A Highly Active Porous Mo2C-Mo2N Heterostructure on Carbon Nanowalls/Diamond for a High-Current Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Materials and Methods
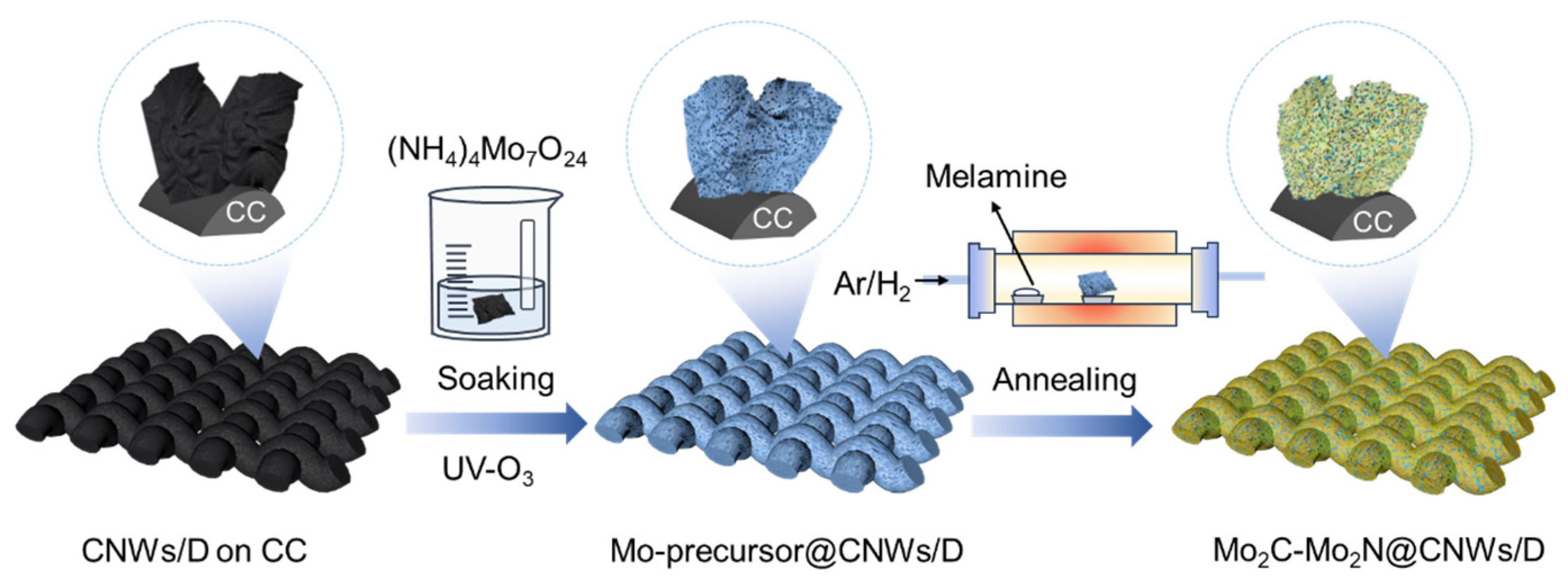
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
2.4. Calculation Method
3. Results and Discussion
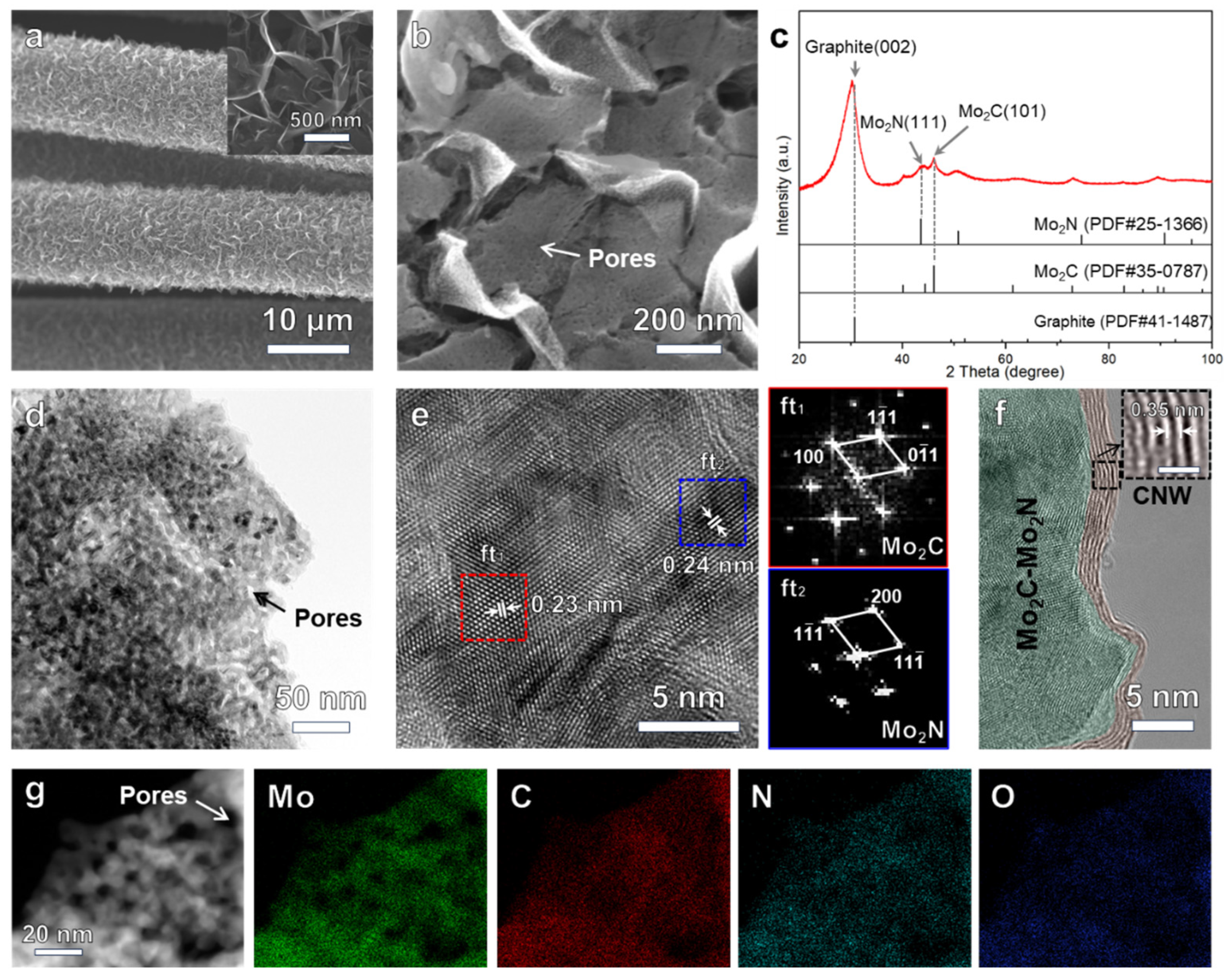
3.1. Microstructure Characterizations
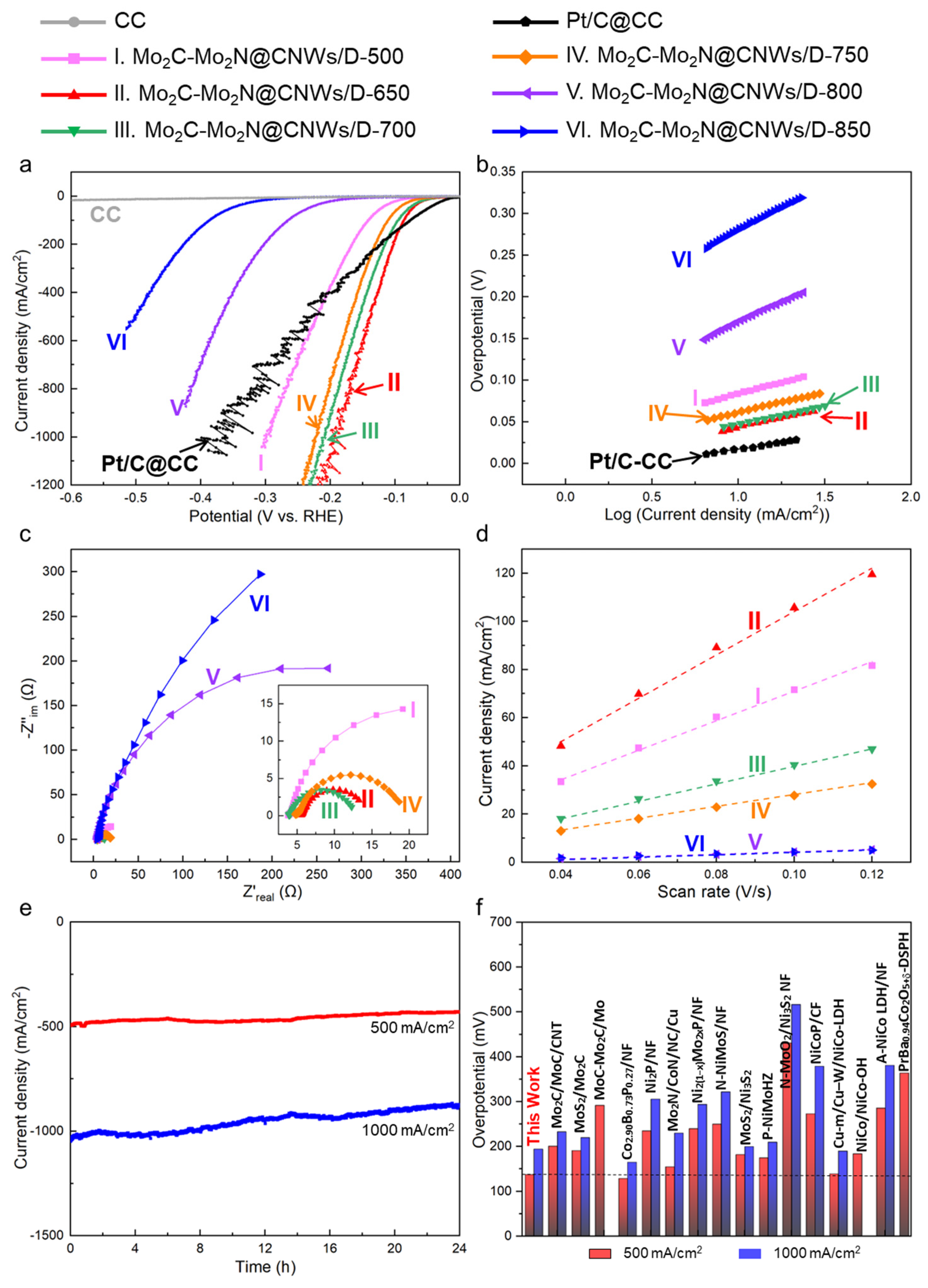
3.2. Performance of Hydrogen Evolution Reaction
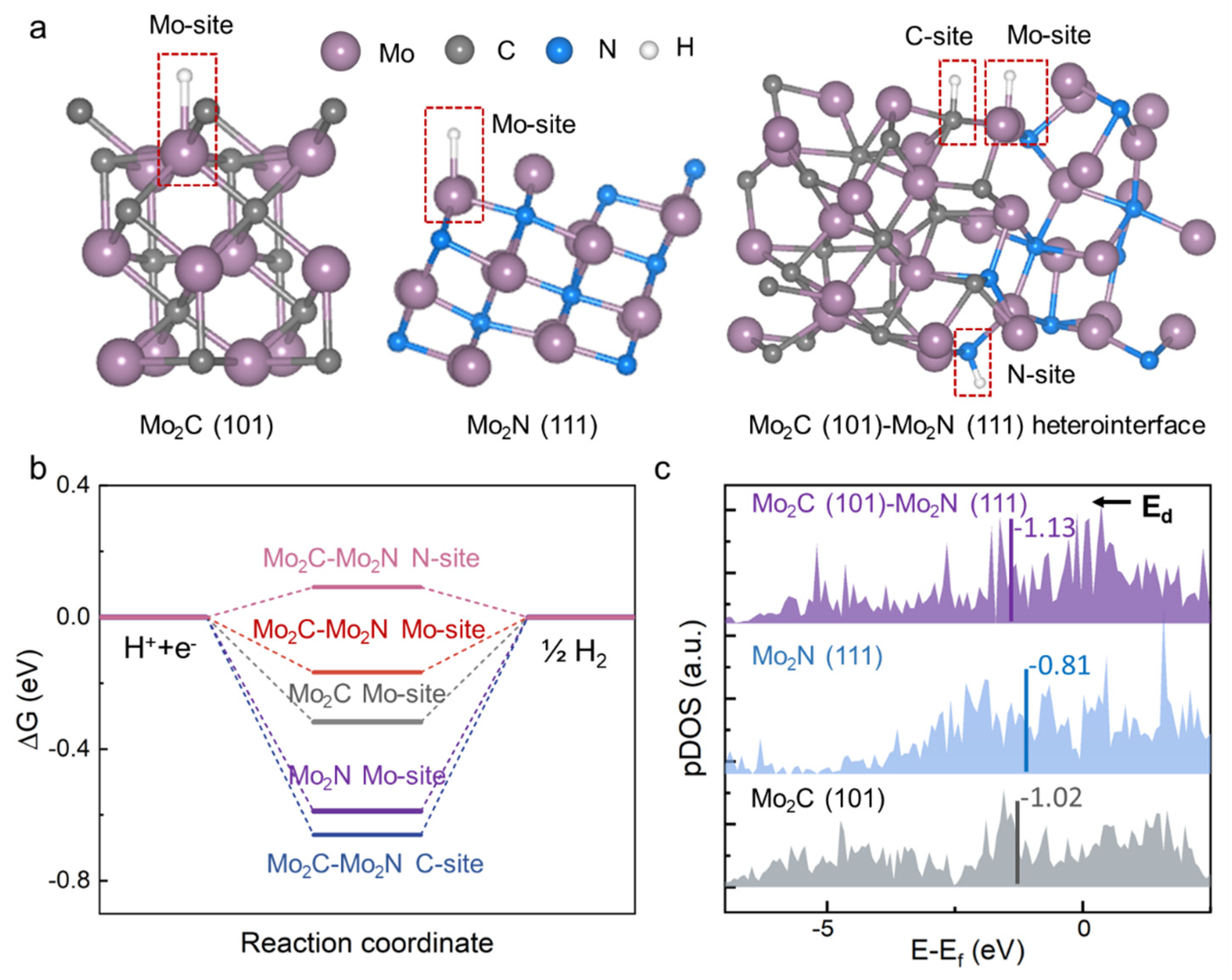
3.3. Dependance of Hydrogen Evolution Reaction Performance on the Surface Chemical State
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lagadec, M.F.; Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 2020, 19, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Ohto, T.; Nagata, Y.; Wakisaka, M.; Aoki, Y.; Fujita, J.I.; Ito, Y. Catalytic activity of graphene-covered non-noble metals governed by proton penetration in electrochemical hydrogen evolution reaction. Nat. Commun. 2021, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, Y.; Wang, D.; Wu, X.; Li, J.; Xi, J. Nickel-copper alloy encapsulated in graphitic carbon shells as electrocatalysts for hydrogen evolution reaction. Adv. Energy Mater. 2018, 8, 1701759. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Li, G.-D.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. Coupling Mo2C with nitrogen-rich nanocarbon leads to efficient hydrogen-evolution electrocatalytic sites. Angew. Chem.-Int. Ed. 2015, 54, 10752–10757. [Google Scholar] [CrossRef]
- Li, J.-S.; Wang, Y.; Liu, C.-H.; Li, S.-L.; Wang, Y.-G.; Dong, L.-Z.; Dai, Z.-H.; Li, Y.-F.; Lan, Y.-Q. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2016, 7, 11204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, G.; Chen, W.; Liu, Y.; Li, G.-D.; Zhu, P.; Tao, Q.; Li, Q.; Liu, J.; Shen, X.; et al. Highly active, nonprecious electrocatalyst comprising borophene subunits for the hydrogen evolution reaction. J. Am. Chem. Soc. 2017, 139, 12370–12373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, E.; An, X.; Hao, X.; Jiang, Z.; Guan, G. Transition metal-based catalysts for electrochemical water splitting at high current density: Current status and perspectives. Nanoscale 2021, 13, 12788–12817. [Google Scholar] [CrossRef]
