Abstract
Single gas sorption experiments with the C4-hydrocarbons n-butane, iso-butane, 1-butene and iso-butene on the flexible MOFs Cu-IHMe-pw and Cu-IHEt-pw were carried out with both thermodynamic equilibrium and overall sorption kinetics. Subsequent static binary gas mixture experiments of n-butane and iso-butane unveil a complex dependence of the overall selectivity on sorption enthalpy, rate of structural transition as well as steric effects. A thermodynamic separation favoring iso-butane as well as kinetic separation favoring n-butane are possible within Cu-IHMe-pw while complete size exclusion of iso-butane is achieved in Cu-IHEt-pw. This proof-of-concept study shows that the structural flexibility offers additional levers for the precise modulation of the separation mechanisms for complex mixtures with similar chemical and physical properties with real selectivities of >10.
1. Introduction
Flexible MOFs or porous coordination polymers represent a unique but large family of crystalline 3D solids built by coordinative bonds between inorganic nodes and organic linkers [1,2,3]. Since their first discovery, an enormous amount of research has been conducted on this material class, utilizing the vast degrees of freedom for the synthesis of new materials, analyzing their properties towards, e.g., gas storage [4,5,6,7], catalysis and even sensor design [8,9] or drug delivery [10,11]. However, given the industrial relevance, energy-efficient gas separation via sorption using MOFs is one of the focus topics within the research community [12,13,14,15,16]. Due to the modularity of the material class, pore geometries are envisioned to be tailored to the specific requirements to achieve good selectivities via size/shape exclusion or kinetic separation as well as for a thermodynamic separation based on differences in adsorbate–surface interactions of at least two concurring gases.
In recent years, remarkable progress was made, e.g., using ultramicroporous MOFs for the separation of propane and propene [15], reaching almost complete exclusion of propane due to size effects and thus a kinetic separation. Another example of the same separation is the MOF ZIF-8, in which the tight pore apertures lead to an enormously decreased diffusivity towards propane as compared to propene [17,18]. In the last two decades, a new subclass of MOFs displaying structurally flexible behavior was found, showing hysteresis in the adsorption isotherm with a large increase in pore volume within a narrow pressure range. Some studies also showcase their potential for separation.
Early work on one of the most prominent flexible MOFs, Mil-53, already showed the potential of this novel material class for CH4/CO2 gas mixture separations due to the higher affinity towards CO2 [19,20,21,22,23]. However, within these studies, it was found that these capabilities are merely based on thermodynamic separation as the species opening the framework (CO2) also allows the second, less preferred species (CH4) to enter due to the wide pore apertures in the MOF. Similar observations were also made by other groups for other materials [18,24,25,26]. While several groups focused on the ability of flexible MOFs to chemically separate very different adsorptives like N2, CH4 and CO2 or specific alkynes [26,27,28,29,30], only a small number of publications deal with the separation of hydrocarbons on flexible MOFs. Van den Bergh et al. analyzed the flexible ZIF-7, a zeolite imidazolate framework, for alkane–alkene separation for C2 and C3 hydrocarbons. It was shown for the first time that flexible MOFs have the ability to separate entirely within breakthrough curve experiments, herein based on the ability to find optimal sites within the pore apertures [31]. On the same material, Chen et al. conducted detailed breakthrough curve analysis for the separation of ethane and ethylene, further verifying the potential of the material class while also proving that experimental results can be sufficiently simulated via DFT calculations [23]. Couck et al. investigated the MOF COMOC-2 for separation of ethane and propane via breakthrough curve experiments with propane selectivity of 2–3 due to the higher affinity for adsorption on the material [32]. The MOFs NJU-Bai8 and NKU-FlexMOF-1 were investigated by Krishna’s group for the separation of propane/propene gas mixtures [33,34] with both showing promising results by experimental breakthrough curves based on a thermodynamic separation mechanism. Cui et al. utilized the ultramicroporous materials MnINA and CuINA, which display flexible behavior, and showed a molecular sieving effect that prefers n-butane over iso-butane via experimental breakthrough curves [35].
Generally, high focus in the literature is placed on the impact of modifications of the linker or the SBU on the thermodynmic equilibrium but less so on the overall sorption kinetics or potential separation mechanism [35,36,37,38,39]. Furthermore, only a few studies have contributed to a consistent view on the specific kinetics of structural transitions in flexible materials to date with a range of different MOFs and sorptives [40,41,42,43]. The possibility for kinetic separations of similar hydrocarbons, even up to practical size exclusion, has only been shown by Cui et al. [35] so far and should be investigated in more detail.
Therefore, the goal of this study is to investigate the governing thermodynamic and kinetic factors for gas separations on flexible MOFs by the individual study of them for a set of MOFs and different hydrocarbon sorptives. The subsequent analysis of static gas mixture experiments allows deeper insights into the molecular mechanism than break-through curves or theoretical assumptions on sorption isotherms only. Furthermore, key indicators for competitive gas separations on flexible materials will be derived in order to promote future research on the topic.
As probe molecules, the C4-hydrocarbons n-butane, iso-butane, 1-butene and iso-butene were used, enabling the analysis of both olefins and paraffins with different spatial demands (kinetical diameters of 4.32–4.72 Å). The gases were probed on the MOFs [Cu2(H-Me-trz-Ia)2] and [Cu2(H-Et-trz-Ia)2], with two isoreticular frameworks which are hereafter referred to as Cu-IHMe-pw and Cu-IHEt-pw for simplicity. These MOF systems have been previously investigated for their thermodynamic [44] and kinetic properties [45]. The linkers are based on triazolyl-isophthalate and only deviate in the alkyl side-chain at the 2-position of the triazol ring. The deviating names thus refer to a methyl and ethyl group, respectively. This further allows the investigation of the impact of the linker size on the separation mechanism. The bridging coordination of the carboxylate groups of the linkers results in a square planar CuO4 environment of the metal ions, leading to the well-known dinuclear paddle-wheel motif. Through coordination of a nitrogen atom of the triazolyl group in the apical positions of the metal centers, a three-dimensional network is assembled. Crystallographic data and CO2 adsorption isotherms were reported by Kobalz et al. [46]. Cu-IHMe-pw exists in three stable phases with two distinct gate-openings while Cu-IHEt-pw exhibits one structural transition only. Crystal structure details for all three phases of Cu-IHMe-pw, denoted as narrow pore (np-phase), medium pore (mp-phase) and large pore phase (lp-phase), were resolved by single-crystal and powder X-ray diffraction experiments. Based on these experiments, Cu-IHMe-pw was shown to have a pore size of around 3–5 Å [46] and thus is within the range of the kinetic diameters of the chosen adsorptives of 4.3 to 5.3 Å [47]. The corresponding crystal structure data of the np- and mp-phases are shown in the Supplementary Materials, Section S4.
