Terbium Removal from Aqueous Solutions Using a In2O3 Nanoadsorbent and Arthrospira platensis Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Precursor of In2O3 NPs
2.2. Adsorption Experiments
2.3. Characterization
2.4. Statistics
3. Results
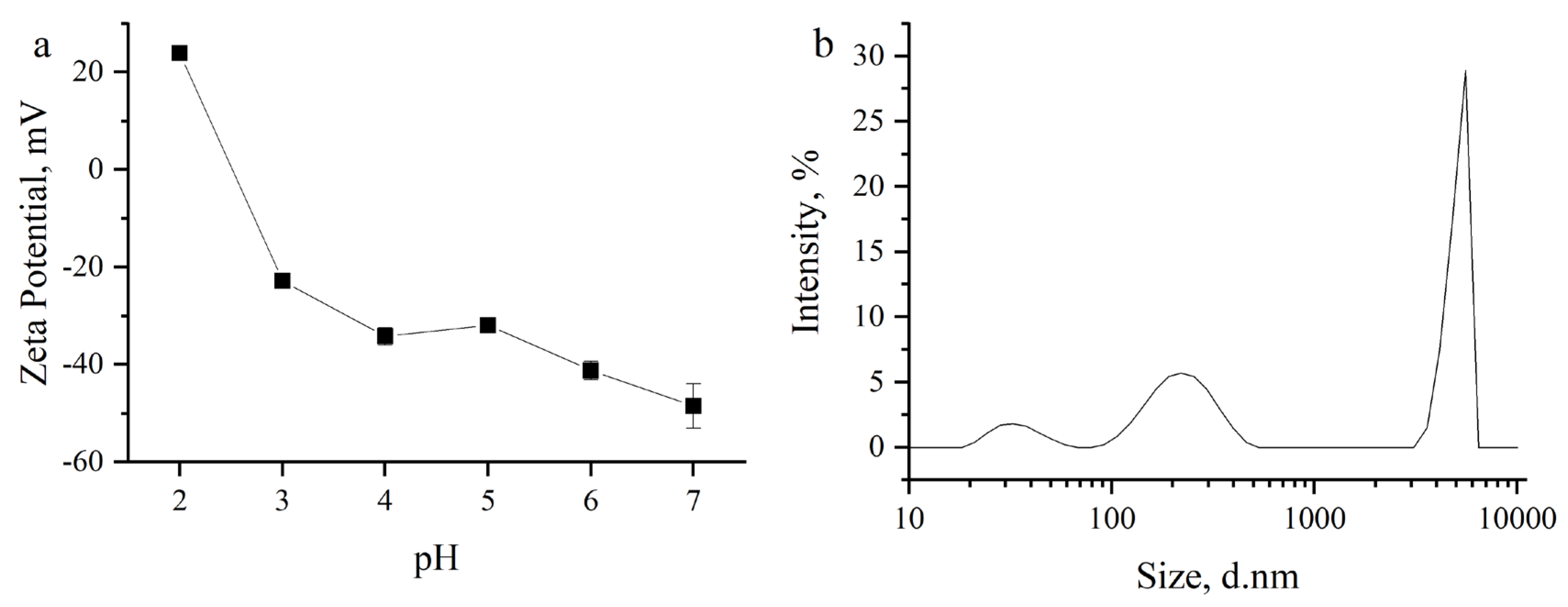
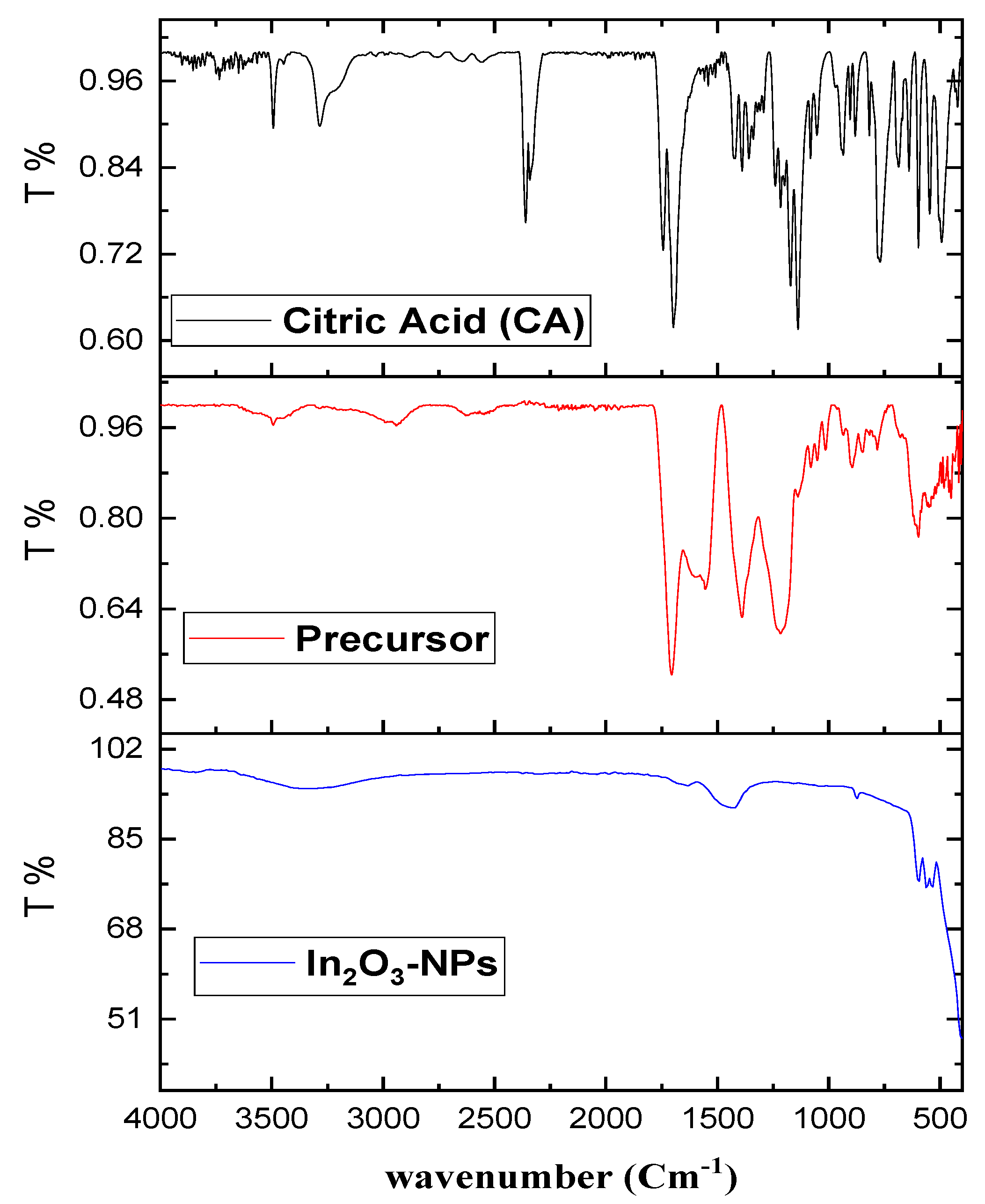
3.1. Adsorbents Characterization
3.2. Effect of pH, Time, and Terbium Concentration on Adsorbents’ Removal Efficiency
3.3. Equilibrium and Kinetics of the Terbium Adsorption Process
3.4. Mechanism of Terbium Ions Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare Earth Elements: Overview of Mining, Mineralogy, Uses, Sustainability and Environmental Impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Paper, M.; Koch, M.; Jung, P.; Lakatos, M.; Nilges, T.; Brück, T.B. Rare Earths Stick to Rare Cyanobacteria: Future Potential for Bioremediation and Recovery of Rare Earth Elements. Front. Bioeng. Biotechnol. 2023, 11, 1130939. [Google Scholar] [CrossRef] [PubMed]
- Maleke, M.; Valverde, A.; Vermeulen, J.-G.; Cason, E.D.; Gómez-Arias, A.; Moloantoa, K.; Coetsee-Hugo, L.; Swart, H.; Van Heerden, E.; Castillo, J. Biomineralization and Bioaccumulation of Europium by a Thermophilic Metal Resistant Bacterium. Front. Microbiol. 2019, 10, 81. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Wu, C.Y.; Hseu, Z.Y. Rare Earth Elements in Tea Garden Soils and Their Bioavailability to Tea Buds in Taiwan. Sci. Total Environ. 2023, 893, 164895. [Google Scholar] [CrossRef]
- Du, X.; Graedel, T.E. Global Rare Earth In-Use Stocks in NdFeB Permanent Magnets. J. Ind. Ecol. 2011, 15, 836–843. [Google Scholar] [CrossRef]
- Pinto, J.; Sousa, J.; Tavares, D.; Henriques, B.; Viana, T.; Ferreira, N.; Rocha, J.; Pereira, E. Removal of Rare-Earth Elements from Aqueous Solutions by Microporous Titanosilicate ETS-4. Microporous Mesoporous Mater. 2023, 357, 112606. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; De Michelis, I.; Medici, F.; Vegliò, F. A Hydrometallurgical Process for the Recovery of Terbium from Fluorescent Lamps: Experimental Design, Optimization of Acid Leaching Process and Process Analysis. J. Environ. Manag. 2016, 184, 552–559. [Google Scholar] [CrossRef]
- Nazarov, M.V.; Jeon, D.Y.; Kang, J.H.; Popovici, E.J.; Muresan, L.E.; Zamoryanskaya, M.V.; Tsukerblat, B.S. Luminescence Properties of Europium–Terbium Double Activated Calcium Tungstate Phosphor. Solid State Commun. 2004, 131, 307–311. [Google Scholar] [CrossRef]
- Kaszewski, J.; Borgstrom, E.; Witkowski, B.S.; Wachnicki, Ł.; Kiełbik, P.; Slonska, A.; Domino, M.A.; Narkiewicz, U.; Gajewski, Z.; Hochepied, J.F.; et al. Terbium Content Affects the Luminescence Properties of ZrO2:Tb Nanoparticles for Mammary Cancer Imaging in Mice. Opt. Mater. 2017, 74, 16–26. [Google Scholar] [CrossRef]
