Abstract
Swarms of self-propelled micromotors can mimic the processes of natural systems and construct artificial intelligent materials to perform complex collective behaviors. Compared to self-propelled Janus micromotors, the isotropic colloid motors, also called micromotors or microswimmers, have advantages in self-assembly to form micromotor swarms, which are efficient in resistance to external disturbance and the delivery of large quantity of cargos. In this minireview, we summarize the fundamental principles and interactions for the assembly of isotropic active particles to generate micromotor swarms. Recent discoveries based on either catalytic or external physical field-stimulated micromotor swarms are also presented. Then, the strategy for the reconstruction and motion control of micromotor swarms in complex environments, including narrow channels, maze, raised obstacles, and high steps/low gaps, is summarized. Finally, we outline the future directions of micromotor swarms and the remaining challenges and opportunities.
1. Introduction
Collective behavior is the autonomous organization of a variety of individuals to form a functional group, which widely exists in nature and ranges in all scales, including crystal molecules, bacteria colonies, macroscopic flocks of animals (fishes, ants, birds, etc.), and galaxies [1,2,3,4]. Compared to the individuals, the collective assembly of natural individuals could be more efficient in working together to accomplish complex tasks [5]. For example, schools of fish can move in an orderly manner or change shape and internal structure suddenly to adapt to their surroundings [6], collectives of ants can stack themselves to build bridges and transport heavy foods together [7], and bacterial colonies are efficient in accessing new sources of nutrients for increasing community size [8]. Similar to natural collective assemblies, artificial systems consisting of simple isotropic units assemble through physical/chemical field-induced interactions, and provide model systems to understand complex collective systems [9,10,11,12,13,14].
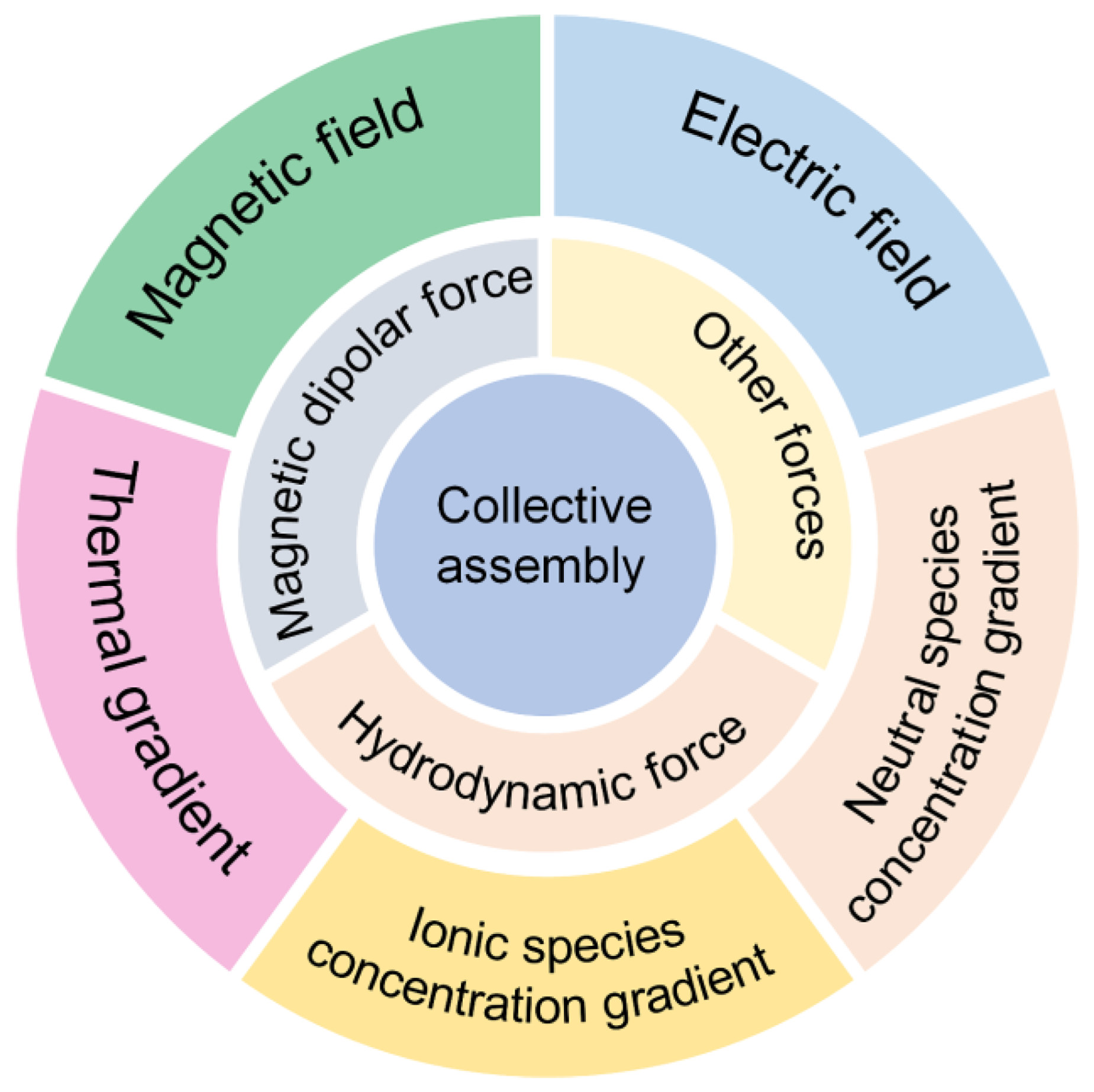
Self-propelled synthetic micromotors are capable of converting the surrounding energy into mechanical motion in fluidic environments, and give a new insight into the understanding of living matter and out-of-equilibrium systems [15,16,17,18,19,20,21]. To break the Scallop rule in a low Reynold regime, the symmetry of micromotors needs to be broken. Typically, Janus micromotors or micromotors of various shapes such as nanorod, nanosphere, core–shell nanowire, and nanotube are designed [18,22,23,24,25,26,27,28]. Among them, isotropic colloidal motors are easy to prepare and can self-assemble into large swarms for the transport of loads, which demonstrates the advantages of collective control [29,30,31]. To date, different reactions and external fields have been applied to generate colloid swarms (Scheme 1), including ionic/neutral species concentration gradients produced by a catalytic or noncatalytic reaction [32], thermal gradients generated by a photothermal conversion [33], and magnetic and electric fields [34,35]. These chemical reactions or external fields can affect microscopic forces among colloidal particles, such as the hydrodynamic force [36], magnetic dipolar force [37], π-π stack force [38], hydrophobic force [39], interfacial tension [40,41,42,43], etc., ultimately leading to the formation of microswarms. Unlike other micromotor swarms based on Janus particles and microtubes, the isotropic character of colloidal particles provides a model system to study the interparticle interaction and clustering mechanism in out-of-equilibrium systems, as well as contributing to larger colloidal clusters for drug delivery, resistance to external disturbance, and high efficiency imaging [44,45,46,47,48,49,50,51,52].
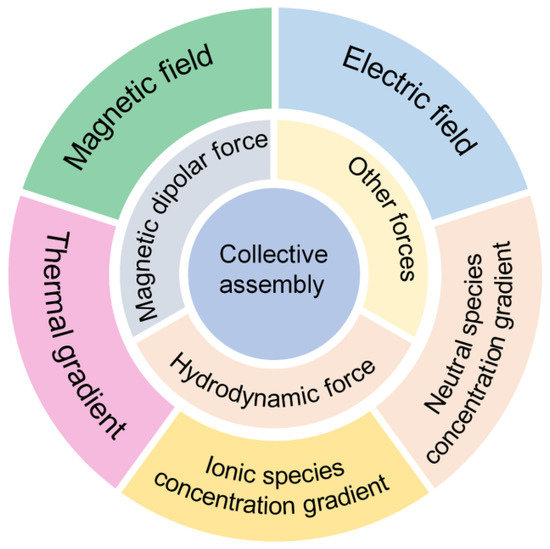
Scheme 1.
Collective assembly of isotropic micromotors via magnetic/hydrodynamic forces under different physical/chemical stimuli.
In this minireview, we summarize the new phenomena and processes of collective assembly of isotropic active colloids, and the reconstruction and motion of the micromotor swarms under external stimuli, and discuss the related fundamental principles and interactions. Then, we discuss the currently developed strategies for the motion control of micromotor swarms in complex environments to meet requirements in real applications. Finally, we outline the challenges and opportunities for the future development of microswarms.
2. Collective Assembly of Isotropic Micromotors
Constructing local fields plays an important role in the design of isotropic micromotor swarms. Because of the isotropy of individual micromotors, the field generated by single colloids cannot be converted to self-propulsion. However, the interactions between single colloids break the symmetry of fields and provide the driving force, such as magnetic forces, hydrodynamic forces, and other forces, for the formation of microswarms [53,54]. Thus, the collective assembly of micromotor swarms can be categorized according to the triggering field. Additionally, the interaction of these local fields has a key influence on the response of the microswarms to the environment or external stimuli. Understanding the coupling of local fields is necessary for realizing a reconfigurable assembly of isotropic micromotors. These local fields can be nonelectrolyte/electrolyte gradients induced by photo- or non-photo-triggered chemical reactions or externally applied stimuli. In this section, the design and assembly mechanisms of isotropic micromotor swarms are briefly described.
2.1. Chemical Gradient-Induced Micromotor Swarm
The chemical gradient field-induced collective assembly of micromotor swarms are usually triggered by a chemical reaction, including a catalytic reaction, ion exchange reaction, and self-dissociation reaction. The generated gradients of ionic/neutral species among individual colloids induce the diffusiophoresis, electrophoresis, and electroosmosis on a charged surface for the assembly of the colloids into a swarm.
2.1.1. Photocatalytic Micromotor Swarms
Isotropic catalytic micromotors made of semiconductors tend to catalyze or react with surrounding molecules symmetrically, resulting in a uniform chemical gradient field around themselves. However, when multiple micromotors are in the proximity of each other, the generation of chemical gradients will be affected by each other on the basis of diffusiophoresis, electrophoresis, hydrodynamic resistance, etc., which can eventually be converted into a non-equilibrium chemical field inside and outside each cluster or swarm [29,55]. As a result, the swarm of isotropic micromotors exhibits multiple collective behaviors including self-assembly, reconstruction, and dispersion [56]. Among them, the photocatalysis of surrounding chemicals (fuels) becomes the main driving force to actuate the collective behavior of isotropic micromotors. In order to obtain light-controlled active micromotor swarms, the reactivity of micromotors, the physicochemical properties of catalyzed species (amount, valence state, diffusion coefficient, etc.), and the surface electrical properties of micromotors and substrates should be mainly evaluated.