2. Materials and Methods
2.1. Synthesis of MOFs
The metal–organic frameworks [Cu2(H-Me-trz-Ia)2] and [Cu2(H-Et-trz-Ia)2] (herein called Cu-IHMe-pw and Cu-IHEt-pw, respectively, for simplicity) were synthesized according to the original procedure reported elsewhere [46].
2.2. Measurement of Equilibrium Sorption Isotherms
The adsorption and desorption isotherms of the C4-hydrocarbons n-butane, iso-butane, 1-butene and iso-butene on the MOF systems were measured in a temperature range from 283 K to 313 K and at pressures up to 300 kPa using a magnetic suspension balance (Fa. Rubotherm GmbH, Bochum, Germany). Three pressure transducers (MKS Instruments Deutschland GmbH, Munich, Germany, Omega Engineering GmbH, Deckenpfronn, Germany) were used to gather accurate data for the whole pressure range up to 300 kPa. In the preparation of the sorption experiments, typically a stainless-steel sample holder was filled with around 0.2 g of a MOF sample. The sample cell was evacuated for at least 12 h at 373 K and a minimum pressure of 0.3 Pa was applied until constant mass was achieved. Subsequently, the gas was dosed into the balance and the pressure was increased stepwise after reaching the equilibrium. Sorption equilibrium was assumed to be reached when no further weight increase and pressure change of less than 1 Pa within 15 min were observed. The temperature was kept constant with an accuracy of 0.5 K. The gases were purchased from Linde (Linde AG, Munich, Germany) with purities of 99.5%. In order to calculate the surface excess mass from the measured weight values, a buoyancy correction was carried out. Furthermore, absolute gas loadings were calculated. Detailed descriptions for these procedures can be found elsewhere [48]. The densities for each gas were calculated with the program FLUIDCAL [49].
2.3. Measurement of Static Gas Mixtures
The static sorption equilibria of gas mixtures were determined by means of a hybrid manometric–gravimetric system built by Lange [50], the schematic structure is shown in Figure S6. Herein, the sample chamber of the magnetic suspension balance was integrated into a manometric arrangement. The manometric part consists of pressure vessels and their piping. The modular design of the apparatus allows three distinct take-aways: First, the gravimetric measurements allow the precise mass calculation of the adsorbent during both activation as well as gas uptake. Second, by deploying mass balance calculations for the manometry, the partial molar loadings of both components on the adsorbent can be calculated. This requires knowledge of the gas phase composition, which was determined externally in a gas chromatograph (GC) with a flame ionization detector (FID). A more detailed description of the approach is provided in the Supplementary Materials, Section S3.3.
3. Results
3.1. Single-Gas Thermodynamic Analysis
For the analysis of the equilibrium sorption isotherms of the studied systems, the Dubinin approach [51,52] is used as in previous studies for the MOF systems under investigation [44,45]. It has the advantage of enabling the analysis of sorption equilibria at different temperatures at once, enabling an easy and quick identification of key differences in sorption equilibria for different sorptive–sorbent systems. Figure 1 presents the band of isotherms for the ad- and desorption of the studied C4-hydrocarbons on Cu-IHMe-pw and Cu-IHEt-pw for the temperatures 283, 298 and 313 K in a characteristic Dubinin plot, each showing the specific pore volume in dependence of the sorption potential . Furthermore, every resulting characteristic curve for each pair of adsorbent and adsorptive as well as adsorption and desorption was fit with a dual-volume Dubinin–Asthakov approach, as was carried out previously [44,45]. These fits build the basis for the analysis in the following. A more detailed description of the fitting equation and methodology is given in the ESI, Supplementary Materials, Section S1.
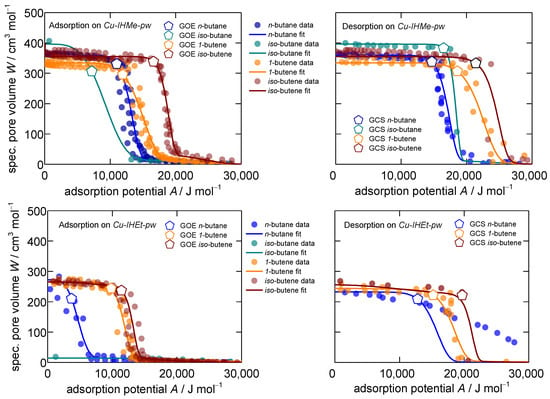
Figure 1.
Characteristic patterns for adsorption (left) and desorption (right) of the C4-hydrocarbons n-butane, iso-butane, 1-butene and iso-butene on Cu-IHMe-pw (top) and Cu-IHEt-pw (bottom) measured at temperatures of 283, 298 and 313 K. The gate-opening end (GOE) and gate-closing start (GCS) potentials are individually marked. All isotherm patterns are modeled with the Dubinin–Asthakov approach [51,52]. Please note two idiosyncrasies here: (1) The adsorption from iso-butane at Cu-IHMe-pw (top left) could not be entirely conducted due to very slow uptakes and thus, only one data-point is provided. (2) The desorption fit of n-butane on Cu-IHEt-pw (bottom right) was derived from a previous work based on the D-UAT and the desorption of propane on the same material [44].
Overall, the measured points of sorption converge into mostly sharp characteristic curves for every single adsorptive–adsorbent system. Three deviations from the expected patterns are observable within the MOF systems. Iso-butane is very slow to open the framework in Cu-IHMe-pw (points in Figure 1 upper left do not resemble equilibrium) and it is not able at all to open Cu-IHEt-pw within a reasonable timeframe (<1 week), indicating that the visualized datapoints do not represent equilibrium states. Thus, this phenomenon is of a kinetic nature and analyzed in more detail within the next section. Furthermore, n-butane does not show a distinct gate-closing within Cu-IHEt-pw, which is an outlier in the dataset and likely due to steric blocking effects of the adsorptive within the framework.
The specific boundaries of the structural transition, meaning the gate-opening end for adsorption and the gate-closing start for desorption, can be determined by applying the ESW theory as described by Adolphs [53] and previous work [44]. All data regarding the gate-opening end and gate-closing start points as sorption potentials as well as the corresponding pressures at 298 K are displayed in Table 1.

Table 1.
Sorption potentials and pressures at 298 K for all gate-opening end (GOE) and gate-closing start (GCS) points for the C4 adsorption on Cu-IHMe-pw and Cu-IHEt-pw.
Due to the flexibility of the frameworks, the characteristic ad- and desorption curves show two distinct sorption regimes: Low sorptive loading within the np-phase and high sorptive loading within the mp-phase of the respective MOF. Furthermore, the sorption patterns show a distinct hysteresis for all the sorptive–sorbent systems studied, which is common for flexible MOFs [1,54,55].