- Temga, J.P.; Sababa, E.; Mamdem, L.E.; Bijeck, M.L.N.; Azinwi, P.T.; Tehna, N.; Zo’o Zame, P.; Onana, V.L.; Nguetnkam, J.P.; Bitom, L.D.; et al. Rare Earth Elements in Tropical Soils, Cameroon Soils (Central Africa). Geoderma Reg. 2021, 25, e00369. [Google Scholar] [CrossRef]
- Prabakaran, K.; Nagarajan, R.; Eswaramoorthi, S.; Anandkumar, A.; Franco, F.M. Environmental Significance and Geochemical Speciation of Trace Elements in Lower Baram River Sediments. Chemosphere 2019, 219, 933–953. [Google Scholar] [CrossRef] [PubMed]
- De Campos, F.F.; Enzweiler, J. Anthropogenic Gadolinium Anomalies and Rare Earth Elements in the Water of Atibaia River and Anhumas Creek, Southeast Brazil. Environ. Monit. Assess. 2016, 188, 281. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, Y.J.A.B.; Cantalice, J.R.B.; Singh, V.P.; Do Nascimento, C.W.A.; Wilcox, B.P.; Bezerra Da Silva, Y.J.A. Heavy Metal Concentrations and Ecological Risk Assessment of the Suspended Sediments of a Multi-Contaminated Brazilian Watershed. Acta Sci. Agron. 2019, 41, e42620. [Google Scholar] [CrossRef]
- Atibu, E.K.; Devarajan, N.; Laffite, A.; Giuliani, G.; Salumu, J.A.; Muteb, R.C.; Mulaji, C.K.; Otamonga, J.P.; Elongo, V.; Mpiana, P.T.; et al. Assessment of Trace Metal and Rare Earth Elements Contamination in Rivers around Abandoned and Active Mine Areas. The Case of Lubumbashi River and Tshamilemba Canal, Katanga, Democratic Republic of the Congo. Geochemistry 2016, 76, 353–362. [Google Scholar] [CrossRef]
- Shimada, H.; Nagano, M.; Funakoshi, T.; Kojima, S. Pulmonary toxicity of systemic terbium chloride in mice. J. Toxicol. Environ. Health Part A 2010, 48, 81–92. [Google Scholar] [CrossRef]
- Hanana, H.; Kowalczyk, J.; André, C.; Gagné, F. Insights on the Toxicity of Selected Rare Earth Elements in Rainbow Trout Hepatocytes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109097. [Google Scholar] [CrossRef]
- Sturla Lompré, J.; Moleiro, P.; De Marchi, L.; Soares, A.M.V.M.; Pretti, C.; Chielini, F.; Pereira, E.; Freitas, R. Bioaccumulation and Ecotoxicological Responses of Clams Exposed to Terbium and Carbon Nanotubes: Comparison between Native (Ruditapes decussatus) and Invasive (Ruditapes philippinarum) Species. Sci. Total Environ. 2021, 784, 146914. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zhou, Q. Roles of Horseradish Peroxidase in Response to Terbium Stress. Biol. Trace Elem. Res. 2014, 161, 130–135. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q.; Zhao, B.; Huang, X. Toxic Effect of Heavy Metal Terbium Ion on Cell Membrane in Horseradish. Chemosphere 2010, 80, 28–34. [Google Scholar] [CrossRef]
- Jena, A.; Pradhan, S.; Mishra, S.; Sahoo, N.K. Evaluation of Europium Biosorption Using Deinococcus radiodurans. Environ. Process. 2021, 8, 251–265. [Google Scholar] [CrossRef]
