2022, 172, 108794. [Google Scholar] [CrossRef]
- Prasad, R.; Patsariya, R.; Dalvi, S.V. Precipitation of Curcumin by Pressure Reduction of CO2-Expanded Acetone. Powder Technol. 2017, 310, 143–153. [Google Scholar] [CrossRef]
- Shashvatt, U.; Benoit, J.; Aris, H.; Blaney, L. CO2-Assisted Phosphorus Extraction from Poultry Litter and Selective Recovery of Struvite and Potassium Struvite. Water Res. 2018, 143, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Waldschmidt, A.; Couvrat, N.; Berton, B.; Dupray, V.; Morin, S.; Petit, S.; Coquerel, G. Impact of Gas Composition in the Mother Liquor on the Formation of Macroscopic Inclusions and Crystal Growth Rates. Case Study with Ciclopirox Crystals. Cryst. Growth Des. 2011, 11, 2463–2470. [Google Scholar] [CrossRef]
- Matsumoto, M.; Morita, Y.; Yoshinaga, M.; Hirose, S.; Onoe, K. Reactive Crystallization of Lithium Carbonate Nanoparticles by Microwave Irradiation of Aqueous Solution Containing CO2 Microbubbles. J. Chem. Eng. Jpn. 2009, 42, s242–s248. [Google Scholar] [CrossRef]
- Huang, J.; Yin, Q.; Ulrich, J. Influence of Dissolved CO2 on Crystallization of Epsomite—Variation of Temperature. J. Cryst. Growth 2017, 469, 18–23. [Google Scholar] [CrossRef]
- Huang, J.; Yin, Q.; Ulrich, J. The Effect of Dissolved Gases as Impurities on Crystallization. Chem. Eng. Technol. 2016, 39, 1213–1218. [Google Scholar] [CrossRef]
- Kimura, T.; Wada, Y.; Kamei, S.; Shirakawa, Y.; Hiaki, T.; Matsumoto, M. Synthesis of CaMg(CO3)2 from Concentrated Brine by CO2 Fine Bubble Injection and Conversion to Inorganic Phosphor. J. Chem. Eng. Jpn. 2020, 53, 555–561. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).