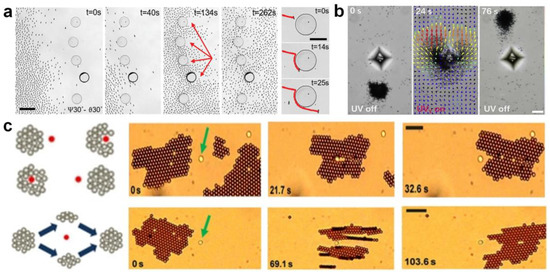
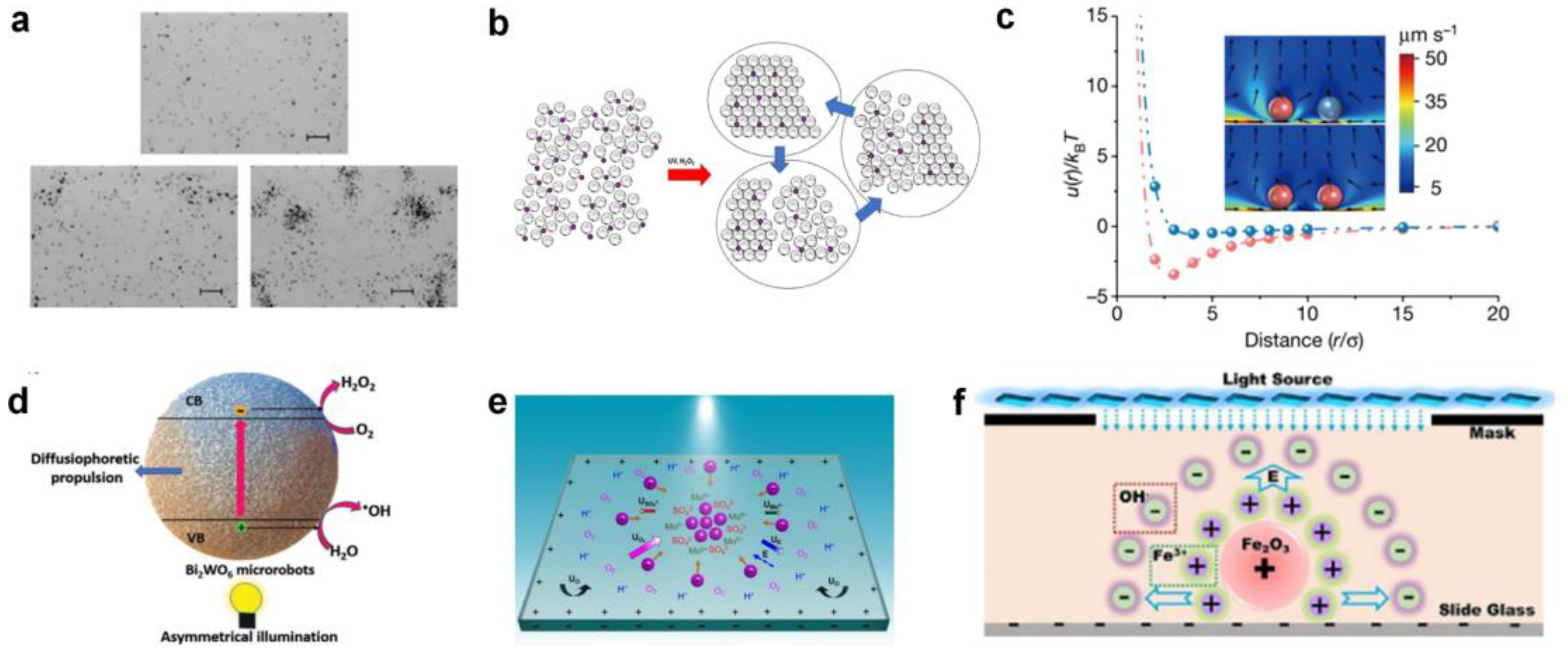
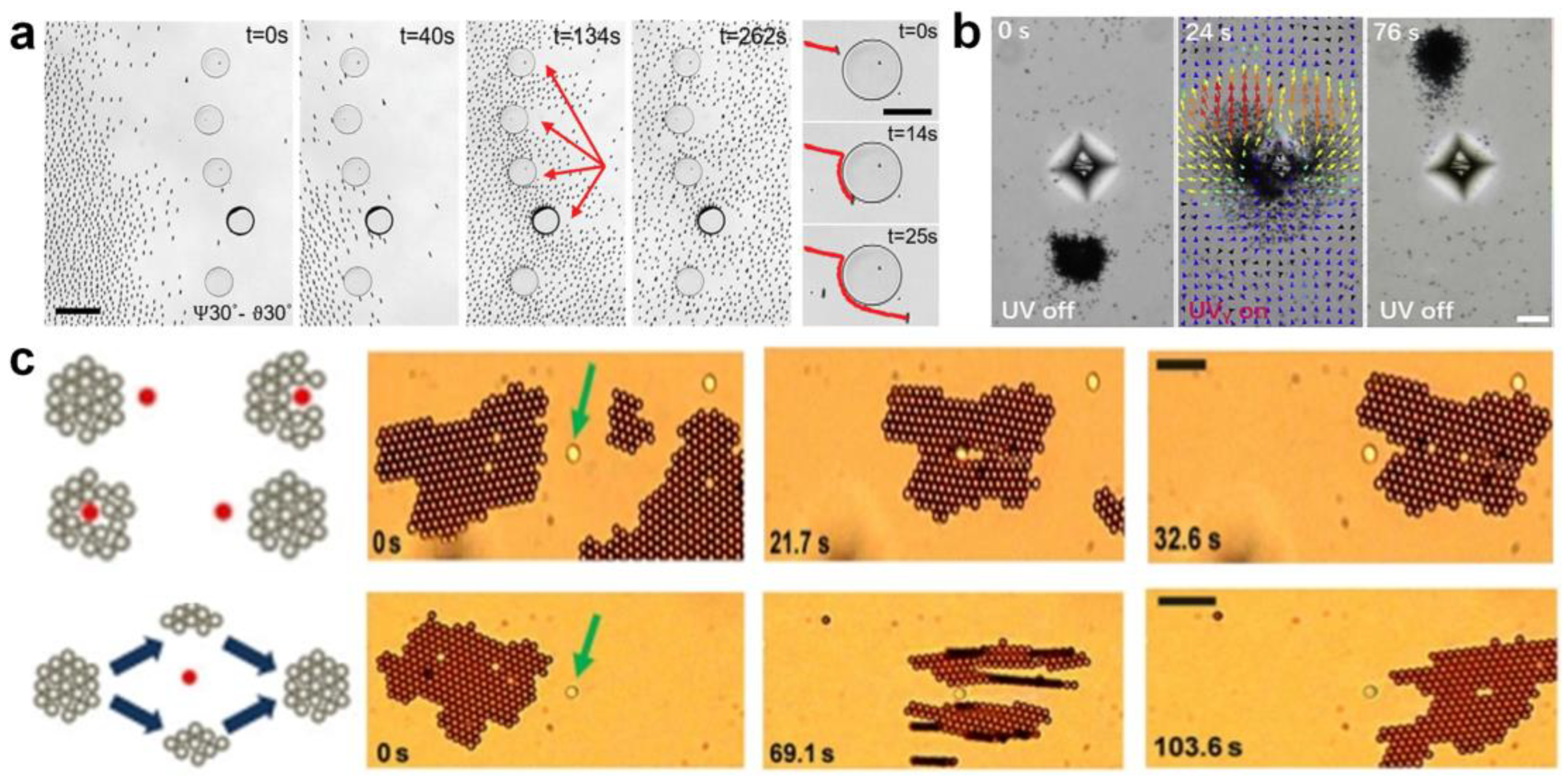
Up to now, many semiconductor-based micromotor swarms have been designed. For example, Ibele et al. [56] reported micrometer-sized silver chloride (AgCl) particles that can secrete H+ and Cl− ions under UV illumination in deionized water (at pH 5). Moving by self-diffusiophoresis, each AgCl particle responds by “schooling” into regions with higher particle concentrations (Figure 1a). Moreover, the photo-inactive silica particles can respond to the ion secretion of AgCl by swimming towards and surrounding individual AgCl particles. Altemose et al. [57] reported the oscillatory waves generated by silver phosphate micromotors which lead to the hexagonal packing of inert silica under UV light and H2O2 (Figure 1b). The colloidal crystals can form/relax under/without UV illumination. The electroosmotic flow generated on the charged substrate dominates the motion and assembly/dispersion of all particles in the system. Zheng et al. [58] recently developed commercial dye-stained TiO2 microspheres to realize phase segregation and interaction tuneability in response to the light of different wavelengths (Figure 1c). At the basis of the photoexcitation of dyes, the redox reaction on TiO2 particles generates chemical gradients, which results in diffusiophoretic flow and effective attractive potential between each other. The particle–particle interaction results in selectively activated colloidal swarms. Villa et al. [30] exhibited self-propelled perovskite-like bismuth tungstate (Bi2WO6) micromotors that can be activated in water under visible-light irradiation (Figure 1d). The activation mechanism involves the generation of electron-hole pairs that react with water and O2 to produce hydroxyl radicals (•OH), protons (H+), hydroxyl groups (OH−), and H2O2 species. The chemical concentration gradient around the spherical microparticles contributes to the swarming behavior of Bi2WO6 micromotors. Chen et al. [59] reported UV light-activated MoS2 colloidal motors which can generate H+, SO42+, and Mo6+ via a photo-corrosion reaction as the surface of MoS2 is oxidized into MoO3 under exposure to UV light (Figure 1e). They found that the collective motion of MoS2 could occur on both negatively charged and positively charged surfaces, which verifies that the diffusiophoresis induced by the locally consumed oxygen gradient dominates the phototaxis of colloidal motors.
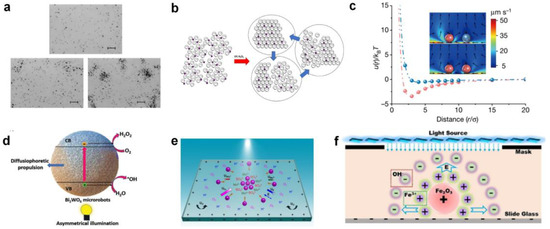
Figure 1.
Photocatalytic micromotor swarms. (a) Collective behaviors of AgCl particles under UV exposure in deionized water. Reproduced from [56]. Copyright 2009 Wiley-VCH GmbH. (b) Self-assembly of silica microspheres in the presence of silver phosphate micromotors under UV light in hydrogen peroxide solution. Reproduced from [57]. Copyright 2020 Wiley-VCH GmbH. (c) Phoretic flow field and effective potential of phase-segregated photosensitive TiO2 colloidal particles. Reproduced form [58]. Copyright 2023 Springer Nature. (d) Motion and swarming mechanism of Bi2WO6 microrobots. Copyright 2020 Wiley-VCH GmbH. Reproduced from [30]. (e) MoS2 colloidal motors (pink particles) undergoing self-diffusiophoresis on a negatively charged substrate to form a swarm. Under illumination of UV light, MoS2 colloid motors at the center of the light consume O2 and generate cations and anions to induce an electric field (E) pointed inwards (blue arrow). Reproduced from [59]. Copyright 2021 Wiley-VCH GmbH. (f) Mechanism of light-induced electrolyte diffusiophoresis caused by Fe2O3 nanomotors with light-irradiation-consuming H2O2 molecules. Reproduced from [60]. Copyright 2019 Wiley-VCH GmbH.
In addition to photoexcited semiconductor materials, micromotors based on Fenton-like reactions are also capable of generating ionic concentration gradients that promote spontaneous self-assembly when stimulated by external light. For example, Zhou et al. [60] employed Fe2O3 nanoparticles as self-assembly units which can react with H2O2 under visible light via a photo-Fenton reaction (Figure 1f). In this catalysis process, ferric ions and hydroxide ions, being different in diffusion coefficients, are released from the surface of the nanomotors. Then, a diffusion-induced electric field is established, which acts not only on the nanomotors, but also on any charge surfaces. As a result, electroosmotic flow is generated to drive the nanomotors towards each other to form the desired shape based on the illumination.
