Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood–Brain Barrier in a µphysiological Model
Abstract
:1. Introduction
2. Results and Discussion
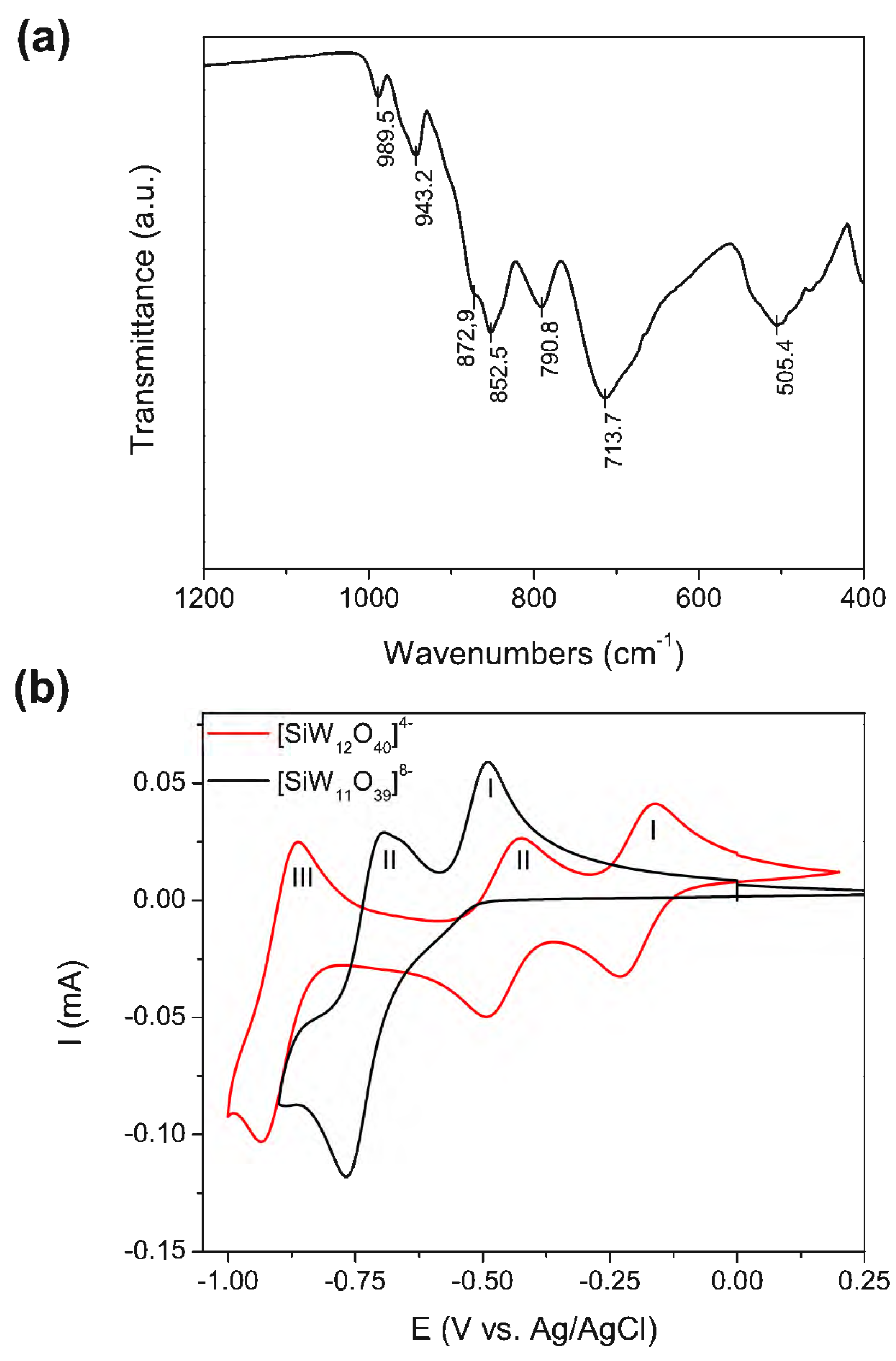
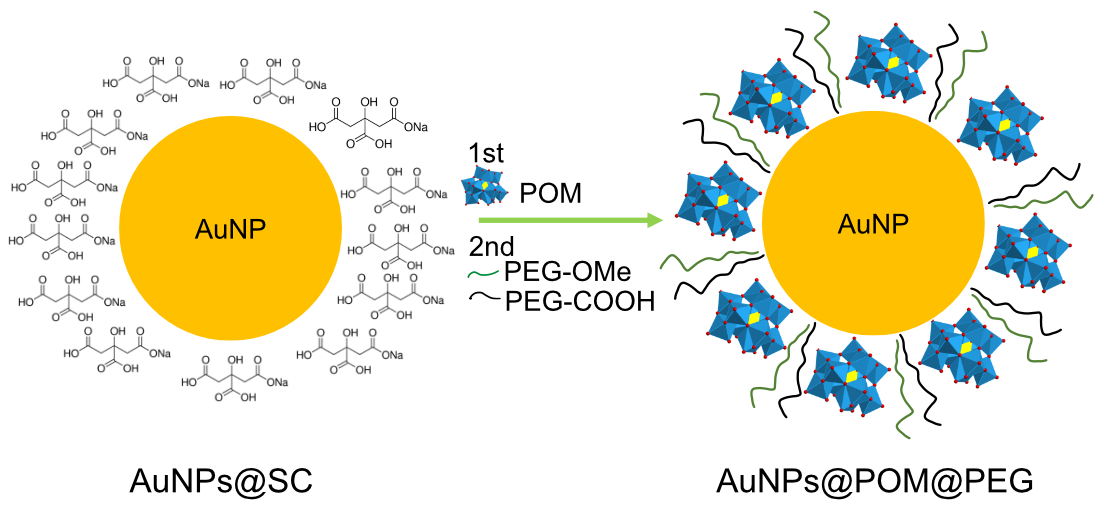
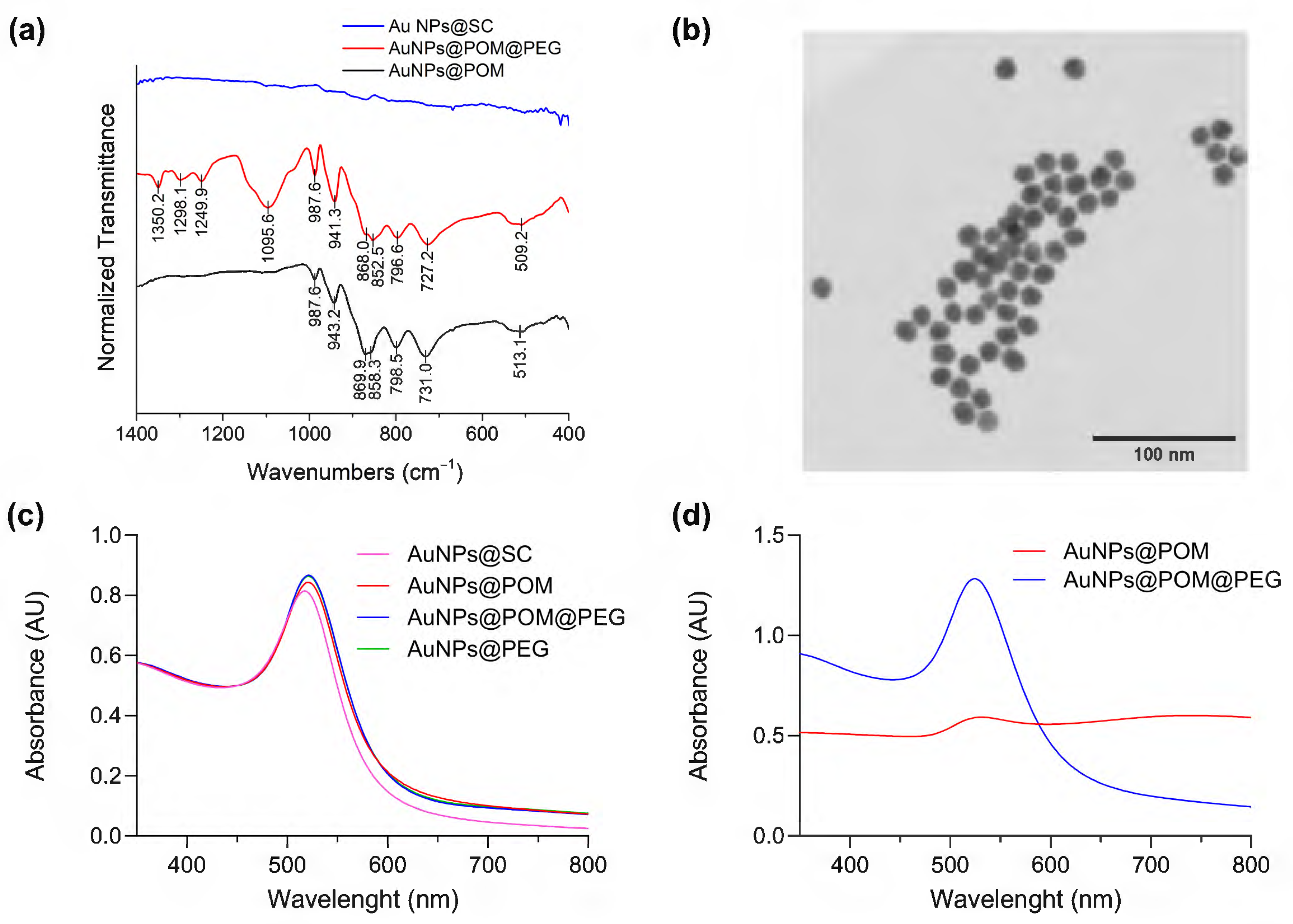
2.1. Synthesis and Characterization of AuNPs@POM@PEG
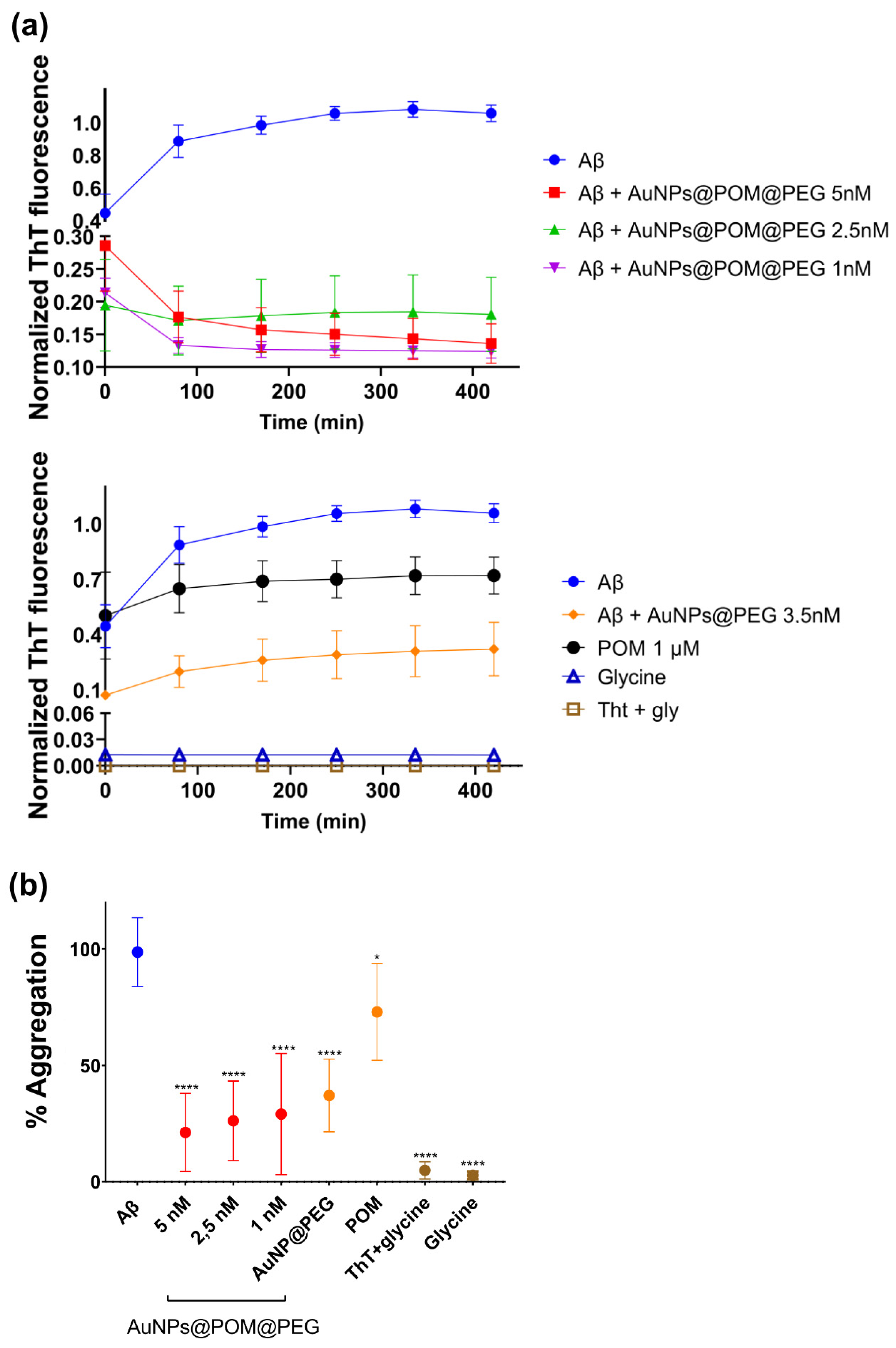
2.2. Evaluation of the Inhibition of Aβ Fibrillization In Vitro by AuNPs@POM@PEG
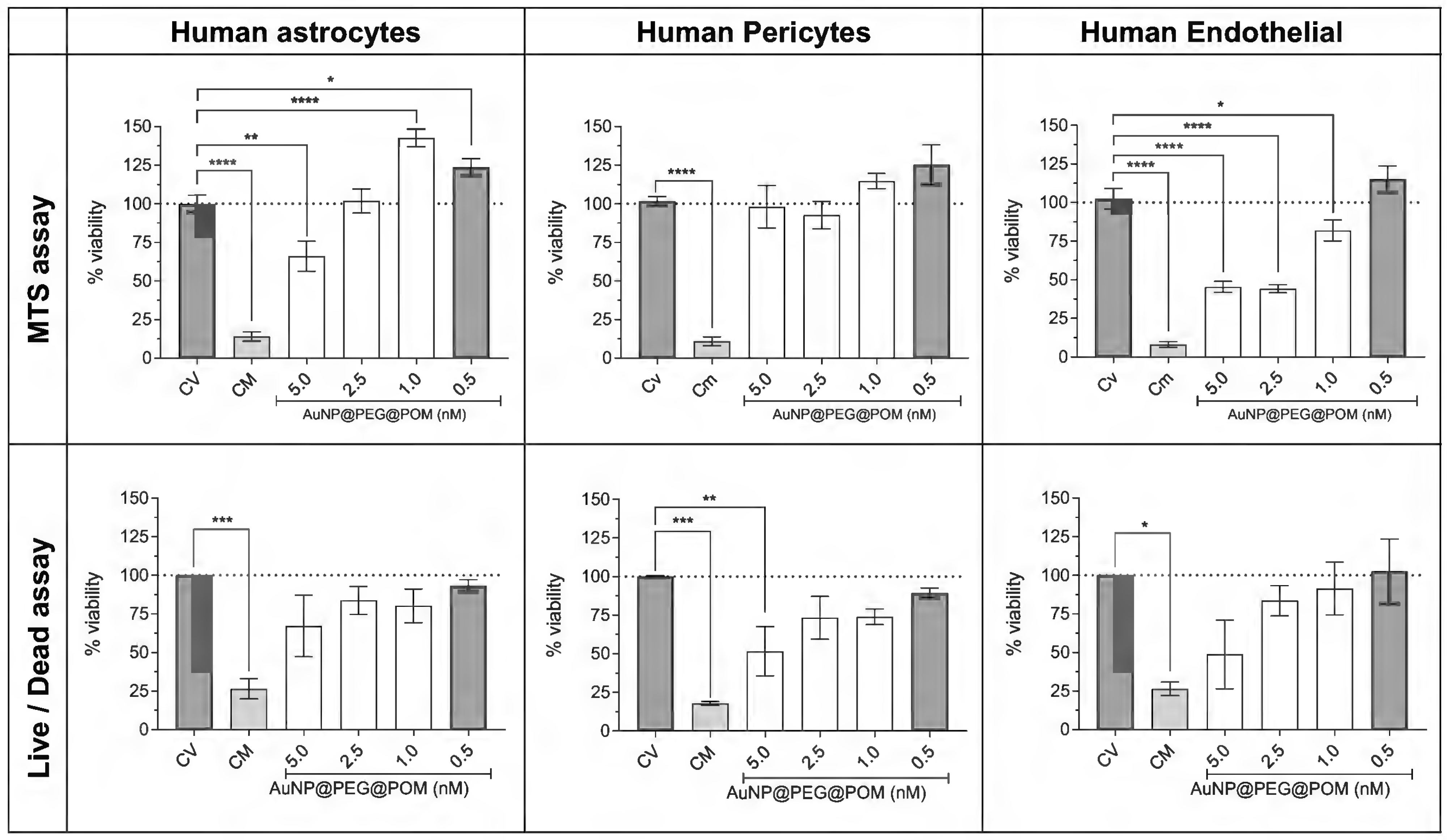
2.3. Evaluation of the Cytotoxicity and Permeability of AuNPs@POM@PEG across the Blood–Brain-Barrier-on-a-Chip (BBB-oC) In Vitro Model