- Balaram, V. Potential Future Alternative Resources for Rare Earth Elements: Opportunities and Challenges. Minerals 2023, 13, 425. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, T.; Pan, Y.; Shi, M.; Ding, D.; Ma, Z.; Liu, J.; Yuan, Y.; Fei, L.; Sun, Y. Recovering Rare Earth Elements via Immobilized Red Algae from Ammonium-Rich Wastewater. Environ. Sci. Ecotechnology 2022, 12, 100204. [Google Scholar] [CrossRef] [PubMed]
- Arunraj, B.; Sathvika, T.; Rajesh, V.; Rajesh, N. Cellulose and Saccharomyces cerevisiae Embark to Recover Europium from Phosphor Powder. ACS Omega 2019, 4, 940–952. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A Comprehensive Review on Conventional and Biological-Driven Heavy Metals Removal from Industrial Wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Jeon, C.; Cha, J.H.; Choi, J.Y. Adsorption and Recovery Characteristics of Phosphorylated Sawdust Bead for Indium(III) in Industrial Wastewater. J. Ind. Eng. Chem. 2015, 27, 201–206. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Lu, W.; Le, X.; Li, P.; Huang, L.; Zhang, J.; Yang, J.; Serpe, M.J.; Chen, D.; et al. Fluorescent Hydrogel-Coated Paper/Textile as Flexible Chemosensor for Visual and Wearable Mercury(II) Detection. Adv. Mater. Technol. 2019, 4, 1800201. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Lopez, F.A.; Rodriguez, O.; Martinez-Ramirez, S.; Garcia-Diaz, I. Sorption of Indium (III) onto Carbon Nanotubes. Ecotoxicol. Environ. Saf. 2016, 130, 81–86. [Google Scholar] [CrossRef]
- Cui, J.; Xie, Y.; Sun, T.; Chen, L.; Zhang, W. Deciphering and engineering photosynthetic cyanobacteria for heavy metal bioremediation. Sci. Total Environ. 2021, 761, 144111. [Google Scholar]
- Fawzy, M.A.; Mohamed, A.K.S.H. Bioremediation of heavy metals from municipal sewage by cyanobacteria and its effects on growth and some metabolites of Beta vulgaris. J. Plant Nutr. 2017, 40, 2550–2561. [Google Scholar] [CrossRef]
- Rugnini, L.; Costa, G.; Congestri, R.; Bruno, L. Testing of two different strains of green microalgae for Cu and Ni removal from aqueous media. Sci. Total Environ. 2017, 601–602, 959–967. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Safonov, A.; Ostalkevich, S.; Gundorina, S.; Nekhoroshkov, P.; Grozdov, D. Metal Ions Removal from Different Type of Industrial Effluents Using Spirulina Platensis Biomass. Int. J. Phytoremediat. 2019, 21, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Gunasundari, E.; Kumar, P.S. Higher Adsorption Capacity of Spirulina Platensis Alga for Cr(VI) Ions Removal: Parameter Optimisation, Equilibrium, Kinetic and Thermodynamic Predictions. IET Nanobiotechnol. 2017, 11, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Purev, O.; Park, C.; Kim, H.; Myung, E.; Choi, N.; Cho, K. Spirulina Platensis Immobilized Alginate Beads for Removal of Pb(II) from Aqueous Solutions. Int. J. Environ. Res. Public Health 2023, 20, 1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, S.; Xie, P.; Gao, Z.; Zou, R. Preparation, Characterization and Photocatalytic Activity of Juglans-like Indium Oxide (In2O3) Nanospheres. Mater. Lett. 2017, 192, 76–79. [Google Scholar] [CrossRef]