- Chi, J.-Q.; Yang, M.; Chai, Y.-M.; Yang, Z.; Wang, L.; Dong, B. Design and modulation principles of molybdenum carbide-based materials for green hydrogen evolution. J. Energy Chem. 2020, 48, 398–423. [Google Scholar] [CrossRef]
- Ledendecker, M.; Mondschein, J.S.; Kasian, O.; Geiger, S.; Göhl, D.; Schalenbach, M.; Zeradjanin, A.; Cherevko, S.; Schaak, R.E.; Mayrhofer, K. Stability and activity of non-noble-metal-based catalysts toward the hydrogen evolution reaction. Angew. Chem.-Int. Ed. 2017, 56, 9767–9771. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Trends in the chemical properties of early transition metal carbide surfaces: A density functional study. Catal. Today 2005, 105, 66–73. [Google Scholar] [CrossRef]
- Liu, Y.; Huo, J.; Guo, J.; Lu, L.; Shen, Z.; Chen, W.; Liu, C.; Liu, H. Hierarchical porous molybdenum carbide based nanomaterials for electrocatalytic hydrogen production. Front. Chem. 2020, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Michalsky, R.; Zhang, Y.-J.; Peterson, A.A. Trends in the hydrogen evolution activity of metal carbide catalysts. ACS Catal. 2014, 4, 1274–1278. [Google Scholar] [CrossRef]
- Kim, S.; Choi, C.; Hwang, J.; Park, J.; Jeong, J.; Jun, H.; Lee, S.; Kim, S.-K.; Jang, J.H.; Jung, Y.; et al. Interaction mediator assisted synthesis of mesoporous molybdenum carbide: Mo-valence state adjustment for optimizing hydrogen evolution. ACS Nano 2020, 14, 4988–4999. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.-Q.; Gao, W.-K.; Lin, J.-H.; Dong, B.; Qin, J.-F.; Liu, Z.-Z.; Liu, B.; Chai, Y.-M.; Liu, C.-G. Porous core-shell N-doped Mo2C@C nanospheres derived from inorganic-organic hybrid precursors for highly efficient hydrogen evolution. J. Catal. 2018, 360, 9–19. [Google Scholar] [CrossRef]
- Jia, J.; Xiong, T.; Zhao, L.; Wang, F.; Liu, H.; Hu, R.; Zhou, J.; Zhou, W.; Chen, S. Ultrathin N-doped Mo2C nanosheets with exposed active sites as efficient electrocatalyst for hydrogen evolution reactions. ACS Nano 2017, 11, 12509–12518. [Google Scholar] [CrossRef]