In a previous study, the sorption thermodynamics in both Cu-IHMe-pw and Cu-IHEt-pw were investigated. It was concluded that the desorption pattern resembles the actual thermodynamic equilibrium more closely [44], an interpretation that is in line with other authors [56,57]. Therefore, the desorption patterns are taken for the thermodynamic analysis of the interaction potentials and energy differences between the two phases.
When the adsorption potential tends to zero, the specific pore volume of the solid occupied by the fluid can be derived at the point of intersection with the y-axis (Figure 1). From Cu-IHMe-pw to Cu-IHEt-pw, the specific pore volume is reduced by one third due to the larger linker size and the resulting smaller pore in the opened mp-form. Regarding Cu-IHMe-pw individually, the accessible pore volume shows a preference firstly towards the branched hydrocarbons and secondly to the paraffins (overall order iso-butane > iso-butene > n-butane > 1-butene). This is likely due to steric reasons as the more compact hydrocarbons may find a denser packing within the opened pores. For Cu-IHEt-pw, the overall loadings of n-butane, iso-butene and 1-butene are almost equal, showing no specific steric preferences while iso-butane is not able to open the framework.
The relative positions of the desorption patterns can give insights into the interaction potentials between the different sorptive–sorbent systems. Generally, the further the pattern is shifted to higher sorption potentials, the higher the specific sorption enthalpy and thus the interaction between guest and host. Herein, both olefins show a much higher interaction potential towards both Cu-IHMe-pw and Cu-IHEt-pw as compared to the paraffins. This is likely due to a stronger interaction of their diffused -orbitals with the polarizing surfaces. Both the higher affinity and denser packing of olefins in MOFs are commonly observed in the literature and a key reason why this material class is considered as promising separation material [58,59,60,61].
A recently published method to normalize gas properties and to subsequently enable the calculation of normalized interaction potentials called D-UAT can be furthermore theoretically confirmed [44]. The reduced interaction potentials of the eight different systems under study are shown in the ESI, emphasizing the higher affinity of both 1-butene and iso-butene towards the MOFs even more (e.g., on Cu-IHEt-pw, iso-butene 103 J mol−1 K−1, n-butane 91 J mol−1 K−1 at half coverage). In order to derive the total energy difference between the np- and mp-phases within the MOFs (), the same approach as utilized in a previous work [44] based on a method by Coudert et al. [62] was applied. Overall, the values of are very consistent within the MOF systems with 17.1 and 19.5 J mol−1 for Cu-IHMe-pw and Cu-IHEt-pw, respectively, and in the same range as calculated within Ref. [44].
3.2. Single-Gas Kinetic Analysis
In a further analysis step, the individual uptake curves of each pressure increase during the isotherm recordings were derived. These were investigated by means of uptake fitting with an LDF approach [63] and, subsequently, effective transport diffusivities via a simplified methodology were derived as was carried out in a previous work of the group [45]. The diffusivities in dependence of the sorptive loading show three distinct regions as can be seen in Figure 2. Fast uptakes were recorded within the bare np- as well as mp-phases, leading to diffusivities of around 5∙10−13 m2 s−1 and above, which is comparable to other measurements for, e.g., n-butane in microporous solids [64]. However, much slower uptakes are recorded during the structural transition, dropping the diffusivity to values of 1∙10−14 and 1∙10−16 m2 s−1 for n-butane on Cu-IHMe-pw and Cu-IHEt-pw, respectively, further exemplifying the kinetic hindrance of the overall process [43,57,65].
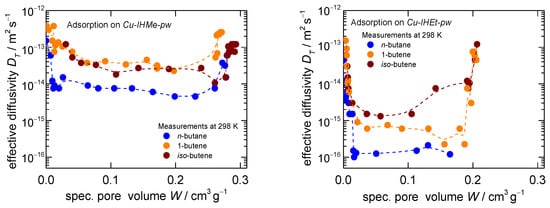
Figure 2.
Transport diffusivities calculated from the individual pressure steps during the gravimetric adsorption measurements for n-butane, 1-butene and iso-butene at 298 K on Cu-IHMe-pw (left) and Cu-IHEt-pw (right). Please note that the adsorption for iso-butane was too slow to be evaluated in both MOFs.
Furthermore, the differences between the two MOFs are significant, most likely caused by the tighter pore space of Cu-IHEt-pw which may slow down the diffusion to the active sites as well as hinder the molecular reorientation of the framework itself.
Within the different adsorptives, it becomes evident that n-butane does open the framework slower as compared to both olefins within both MOFs. While no big difference between the olefins is recognizable in Cu-IHMe-pw, iso-butene seems to trigger the overall process the fastest of all adsorptives within Cu-IHEt-pw. An evaluation of the transport diffusivities of iso-butane was not possible as the process is too time-intensive to ensure a proper measurement in either MOF. This is exemplified by the uptake curves for a larger pressure step on Cu-IHMe-pw as shown in Figure 3. Herein, the uptake curves for pressure jumps up to 20 kPa and thus beyond the respective gate-opening points were recorded. The iso-butane uptake takes more than 1000 times longer as compared to n-butane, while both olefin uptakes are completed much quicker.
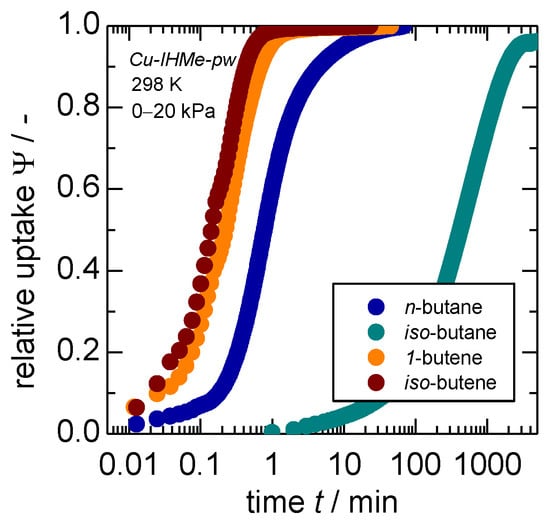
Figure 3.
Individual gas uptakes of C4-hydrocarbons on Cu-IHMe-pw at 298 K for pressure jumps of 0–20 kPa. Please note that due to the slow uptake of iso-butane, another gravimetric set-up was used with a temporal resolution of 1 min as compared to the other measurements with a resolution of 0.77 s.
In a previous study of the system n-butane/Cu-IHMe-pw [45] and a recent publication by Miura et al. [43], it was concluded that the overall rate of structural transition is dependent on the difference of the sorption potential A at the gate-opening pressure and the pressure point being set. Herein, the points of the gate-closing starts (GCSs) from the desorption patterns are taken as reference points and the corresponding potential is set as the minimum potential that has to be overshot to ensure a nearly complete structural transition. The calculated potential difference for each adsorptive can be defined as the driving force of the structural transition as a guide (please see Supplementary Materials Section S3.2 for further detail).