- Smok, W.; Zaborowska, M.; Tański, T.; Radoń, A. Novel In2O3/SnO2 Heterojunction 1D Nanostructure Photocatalyst for MB Degradation. Opt. Mater. 2023, 139, 113757. [Google Scholar] [CrossRef]
- He, Y.Y.; Zhao, X.; Cao, Y.; Zou, X.X.; Li, G.D. Facile Synthesis of In2O3 Nanospheres with Excellent Sensitivity to Trace Explosive Nitro-Compounds. Sens. Actuators B Chem. 2016, 228, 295–301. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.; Bai, J.; Li, X.; Li, F.; Liu, L. Controlled Synthesis of Rh-In2O3 Nanostructures with Different Morphologies for Efficient Photocatalytic Degradation of Oxytetracycline. Appl. Surf. Sci. 2019, 464, 115–124. [Google Scholar] [CrossRef]
- Tseng, T.T.; Uan, J.Y.; Tseng, W.J. Synthesis, Microstructure, and Photocatalysis of In2O3 Hollow Particles. Ceram. Int. 2011, 37, 1775–1780. [Google Scholar] [CrossRef]
- Chen, H.; Blatnik, M.A.; Ritterhoff, C.L.; Sokolović, I.; Mirabella, F.; Franceschi, G.; Riva, M.; Schmid, M.; Čechal, J.; Meyer, B.; et al. Water Structures Reveal Local Hydrophobicity on the In2O3(111) Surface. ACS Nano 2022, 16, 21163–21173. [Google Scholar] [CrossRef]
- Wagner, M.; Meyer, B.; Setvin, M.; Schmid, M.; Diebold, U. Direct Assessment of the Acidity of Individual Surface Hydroxyls. Nature 2021, 592, 722–725. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Su, Y.; Liu, Z.; Du, C. Indium Oxide/Halloysite Composite as Highly Efficient Adsorbent for Tetracycline Removal: Key Roles of Hydroxyl Groups and Interfacial Interaction. Appl. Surf. Sci. 2021, 566, 150708. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Grozdov, D.; Yushin, N.; Abdusamadzoda, D.; Gundorina, S.; Rodlovskaya, E.; Kristavchuk, O. Metal Removal from Chromium Containing Synthetic Effluents by Saccharomyces Cerevisiae. Desalin. Water Treat. 2020, 178, 254–270. [Google Scholar] [CrossRef]
- Gomaa, I.; Abdel-Salam, A.I.; Khalid, A.; Soliman, T.S. Fabrication, Structural, Morphological, and Optical Features of Mn2O3 Polyhedron Nano-Rods and Mn2O3/Reduced Graphene Oxide Hybrid Nanocomposites. Opt. Laser Technol. 2023, 161, 109126. [Google Scholar] [CrossRef]
- Braiek, Z.; Gannouni, M.; Assaker, I.B.; Bardaoui, A.; Lamouchi, A.; Brayek, A.; Chtourou, R. Correlation between physical properties and growth mechanismof In2S3 thin films fabricated by electrodeposition technique with different deposition times. Eur. Phys. J. Appl. Phys. 2015, 72, 10302. [Google Scholar] [CrossRef]