- Shi, Z.; Nie, K.; Shao, Z.-J.; Gao, B.; Lin, H.; Zhang, H.; Liu, B.; Wang, Y.; Zhang, Y.; Sun, X.; et al. Phosphorus-Mo2C@carbon nanowires toward efficient electrochemical hydrogen evolution: Composition, structural and electronic regulation. Energy Environ. Sci. 2017, 10, 1262–1271. [Google Scholar] [CrossRef]
- Xiong, K.; Li, L.; Zhang, L.; Ding, W.; Peng, L.; Wang, Y.; Chen, S.; Tan, S.; Wei, Z. Ni-doped Mo2C nanowires supported on Ni foam as a binder-free electrode for enhancing the hydrogen evolution performance. J. Mater. Chem. A 2015, 3, 1863–1867. [Google Scholar] [CrossRef]
- Lin, H.; Liu, N.; Shi, Z.; Guo, Y.; Tang, Y.; Gao, Q. Cobalt-doping in molybdenum-carbide nanowires toward efficient electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2016, 26, 5590–5598. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Wang, F.; Du, K.; Zhang, Z.; Guo, Y.; Yin, H.; Wang, D. A durable and pH-universal self-standing MoC-Mo2C heterojunction electrode for efficient hydrogen evolution reaction. Nat. Commun. 2021, 12, 6776. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Liu, M.; Wang, E.; Wang, B.; Xu, L.; Jiang, K.; Fan, S.; Sun, Y.; Li, J.; et al. Ultrafast self-heating synthesis of robust heterogeneous nanocarbides for high current density hydrogen evolution reaction. Nat. Commun. 2022, 13, 3338. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Jiao, Y.; Wu, A.; Tian, C.; Zhang, X.; Wang, L.; Fu, H. Holey reduced graphene oxide coupled with an Mo2N-Mo2C heterojunction for efficient hydrogen evolution. Adv. Mater. 2018, 30, 1704156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, H.; Chen, Q.; Chen, H.; He, G. ZIF-67 derived Mo2N/Mo2C heterostructure as high-efficiency electrocatalyst for hydrogen evolution reaction. J. Alloys Compd. 2022, 922, 166216. [Google Scholar] [CrossRef]
- Song, H.; Guo, S.; Zhang, X.; Yang, Y.; Gao, B.; Pi, Y.; Pi, C.; Chu, P.K.; Huo, K. In-situ and controllable construction of Mo2N embedded Mo2C nanobelts as robust electrocatalyst for superior pH-universal hydrogen evolution reaction. J. Alloys Compd. 2022, 918, 165611. [Google Scholar] [CrossRef]
- Xia, M.; Li, S.; Zhang, X.; Xie, Z. Guanosine-assisted synthesis of a core–shell Mo2N/Mo2C/C structure for enhanced hydrogen evolution reaction. Inorg. Chem. Front. 2023, 10, 7018–7027. [Google Scholar] [CrossRef]