However, given the desorption patterns of n- and iso-butane, the latter has a larger potential difference for the described pressure step but has a much slower rate of structural transition. This effect can likely be ascribed to steric effects of iso-butane within the framework, with two potential explanations that have to be further analyzed. As evident by the equilirbium data, iso-butane can enter the opened framework but the diffusion through the opened pores might be hampered due to the larger spatial demand of the sorptive with 5.3 Å.
However, it is also possible that the re-orientation of the framework is sterically blocked by the sorptive, leading to a very high framework energy of the intermediate state. This activation energy within the free energy profile would make the overall transition become less likely and thus requires more time to complete. While both hypotheses may hold true, a more in-depth explanation can be found within the Supplementary Materials, Section S3.3. Thus, there stands the question whether these kinetic observations can be utilized for potential gas separations.
3.3. Binary Gas Mixture—Static Measurements
Within this section, the general applicability of Cu-IHMe-pw and Cu-IHEt-pw for the separation of binary C4-hydrocarbons is investigated. By utilizing a novel hybrid manometric–gravimetric apparatus set-up [50], it is possible to analyze both the overall kinetic gas uptake as well as the gas composition within the sample chamber in dependence of time. This allows the precise investigation of the complex interplay of thermodynamic and kinetic relations between both gases and the host under static conditions, which has not been conducted so far to the best of our knowledge. The investigation was conducted as follows:
- (1)
- The overall gas uptakes recorded via the gravimetric suspension balance of the gas mixture on the MOFs were fit with the aid of the kinetic gate-opening model (“GO” model) by Tanaka et al. [66].
- (2)
- A coupled mass balance incorporating the pre-determined overall available gas volume as well as the gas composition monitored during the experiment via gas chromatography enables the calculation of the mass of the adsorbed phase per gas species.
- (3)
- An additional fit for the uptake of the slower gas species allows the detailed modeling of the gas phase composition over time as well and thus the evaluation of the selectivity over the whole measurement time.
The overall modeling approach is explained in more detail within the Supplementary Materials, Section S3.2. Given the idiosyncrasies of iso-butane, the paraffin separation of n-butane and iso-butane is examined first. For this experiment, a molar 50:50 mix of n-butane and iso-butane was released to the sample chamber leading to a final pressure of 40 kPa after adsorption. The subsequent partial pressures of 20 kPa each are identical to the single-gas uptakes presented in Figure 3, thus both are overshooting the minimum necessary pressure for a complete gate-opening considerably with 0.6 and 0.5 kPa, respectively. The resulting kinetic gas uptakes, the gas phase composition and separation selectivity are shown in Figure 4.
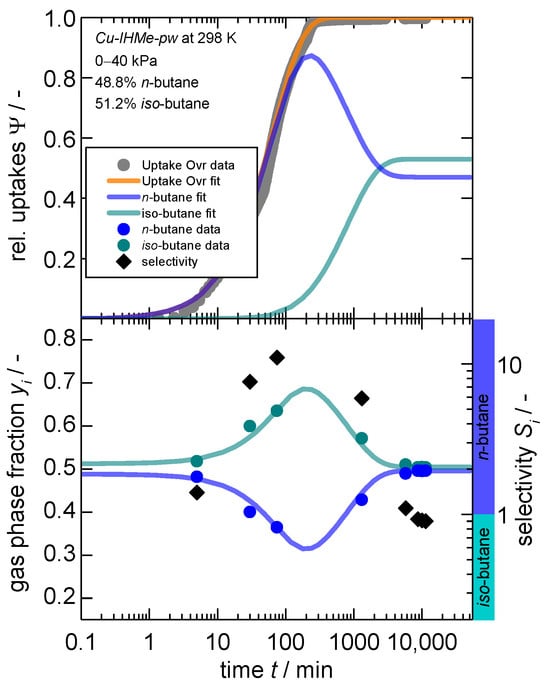
Figure 4.
Kinetic gas uptake (top), gas phase composition (bottom, left axis) and separation selectivity (bottom, right axis) in dependence of time for a “50:50” mixture1 of n-butane and iso-butane on Cu-IHMe-pw for a pressure jump of 0–40 kPa. Additionally, the overall gas phase compositions and sorption kinetics are modeled via the “GO” model [66] and a mass balance. Within the bottom graph, a selectivity larger than 1 indicates a preference for n-butane as indicated by the additional colored ribbons. 1. Please note that a 50:50 molar mixture was aimed for, but actual results show slight deviations with 48.8:51.2.
Herein, it becomes evident that the overall gas uptake is slower for the gas mixture as compared to the single-gas uptake of n-butane with the same partial pressure step, although much faster than compared to the bare iso-butane adsorption (half coverage is reached after 40 min for the mixture, 1 min for n-butane and ~1000 min for iso-butane individually). From the evolution of the gas phase composition, it can be seen that n-butane is predominantly adsorbed at the beginning, reducing the total gas phase fraction to about 33%, resulting in a total separation factor of a maximum of 10 (at time 200 min). Beyond that, iso-butane does continously enter the opened framework, exchanges the adsorbed n-butane and incorporates itself within the framework. This leads to a subsequent increase in the gas phase fraction of n-butane and a final separation factor after 10,000 min (~7 days) of 0.9 for n-butane, meaning iso-butane is slightly preferred.
It can be concluded that, although iso-butane is preferentially adsorbed under equilibrium conditions (see thermodynamic section), n-butane is predominantly adsorbed first and initiates the structural transition. Thus, n-butane simultaneously forces a penetration on its part into the first opened pore regions of the mp-phase and occupies them immediately, indicating a cascading effect that continuously discriminates the slower species. This is the first time that such a complex interplay of thermodynamic and kinetic separation mechanisms has been observed within flexible MOFs to the best of our knowledge. However, as the maximum separation factor reaches 10, a complete exclusion of iso-butane is not observed. This is probably due to the preferential adsorption of iso-butane in the pore entries of the particles. The consequent slow re-exchange also shows that the pores are wide enough to enable such a counter diffusion. It can therefore be concluded that the framework offers both the potential for a thermodynamic separation as well as a kinetic separation, whilst the structural flexibility offers the fine-tuning of both.
Based on these results, it needs to be clarified whether the effect of kinetic preference could be even more harnessed utilizing Cu-IHEt-pw with its tighter pore spaces. Therefore, a similar experiment was conducted for the same mixtures albeit with the latter MOF. Herein, the overall pressure step was set to 0–200 kPa, a pressure that largely overshoots the minimum pressure necessary to open the framework for n-butane to ensure a complete uptake within an acceptable timeframe. The results can be seen in Figure 5 (please note that the overall sample masses between the experiments presented in Figure 4 and Figure 5 deviate and, thus, the bare gas phase compositions cannot be compared).