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. POM Synthesis
4.3. POM Characterization
4.4. Gold Nanoparticle Synthesis
4.5. AuNPs@POM@PEG Functionalization
4.6. Alexa 647 Functionalization
4.7. Characterization of AuNPs@PEG@POM
4.8. βamyloid Aggregation Inhibition
4.9. Cell Culture
4.10. Cytotoxicity Assays
4.11. BBB-on-a-Chip Device Fabrication
4.12. BBB-oC Cell Seeding
4.13. AuNPs@POM@PEG Permeability Assays in BBB-oC
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godyń, J.; Jończyk, J.; Panek, D.; Malawska, B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol. Rep. 2016, 68, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science (1979) 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Cell biology of protein misfolding: The examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol. 2004, 6, 1054–1061. [Google Scholar] [CrossRef]
- Stains, C.I.; Mondal, K.; Ghosh, I. Molecules that target beta-amyloid. ChemMedChem 2007, 2, 1674–1692. [Google Scholar] [CrossRef]
- Hung, S.-Y.; Fu, W.-M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017, 24, 47. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, S.I.; Kim, Y. Nanotherapeutics engineered to cross the blood-brain barrier for advanced drug delivery to the central nervous system. J. Ind. Eng. Chem. 2019, 73, 8–18. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Dal-Ré, R. La Agencia Europea de Medicamentos rechaza la autorización del aducanumab para la enfermedad de Alzheimer. Rev. Neurol. 2022, 74, 207–208. [Google Scholar]
- Investor Relations. Lecanemab Confirmatory Phase 3 Clarity ad Study Met Primary Endpoint, Showing Highly Statistically Significant Reduction of Clinical Decline in Large Global Clinical Study of 1795 Participants with Early Alzheimer’s Disease. 2022. Available online: https://investors.biogen.com/news-releases/news-release-details/lecanemab-confirmatory-phase-3-clarity-ad-study-met-primary (accessed on 30 September 2023).