- Bang, J.; Moon, I.K.; Kim, Y.-K.; Oh, J. Heterostructured Mo2N–Mo2C nanoparticles coupled with N-doped carbonized wood to accelerate the hydrogen evolution reaction. Small Struct. 2023, 4, 2200283. [Google Scholar] [CrossRef]
- Shi, H.; Dai, T.Y.; Wan, W.B.; Wen, Z.; Lang, X.Y.; Jiang, Q. Mo-/Co-N-C hybrid nanosheets oriented on hierarchical nanoporous Cu as versatile electrocatalysts for efficient water splitting. Adv. Funct. Mater. 2021, 31, 2102285. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. A Step-by-Step Guide to Perform X-ray Photoelectron Spectroscopy. J. Appl. Phys. 2022, 132, 11101. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. Vaspkit: A user-friendly interface facilitating high-throughput computing and analysis using vasp code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Le, H.T.; Tran, D.T.; Kim, N.H.; Lee, J.H. Worm-Like Gold Nanowires Assembled Carbon Nanofibers-CVD Graphene Hybrid as Sensitive and Selective Sensor for Nitrite Detection. J. Colloid Interface Sci. 2021, 583, 425–434. [Google Scholar] [CrossRef]
- Kim, H.; Huang, X.; Guo, X.; Wang, Y.; Cui, S.; Wen, Z.; Chen, J. Novel Hybrid Si Film/Highly Branched Graphene Nanosheets for Anode Materials in Lithium-Ion Batteries. J. Phys. D-Appl. Phys. 2019, 52, 345201. [Google Scholar] [CrossRef]
- Zhai, Z.; Leng, B.; Yang, N.; Yang, B.; Liu, L.; Huang, N.; Jiang, X. Rational construction of 3D-networked carbon nanowalls/diamond supporting CuO architecture for high-performance electrochemical biosensors. Small 2019, 15, 1901527. [Google Scholar] [CrossRef] [PubMed]
- Jansonius, R.P.; Schauer, P.A.; Dvorak, D.J.; MacLeod, B.P.; Fork, D.K.; Berlinguette, C.P. Strain influences the hydrogen evolution activity and absorption capacity of palladium. Angew. Chem.-Int. Ed. 2020, 59, 12192–12198. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Liu, Z.; Ma, C.; Cheng, H.-M.; Ren, W. A highly active and durable electrocatalyst for large current density hydrogen evolution reaction. Sci. Bull. 2020, 65, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Guo, T.; Liu, T.; Wu, Z.; Cavallo, L.; Cao, Z.; Wang, D. Structure and phase regulation in MoxC (alpha-MoC1-x/beta-Mo2C) to enhance hydrogen evolution. Appl. Catal. B-Environ. 