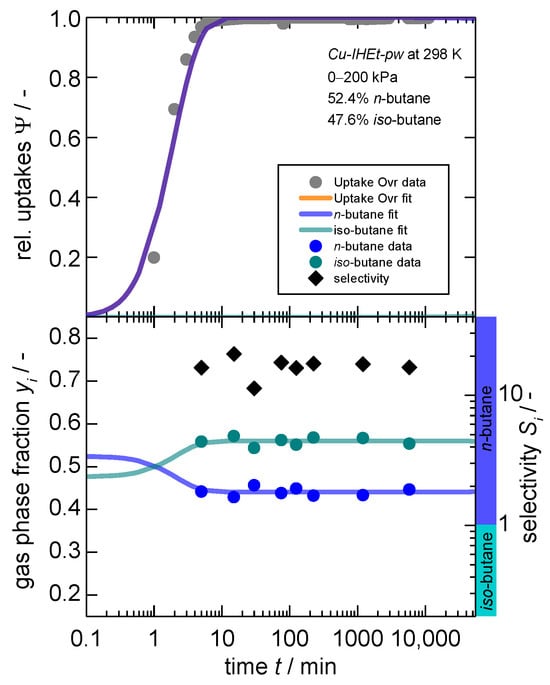
Figure 5.
Kinetic gas uptake (top), gas phase composition (bottom, left axis) and separation selectivity (bottom, right axis) in dependence of time for a “50:50” mixture1 of n-butane and iso-butane on Cu-IHEt-pw for a pressure jump of 0–200 kPa. Within the bottom graph, a selectivity larger than 1 indicates a preference for n-butane as indicated by the additional colored ribbons. 1. Please note two observations: (1) the fit for the overall uptake as well as for n-butane are overlapping as almost no iso-butane is adsorbed. (2) A 50:50 molar mixture was aimed for, but actual results show slight deviations with 52.4:47.6.
Overall, similar observations can be made as for the previous experiment. The sorptive n-butane is overall kinetically preferred within this binary mixture, leading to a separation factor of around 11. However, the re-exchange of iso-butane cannot be observed even after around 5000 min. This is likely caused by the tighter pore space within Cu-IHEt-pw and the subsequent kinetic hindrance of the exchange.
This clearly shows that the overall separation mechanism switches herein from a kinetic separation towards a size exclusion effect and the overall dependence on the linker size. An additional experimental set-up includes a mixture of iso-butane and iso-butene. The latter is both thermodynamically and kinetically preferred considering single-gas equilibrium and uptake data. The gas composition and the subsequent separation factor are displayed in Figure 6. Herein, an almost complete exclusion of iso-butane can be observed, leading to a separation factor of above 50 since the adsorption sites on the pore entries and particle surface also seem to have a higher affinity towards iso-butene.
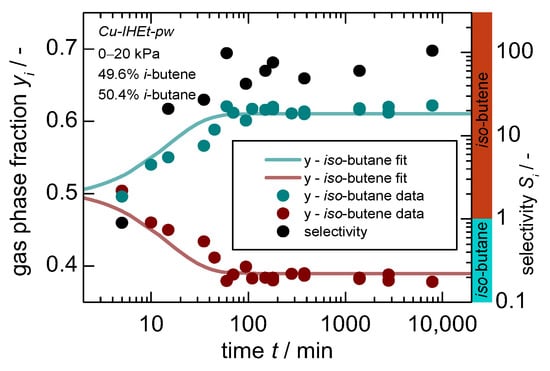
Figure 6.
Gas phase composition (left axis) and separation factor (right axis) in dependence of time for a “50:50” mixture1 of iso-butene and iso-butane on Cu-IHEt-pw for a pressure jump of 0–20 kPa. Within the bottom graph, a selectivity larger than 1 indicates a preference for iso-butene as indicated by the additional colored ribbons. 1. Please note that a 50:50 molar mixture was aimed for but actual results show slight deviations with 49.6:50.4.
In order to show that a sufficient separation of a gas mixture with much similar thermodynamic affinities and rates of sorption, a 50:50 mixture of 1-butene and iso-butene is shown in Figure S8. Herein, a separation factor of 4–5 was reached without a subsequent re-exchange of gases, which can likely also be ascribed to a hybrid thermodynamic as well as kinetic separation pathway with iso-butene having both higher sorption enthalpy and a higher rate of structural transition.
However, there stands the question to what degree the latter is dependent on the former. A high sorption enthalpy per mole of adsorbate may trigger a faster provision of energy to close the energetic gap between both structures. Additional parameters like the framework energy of the intermediate state as well as size exclusion effects may play a pivotal role in the precise modulation of separation mechanisms. These thermodynamic relations are intrinsically dependent on the precise energy profile of the host–guest system. Although computationally very expensive, the calculations of such profiles are major focus topics of the MOF community now [67,68,69]. A subsequent calculation of the rate of structural transition via the transition state theory (TST, more detailed description within Supplementary Materials S3.3) may be possible in similar ways as conducted by Camp et al. [70], which would subsequently allow a complete in silico screening of the separation ability of flexible porous solids. Indeed, computational studies concerning large-scale screenings of a vast number of potential porous solids have shown to be very promising, taking into account pore geometries as well as molecular interactions [71,72,73,74,75].
4. Conclusions
Within this study, the potential of flexible MOFs for gas separations was investigated by analyzing the governing thermodynamic and kinetic properties individually during static gas mixture experiments. The model adsorbents Cu-IHMe-pw and Cu-IHEt-pw both show flexible behavior, with the latter having higher equilibrium pressures for the gate-opening and -closing of the structural transition than the former. This is due to a slightly higher energy difference between the two concurring structural conformations (np- and mp-phase). Probing both frameworks with C4-hydrocarbons in single-gas experiments individually shows a strong preference for the olefins while experiments with iso-butane inidicate steric effects with slow diffusion or even size exclusion due to the tight pore widths. To gain deeper insights into the precise interplay of sorption enthalpy and rate of structural transition in dependence of the pressure step, computational methods could be applied in the future. Recent advances in the development of free-energy profiles coupled with the transition state theory could be harnessed in order to allow for large-scale screenings of MOFs for specific separation purposes.
The proof of concept presented here shows that the structural flexibility provides an additional tool for the design of selective adsorbents and the modulation of the separation mechanism from thermodynamic to kinetic or even size exclusion with the alteration of the linker size and pore width. Further investigations will focus on the influence of different pressure steps as well as breakthrough curve analysis in order to verify the effective potential regarding olefin/paraffin separation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano14030241/s1. The supporting material is structured as follows: Section S1—Thermodynamic Analysis, Section S2—Kinetic Analysis, Section S3—Static Gas Mixture Measurements and Model Development, Section S4—Structure Information. References [76,77] are cited in the Supplementary Materials.