- Derek Lowe’s. A Positive Amyloid Trial, Finally? 2022. Available online: https://www.science.org/content/blog-post/positive-amyloid-trial-finally (accessed on 30 September 2023).
- Drugs@FDA: FDA-Approved Drugs. (Online). 2023. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761269 (accessed on 22 September 2023).
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771–1799. [Google Scholar] [CrossRef]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Spadavecchia, J.; Movia, D.; Moore, C.; Maguire, C.M.; Moustaoui, H.; Casale, S.; Volkov, Y.; Prina-Mello, A. Targeted polyethylene glycol gold nanoparticles for the treatment of pancreatic cancer: From synthesis to proof-of-concept in vitro studies. Int. J. Nanomed. 2016, 11, 791. [Google Scholar] [CrossRef]
- Liao, Y.; Chang, Y.; Yoshiike, Y.; Chang, Y.; Chen, Y. Negatively Charged Gold Nanoparticles Inhibit Alzheimer’s Amyloid-β Fibrillization, Induce Fibril Dissociation, and Mitigate Neurotoxicity. Small 2012, 8, 3631–3639. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; Rotello, V.M. Functionalized gold nanoparticles for drug delivery. Nanomedicine 2007, 2, 113–123. [Google Scholar] [CrossRef]
- Manson, J.; Kumar, D.; Meenan, B.J.; Dixon, D. Polyethylene glycol functionalized gold nanoparticles: The influence of capping density on stability in various media. Gold. Bull. 2011, 44, 99–105. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernandez, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef]
- Surman, A.J.; Robbins, P.J.; Ujma, J.; Zheng, Q.; Barran Perdita, E.; Cronin, L. Sizing and Discovery of Nanosized Polyoxometalate Clusters by Mass Spectrometry. J. Am. Chem. Soc. 2016, 138, 3824–3830. [Google Scholar] [CrossRef]
- Pope, M.T.; Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Gómez-Romero, P. Polyoxometalates as photoelectrochemical models for quantum-sized colloidal semiconducting oxides. Solid. State Ion. 1997, 101–103, 243–248. [Google Scholar] [CrossRef]
- Gabas, I.M.; Stepien, G.; Moros, M.; Mitchell, S.G.; Jesús, M. In vitro cell cytotoxicity profile and morphological response to polyoxometalate-stabilised gold nanoparticles. New J. Chem. 2016, 40, 1039–1047. [Google Scholar] [CrossRef]
- Yamase, T. Anti-tumor,-viral, and-bacterial activities of polyoxometalates for realizing an inorganic drug. J. Mater. Chem. 2005, 15, 4773–4782. [Google Scholar] [CrossRef]
- Geng, J.; Li, M.; Ren, J.; Wang, E.; Qu, X. Polyoxometalates as inhibitors of the aggregation of amyloid β peptides associated with Alzheimer’s disease. Angew. Chem.-Int. Ed. 2011, 50, 4184–4188. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, L.; Han, F.; Zhang, G.; Ma, Y.; Yao, J.; Keita, B.; De Oliveira, P.; Nadjo, L. Inhibition of amyloid-β protein fibrillization upon interaction with polyoxometalates nanoclusters. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 97–101. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Duan, T.; Xu, C.; Qu, X. Transition-metal-substituted polyoxometalate derivatives as functional anti-amyloid agents for Alzheimer’s disease. Nat. Commun. 2014, 5, 3422. [Google Scholar] [CrossRef]
- Gao, N.; Du, Z.; Guan, Y.; Dong, K.; Ren, J.; Qu, X. Chirality-Selected Chemical Modulation of Amyloid Aggregation. J. Am. Chem. Soc. 2019, 2019 141, 6915–6921. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-nanoparticle-based multifunctional amyloid-β inhibitor against Alzheimer’s disease. Chem.-A Eur. J. 2015, 21, 829–835. [Google Scholar] [CrossRef]
- Li, M.; Guan, Y.; Zhao, A.; Ren, J.; Qu, X. Using multifunctional peptide conjugated Au nanorods for monitoring β-amyloid aggregation and chemo-photothermal treatment of Alzheimer’s disease. Theranostics 2017, 7, 2996. [Google Scholar] [CrossRef]