2019, 247, 78–85. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, N.; Zhuang, H.; Liu, L.; Yang, B.; Wang, C.; Gai, Z.; Guo, F.; Li, Z.; Jiang, X. A diamond/graphite nanoplatelets electrode for anodic stripping voltammetric trace determination of Zn(II), Cd(II), Pb(II) and Cu(II). Appl. Surf. Sci. 2018, 457, 1192–1201. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, A.; Jiao, Y.; Zheng, H.; Wang, X.; Xie, Y.; Wang, L.; Tian, C.; Fu, H. Two-dimensional porous molybdenum phosphide/nitride heterojunction nanosheets for pH-universal hydrogen evolution reaction. Angew. Chem.-Int. Ed. 2021, 60, 6673–6681. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.-M.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Yan, Z.; Chen, X.; Jiao, L.; Cheng, F.; Chen, J. Superhydrophilic amorphous Co–B–P nanosheet electrocatalysts with Pt-like activity and durability for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 22062–22069. [Google Scholar] [CrossRef]
- Yu, X.; Yu, Z.-Y.; Zhang, X.-L.; Zheng, Y.-R.; Duan, Y.; Gao, Q.; Wu, R.; Sun, B.; Gao, M.-R.; Wang, G.; et al. “Superaerophobic” nickel phosphide nanoarray catalyst for efficient hydrogen evolution at ultrahigh current densities. J. Am. Chem. Soc. 2019, 141, 7537–7543. [Google Scholar] [CrossRef]
- Yu, L.; Mishra, I.K.; Xie, Y.; Zhou, H.; Sun, J.; Zhou, J.; Ni, Y.; Luo, D.; Yu, F.; Yu, Y.; et al. Ternary Ni2(1-x)Mo2xP nanowire arrays toward efficient and stable hydrogen evolution electrocatalysis under large-current-density. Nano Energy 2018, 53, 492–500. [Google Scholar] [CrossRef]
- Huang, C.; Yu, L.; Zhang, W.; Xiao, Q.; Zhou, J.; Zhang, Y.; An, P.; Zhang, J.; Yu, Y. N-doped Ni-Mo based sulfides for high-efficiency and stable hydrogen evolution reaction. Appl. Catal. B-Environ. 2020, 276, 119137. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Song, E.; Wang, N.; Dong, J.; Zhang, X.; Ajayan, P.M.; Yao, W.; Wang, C.; Liu, J.; et al. Manipulation on active electronic states of metastable phase β-NiMoO4 for large current density hydrogen evolution. Nat. Commun. 2021, 12, 5960. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, J.; Lei, C.; Dai, Q.; Yang, B.; Li, Z.; Zhang, X.; Yuan, C.; Lei, L.; Hou, Y. Strongly coupled 3D N-doped MoO2/Ni3S2 hybrid for high current density hydrogen evolution electrocatalysis and biomass upgrading. ACS Appl. Mater. Interfaces 2019, 11, 27743–27750. [Google Scholar] [CrossRef]