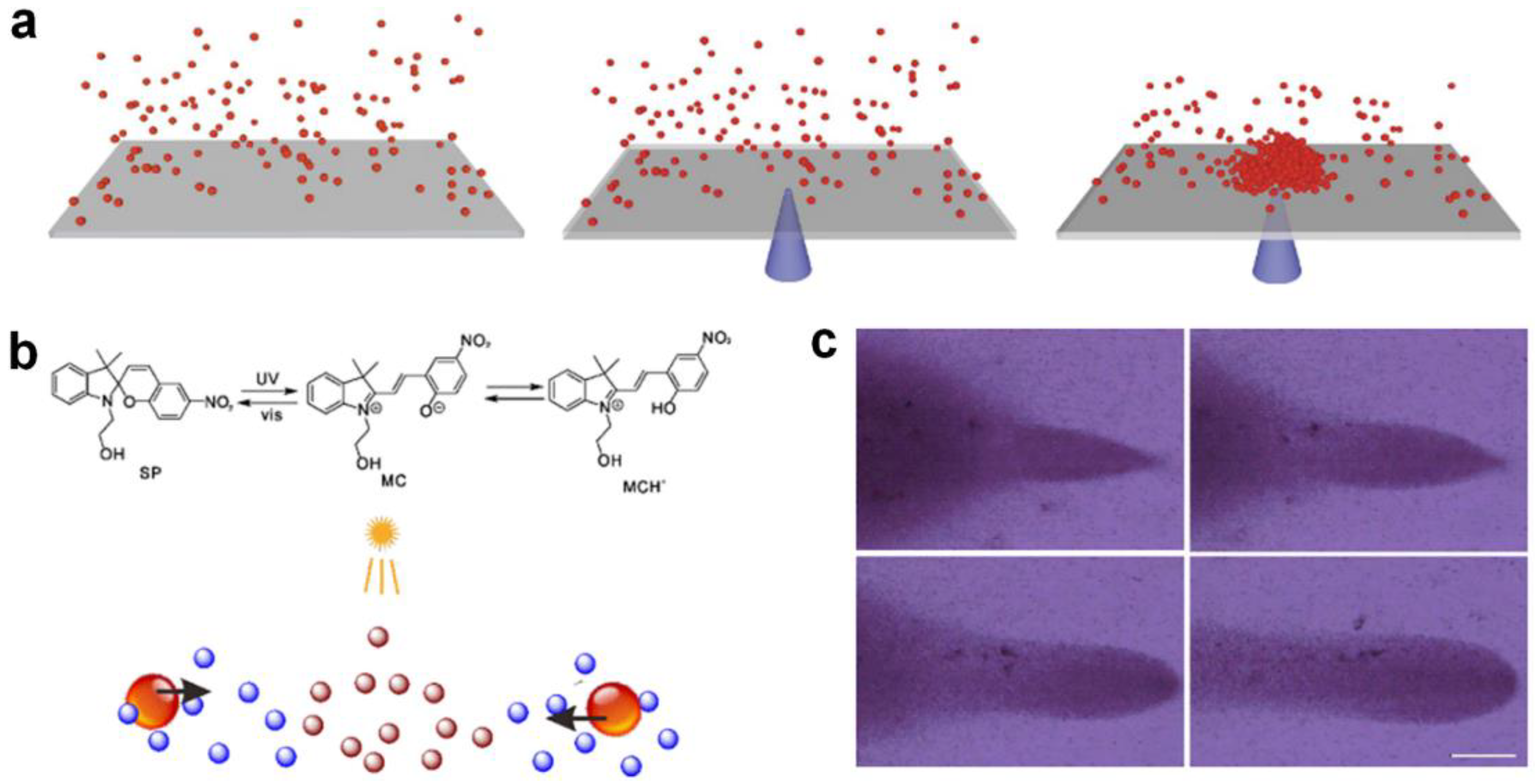
Catalytically or photo-chemically consuming environmental chemical compounds can also lead to microparticle agglomeration. This micro-nano cluster assembly method does not depend on certain specific active micro-nanoparticles; it therefore greatly expands the range of candidates for the formation of micromotor swarms, and can be used to assemble chemically inert micro-nano particles. Kim et al. [61] reported the photo-induced assembly of PMMA particles on conductive or semi-conductive substrates (for example, ITO- or gold-coated substrates) under the illumination of laser of varying wavelengths (Figure 2a). They found that at the basis of illumination, a local electric field could be established on an absorptive, conductive substrate such as ITO to direct colloid motion and lead to colloid assembly. The photo-induced assembly is strongly affected by solvent conditions and the surface functionalization of PMMA particles, indicating the electrophoresis-dominated assembly process. Wu et al. [36] investigated the transport of aggregated silica microspheres via the photoisomerization of spiropyran in solution (Figure 2b). Under the illumination of UV spot sources, the silica microspheres moved and aggregated at the center of the light spot and finally formed three-dimensional aggregates (Figure 2c). The researchers found that the spiropyran concentration as well as the chemical gradient derived from UV irradiation play key roles in silica microsphere aggregation. Based on the strength of osmotic flow, other inert colloidal particles such as PS microspheres can also be transported to the light spot.
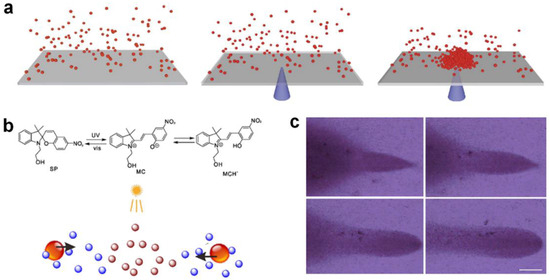
Figure 2.
Assembly by environmental matter catalysis. (a) Assembly of poly(12-hydroxy-stearic acid) (PHSA)-stabilized poly(methyl methacrylate) (PMMA) colloids on the coupling of electromagnetic illumination to a ITO-coated glass substrate. Reproduced from [61]. Copyright 2014 Springer Nature. (b) Photoisomerization causing chemical gradients under UV spot irradiation. (c) Aggregated silica particles following a moving UV spot. Reproduced from [36]. Copyright 2020 ACS publication.
2.1.2. Non-Catalytic Micromotor Swarms
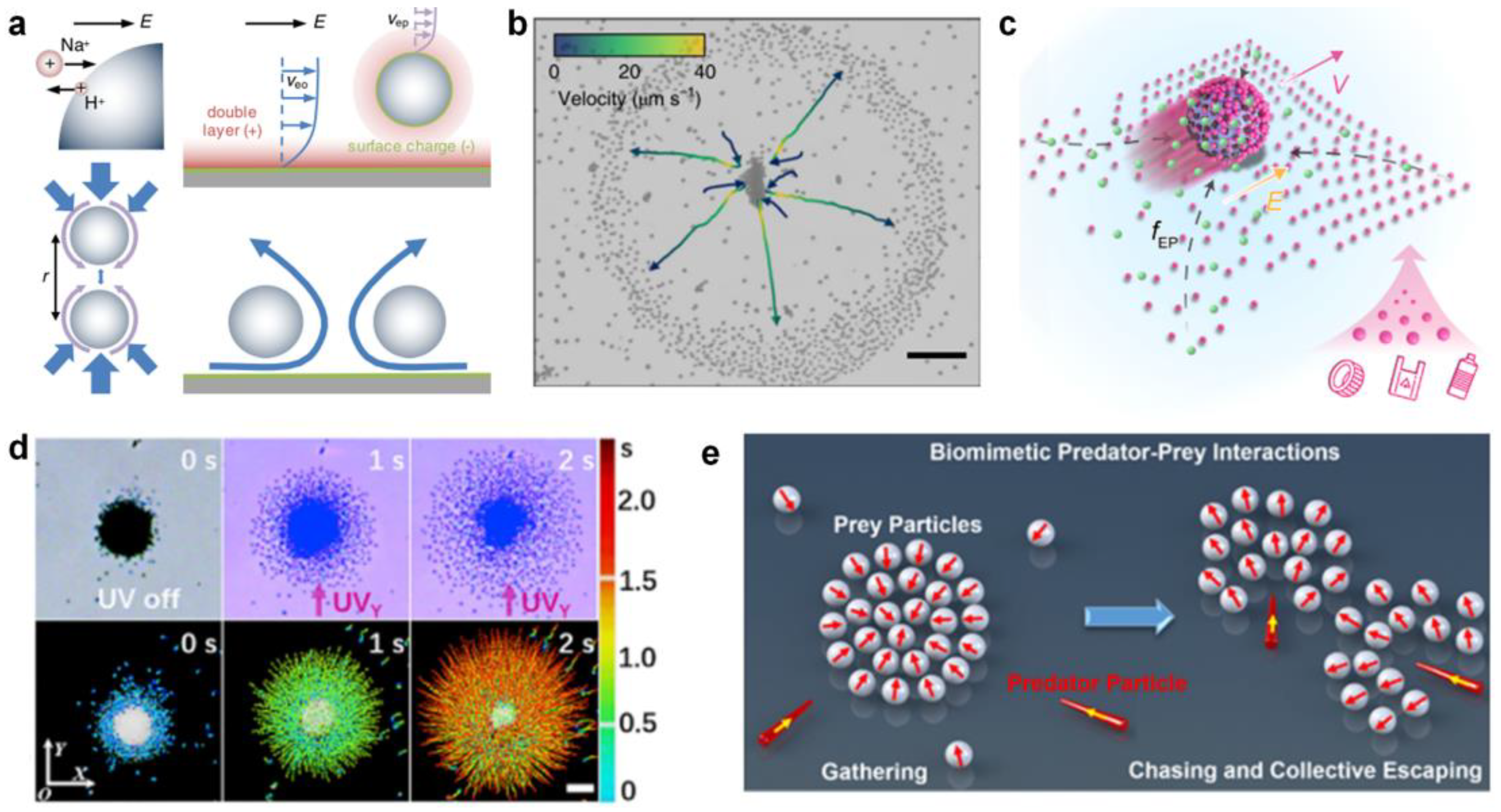
The above research on light-triggered swarms greatly improves the understanding of the formation mechanism of micromotor clusters, and lays a foundation for constructing single-component isotropic micromotor swarms with an on-off control. While the non-catalytic reaction-triggered swarms often involve spontaneous interactions of multiple components in a system, inducing complex phase behaviors that exceed those of single-component clusters, such as predator–prey interactions. This multi-component phase separation often results from the exchange of ions between each other. Michelin et al. proved that asymmetries in geometry are sufficient to induce chemical gradients and swimming [62]. A typical example is ion-exchange resin particles, which can exchange cations for protons and generate pH gradient and long-rang electroosmotic flow. Niu et al. [31] first demonstrated the long-range flow and its role in acting as a long-range attractive interaction between two particles, which induces the self-assembly of colloidal molecules (Figure 3a). Later on, the Tang group [63] demonstrated the “waste” ion exchange-induced quorum sensing of the ZnO-sulfonated PS complex (Figure 3b). Briefly, the ZnO nanorod serves as a Zn2+ source for the ion exchange of sulfonated PS, and the H+ released from the sulfonated PS accelerates the dissolving of ZnO in turn. The migration speeds of the system are greatly enhanced where the two active particle species are regulated by the nearby counter particles. At an equilibrium position of the chemotaxis attraction and surface slipping flow, the ZnO-sulfonated PS complex is assembled. Then, the researchers explored the application of the long-range attractive flow in microplastic removal in nonmarine water (Figure 3c) [64].