- Morsy, M.; Gomaa, I.; Mokhtar, M.M.; ElHaes, H.; Ibrahim, M. Design and Implementation of Humidity Sensor Based on Carbon Nitride Modified with Graphene Quantum Dots. Sci. Rep. 2023, 13, 2891. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhong, W.; Zhao, J.; Suib, S.L.; Lei, Y. “Self-Heating” Enabled One-Pot Synthesis of Fluorescent Carbon Dots. Eng. Sci. 2020, 9, 44–49. [Google Scholar] [CrossRef]
- Salem, M.A.; El-Damanhoury, M.A.; Omer, A.M.; El-Ghobashy, M.A. In2O3/Polyaniline Nanocomposite as an Innovative and Effective Adsorbent for Removing Acid Blue 25 from Wastewater. Delta J. Sci. 2023, 46, 49–72. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Zhang, N.; Wang, X.; Dang, D.; Jing, X.; Xi, D.; Hao, Y.; Tang, B.Z.; Meng, L. Deep-Red Fluorescent Organic Nanoparticles with High Brightness and Photostability for Super-Resolution in Vitro and in Vivo Imaging Using STED Nanoscopy. ACS Appl. Mater. Interfaces 2020, 12, 6814–6826. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Shi, S.; Li, Y.; Luo, Y.; Huang, Z.; Xiao, W.; Wang, S.; Zhang, P.; Shu, Y.; et al. Embedding Hierarchical Pores by Mechanochemistry in Carbonates with Superior Chemoselective Catalysis and Stability. Inorg. Chem. 2023. [Google Scholar] [CrossRef]
- Brian, D.; Benjamin, E. Evaluation of the Tauc Method for Optical Absorption Edge Determination: ZnO Thin Films as a Model System. Phys. Status Solidi 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Rajamani, S.; Arora, K.; Konakov, A.; Belov, A.; Korolev, D.; Nikolskaya, A.; Mikhaylov, A.; Surodin, S.; Kryukov, R.; Nikolitchev, D.; et al. Deep UV Narrow-Band Photodetector Based on Ion Beam Synthesized Indium Oxide Quantum Dots in Al2O3 Matrix. Nanotechnology 2018, 29, 305603. [Google Scholar] [CrossRef] [PubMed]
- Razeghi, M.; Rogalski, A. Semiconductor Ultraviolet Detectors. J. Appl. Phys. 1996, 79, 7433–7473. [Google Scholar] [CrossRef]
- Monroy, E.; Omnès, F.; Calle, F. Wide-Bandgap Semiconductor Ultraviolet Photodetectors. Semicond. Sci. Technol. 2003, 18, R33–R51. [Google Scholar] [CrossRef]
- Abbar, B.; Alem, A.; Marcotte, S.; Pantet, A.; Ahfir, N.D.; Bizet, L.; Duriatti, D. Experimental Investigation on Removal of Heavy Metals (Cu2+, Pb2+, and Zn2+) from Aqueous Solution by Flax Fibres. Process Saf. Environ. Prot. 2017, 109, 639–647. [Google Scholar] [CrossRef]
- Al Moharbi, S.S.; Devi, M.G.; Sangeetha, B.M.; Jahan, S. Studies on the Removal of Copper Ions from Industrial Effluent by Azadirachta Indica Powder. Appl. Water Sci. 2020, 10, 23. [Google Scholar] [CrossRef]
- Vasylechko, V.O.; Gryshchouk, G.V.; Zakordonskiy, V.P.; Patsay, I.O.; Len, N.V.; Vyviurska, O.A. Sorption of Terbium on Transcarpathian Clinoptilolite. Microporous Mesoporous Mater. 2013, 167, 155–161. [Google Scholar] [CrossRef]