Author Contributions
The study was planned and designed by H.P.-K. with major contributions regarding the data analysis by J.M. and M.L. The theoretical models were developed mainly by H.P.-K., structure analysis was conducted by O.E., M.K. and H.K. Gravimetric measurements were conducted by H.P.-K. and M.L.; H.P.-K., R.G., J.M. and H.K. discussed the results and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG GL 290/10-1/KR 1675/8-1 und 12-1). Furthermore, we also gratefully acknowledge financial support by Universität Leipzig (Forschungsprofilbereich Komplexe Materie). The publication was funded by the Open Access Publishing Fund of Leipzig University supported by the German Research Foundation within the program Open Access Publication Funding.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Mason, J.A.; Oktawiec, J.; Taylor, M.K.; Hudson, M.R.; Rodriguez, J.; Bachman, J.E.; Gonzalez, M.I.; Cervellino, A.; Guagliardi, A.; Brown, C.M.; et al. Methane storage in flexible metal-organic frameworks with intrinsic thermal management. Nature 2015, 527, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal–organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Getman, R.B.; Bae, Y.-S.; Wilmer, C.E.; Snurr, R.Q. Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal-organic frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045–17060. [Google Scholar] [CrossRef]
- Stassen, I.; Burtch, N.; Talin, A.; Falcaro, P.; Allendorf, M.; Ameloot, R. An updated roadmap for the integration of metal-organic frameworks with electronic devices and chemical sensors. Chem. Soc. Rev. 2017, 46, 3185–3241. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef]
- Allan, P.K.; Wheatley, P.S.; Aldous, D.; Mohideen, M.I.; Tang, C.; Hriljac, J.A.; Megson, I.L.; Chapman, K.W.; de Weireld, G.; Vaesen, S.; et al. Metal-organic frameworks for the storage and delivery of biologically active hydrogen sulfide. Dalton Trans. 2012, 41, 4060–4066. [Google Scholar] [CrossRef] [PubMed]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef] [PubMed]
- Herm, Z.R.; Bloch, E.D.; Long, J.R. Hydrocarbon separations in metal–organic frameworks. Chem. Mater. 2014, 26, 323–338. [Google Scholar] [CrossRef]
- Seoane, B.; Coronas, J.; Gascon, I.; Etxeberria Benavides, M.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal-organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar] [CrossRef] [PubMed]
- Cadiau, A.; Adil, K.; Bhatt, P.M.; Belmabkhout, Y.; Eddaoudi, M. A metal-organic framework–based splitter for separating propylene from propane. Science 2016, 353, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Siegelman, R.L.; McDonald, T.M.; Gonzalez, M.I.; Martell, J.D.; Milner, P.J.; Mason, J.A.; Berger, A.H.; Bhown, A.S.; Long, J.R. Controlling Cooperative CO2 Adsorption in Diamine-Appended Mg2(dobpdc) Metal-Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 10526–10538. [Google Scholar] [CrossRef] [PubMed]
- Chmelik, C.; Kärger, J. The predictive power of classical transition state theory revealed in diffusion studies with MOF ZIF-8. Microporous Mesoporous Mater. 2016, 225, 128–132. [Google Scholar] [CrossRef]
- Pimentel, B.R.; Lively, R.P. Enabling Kinetic Light Hydrocarbon Separation via Crystal Size Engineering of ZIF-8. Ind. Eng. Chem. Res. 2016, 55, 12467–12476. [Google Scholar] [CrossRef]
- Ortiz, A.U.; Springuel-Huet, M.-A.; Coudert, F.-X.; Fuchs, A.H.; Boutin, A. Predicting mixture coadsorption in soft porous crystals: Experimental and theoretical Study of CO2/CH4 in MIL-53(Al). Langmuir 2012, 28, 494–498. [Google Scholar] [CrossRef]
- Coudert, F.-X. The osmotic framework adsorbed solution theory: Predicting mixture coadsorption in flexible nanoporous materials. Phys. Chem. Chem. Phys. 2010, 12, 10904–10913. [Google Scholar] [CrossRef]
- Couck, S.; Denayer, J.F.M.; Baron, G.V.; Rémy, T.; Gascon, J.; Kapteijn, F. An amine-functionalized MIL-53 metal-organic framework with large separation power for CO2 and CH4. J. Am. Chem. Soc. 2009, 131, 6326–6327. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Mekala, S.; Dreisbach, F.; Mandal, B.; Gumma, S. Adsorption of CO2, CO, CH4 and N2 on a zinc based metal organic framework. Sep. Purif. Technol. 2012, 94, 124–130. [Google Scholar] [CrossRef]
- Chen, D.-L.; Wang, N.; Xu, C.; Tu, G.; Zhu, W.; Krishna, R. A combined theoretical and experimental analysis on transient breakthroughs of C2H6/C2H4 in fixed beds packed with ZIF-7. Microporous Mesoporous Mater. 2015, 208, 55–65. [Google Scholar] [CrossRef]
- Schneemann, A.; Bloch, E.D.; Henke, S.; Llewellyn, P.L.; Long, J.R.; Fischer, R.A. Influence of Solvent-Like Sidechains on the Adsorption of Light Hydrocarbons in Metal–Organic Frameworks. Chem. Eur. J. 2015, 21, 18764–18769. [Google Scholar] [CrossRef]
- Chen, D.-L.; Wang, N.; Wang, F.-F.; Xie, J.; Zhong, Y.; Zhu, W.; Johnson, J.K.; Krishna, R. Utilizing the Gate-Opening Mechanism in ZIF-7 for Adsorption Discrimination between N2O and CO2. J. Phys. Chem. C 2014, 118, 17831–17837. [Google Scholar] [CrossRef]
- Noro, S.-I.; Ochi, R.; Inubushi, Y.; Kubo, K.; Nakamura, T. CH4/CO2 and CH4/C2H6 gas separation using a flexible one-dimensional copper(II) porous coordination polymer. Microporous Mesoporous Mater. 2015, 216, 92–96. [Google Scholar] [CrossRef]
- Foo, M.L.; Matsuda, R.; Hijikata, Y.; Krishna, R.; Sato, H.; Horike, S.; Hori, A.; Duan, J.; Sato, Y.; Kubota, Y.; et al. An Adsorbate Discriminatory Gate Effect in a Flexible Porous Coordination Polymer for Selective Adsorption of CO2 over C2H2. J. Am. Chem. Soc. 2016, 138, 3022–3030. [Google Scholar] [CrossRef]