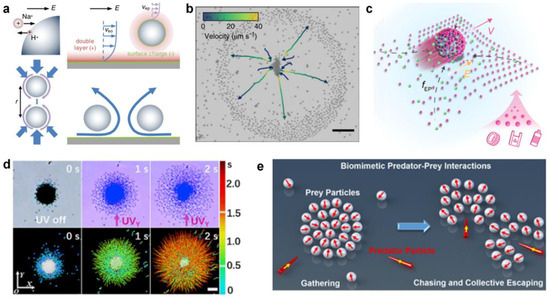
Figure 3.
Non-catalytic reaction-triggered micromotor swarms. (a) Clustering and phototactic motion behaviors of TiO2 micromotors with high-surface acidic bridging hydroxyls. Reproduced from [31]. Copyright 2017 PRL. (b) Schematic demonstration of biomimetic predator-prey interactions based on the binary repulsive−attractive diffusiophoretic interactions. Reproduced from [63]. Copyright 2021 Springer Nature. (c) Long-range interaction between ion-exchange resin colloids through electroosmotic flow. Reproduced from [64]. Copyright 2022 AAAS. (d) The ion-exchange interaction of the ZnO-sulfonated PS complex. Reproduced from [32]. Copyright 2019 ScienceDirect. (e) Self-driven magnetorobot (SMR) consisting of ion-exchange resin microsphere-enabling adsorption of MNPs from the aqueous environment. Reproduced from [65]. Copyright 2020 ACS Publicaton.
Another example is anatase TiO2 micromotors with abundant hydroxyl groups. Mou et al. [32] demonstrated that the surface hydroxyl groups on TiO2 can help to secrete H+ ions from surface acidic bridging hydroxyls and OH− ions from basic terminal hydroxyls by dissociating water (Figure 3d). Then, a converging electroosmotic flow was established to drive the aggregation of particles due to the migration of cations in the electrical double layer of micromotors and glass substrate. However, when UV light was applied, the TiO2 flocks catalyzed environmental H2O2 to produce a cloud of O2 molecules around the micromotors, which induced the dilatational phototaxis. Recently, Mou et al. [29] demonstrated that the micromotor predator–prey system, containing diffusiophoretic repulsive and attractive micromotors, could be established without additional chemical fuels (Figure 3e). The two species of microparticles, including active Ag3PO4-TiO2, ZnO-AgBr, and ZnO-TiO2 micromotor systems, show dynamic group reconfigurations based on local repulsion.
2.2. Photo-Thermal Effects-Caused Self-Assembly
By utilizing various thermal forces such as thermophoresis, photophoresis, and convective flows in a light-controlled temperature field, researchers have endowed micromotors with versatile manipulation methods. The thermophoresis-actuated micro/nano motors and swarms have been used in the fields of nanomedicine, chemical and biological sensing, and micro patterns [65,66]. Here, the heat-mediated assembly of micromotors and its formation mechanism are briefly introduced.
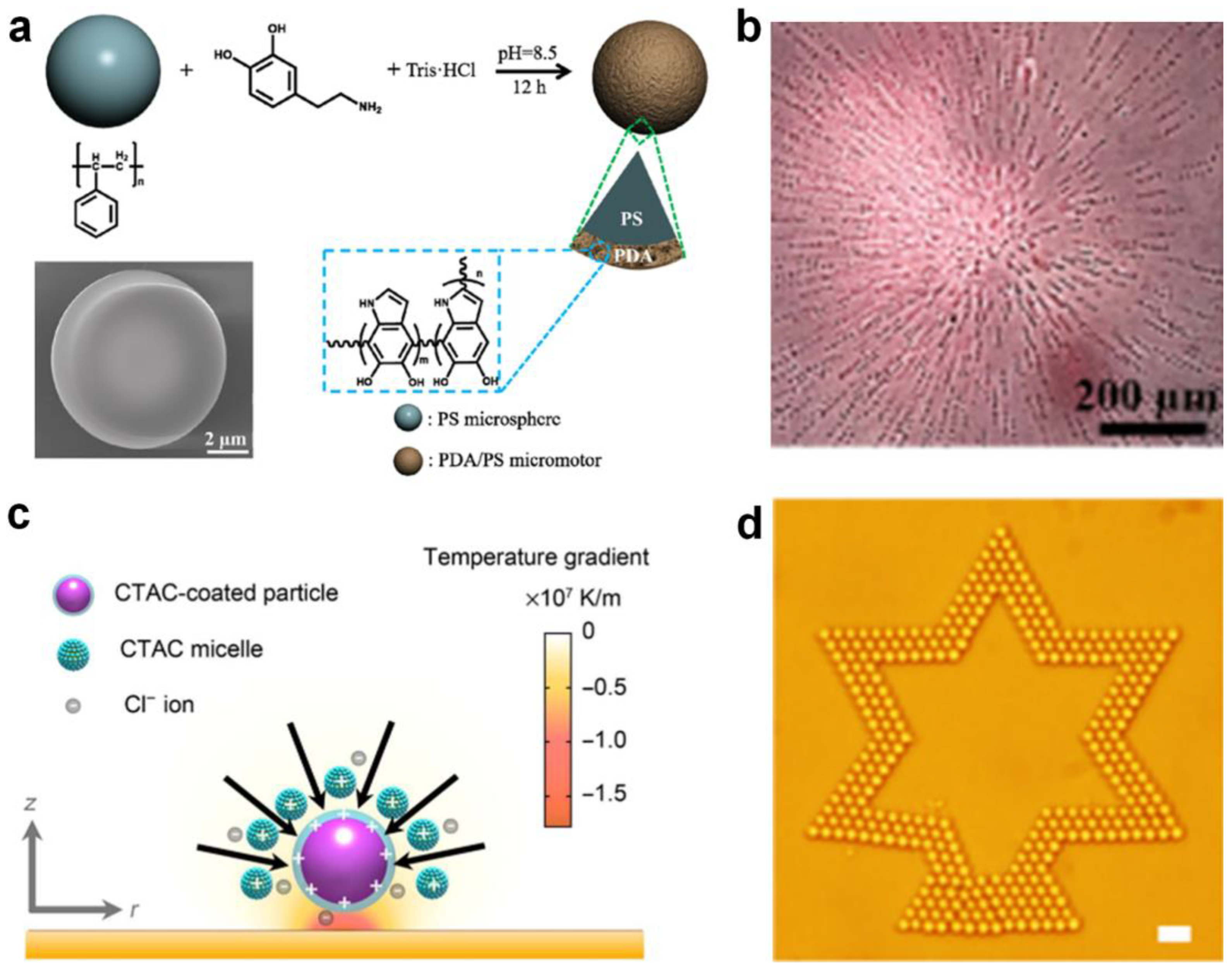
Sun et al. [33] demonstrated a novel micromotor consisting of a polystyrene microsphere core and a polydopamine shell (PS@PDA) which can be activated by NIR light (Figure 4a). Interestingly, the micromotor demonstrates a concentration-dependent motion direction reversal, i.e., a single micromotor exhibits negative phototaxis, whereas a group of micromotors shows positive phototaxis-induced aggregation near the NIR light spot, which is tunable in shape via the light irradiation position (Figure 4b). The differences can be attributed to the competition between the thermophoretic force and hydrodynamic drag caused by the thermal buoyancy. Lin et at. [67] demonstrated that ionic surfactant cetyltrimethylammonium chloride (CTAC) micelles can be used as positive macro ions to create a thermoelectric field for the optical trapping of colloidal particles (Figure 4c). They found that the added-CTAC molecules and micelles would adsorb onto the colloidal particles through electrostatic interaction and/or hydrophobic interaction. Then, a temperature gradient along the z axis was established through the irradiation of a laser beam, which could drive both the CTAC micelles and Cl− ions to the cold side. As a result, a thermoelectric field was established which can manipulate the assembly of colloidal particles into two-dimensional (2D) colloidal matter (Figure 4d). In this way, colloids of various sizes and materials can be efficiently assembled, which is applicable to a range of particles.
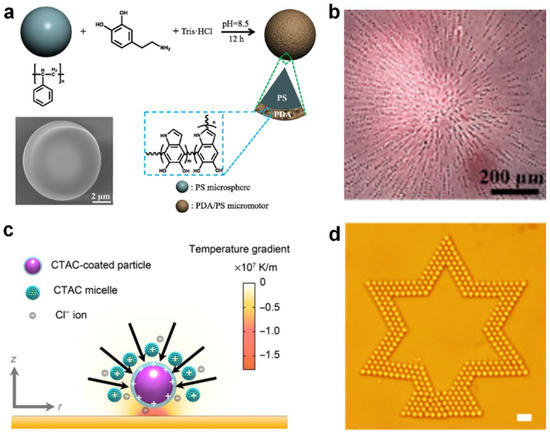
Figure 4.
Self-assembly by photothermal conversion-induced thermal gradient. (a) Fabrication and (b) optical microscopic image of NIR light-driven positive phototaxis of PS@PDA micromotors. Reproduced from [33]. Copyright 2019 ACS Publication. (c) Light-directed thermoelectric field around colloid particle and (d) 2D assembly of a star pattern of 2-μm silica beads. Reproduced from [67]. Copyright 2017 AAAS.
2.3. Magnetic Field-Induced Self-Assembly
Magnetism, as an external stimulus, is commonly employed to provide additional magnetic dipole–dipole force beyond the traditional electrostatic, gravity, and hydrodynamics forces for the control of micromotors. The advantages of applying a magnetic field force include the capacity for high temporal and spatial control, and superior non-invasive and harmless characteristics. Moreover, the magnetic field can promote the formation of micromotor swarms that is easy to control. Up to now, a large number of studies on the swarm behaviors of magnetic micromotors have been reported.