- Yushin, N.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Rudi, L.; Grozdov, D. Application of Cyanobacteria Arthospira Platensis for Bioremediation of Erbium-Contaminated Wastewater. Materials 2022, 15, 6101. [Google Scholar] [CrossRef]
- Qadeer, R.; Hanif, J. Adsorption of Dysprosium Ions on Activated Charcoal from Aqueous Solutions. Carbon N. Y. 1995, 33, 215–220. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, Z.; Khan, A.A.; Rehman, N.U. Terbium Extraction by Functionalized Surface: Experimental and DFT Approach. Adsorption 2020, 26, 117–125. [Google Scholar] [CrossRef]
- Chang, Q.; Li, P.; Han, Y.; Guan, X.; Xiong, J.; Li, Q.; Zhang, H.; Huang, K.; Zhang, X.; Xie, H.; et al. Ultra-Low Concentration Terbium (Tb) Adsorption on Garlic Peels Biosorbent and Its Application for Nd-Fe-B Scraps Recovery. J. Environ. Chem. Eng. 2023, 11, 109997. [Google Scholar] [CrossRef]
- Alcaraz, L.; Saquinga, D.N.; Alguacil, F.J.; Escudero, E.; López, F.A. Application of Activated Carbon Obtained from Spent Coffee Ground Wastes to Effective Terbium Recovery from Liquid Solutions. Metals 2021, 11, 630. [Google Scholar] [CrossRef]
- Rabie, A.M.; Abd El-Salam, H.M.; Betiha, M.A.; El-Maghrabi, H.H.; Aman, D. Mercury Removal from Aqueous Solution via Functionalized Mesoporous Silica Nanoparticles with the Amine Compound. Egypt. J. Pet. 2019, 28, 289–296. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Bartczak, P.; Jesionowski, T. Enhanced Removal of Hazardous Dye Form Aqueous Solutions and Real Textile Wastewater Using Bifunctional Chitin/Lignin Biosorbent. Int. J. Biol. Macromol. 2017, 99, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Chiban, M.; Carja, G.; Lehutu, G.; Sinan, F. Equilibrium and Thermodynamic Studies for the Removal of As(V) Ions from Aqueous Solution Using Dried Plants as Adsorbents. Arab. J. Chem. 2016, 9, S988–S999. [Google Scholar] [CrossRef]
- Samadi, N.; Hasanzadeh, R.; Rasad, M. Adsorption Isotherms, Kinetic, and Desorption Studies on Removal of Toxic Metal Ions from Aqueous Solutions by Polymeric Adsorbent. J. Appl. Polym. Sci. 2015, 132, 41642. [Google Scholar] [CrossRef]
- Hosny, N.M.; Gomaa, I.; Abd El-Moemen, A.; Anwar, Z.M. Adsorption of Ponceau Xylidine Dye by Synthesised Mn2O3 Nanoparticles. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Abdusamadzoda, D.; Grozdov, D.; Shvetsova, M. Efficient Removal of Metals from Synthetic and Real Galvanic Zinc–Containing Effluents by Brewer’s Yeast Saccharomyces Cerevisiae. Materials 2020, 13, 3624. [Google Scholar] [CrossRef]
- Özer, A.; Özer, D. Comparative Study of the Biosorption of Pb(II), Ni(II) and Cr(VI) Ions onto S. Cerevisiae: Determination of Biosorption Heats. J. Hazard. Mater. 2003, 100, 219–229. [Google Scholar] [CrossRef]