- Guan, Y.; Li, M.; Dong, K.; Gao, N.; Ren, J.; Zheng, Y.; Qu, X. Ceria/POMs hybrid nanoparticles as a mimicking metallopeptidase for treatment of neurotoxicity of amyloid-β peptide. Biomaterials 2016, 98, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, M.; do Carmo, M.A.P.; van der Helm, M.W.; de Graaf, M.N.S.; Orlova, V.; van den Berg, A.; van der Meer, A.D.; Broersen, K.; Segerink, L.I. Multiplexed blood–brain barrier organ-on-chip. Lab. Chip 2020, 20, 3132–3143. [Google Scholar] [CrossRef] [PubMed]
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in microfluidic blood–brain barrier (BBB) models. Trends Biotechnol. 2019, 37, 1295–1314. [Google Scholar] [CrossRef] [PubMed]
- Palma-Florez, S.; Lopez-Canosa, A.; Morales-Zavala, F.; Castaño, O.; Kogan, M.J.; Samitier, J.; Lagunas, A.; Mir, M. BBB-on-a-chip with Integrated micro-TEER for permeability evaluation of multi-functionalized gold nanorods against Alzheimer’s disease. J. Nanobiotechnol. 2023, 21, 115. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size control of gold nanocrystals in citrate reduction: The third role of citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef]
- Chermette, H.; Lefebvre, F. Theoretical study of the four isomers of [Siw11o39]8−: Structure, stability and physical properties. Comptes Rendus Chim. 2012, 15, 143–151. [Google Scholar]
- Tézé, A.; Hervé, G. Formation et isomerisation des undeca et dodeca tungstosilicates et germanates isomeres. J. Inorg. Nucl. Chem. 1977, 39, 999–1002. [Google Scholar] [CrossRef]
- Rocchiccioli-Deltcheff, C.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum(VI) and tungsten(VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207–216. [Google Scholar] [CrossRef]
- Dong, S.; Xi, X.; Tian, M. Study of the electrocatalytic reduction of nitrite with silicotungstic heteropolyanion. J. Electroanal. Chem. 1995, 385, 227–233. [Google Scholar] [CrossRef]
- Guo, S.-X.; Mariotti, A.W.A.; Schlipf, C.; Bond, A.M.; Wedd, A.G. A Systematic approach to the simulation of the voltammetric reduction of [α-SiW12O40]4− in buffered aqueous electrolyte media and acetonitrile. J. Electroanal. Chem. 2006, 591, 7–18. [Google Scholar] [CrossRef]
- Guo, S.-X.; Mariotti, A.W.A.; Schlipf, C.; Bond, A.M.; Wedd, A.G. Investigation of the Pronounced Medium Effects Observed in the Voltammetry of the Highly Charged Lacunary Anions [α-SiW11O39]8− and [α-PW11O39]7−. Inorg. Chem. 2006, 45, 8563–8574. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chem. Mater. 2016, 28, 1066–1075. [Google Scholar] [CrossRef]
- Lica, G.C.; Browne, K.P.; Tong, Y. Interactions between Keggin-type lacunary polyoxometalates and Ag nanoparticles: A surface-enhanced Raman scattering spectroscopic investigation. J. Clust. Sci. 2006, 17, 349–359. [Google Scholar] [CrossRef]
- Mayer, C.R.; Neveu, S.; Cabuil, V. A nanoscale hybrid system based on gold nanoparticles and heteropolyanions. Angew. Chem.-Int. Ed. 2002, 41, 501–503. [Google Scholar] [CrossRef]
- Ernst, A.Z.; Sun, L.; Wiaderek, K.; Kolary, A.; Zoladek, S.; Kulesza, P.J.; Cox, J.A. Synthesis of polyoxometalate-protected gold nanoparticles by a ligand-exchange method: Application to the electrocatalytic reduction of bromate. Electroanalysis 2007, 19, 2103–2109. [Google Scholar] [CrossRef]
- Wang, Y.; Neyman, A.; Arkhangelsky, E.; Gitis, V.; Meshi, L.; Weinstock, I.A. Self-assembly and structure of directly imaged inorganic-anion monolayers on a gold nanoparticle. J. Am. Chem. Soc. 2009, 131, 17412–17422. [Google Scholar] [CrossRef]