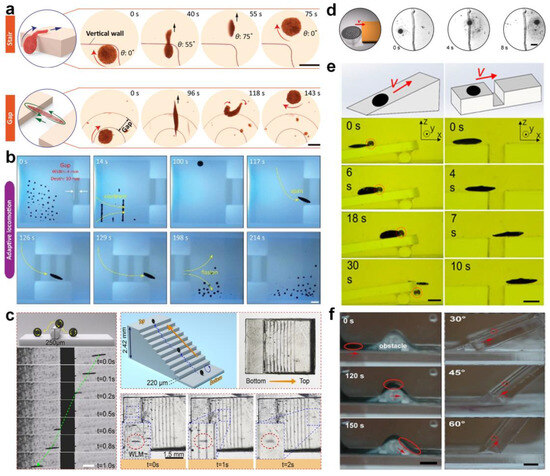
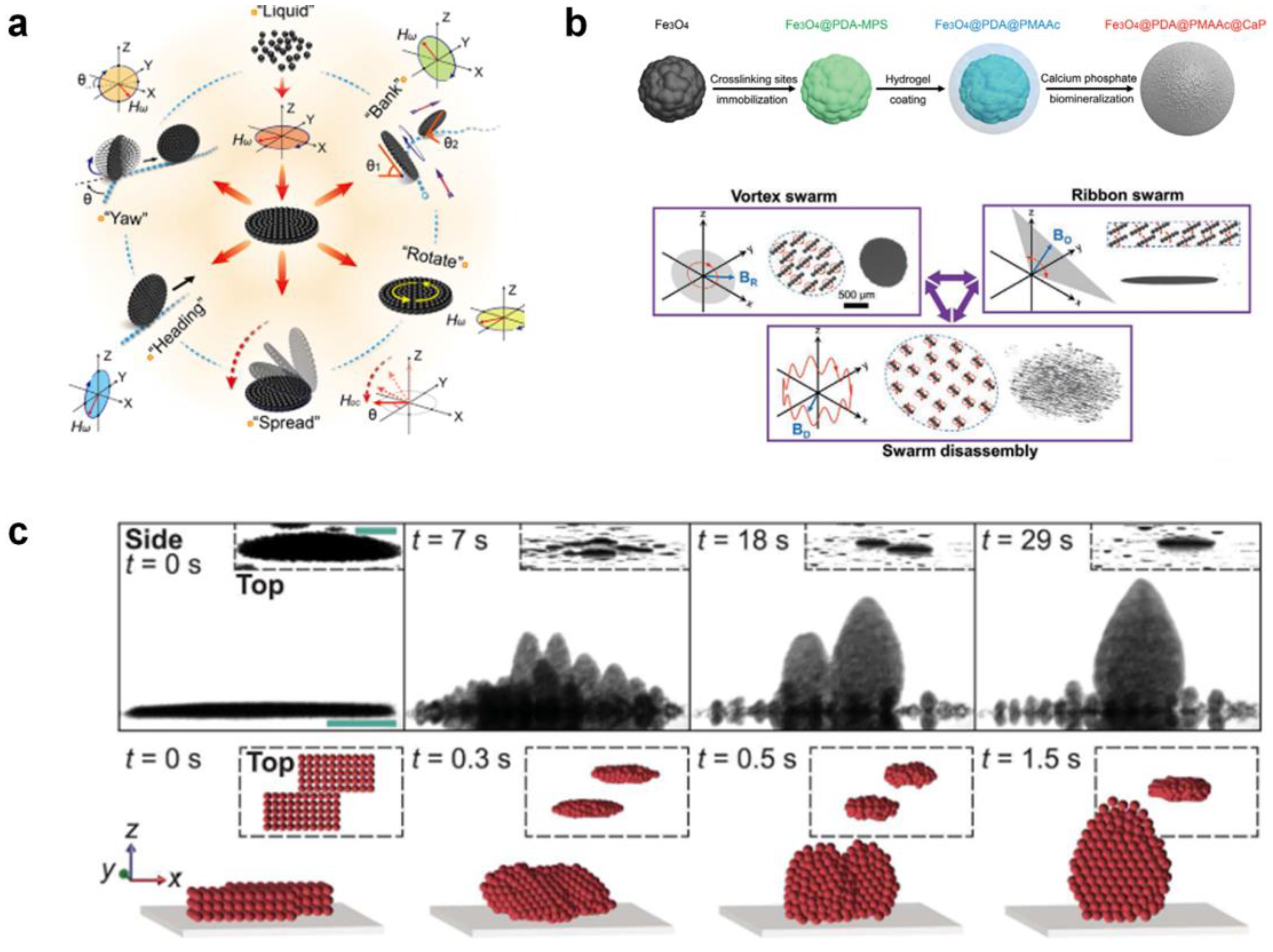
Li et al. [68] reported a ferromagnetic nanoparticle-assembled wheel-like micromotor swarm which maintains dense and stable morphology (Figure 5a). They employed a “packing under rolling” method to assemble the micromotor swarms with variable aspect ratios via integrating AC and DC magnetic field control. The frequency and strength of the external magnetic field were tuned to fabricate swarms and to achieve five switchable motion modes (Figure 5b). Moreover, the micromotor swarms can navigate along the intestinal inner wall with controllable direction, intending to repair microscale intestinal perforation in the future. Jin et al. [34] fabricated core–shell-structured paramagnetic nanoparticle@ calcium phosphate (Fe3O4@PDA@PMAAc@CaP) to achieve magnetic swarm control and targeted drug delivery (Figure 5c). The swarm patterns of Fe3O4@PDA@PMAAc@CaP could be reconfigured by changing the types of superposed magnetic fields in a reversibly way. Moreover, by programming the external magnetic fields, the swarms can serve as the drug delivery vehicle with on-demand cargo loading and release. Law et al. [69] conducted pioneering work in presenting a strategy to overcome the self-assembly of dynamic colloidal structures along the vertical direction via a combination of dipole–dipole interactions, gravity, and hydrodynamic drag forces. The 3 μm diameter paramagnetic particles with hydrophilic surface were suspended in 0.1% Tween 20 solution to prevent nonspecific aggregations. The dual-axis-oscillating magnetic field was applied to assemble magnetic particles against gravity into vertical collectives. Moreover, the researchers found that the self-assembled swarms can perform controllable changing in shape, orientation, inclination, and locomotion, which can be used to mimic ant colonies in multi-dimensional scales.
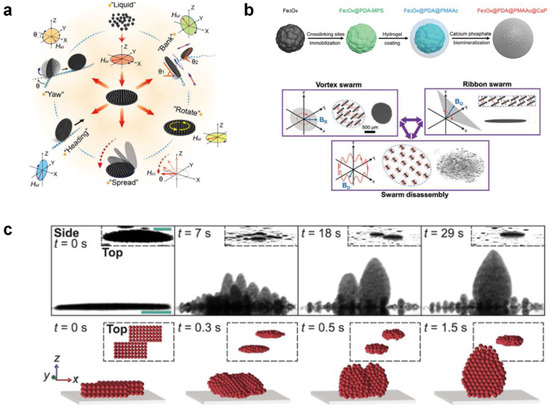
Figure 5.
Self-assembly by external magnetic fields. (a) Magnetic-driven wheel-like microswarm (WLM) of multiple switchable motion modes. Reproduced from [68]. Copyright 2022 ACS publication. (b) Fabrication and manipulation of magnetic colloidal microswarms with three kinds of collective behaviors. Reproduced from [34]. Copyright 2021 Wiley-VCH GmbH. (c) Magnetic generation of vertical collectives from dispersed colloidal particles. Reproduced from [69]. Copyright 2022 AAAS.
2.4. Electric Field-Caused Self-Assembly
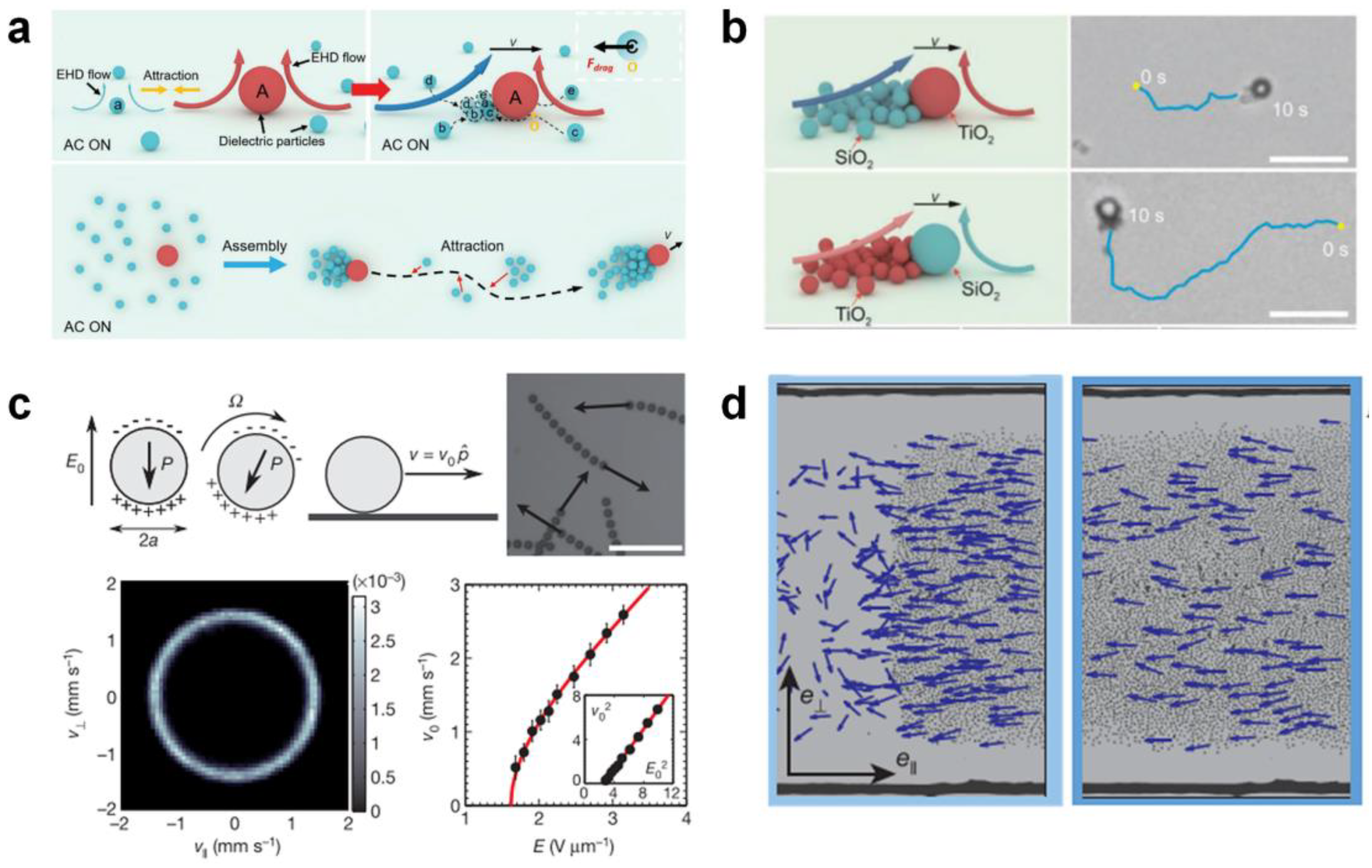
Electric fields can trigger swarm behaviors based on dipole–dipole interactions, dielectrophoresis forces, and hydrodynamic interactions. Liang et al. [54] reported a self-organized swarm of dissimilar microparticles by applying AC electric field (Figure 6a). The dissimilar dielectric microparticles can self-assemble into leader–follower-like microswarms hierarchically in an AC electric field, due to the unbalanced surrounding local electrohydrodynamic (EHD) flows generated around different microparticles and diffusiophoretic interactions. Moreover, the microswarms can exhibit reversible states between stop and go in response to vertical UV irradiation, which may open remarkable opportunities in biomedical intelligent microswarms (Figure 6b). Bricard et al. [35] reported self-organized colloidal roller populations which show coherent motion in a unique direction (Figure 6c). The unstable charge distribution at the sphere’s surface above a critical electric field induces a spontaneous symmetry breaking and a net electrostatic torque, leading to the well-known Quincke rotation. The researchers suggested that the hydrodynamic interactions between Quincke rollers lead to the emergence of collective motion (Figure 6d).
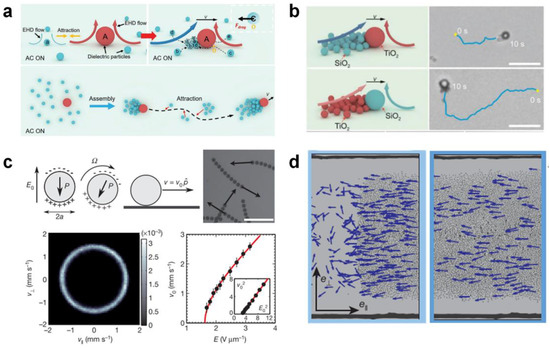
Figure 6.