- Ma, D.; Zou, X.; Li, R.; Chen, P.; Wang, Y.; Chen, T.; Zhang, Q.; Liu, H.; Chen, Y.; Lv, W.; et al. Highly Efficient Adsorption of Pb(II) by Cubic Nanocrystals in Aqueous Solution: Behavior and Mechanism. Arab. J. Chem. 2020, 13, 5229–5240. [Google Scholar] [CrossRef]
- Neag, E.; Török, A.I.; Tanaselia, C.; Aschilean, I.; Senila, M. Kinetics and Equilibrium Studies for the Removal of Mn and Fe from Binary Metal Solution Systems Using a Romanian Thermally Activated Natural Zeolite. Water 2020, 12, 1614. [Google Scholar] [CrossRef]
- Diaz De Tuesta, J.L.; Roman, F.F.; Marques, V.C.; Silva, A.S.; Silva, A.P.F.; Bosco, T.C.; Shinibekova, A.A.; Aknur, S.; Kalmakhanova, M.S.; Massalimova, B.K.; et al. Performance and Modeling of Ni(II) Adsorption from Low Concentrated Wastewater on Carbon Microspheres Prepared from Tangerine Peels by FeCl3-Assisted Hydrothermal Carbonization. J. Environ. Chem. Eng. 2022, 10, 108143. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Boldyrev, K.; Rodlovskaya, E.; Ostrovnaya, T. Removal of Metals from Synthetic and Real Galvanic Nickel-Containing Effluents by Saccharomyces cerevisiae. Chem. Ecol. 2020, 37, 83–103. [Google Scholar] [CrossRef]
- Majeed Khan, M.A.; Khan, W.; Ahamed, M.; Alsalhi, M.S.; Ahmed, T. Crystallite Structural, Electrical and Luminescent Characteristics of Thin Films of In2O3 Nanocubes Synthesized by Spray Pyrolysis. Electron. Mater. Lett. 2013, 9, 53–57. [Google Scholar] [CrossRef]
- Wagner, M.; Hofinger, J.; Setvín, M.; Boatner, L.A.; Schmid, M.; Diebold, U. Prototypical Organic-Oxide Interface: Intramolecular Resolution of Sexiphenyl on In2O3(111). ACS Appl. Mater. Interfaces 2018, 10, 14175–14182. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, C.; Ge, Q. DFT Study of CO2 Adsorption and Hydrogenation on the In2O3 Surface. J. Phys. Chem. C 2012, 116, 7817–7825. [Google Scholar] [CrossRef]
- Bataller, B.G.; Capareda, S.C. A Rapid and Non-Destructive Method for Quantifying Biomolecules in Spirulina Platensis via Fourier Transform Infrared—Attenuated Total Reflectance Spectroscopy. Algal Res. 2018, 32, 341–352. [Google Scholar] [CrossRef]
- Çelekli, A.; Yavuzatmaca, M.; Bozkurt, H. An Eco-Friendly Process: Predictive Modelling of Copper Adsorption from Aqueous Solution on Spirulina Platensis. J. Hazard. Mater. 2010, 173, 123–129. [Google Scholar] [CrossRef]
- Boyaci, I.H.; Temiz, H.T.; Geniş, H.E.; Soykut, E.A.; Yazgan, N.N.; Güven, B.; Uysal, R.S.; Bozkurt, A.G.; Ilaslan, K.; Torun, O.; et al. Dispersive and FT-Raman Spectroscopic Methods in Food Analysis. RSC Adv. 2015, 5, 56606–56624. [Google Scholar] [CrossRef]
- Peng, X.; Mo, S.; Li, R.; Li, J.; Tian, C.; Liu, W.; Wang, Y. Effective Removal of the Rare Earth Element Dysprosium from Wastewater with Polyurethane Sponge-Supported Graphene Oxide–Titanium Phosphate. Environ. Chem. Lett. 2021, 19, 719–728. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Peshkova, A.; Vergel, K.; Rodlovskaya, E. The Remediation of Dysprosium-Containing Effluents Using Cyanobacteria Spirulina platensis and Yeast Saccharomyces cerevisiae. Microorganisms 2023, 11, 2009. [Google Scholar] [CrossRef]