- Shameli, K.; bin Ahmad, M.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and Characterization of Polyethylene Glycol Mediated Silver Nanoparticles by the Green Method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef] [PubMed]
- Hendel, T.; Wuithschick, M.; Kettemann, F.; Birnbaum, A.; Rademann, K.; Polte, J. In situ determination of colloidal gold concentrations with uv-vis spectroscopy: Limitations and perspectives. Anal. Chem. 2014, 86, 11115–11124. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jin, R.; Mirkin, C.A.; Letsinger, R.L. Multiple thiol-anchor capped DNA-gold nanoparticle conjugates. Nucleic Acids Res. 2002, 30, 1558–1562. [Google Scholar] [CrossRef]
- Navarro, J.R.G.; Plugge, M.; Loumaigne, M.; Sanchez-Gonzalez, A.; Mennucci, B.; Débarre, A.; Brouwer, A.M.; Werts, M.H.V. Probing the interactions between disulfide-based ligands and gold nanoparticles using a functionalised fluorescent perylene-monoimide dye. Photochem. Photobiol. Sci. 2010, 9, 1042–1054. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Zhang, G.; Ma, Y.; Yao, J.; Keita, B.; Louis, N.; Zhao, H. Green chemical decoration of multiwalled carbon nanotubes with polyoxometalate-encapsulated gold nanoparticles: Visible light photocatalytic activities. J. Mater. Chem. 2011, 21, 2282–2287. [Google Scholar] [CrossRef]
- Gade Malmos, K.; Blancas-Mejia, L.M.; Weber, B.; Buchner, J.; Ramirez-Alvarado, M.; Naiki, H.; Otzen, D. ThT 101: A primer on the use of thioflavin T to investigate amyloid formation. Amyloid 2017, 24, 1–16. [Google Scholar] [CrossRef]
- Rovnyagina, N.R.; Budylin, G.S.; Vainer, Y.G.; Tikhonova, T.N.; Vasin, S.L.; Yakovlev, A.A.; Kompanets, V.O.; Chekalin, S.V.; Priezzhev, A.V.; Shirshin, E.A. Fluorescence lifetime and intensity of thioflavin T as reporters of different fibrillation stages: Insights obtained from fluorescence up-conversion and particle size distribution measurements. Int. J. Mol. Sci. 2020, 21, 6169. [Google Scholar] [CrossRef]
- Finder, V.H.; Glockshuber, R. Amyloid-beta aggregation. Neurodegener. Dis. 2007, 4, 13–27. [Google Scholar] [CrossRef]
- Brudar, S.; Hribar-Lee, B. The Role of Buffers in Wild-Type HEWL Amyloid Fibril Formation Mechanism. Biomolecules 2019, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.M.D.; Hussain, T.; Ahmed, A.B.F.; Thamir; Alshammari, M.; Moin, A.; Ahmed, M.Q.; George; Barreto, E.; Kamal, M.A.; et al. Gold nanoparticles: A plausible tool to combat neurological bacterial infections in humans. Biomed. Pharmacother. 2018, 107, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef]
- Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. JAT 2010, 30, 212–217. [Google Scholar] [PubMed]
- Nativo, P.; Prior, I.A.; Brust, M. Uptake and intracellular fate of surface-modified gold nanoparticles. ACS Nano 2008, 2, 1639–1644. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Schleh, C.; Semmler-Behnke, M.; Lipka, J.; Wenk, A.; Hirn, S.; Schäffler, M.; Schmid, G.; Simon, U.; Kreyling, W.G. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology 2012, 6, 36–46. [Google Scholar] [CrossRef]
- Cho, E.C.; Xie, J.; Wurm, P.A.; Xia, Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Berry, S.A.; Duthie, K.; Han, G.; Rotello, V.M.; McNerny, D.Q.; Baker, J.R., Jr.; Orr, B.G.; Holl, M.M. Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Lett. 2008, 8, 420–424. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of lipid membranes by gold nanoparticles: Insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; 2004; pp. 1–31. [Google Scholar]
- Invitrogen Molecular Probes. LIVE/DEAD Viability/Cytotoxicity Kit for Mammalian Cells. Product Information, Catalog Number: MP 03224. 2005, pp. 1–7. Available online: https://www.thermofisher.com/order/catalog/product/L3224 (accessed on 1 October 2023).