Self-assembly in external electric fields. (a) Schematic illustration of the assembly and (b) actuation of hierarchical micromotor swarms by applying AC electric field. Reproduced from [54]. Copyright Wiley-VCH GmbH. (c) Population mechanism of self-propelled colloidal rollers and (d) the formed macroscopic band propagating along the racetrack via electric polarization. Reproduced from [35]. Copyright 2013 Spring Nature.
4. Outlook and Conclusions
Although synthetic micromotors have attracted great interest and been widely investigated during the past two decades, the study of isotropic colloids as active matter and assembly units to construct swarms is just beginning. Isotropic colloids are driven by magnetic dipole–dipole, hydrodynamic, and other forces to form swarms. Here, we focused on some of the well-understood fields/strategies that can lead to collective assembly and are used for motion control. However, faster, more precise, and more programmable strategies for the motion control of swarms are still needed for real applications.
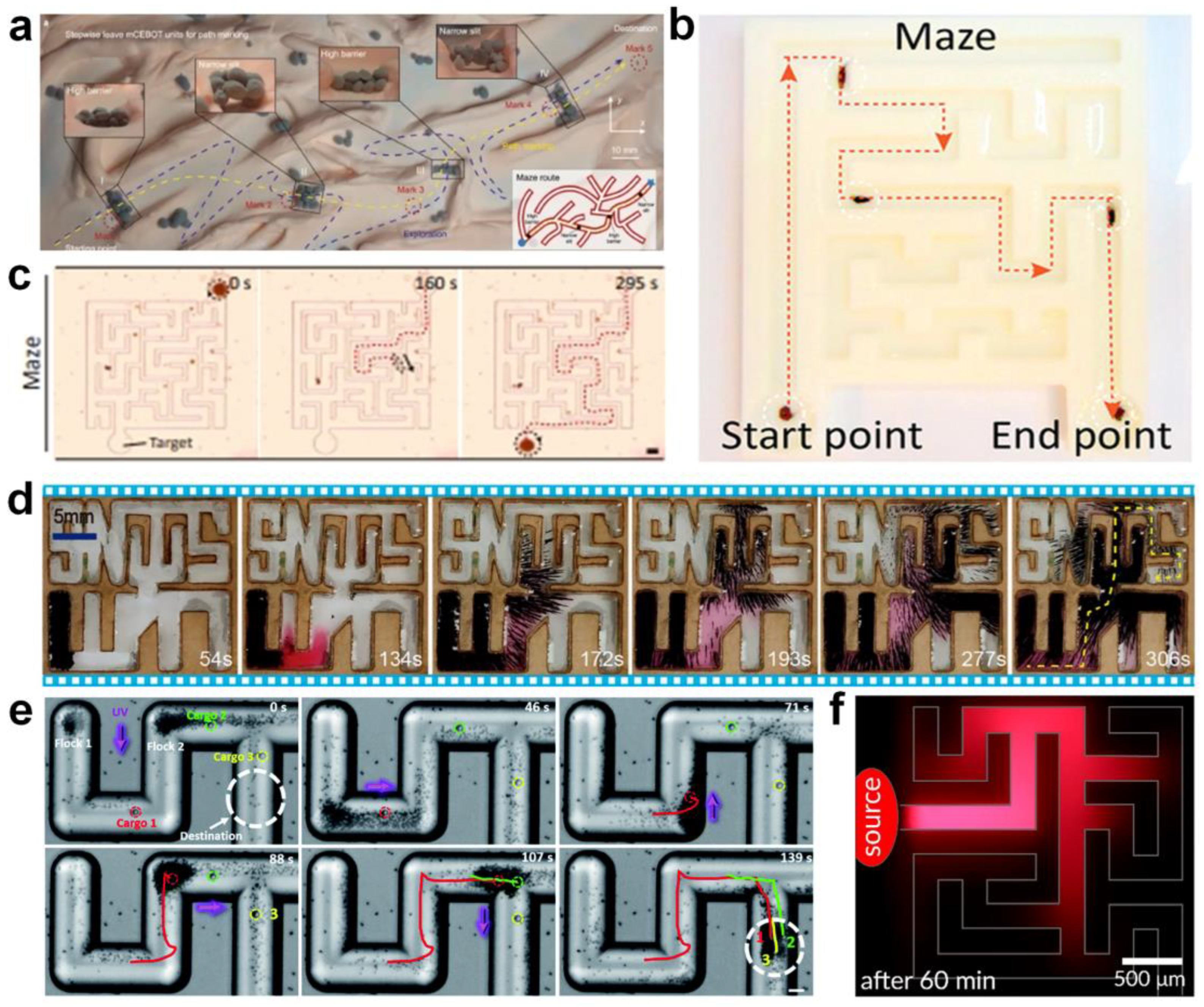
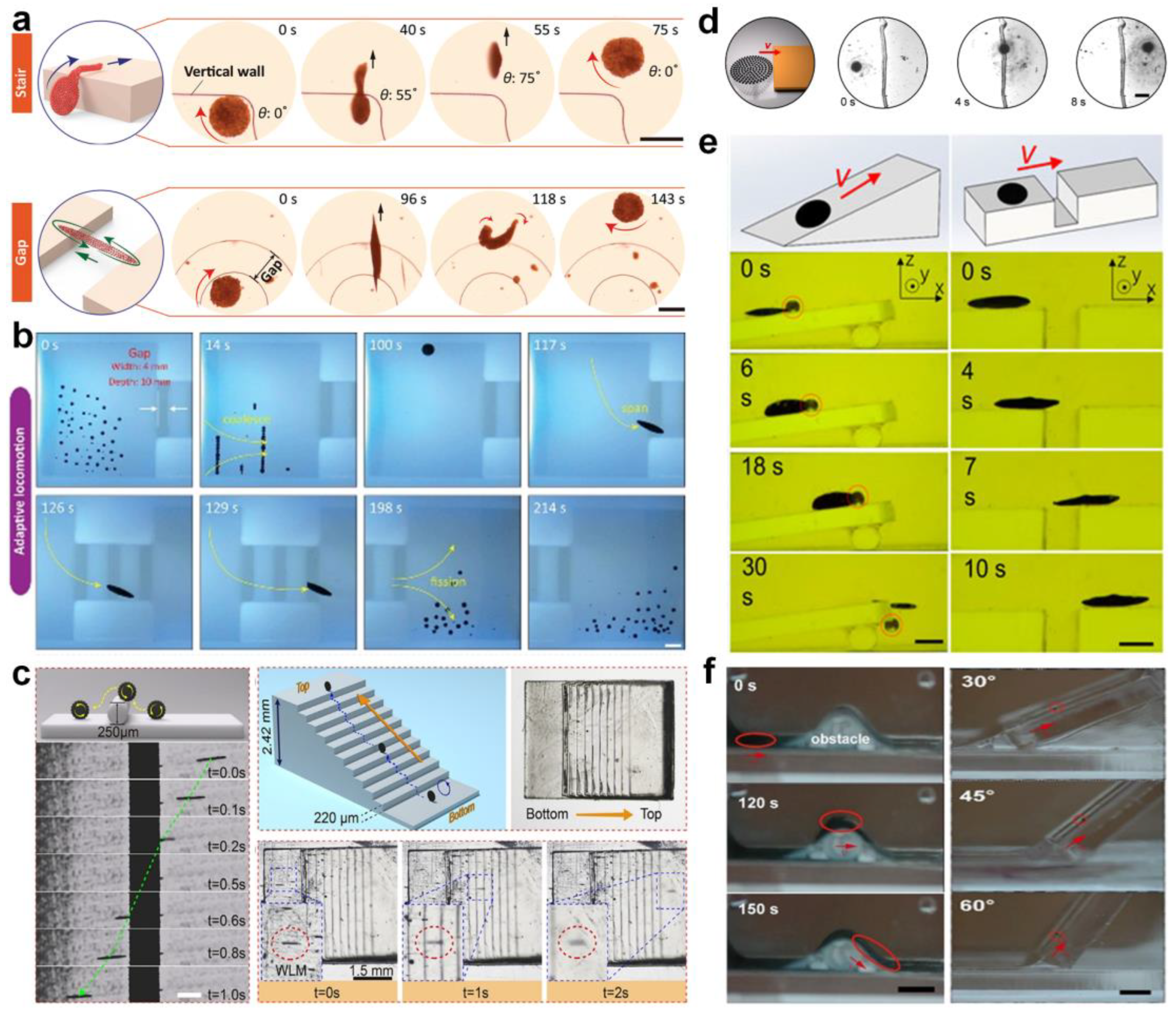
The exploration of micromotor swarms in complex environments is also at an early stage. With the development of applications of micromotor swarms in drug delivery, environmental remediation, sensing, and material science, more real challenging environments will emerge for micromotor swarms to confront. One of the biggest challenges would be the strong fluidic environment in blood vessels. Other challenges include narrow spaces, stents in the vessels, or other implants. For this, the deformation of the swarm as well as path planning are vital for success. For future applications, one challenging task would be how to control micromotor swarms to perform different functions at different stages in a targeted position. More importantly, the development of machine learning is shining new light on active matter. Current progress in this field has been summarized in recent reviews [90,91,92]. The opportunities that machine learning has brought, such as data analysis and the classification of experimental data, come with challenges. Machine learning is a black box to be revealed to promote the development of active matter.