- Lin, R.-B.; Li, L.; Wu, H.; Arman, H.; Li, B.; Lin, R.-G.; Zhou, W.; Chen, B. Optimized Separation of Acetylene from Carbon Dioxide and Ethylene in a Microporous Material. J. Am. Chem. Soc. 2017, 139, 8022–8028. [Google Scholar] [CrossRef]
- Xue, D.-X.; Cairns, A.J.; Belmabkhout, Y.; Wojtas, L.; Liu, Y.; Alkordi, M.H.; Eddaoudi, M. Tunable rare-earth fcu-MOFs: A platform for systematic enhancement of CO2 adsorption energetics and uptake. J. Am. Chem. Soc. 2013, 135, 7660–7667. [Google Scholar] [CrossRef]
- Taylor, M.K.; Runčevski, T.; Oktawiec, J.; Bachman, J.E.; Siegelman, R.L.; Jiang, H.; Mason, J.A.; Tarver, J.D.; Long, J.R. Near-Perfect CO2/CH4 Selectivity Achieved through Reversible Guest Templating in the Flexible Metal-Organic Framework Co(bdp). J. Am. Chem. Soc. 2018, 140, 10324–10331. [Google Scholar] [CrossRef]
- van den Bergh, J.; Gücüyener, C.; Pidko, E.A.; Hensen, E.J.M.; Gascon, J.; Kapteijn, F. Understanding the anomalous alkane selectivity of ZIF-7 in the separation of light alkane/alkene mixtures. Chem. Eur. J. 2011, 17, 8832–8840. [Google Scholar] [CrossRef] [PubMed]
- Couck, S.; van Assche, T.R.C.; Liu, Y.-Y.; Baron, G.V.; van der Voort, P.; Denayer, J.F.M. Adsorption and separation of small hydrocarbons on the flexible, vanadium-containing MOF, COMOC-2. Langmuir 2015, 31, 5063–5070. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Krishna, R.; Li, L.; Wang, B.; He, T.; Zhang, Y.-Z.; Li, J.-R.; Li, J. Guest-dependent pressure induced gate-opening effect enables effective separation of propene and propane in a flexible MOF. Chem. Eng. J. 2018, 346, 489–496. [Google Scholar] [CrossRef]
- Yu, M.-H.; Space, B.; Franz, D.; Zhou, W.; He, C.; Li, L.; Krishna, R.; Chang, Z.; Li, W.; Hu, T.-L.; et al. Enhanced Gas Uptake in a Microporous Metal-Organic Framework via a Sorbate Induced-Fit Mechanism. J. Am. Chem. Soc. 2019, 141, 17703–17712. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, Z.; Tan, B.; Zhang, Y.; Wang, P.; Cui, X.; Xing, H. Efficient Separation of n-Butene and iso-Butene by Flexible Ultramicroporous Metal-Organic Frameworks with Pocket-like Cavities. Chem. Asian J. 2019, 14, 3572–3576. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.K.; Runčevski, T.; Oktawiec, J.; Gonzalez, M.I.; Siegelman, R.L.; Mason, J.A.; Ye, J.; Brown, C.M.; Long, J.R. Tuning the Adsorption-Induced Phase Change in the Flexible Metal-Organic Framework Co(bdp). J. Am. Chem. Soc. 2016, 138, 15019–15026. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, R.; Hou, S.; Chen, W.; Huang, H.; Wang, Y.; Wu, Y.-N.; Li, F. Common but differentiated flexible MIL-53(Al): Role of metal sources in synthetic protocol for tuning the adsorption characteristics. J. Mater. Sci. 2019, 54, 6174–6185. [Google Scholar] [CrossRef]
- Senkovska, I.; Bon, V.; Abylgazina, L.; Mendt, M.; Berger, J.; Kieslich, G.; Petkov, P.; Luiz Fiorio, J.; Joswig, J.-O.; Heine, T.; et al. Understanding MOF Flexibility: An Analysis Focused on Pillared Layer MOFs as a Model System. Angew. Chem. Int. Ed. 2023, 62, e202218076. [Google Scholar] [CrossRef]
- Schneemann, A.; Henke, S.; Schwedler, I.; Fischer, R.A. Targeted manipulation of metal-organic frameworks to direct sorption properties. ChemPhysChem 2014, 15, 823–839. [Google Scholar] [CrossRef]
- van Speybroeck, V.; Vandenhaute, S.; Hoffman, A.E.J.; Rogge, S.M.J. Towards modeling spatiotemporal processes in metal–organic frameworks. Trends Chem. 2021, 3, 605–619. [Google Scholar] [CrossRef]
- Cerasale, D.J.; Ward, D.C.; Easun, T.L. MOFs in the time domain. Nat. Rev. Chem. 2022, 6, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Milić, J.V. Functional dynamics in framework materials. Commun. Chem. 2023, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Bon, V.; Senkovska, I.; Ehrling, S.; Bönisch, N.; Mäder, G.; Grünzner, S.; Khadiev, A.; Novikov, D.; Maity, K.; et al. Spatiotemporal Design of the Metal-Organic Framework DUT-8(M). Adv. Mater. Weinheim 2023, 35, e2207741. [Google Scholar] [CrossRef] [PubMed]
- Preißler-Kurzhöfer, H.; Lange, M.; Kolesnikov, A.; Möllmer, J.; Erhart, O.; Kobalz, M.; Krautscheid, H.; Gläser, R. Hydrocarbon Sorption in Flexible MOFs-Part I: Thermodynamic Analysis with the Dubinin-Based Universal Adsorption Theory (D-UAT). Nanomaterials 2022, 12, 2415. [Google Scholar] [CrossRef] [PubMed]
- Preißler-Kurzhöfer, H.; Kolesnikov, A.; Lange, M.; Möllmer, J.; Erhart, O.; Kobalz, M.; Hwang, S.; Chmelik, C.; Krautscheid, H.; Gläser, R. Hydrocarbon Sorption in Flexible MOFs-Part II: Understanding Adsorption Kinetics. Nanomaterials 2023, 13, 601. [Google Scholar] [CrossRef] [PubMed]
- Kobalz, M.; Lincke, J.; Kobalz, K.; Erhart, O.; Bergmann, J.; Lässig, D.; Lange, M.; Möllmer, J.; Gläser, R.; Staudt, R.; et al. Paddle wheel based triazolyl isophthalate MOFs: Impact of linker modification on crystal structure and gas sorption properties. Inorg. Chem. 2016, 55, 3030–3039. [Google Scholar] [CrossRef] [PubMed]
- Sircar, S.; Myers, A.L. Handbook of Zeolite Science and Technology: Gas Separation by Zeolites, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Keller, J.U.; Staudt, R. Gas Adsorption Equilibria: Experimental Methods and Adsorption Isotherms, 3rd ed.; Springer: New York, NY, USA, 2004. [Google Scholar]
- Wagner, W. Program FLUIDCAL; R.uhr-Universität Bochum: Bochum, Germany, 2017. [Google Scholar]
- Lange, M. Sorption von C4-Kohlenwasserstoffen and Strukturell Flexiblen, Porösen Koordinationspolymeren. Ph.D. Thesis, Universität Leipzig, Leipzig, Germany, 2015. [Google Scholar]