- Pei, Y.; Huang, L.; Han, L.; Zhang, H.; Dong, L.; Jia, Q.; Zhang, S. NiCoP/NiOOH nanoflowers loaded on ultrahigh porosity Co foam for hydrogen evolution reaction under large current density. Green Energy Environ. 2022, 7, 467–476. [Google Scholar] [CrossRef]
- Parvin, S.; Kumar, A.; Ghosh, A.; Bhattacharyya, S. An earth-abundant bimetallic catalyst coated metallic nanowire grown electrode with platinum-like pH-universal hydrogen evolution activity at high current density. Chem. Sci. 2020, 11, 3893–3902. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, W.; Yu, H.; Zeng, Y.; Ming, F.; Liang, H.; Wang, Z. NiCo/NiCo–OH and NiFe/NiFe–OH core shell nanostructures for water splitting electrocatalysis at large currents. Appl. Catal. B-Environ. 2020, 278, 119326. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Guo, P.; Fei, B.; Wu, R. B-doping-induced amorphization of LDH for large-current-density hydrogen evolution reaction. Appl. Catal. B-Environ. 2020, 261, 118240. [Google Scholar] [CrossRef]
- Liu, Y.; Dou, Y.; Li, S.; Xia, T.; Xie, Y.; Wang, Y.; Zhang, W.; Wang, J.; Huo, L.; Zhao, H. Synergistic interaction of double/simple perovskite heterostructure for efficient hydrogen evolution reaction at high current density. Small Methods 2021, 5, 2000701. [Google Scholar] [CrossRef]
- Li, P.; Wu, D.; Dai, C.; Huang, X.; Li, C.; Yin, Z.; Zhou, S.; Lv, Z.; Cheng, D.; Zhu, J.; et al. Controlled synthesis of copper-doped molybdenum carbide catalyst with enhanced activity and stability for hydrogen evolution reaction. Catal. Lett. 2019, 149, 1368–1374. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Sagaltchik, A.; Pachfule, P.; Zhao, C.; Thomas, A. Metal-organic precursor-derived mesoporous carbon spheres with homogeneously distributed molybdenum carbide/nitride nanoparticles for efficient hydrogen evolution in alkaline media. Adv. Funct. Mater. 2019, 29, 1807419. [Google Scholar] [CrossRef]
- Wei, Z.B.Z.; Grange, P.; Delmon, B. XPS and XRD studies of fresh and sulfided Mo2N. Appl. Surf. Sci. 1998, 135, 107–114. [Google Scholar] [CrossRef]