Author Contributions
K.F. and L.C.: conceptualization, investigation, and writing of original draft version; X.Z.: data curation; J.G.: review and editing; J.Q. and R.N.: funding acquisition, and review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The present work is supported by the National Natural Science Foundation of China (No. 22102059) and the Opening Project of Guangdong Provincial Key Laboratory of Technique and Equipment for Macromolecular Advanced Manufacturing, South China University of Technology, China (No. 2022kfkt03).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anderson, C.; Theraulaz, G.; Deneubourg, J.L. Self-assemblages in insect societies. Insect. Soc. 2002, 49, 99–110. [Google Scholar] [CrossRef]
- Sumino, Y.; Nagai, K.H.; Shitaka, Y.; Tanaka, D.; Yoshikawa, K.; Chaté, H.; Oiwa, K. Large-scale vortex lattice emerging from collectively moving microtubules. Nature 2012, 483, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Vicsek, T.; Zafeiris, A. Collective motion. Phys. Rep. 2012, 517, 71–140. [Google Scholar] [CrossRef]
- Poli, R.; Kennedy, J.; Blackwell, T. Particle swarm optimization. Swarm Intell. 2007, 1, 33–57. [Google Scholar] [CrossRef]
- Zhang, J.; Luijten, E.; Grzybowski, B.A.; Granick, S. Active colloids with collective mobility status and research opportunities. Chem. Soc. Rev. 2017, 46, 5551–5569. [Google Scholar] [CrossRef]
- Lopez, U.; Gautrais, J.; Couzin, I.D.; Theraulaz, G. From behavioural analyses to models of collective motion in fish schools. Interface Focus 2012, 2, 693–707. [Google Scholar] [CrossRef]
- Reid, C.R.; Lutz, M.J.; Powell, S.; Kao, A.B.; Couzin, I.D.; Garnier, S. Army ants dynamically adjust living bridges in response to a cost-benefit trade-off. Proc. Natl. Acad. Sci. USA 2015, 112, 15113–15118. [Google Scholar] [CrossRef]
- Zhang, H.P.; Be’er, A.; Florin, E.L.; Swinney, H.L. Collective motion and density fluctuations in bacterial colonies. Proc. Natl. Acad. Sci. USA 2010, 107, 13626–13630. [Google Scholar] [CrossRef]
- Law, J.; Yu, J.; Tang, W.; Gong, Z.; Wang, X.; Sun, Y. Micro/Nanorobotic swarms: From fundamentals to functionalities. ACS Nano 2023, 17, 12971–12999. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Mou, F.; Guan, J. Light-controlled swarming and assembly of colloidal particles. Micromachines 2018, 9, 88. [Google Scholar] [CrossRef]
- Zhang, J.; Laskar, A.; Song, J.; Shklyaev, O.E.; Mou, F.; Guan, J.; Balazs, A.C.; Sen, A. Light-powered, fuel-free oscillation, migration, and reversible manipulation of multiple cargo types by micromotor swarms. ACS Nano 2022, 17, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Gao, Y.; Yang, J.; Li, Y.C.; Shao, G.; Zhang, G.; Li, T.; Li, L. Light-ultrasound driven collective “firework” behavior of nanomotors. Adv. Sci. 2018, 5, 1800122. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Soto, F.; Gao, W.; Dong, R.; Garcia-Gradilla, V.; Magaña, E.; Zhang, X.; Wang, J. Reversible swarming and separation of self-propelled chemically powered nanomotors under acoustic fields. J. Am. Chem. Soc. 2015, 137, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Kokot, G.; Snezhko, A. Manipulation of emergent vortices in swarms of magnetic rollers. Nat. Commun. 2018, 9, 2344. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Duan, W.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small power: Autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 2013, 8, 531–554. [Google Scholar] [CrossRef]
- Moo, J.G.; Pumera, M. Chemical energy powered nano/micro/macromotors and the environment. Chem.—Eur. J. 2015, 21, 58–72. [Google Scholar] [CrossRef]
- Villa, K.; Novotny, F.; Zelenka, J.; Browne, M.P.; Ruml, T.; Pumera, M. Visible-light-driven single-component BiVO4 micromotors with the autonomous ability for capturing microorganisms. ACS Nano 2019, 13, 8135–8145. [Google Scholar] [CrossRef]
- Xiao, Z.; Duan, S.; Xu, P.; Cui, J.; Zhang, H.; Wang, W. Synergistic speed enhancement of an electric-photochemical hybrid micromotor by tilt rectification. ACS Nano 2020, 14, 8658–8667. [Google Scholar] [CrossRef]
- Chen, C.; Mou, F.; Xu, L.; Wang, S.; Guan, J.; Feng, Z.; Wang, Q.; Kong, L.; Li, W.; Wang, J.; et al. Light-steered isotropic semiconductor micromotors. Adv. Mater. 2017, 29, 1603374. [Google Scholar] [CrossRef]
- Dai, J.; Cheng, X.; Li, X.; Wang, Z.; Wang, Y.; Zheng, J.; Liu, J.; Chen, J.; Wu, C.; Tang, J. Solution-synthesized multifunctional Janus nanotree microswimmer. Adv. Funct. Mater. 2021, 31, 2106204. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Villa, K.; Manzanares Palenzuela, C.L.; Ying, Y.; Sofer, Z.; Pumera, M. Catalytic and light-driven ZnO/Pt Janus nano/micromotors: Switching of motion mechanism via interface roughness and defect tailoring at the nanoscale. Adv. Funct. Mater. 2019, 29, 1808678. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Z.; Hu, S.; Huang, N.; Lee, H.; Liu, Y.; Yang, J.; Huan, X.; Xu, Z.; Cao, S.; et al. 3D Printing of arbitrary perovskite nanowire heterostructures. Adv. Funct. Mater. 2023, 29, 1808678. [Google Scholar] [CrossRef]
- Feng, K.; Gong, J.; Qu, J.; Niu, R. Dual-mode-driven micromotor based on foam-like carbon nitride and Fe3O4 with improved manipulation and photocatalytic performance. ACS Appl. Mater. Interfaces 2022, 14, 44271–44281. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Zhang, L.; Gong, J.; Qu, J.; Niu, R. Visible light triggered exfoliation of COF micro/nanomotors for efficient photocatalysis. Green Energy Environ. 2021, 8, 567–578. [Google Scholar] [CrossRef]
- Feuerstein, L.; Biermann, C.G.; Xiao, Z.; Holm, C.; Simmchen, J. Highly efficient active colloids driven by galvanic exchange reactions. J. Am. Chem. Soc. 2021, 143, 17015–17022. [Google Scholar] [CrossRef]
- Xie, L.; Yan, M.; Liu, T.; Gong, K.; Luo, X.; Qiu, B.; Zeng, J.; Liang, Q.; Zhou, S.; He, Y.; et al. Kinetics-controlled super-assembly of asymmetric porous and hollow carbon nanoparticles as light-sensitive smart nanovehicles. J. Am. Chem. Soc. 2022, 144, 1634–1646. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, H.P.; Tang, J.; Wang, W. Photochemically powered AgCl Janus micromotors as a model system to understand ionic self-diffusiophoresis. Langmuir 2018, 34, 3289–3295. [Google Scholar] [CrossRef]
- Urso, M.; Ussia, M.; Novotny, F.; Pumera, M. Trapping and detecting nanoplastics by MXene-derived oxide microrobots. Nat. Commun. 2022, 13, 3573. [Google Scholar] [CrossRef]
- Mou, F.; Li, X.; Xie, Q.; Zhang, J.; Xiong, K.; Xu, L.; Guan, J. Active micromotor systems built from passive particles with biomimetic predator–prey interactions. ACS Nano 2019, 14, 406–414. [Google Scholar] [CrossRef]
- Villa, K.; Děkanovský, L.; Plutnar, J.; Kosina, J.; Pumera, M. Swarming of perovskite-like Bi2WO6 microrobots destroy textile fibers under visible light. Adv. Funct. Mater. 2020, 30, 2007073. [Google Scholar] [CrossRef]
- Niu, R.; Palberg, T.; Speck, T. Self-assembly of colloidal molecules due to self-generated flow. Phys. Rev. Lett. 2017, 119, 028001. [Google Scholar] [CrossRef]
- Mou, F.; Zhang, J.; Wu, Z.; Du, S.; Zhang, Z.; Xu, L.; Guan, J. Phototactic flocking of photochemical micromotors. iScience 2019, 19, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Zhang, D.; Zhang, H.; Jiang, J.; Duan, R.; Xiao, J.; Xing, J.; Zhang, D.; Dong, B. Calligraphy/painting based on a bioinspired light-driven micromotor with concentration-dependent motion direction reversal and dynamic swarming behavior. ACS Appl. Mater. Interfaces 2019, 11, 40533–40542. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Yuan, K.; Du, X.; Wang, Q.; Wang, S.; Zhang, L. Domino reaction encoded heterogeneous colloidal microswarm with on-demand morphological adaptability. Adv. Mater. 2021, 33, 2100070. [Google Scholar] [CrossRef] [PubMed]
- Bricard, A.; Caussin, J.-B.; Desreumaux, N.; Dauchot, O.; Bartolo, D. Emergence of macroscopic directed motion in populations of motile colloids. Nature 2013, 503, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xue, X.; Wang, J.; Liu, H. Phototropic aggregation and light-guided long-distance collective transport of colloidal particles. Langmuir 2020, 36, 6819–6827. [Google Scholar] [CrossRef]
- Soni, V.; Bililign, E.S.; Magkiriadou, S.; Sacanna, S.; Bartolo, D.; Shelley, M.J.; Irvine, W.T.M. The odd free surface flows of a colloidal chiral fluid. Nat. Phys. 2019, 15, 1188–1194. [Google Scholar] [CrossRef]
- Hermanová, S.; Pumera, M. Micromachines for microplastics treatment. ACS Nanosci. 2022, 2, 225–232. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, B.R.; Wang, Y.; Pan, X.X.; Qu, Z.H.; Mei, Y.F. Self-powered locomotion of a hydrogel water strider. Sci. Robot. 2021, 6, eabe7925. [Google Scholar] [CrossRef]
- Feng, K.; Ureña Marcos, J.C.; Mukhopadhyay, A.K.; Niu, R.; Zhao, Q.; Qu, J.; Liebchen, B. Self-solidifying active droplets showing memory-induced chirality. Adv. Sci. 2023, 10, 2300866. [Google Scholar] [CrossRef]
- Kruger, C.; Klos, G.; Bahr, C.; Maass, C.C. Curling liquid crystal microswimmers: A cascade of spontaneous symmetry breaking. Phys. Rev. Lett. 2016, 117, 048003. [Google Scholar] [CrossRef] [PubMed]
- Suda, S.; Suda, T.; Ohmura, T.; Ichikawa, M. Straight-to-curvilinear motion transition of a swimming droplet caused by the susceptibility to fluctuations. Phys. Rev. Lett. 2021, 127, 088005. [Google Scholar] [CrossRef] [PubMed]
- Hokmabad, B.V.; Dey, R.; Jalaal, M.; Mohanty, D.; Almukambetova, M.; Baldwin, K.A.; Lohse, D.; Maass, C.C. Emergence of bimodal motility in active droplets. Phys. Rev. X 2021, 11, 011043. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L. External power-driven microrobotic swarm: From fundamental understanding to imaging-guided delivery. ACS Nano 2021, 15, 149–174. [Google Scholar] [CrossRef]