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, A.P. Inorganic Syntheses; John Wiley & Sons: Hoboken, NJ, USA, 1991; Volume 27. [Google Scholar]
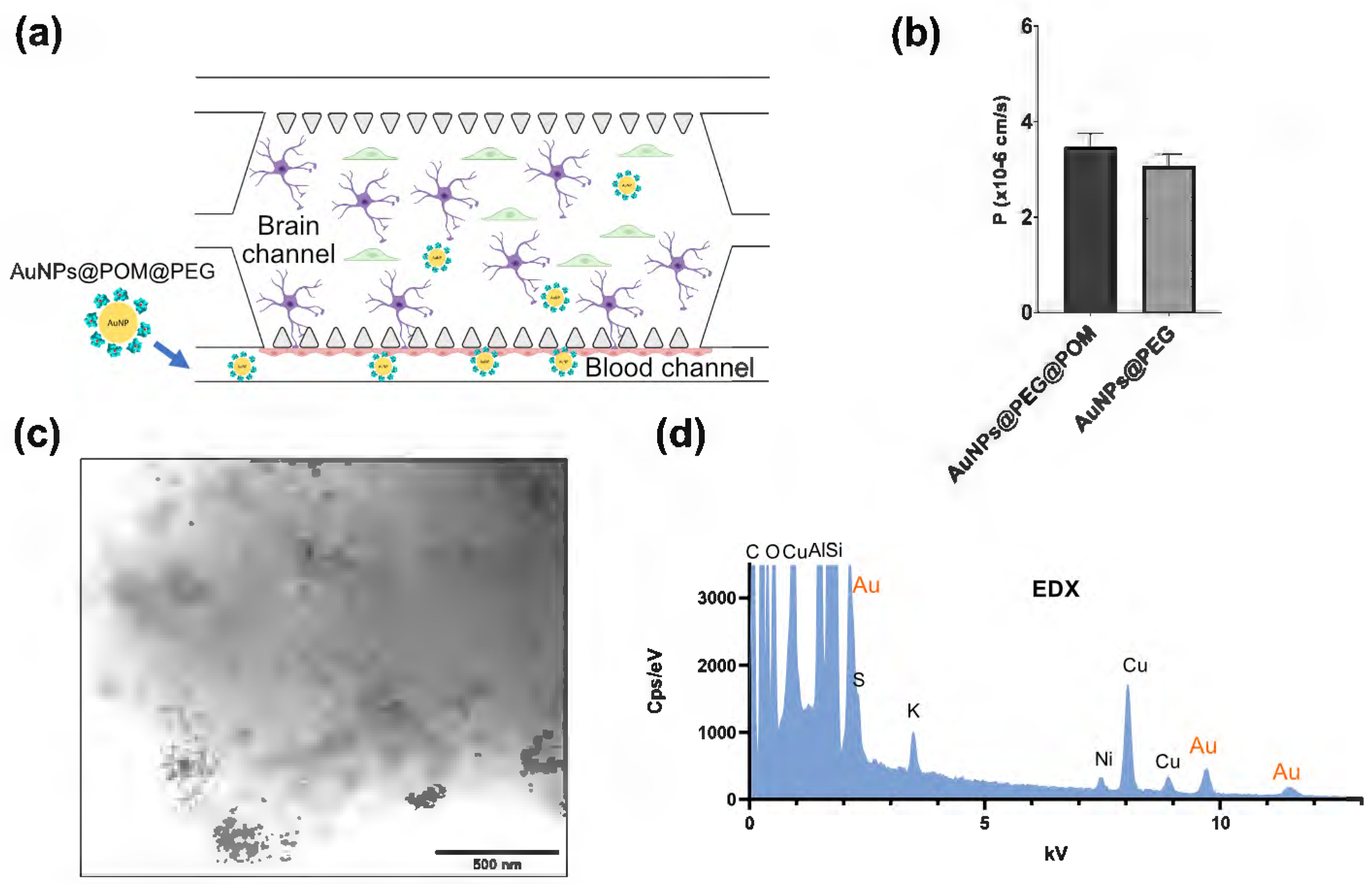
| AuNPs@SC | AuNPs@PEG | AuNPs@POM | AuNPs@POM@PEG | |
|---|---|---|---|---|
| Dh (nm) ± SD | 33 ± 1.50 | 50 ± 0.50 | - | 60 ± 1.5 |
| Pdi | 0.45 | 0.23 | - | 0.27 |
| pZ (mV) ± SD | −43.97 ± 0.21 | −40.80 ± 2.00 | −60.07 ± 1.52 | −57.30 ± 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perxés Perich, M.; Palma-Florez, S.; Solé, C.; Goberna-Ferrón, S.; Samitier, J.; Gómez-Romero, P.; Mir, M.; Lagunas, A. Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood–Brain Barrier in a µphysiological Model. Nanomaterials 2023, 13, 2697. https://doi.org/10.3390/nano13192697
Perxés Perich M, Palma-Florez S, Solé C, Goberna-Ferrón S, Samitier J, Gómez-Romero P, Mir M, Lagunas A. Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood–Brain Barrier in a µphysiological Model. Nanomaterials. 2023; 13(19):2697. https://doi.org/10.3390/nano13192697
Chicago/Turabian StylePerxés Perich, Marta, Sujey Palma-Florez, Clara Solé, Sara Goberna-Ferrón, Josep Samitier, Pedro Gómez-Romero, Mònica Mir, and Anna Lagunas. 2023. "Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood–Brain Barrier in a µphysiological Model" Nanomaterials 13, no. 19: 2697. https://doi.org/10.3390/nano13192697
APA StylePerxés Perich, M., Palma-Florez, S., Solé, C., Goberna-Ferrón, S., Samitier, J., Gómez-Romero, P., Mir, M., & Lagunas, A. (2023). Polyoxometalate-Decorated Gold Nanoparticles Inhibit β-Amyloid Aggregation and Cross the Blood–Brain Barrier in a µphysiological Model. Nanomaterials, 13(19), 2697. https://doi.org/10.3390/nano13192697