| Sample Name | (hkl) | d-Spacing | βD (rad) | D (Scherrer eq.) (nm) | δ (nm 2) | ε% |
|---|---|---|---|---|---|---|
| Precursor | 2 2 0 | 0.4 | 0.1899 | 0.743 | 1.8 | 24.2 |
| In2O3-NPs | 2 2 2 | 0.29 | 0.005031 | 28.5 | 0.00122 | 0.4582 |
| Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qm, mg/g | b, L/mg | R2 | Kf, mg/g | n | R2 | aT, L/g | bT, J/mol | R2 | |
| In2O3-NPs | 94.7 ± 13.6 | 0.01 ± 0.004 | 0.98 | 3.69 ± 0.3 | 1.66 ± 0.24 | 0.95 | 0.14 ± 0.02 | 116.7 ± 8.8 | 0.97 |
| S. platensis | 212 ± 35 | 0.004 ± 0.008 | 0.81 | 0.03 ± 0.003 | 0.59 ± 0.06 | 0.97 | 0.072 ± 0.02 | 82.1 ± 22.3 | 0.73 |
| Sorbent | q, mg/g | Reference |
|---|---|---|
| Spirulina platensis | 212 | Present work |
| In2O3-NPs | 94.7 | Present work |
| natural clinoptilolite | 1.67–3.85 | [56] |
| 1-(2-pyridylazo)-2-naphthol (PAN)-doped hybrid silica | 123.6 | [59] |
| Ca(Ⅱ)-modified garlic peels | 0.06 μg/g | [60] |
| Activated Carbon | 14.9 | [61] |
| Pseudo-First-Order | Pseudo-Second-Order | Elovich | |||||||
|---|---|---|---|---|---|---|---|---|---|
| qe, mg/g | k1, 1/min | R2 | qe, mg/g | k2, g/mg·min | R2 | α, g/mg∙min | Β, g/mg | R2 | |
| In2O3 | 7.27 ± 0.03 | 0.96 ± 0.07 | 0.97 | 7.32 ± 0.05 | 0.78 ± 0.24 | 0.99 | 2.64 × 1038 ± 1.50 × 1034 | 13.0 ± 8.03 | 0.99 |
| S. platensis | 5.23 ± 0.11 | 6.5 ± 0.07 | 0.98 | 5.23 ± 0.11 | 0.75±0.17 | 0.98 | 2.22 × 1043 ± 1.73 × 1040 | 20.3 ± 4.8 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Bagawi, A.H.; Yushin, N.; Hosny, N.M.; Gomaa, I.; Ali, S.; Boyd, W.C.; Kalil, H.; Zinicovscaia, I. Terbium Removal from Aqueous Solutions Using a In2O3 Nanoadsorbent and Arthrospira platensis Biomass. Nanomaterials 2023, 13, 2698. https://doi.org/10.3390/nano13192698
Al-Bagawi AH, Yushin N, Hosny NM, Gomaa I, Ali S, Boyd WC, Kalil H, Zinicovscaia I. Terbium Removal from Aqueous Solutions Using a In2O3 Nanoadsorbent and Arthrospira platensis Biomass. Nanomaterials. 2023; 13(19):2698. https://doi.org/10.3390/nano13192698
Chicago/Turabian StyleAl-Bagawi, Amal H., Nikita Yushin, Nasser Mohammed Hosny, Islam Gomaa, Sabah Ali, Warren Christopher Boyd, Haitham Kalil, and Inga Zinicovscaia. 2023. "Terbium Removal from Aqueous Solutions Using a In2O3 Nanoadsorbent and Arthrospira platensis Biomass" Nanomaterials 13, no. 19: 2698. https://doi.org/10.3390/nano13192698
APA StyleAl-Bagawi, A. H., Yushin, N., Hosny, N. M., Gomaa, I., Ali, S., Boyd, W. C., Kalil, H., & Zinicovscaia, I. (2023). Terbium Removal from Aqueous Solutions Using a In2O3 Nanoadsorbent and Arthrospira platensis Biomass. Nanomaterials, 13(19), 2698. https://doi.org/10.3390/nano13192698