- Xie, H.; Sun, M.; Fan, X.; Lin, Z.; Chen, W.; Wang, L.; Dong, L.; He, Q. Reconfigurable magnetic microrobot swarm: Multimode transformation, locomotion, and manipulation. Sci. Robot. 2019, 4, eaav8006. [Google Scholar] [CrossRef]
- Hokmabad, B.V.; Nishide, A.; Ramesh, P.; Kruger, C.; Maass, C.C. Spontaneously rotating clusters of active droplets. Soft Mater. 2022, 18, 2731–2741. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, H.; Xu, T.; Yu, J. An overview of micronanoswarms for biomedical applications. ACS Nano 2021, 15, 15625–15644. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Lou, K.; Peng, Y.; Zhou, C.; Zhang, H.P.; Wang, W. Programmable, spatiotemporal control of colloidal motion waves via structured light. ACS Nano 2022, 16, 12755–12766. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, L.; Lv, W.; Liu, X.; Ren, J.; Qu, X. Biological mediator-propelled nanosweeper for nonpharmaceutical thrombus therapy. ACS Nano 2021, 15, 6604–6613. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Zhao, D.; Liu, X.; Wang, Y.; Chen, Y.; Ma, X. Enzyme-powered hollow nanorobots for active microsampling enabled by thermoresponsive polymer gating. ACS Nano 2022, 16, 10354–10363. [Google Scholar] [CrossRef]
- Shao, J.; Abdelghani, M.; Shen, G.; Cao, S.; Williams, D.S.; van Hest, J.C.M. Erythrocyte membrane modified janus polymeric motors for thrombus therapy. ACS Nano 2018, 12, 4877–4885. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, J.; Pan, X.; Sanchez, S.; Yan, X.; Ma, X. Enzyme-powered liquid metal nanobots endowed with multiple biomedical functions. ACS Nano 2021, 15, 11543–11554. [Google Scholar] [CrossRef]
- Niu, R.; Fischer, A.; Palberg, T.; Speck, T. Dynamics of binary active clusters driven by ion-exchange particles. ACS Nano 2018, 12, 10932–10938. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mou, F.; Huang, Z.; Zhang, J.; You, M.; Xu, L.; Luo, M.; Guan, J. Hierarchical microswarms with leader-follower-like structures: Electrohydrodynamic self-organization and multimode collective photoresponses. Adv. Funct. Mater. 2020, 30, 1908602. [Google Scholar] [CrossRef]
- Wang, W.; Duan, W.; Ahmed, S.; Sen, A.; Mallouk, T.E. From one to many: Dynamic assembly and collective behavior of self-propelled colloidal motors. Acc. Chem. Res. 2015, 48, 1938–1946. [Google Scholar] [CrossRef]
- Ibele, M.; Mallouk, T.E.; Sen, A. Schooling behavior of light-powered autonomous micromotors in water. Angew. Chem. Int. Ed. 2009, 48, 3308–3312. [Google Scholar] [CrossRef]
- Altemose, A.; Harris, A.J.; Sen, A. Autonomous formation and annealing of colloidal crystals induced by light-powered oscillations of active particles. ChemSystemsChem 2019, 2, e1900061. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Jin, Y.; Wen, Y.; Mu, Y.; Wu, C.; Wang, Y.; Tong, P.; Li, Z.; Hou, X.; et al. Photochromism from wavelength-selective colloidal phase segregation. Nature 2023, 617, 499–506. [Google Scholar] [CrossRef]
- Chen, M.; Lin, Z.; Xuan, M.; Lin, X.; Yang, M.; Dai, L.; He, Q. Programmable dynamic shapes with a swarm of light-powered colloidal motors. Angew. Chem. Int. Ed. 2021, 60, 16674–16679. [Google Scholar] [CrossRef]
- Zhou, D.; Gao, Y.; Liu, H.; Zhang, G.; Li, L. Light-induced patterned self-assembly behavior of isotropic semiconductor nanomotors. Chem. Asian J. 2019, 14, 2445–2449. [Google Scholar] [CrossRef]
- Kim, Y.; Shah, A.A.; Solomon, M.J. Spatially and temporally reconfigurable assembly of colloidal crystals. Nat. Commun. 2014, 5, 3676. [Google Scholar] [CrossRef] [PubMed]
- Michelin, S.; Lauga, E. Autophoretic locomotion from geometric asymmetry. Eur. Phys. J. E 2015, 38, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dai, J.; Li, X.; Gao, L.; Wang, J.; Liu, J.; Zheng, J.; Zhan, X.; Chen, J.; Cheng, X.; et al. Ion-exchange enabled synthetic swarm. Nat. Nanotechnol. 2021, 16, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, C.; Xiong, Z.; Liang, C.; Li, Z.; Liu, B.; Cao, Q.; Wang, J.; Tang, J.; Li, D. Self-driven magnetorobots for recyclable and scalable micro/nanoplastic removal from nonmarine waters. Sci. Adv. 2022, 8, eade1731. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Zheng, Y. Heat-mediated optical manipulation. Chem. Rev. 2022, 122, 3122–3179. [Google Scholar] [CrossRef]
- Wu, Y.; Si, T.; Shao, J.; Wu, Z.; He, Q. Near-infrared light-driven Janus capsule motors: Fabrication, propulsion, and simulation. Nano Res. 2016, 9, 3747–3756. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Peng, X.; Wu, Z.; Coughlan, A.C.H.; Mao, Z.; Bevan, M.A.; Zheng, Y. Opto-thermophoretic assembly of colloidal matter. Sci. Adv. 2017, 3, e1700458. [Google Scholar] [CrossRef]
- Yue, H.; Chang, X.; Liu, J.; Zhou, D.; Li, L. Wheel-like magnetic-driven microswarm with a band-aid imitation for patching up microscale intestinal perforation. ACS Appl. Mater. Interfaces 2022, 14, 8743–8752. [Google Scholar] [CrossRef]
- Law, J.; Chen, H.; Wang, Y.; Yu, J.; Sun, Y. Gravity-resisting colloidal collectives. Sci. Adv. 2022, 8, eade3161. [Google Scholar] [CrossRef]
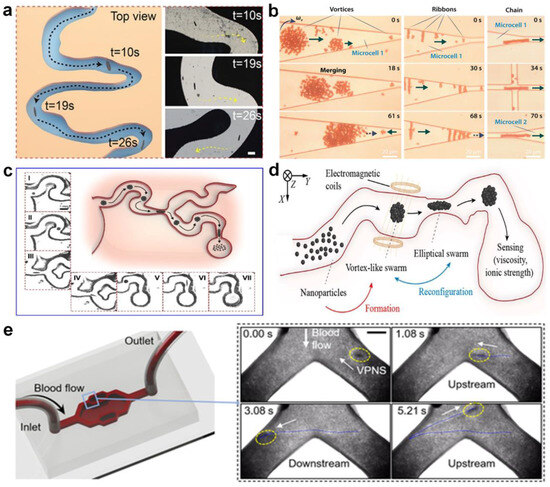
- Chen, H.; Wang, Y.; Liu, Y.; Zou, Q.; Yu, J. Sensing of fluidic features using colloidal microswarms. ACS Nano 2022, 16, 16281–16291. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Sun, H.; Ji, Y.; Song, L.; Jia, L.; Wang, C.; Li, C.; Zhang, D.; Xu, Y.; et al. Reconfigurable vortex-like paramagnetic nanoparticle swarm with upstream motility and high body-length ratio velocity. Research 2023, 6, 0088. [Google Scholar] [CrossRef] [PubMed]
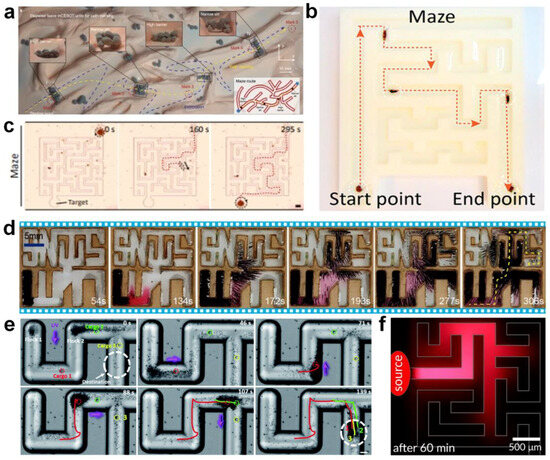
- Yu, J.; Yang, L.; Du, X.; Chen, H.; Xu, T.; Zhang, L. Adaptive pattern and motion control of magnetic microrobotic swarms. IEEE Trans. Robot. 2022, 38, 1552–1570. [Google Scholar] [CrossRef]
- Xie, H.; Fan, X.; Sun, M.; Lin, Z.; He, Q.; Sun, L. Programmable generation and motion control of a snakelike magnetic microrobot swarm. IEEE ASME Trans. Mechatron. 2019, 24, 902–912. [Google Scholar] [CrossRef]
- Zheng, L.; Ji, H.; Sun, D. Automated manipulation of microswarms without real-time image feedback using magnetic tweezers. IEEE ASME Trans. Mechatron. 2022, 27, 5712–5723. [Google Scholar] [CrossRef]
- Yang, X.; Tan, R.; Lu, H.; Fukuda, T.; Shen, Y. Milli-scale cellular robots that can reconfigure morphologies and behaviors simultaneously. Nat. Commun. 2022, 13, 4156. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chan, K.F.; Zhang, Z.; Wang, L.; Wang, Q.; Yang, S.; Chan, S.M.; Chiu, P.W.Y.; Sung, J.J.Y.; Zhang, L. Magnetic microswarm and fluoroscopy-guided platform for biofilm eradication in biliary stents. Adv. Mater. 2022, 34, 2201888. [Google Scholar] [CrossRef]
- Sun, M.; Fan, X.; Tian, C.; Yang, M.; Sun, L.; Xie, H. Swarming microdroplets to a dexterous micromanipulator. Adv. Funct. Mater. 2021, 31, 2011193. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, Q. Collective behaviors of magnetic microparticle swarms: From dexterous tentacles to reconfigurable carpets. ACS Nano 2022, 16, 13728–13739. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, F.; Wu, Z.; Song, J.; Kauffman, J.E.; Sen, A.; Guan, J. Cooperative transport by flocking phototactic micromotors. Nanoscale Adv. 2021, 3, 6157–6163. [Google Scholar] [CrossRef]
- Jin, C.; Krüger, C.; Maass, C.C. Chemotaxis and autochemotaxis of self-propelling droplet swimmers. Proc. Natl. Acad. Sci. USA 2017, 114, 5089–5094. [Google Scholar] [CrossRef]
- Yigit, B.; Alapan, Y.; Sitti, M. Programmable collective behavior in dynamically self-assembled mobile microrobotic swarms. Adv. Sci. 2019, 6, 1801837. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pedrero, F.; Tierno, P. Magnetic propulsion of self-assembled colloidal carpets: Efficient cargo transport via a conveyor-belt effect. Phys. Rev. Appl. 2015, 3, 051003. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Zhang, L. Micromanipulation using reconfigurable self-assembled magnetic droplets with needle guidance. IEEE Trans. Autom. Sci. Eng. 2022, 19, 759–771. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Zou, Q.; Du, X.; Wang, Y.; Yu, J. Automatic navigation of microswarms for dynamic obstacle avoidance. IEEE Trans. Robot. 2023, 39, 2770–2785. [Google Scholar] [CrossRef]
- Sun, M.; Yang, S.; Jiang, J.; Zhang, L. Horizontal and vertical coalescent microrobotic collectives using ferrofluid droplets. Adv. Mater. 2023, 35, 2300521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Yang, H.; Song, W.; Dai, L.; Yu, S.; Liu, X.; Li, T. Magnetic microswarm for MRI contrast enhancer. Chem. Asian J. 2022, 17, e202200561. [Google Scholar] [CrossRef]
- Wang, L.; Song, L.; Sun, H.; Ji, Y.; Dai, Y.; Feng, L. Multi-mode motion control of reconfigurable vortex-shaped microrobot swarms for targeted tumor therapy. IEEE Robot. Autom. Lett. 2022, 7, 3578–3583. [Google Scholar] [CrossRef]
- Li, M.; Zhang, T.; Zhang, X.; Mu, J.; Zhang, W. Vector-controlled wheel-like magnetic swarms with multimodal locomotion and reconfigurable capabilities. Front. Bioeng. Biotechnol. 2022, 10, 877964. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiong, H.; Li, Y.; Gao, W.; Hua, C.; Wu, J.; Fan, C.; Cai, X.; Zheng, Y. Magnetically actuated reactive oxygen species scavenging nano-robots for targeted treatment. Adv. Intell. Syst. 2022, 4, 2200061. [Google Scholar] [CrossRef]
- Cichos, F.; Gustavsson, K.; Mehlig, B.; Volpe, G. Machine learning for active matter. Nat. Mach. Intell. 2020, 2, 94–103. [Google Scholar] [CrossRef]
- Tsang, A.C.; Demir, E.; Ding, Y.; Pak, O.S. Roads to smart artificial microswimmers. Adv. Intell. Syst. 2020, 2, 1900137. [Google Scholar] [CrossRef]
- Nasiri, M.; Lowen, H.; Liebchen, B. Optimal active particle navigation meets machine learning. Europhys. Lett. 2023, 142, 17001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).