- Burevski, D. The application of the Dubinin-Astakhov equation to the characterization of microporous carbons. Colloid. Polym. Sci. 1982, 260, 623–627. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 235–242. [Google Scholar] [CrossRef]
- Adolphs, J. Excess surface work?: A modelless way of getting surface energies and specific surface areas directly from sorption isotherms. Appl. Surf. Sci. 2007, 253, 5645–5649. [Google Scholar] [CrossRef]
- Férey, G. Structural flexibility in crystallized matter: From history to applications. Dalton Trans. 2016, 45, 4073–4089. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeoung, S.; Chung, Y.G.; Moon, H.R. Elucidation of flexible metal-organic frameworks: Research progresses and recent developments. Coord. Chem. Rev. 2019, 389, 161–188. [Google Scholar] [CrossRef]
- Numaguchi, R.; Tanaka, H.; Watanabe, S.; Miyahara, M.T. Simulation study for adsorption-induced structural transition in stacked-layer porous coordination polymers: Equilibrium and hysteretic adsorption behaviors. J. Chem. Phys. 2013, 138, 54708. [Google Scholar] [CrossRef] [PubMed]
- Numaguchi, R.; Tanaka, H.; Watanabe, S.; Miyahara, M.T. Dependence of adsorption-induced structural transition on framework structure of porous coordination polymers. J. Chem. Phys. 2014, 140, 44707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Q.; Cui, X.; Yang, L.; Bao, Z.; Ren, Q.; Xing, H. Sorting of C4 Olefins with Interpenetrated Hybrid Ultramicroporous Materials by Combining Molecular Recognition and Size-Sieving. Angew. Chem. Int. Ed. 2017, 56, 1–7. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal-organic frameworks for separation. Adv. Mater. 2018, 30, 1705189–1705223. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.R.; Gonzalez, M.I.; Long, J.R. Recent Progress Towards Light Hydrocarbon Separations Using Metal–Organic Frameworks. Trends Chem. 2019, 1, 159–171. [Google Scholar] [CrossRef]
- Cui, W.-G.; Hu, T.-L.; Bu, X.-H. Metal-Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv. Mater. 2020, 32, 1806445–1806468. [Google Scholar] [CrossRef]
- Coudert, F.-X.; Jeffroy, M.; Fuchs, A.H.; Boutin, A.; Mellot-Draznieks, C. Thermodynamics of guest-induced structural transitions in hybrid organic-inorganic frameworks. J. Am. Chem. Soc. 2008, 130, 14294–14302. [Google Scholar] [CrossRef]
- Glueckauf, E.; Coates, J.J. Theory of chromatography; the influence of incomplete equilibrium on the front boundary of chromatograms and on the effectiveness of separation. J. Chem. Soc. 1947, 1315–1321. [Google Scholar] [CrossRef]
- Möller, A.; Staudt, R. Gravimetrische Messungen von Transportkoeffizienten von n-Alkanen am 5A-Zeolith. Chem. Ing. Tech. 2008, 80, 593–598. [Google Scholar] [CrossRef]
- Chmelik, C. Characteristic features of molecular transport in MOF ZIF-8 as revealed by IR microimaging. Microporous Mesoporous Mater. 2015, 216, 138–145. [Google Scholar] [CrossRef]
- Tanaka, D.; Nakagawa, K.; Higuchi, M.; Horike, S.; Kubota, Y.; Kobayashi, T.C.; Takata, M.; Kitagawa, S. Kinetic gate-opening process in a flexible porous coordination polymer. Angew. Chem. Int. Ed. 2008, 47, 3914–3918. [Google Scholar] [CrossRef] [PubMed]
- Demuynck, R.; Rogge, S.M.J.; Vanduyfhuys, L.; Wieme, J.; Waroquier, M.; van Speybroeck, V. Efficient Construction of Free Energy Profiles of Breathing Metal-Organic Frameworks Using Advanced Molecular Dynamics Simulations. J. Chem. Theory Comput. 2017, 13, 5861–5873. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Miyahara, M.T. Free energy calculations for adsorption-induced deformation of flexible metal–organic frameworks. Curr. Opin. Chem. Eng. 2019, 24, 19–25. [Google Scholar] [CrossRef]
- Vanduyfhuys, L.; Rogge, S.M.J.; Wieme, J.; Vandenbrande, S.; Maurin, G.; Waroquier, M.; van Speybroeck, V. Thermodynamic insight into stimuli-responsive behaviour of soft porous crystals. Nat. Commun. 2018, 9, 204. [Google Scholar] [CrossRef]
- Camp, J.S.; Sholl, D.S. Transition State Theory Methods To Measure Diffusion in Flexible Nanoporous Materials: Application to a Porous Organic Cage Crystal. J. Phys. Chem. C 2016, 120, 1110–1120. [Google Scholar] [CrossRef]
- Li, S.; Chung, Y.G.; Simon, C.M.; Snurr, R.Q. High-Throughput Computational Screening of Multivariate Metal-Organic Frameworks (MTV-MOFs) for CO2Capture. J. Phys. Chem. Lett. 2017, 8, 6135–6141. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, K.; Jiang, J. In silico screening of 4764 computation-ready, experimental metal–organic frameworks for CO2 separation. J. Mater. Chem. A 2016, 4, 2105–2114. [Google Scholar] [CrossRef]
- McDaniel, J.G.; Li, S.; Tylianakis, E.; Snurr, R.Q.; Schmidt, J.R. Evaluation of Force Field Performance for High-Throughput Screening of Gas Uptake in Metal–Organic Frameworks. J. Phys. Chem. C 2015, 119, 3143–3152. [Google Scholar] [CrossRef]
- Watanabe, T.; Sholl, D.S. Accelerating applications of metal-organic frameworks for gas adsorption and separation by computational screening of materials. Langmuir 2012, 28, 14114–14128. [Google Scholar] [CrossRef]
- Wilmer, C.E.; Leaf, M.; Lee, C.Y.; Farha, O.K.; Hauser, B.G.; Hupp, J.T.; Snurr, R.Q. Large-scale screening of hypothetical metal-organic frameworks. Nat. Chem. 2011, 4, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L. Thermodynamics of adsorption in porous materials. AIChE J. 2002, 48, 145–160. [Google Scholar] [CrossRef]
- Ghysels, A.; Vanduyfhuys, L.; Vandichel, M.; Waroquier, M.; van Speybroeck, V.; Smit, B. On the thermodynamics of framework breathing: A Free Energy Model for Gas Adsorption in MIL-53. J. Phys. Chem. C 2013, 117, 11540–11554. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).