- Wei, H.; Xi, Q.; Chen, X.; Guo, D.; Ding, F.; Yang, Z.; Wang, S.; Li, J.; Huang, S. Molybdenum carbide nanoparticles coated into the graphene wrapping N-doped porous carbon microspheres for highly efficient electrocatalytic hydrogen evolution both in acidic and alkaline media. Adv. Sci. 2018, 5, 1700733. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Chen, L.; Ma, B.; Wang, J.; Song, W.; Fan, X.; Li, Y.; Zhang, F.; Peng, W. Ultra-small Mo2C nanodots encapsulated in nitrogen-doped porous carbon for pH-universal hydrogen evolution: Insights into the synergistic enhancement of her activity by nitrogen doping and structural defects. J. Mater. Chem. A 2019, 7, 4734–4743. [Google Scholar] [CrossRef]
- Li, H.; Hu, M.; Zhang, L.; Huo, L.; Jing, P.; Liu, B.; Gao, R.; Zhang, J.; Liu, B. Hybridization of bimetallic molybdenum-tungsten carbide with nitrogen-doped carbon: A rational design of super active porous composite nanowires with tailored electronic structure for boosting hydrogen evolution catalysis. Adv. Funct. Mater. 2020, 30, 2003198. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, Y.; Chen, Y.; Li, P.; Liu, Q.; Wang, J. Ultrafine molybdenum carbide nanoparticles composited with carbon as a highly active hydrogen-evolution electrocatalyst. Angew. Chem.-Int. Ed. 2015, 54, 14723–14727. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, N.; Zhai, Z.; Liu, L.; Chen, B.; Yang, B.; Jiang, X.; Yang, N. Bifunctional oxygen electrocatalyst of Co4N and nitrogen-doped carbon nanowalls/diamond for high-performance flexible zinc–air batteries. Adv. Energy Mater. 2023, 13, 2301749. [Google Scholar] [CrossRef]

| Sample | η10 (mV) | η500 (mV) | η1000 (mV) | Tafel Slope (mV/dec) | Rct (Ω) | Capacitance (mF/cm2) |
|---|---|---|---|---|---|---|
| Mo2C-Mo2N@CNWs/D-500 | 83.7 | 221.5 | 299.8 | 54.3 | 33.94 | 603 |
| Mo2C-Mo2N@CNWs/D-650 | 42.8 | 137.8 | 194.4 | 45.6 | 8.59 | 891 |
| Mo2C-Mo2N@CNWs/D-700 | 47.9 | 154.8 | 207.8 | 46.5 | 8.64 | 359 |
| Mo2C-Mo2N@CNWs/D-750 | 62.6 | 169.4 | 222.9 | 48.8 | 13.95 | 243 |
| Mo2C-Mo2N@CNWs/D-800 | 169.3 | 369.1 | - | 97.4 | 464.65 | 46 |
| Mo2C-Mo2N@CNWs/D-850 | 281.7 | 502.2 | - | 109.2 | 1214.90 | 40 |
| Pt/C@CC | 16.8 | 228.5 | 359.3 | 32.5 | - | - |
| Catalysts | η10 (mV) | η500 (mV) | η1000 (mV) | Ref. |
|---|---|---|---|---|
| Mo2C-Mo2N@CNWs/D-650 | 42.8 | 137.8 | 194.4 | This work |
| Mo2C-Mo2N/HGr | 154 | - | - | [21] |
| MoxC | 116 | - | - | [13] |
| P-MoP/Mo2N | 89 | - | - | [36] |
| Mo2C/MoC/CNT | 82 | 201 | 233 | [20] |
| MoS2/Mo2C | - | 191 | 220 | [37] |
| MoC-Mo2C/Mo | 98.2 | 292 | - | [19] |
| Co2.90B0.73P0.27/NF | 42 | 129 | 165 | [38] |
| Ni2P/NF | - | 235 | 306 | [39] |
| Mo2N/CoN/NC/Cu | 22 | 155 | 230 | [26] |
| Ni2(1−x)Mo2xP/NF | 72 | 240 | 294 | [40] |
| N-NiMoS/NF | 68 | 250 | 322 | [41] |
| MoS2/Ni3S2 | 70 | 182 | 200 | [33] |
| P-NiMoHZ | 23 | 175 | 210 | [42] |
| N-MoO2/Ni3S2 NF | - | 431 | 517 | [43] |
| NiCoP/CF | 47 | 273 | 379 | [44] |
| Cu-m/Cu–W/NiCo-LDH | 21 | 139 | 190 | [45] |
| NiCo/NiCo-OH | 19 | 184 | - | [46] |
| A-NiCo LDH/NF | 36 | 286 | 381 | [47] |
| PrBa0.94Co2O5+δ-DSPH | 186 | 364 | - | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Zhang, C.; Chen, B.; Liu, L.; Song, H.; Yang, B.; Zheng, Z.; Li, J.; Jiang, X.; Huang, N. A Highly Active Porous Mo2C-Mo2N Heterostructure on Carbon Nanowalls/Diamond for a High-Current Hydrogen Evolution Reaction. Nanomaterials 2024, 14, 243. https://doi.org/10.3390/nano14030243
Zhai Z, Zhang C, Chen B, Liu L, Song H, Yang B, Zheng Z, Li J, Jiang X, Huang N. A Highly Active Porous Mo2C-Mo2N Heterostructure on Carbon Nanowalls/Diamond for a High-Current Hydrogen Evolution Reaction. Nanomaterials. 2024; 14(3):243. https://doi.org/10.3390/nano14030243
Chicago/Turabian StyleZhai, Zhaofeng, Chuyan Zhang, Bin Chen, Lusheng Liu, Haozhe Song, Bing Yang, Ziwen Zheng, Junyao Li, Xin Jiang, and Nan Huang. 2024. "A Highly Active Porous Mo2C-Mo2N Heterostructure on Carbon Nanowalls/Diamond for a High-Current Hydrogen Evolution Reaction" Nanomaterials 14, no. 3: 243. https://doi.org/10.3390/nano14030243
APA StyleZhai, Z., Zhang, C., Chen, B., Liu, L., Song, H., Yang, B., Zheng, Z., Li, J., Jiang, X., & Huang, N. (2024). A Highly Active Porous Mo2C-Mo2N Heterostructure on Carbon Nanowalls/Diamond for a High-Current Hydrogen Evolution Reaction. Nanomaterials, 14(3), 243. https://doi.org/10.3390/nano14030243








