Nanodiamond Decorated PEO Oxide Coatings on NiTi Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanodiamonds Functionalization
2.2. Nanodiamond Assessment
2.3. Cytotoxicity of ND
2.4. Surface Modification of the NiTi Alloy
2.5. Biocompatibility of Nitinol-PEO-ND
3. Results
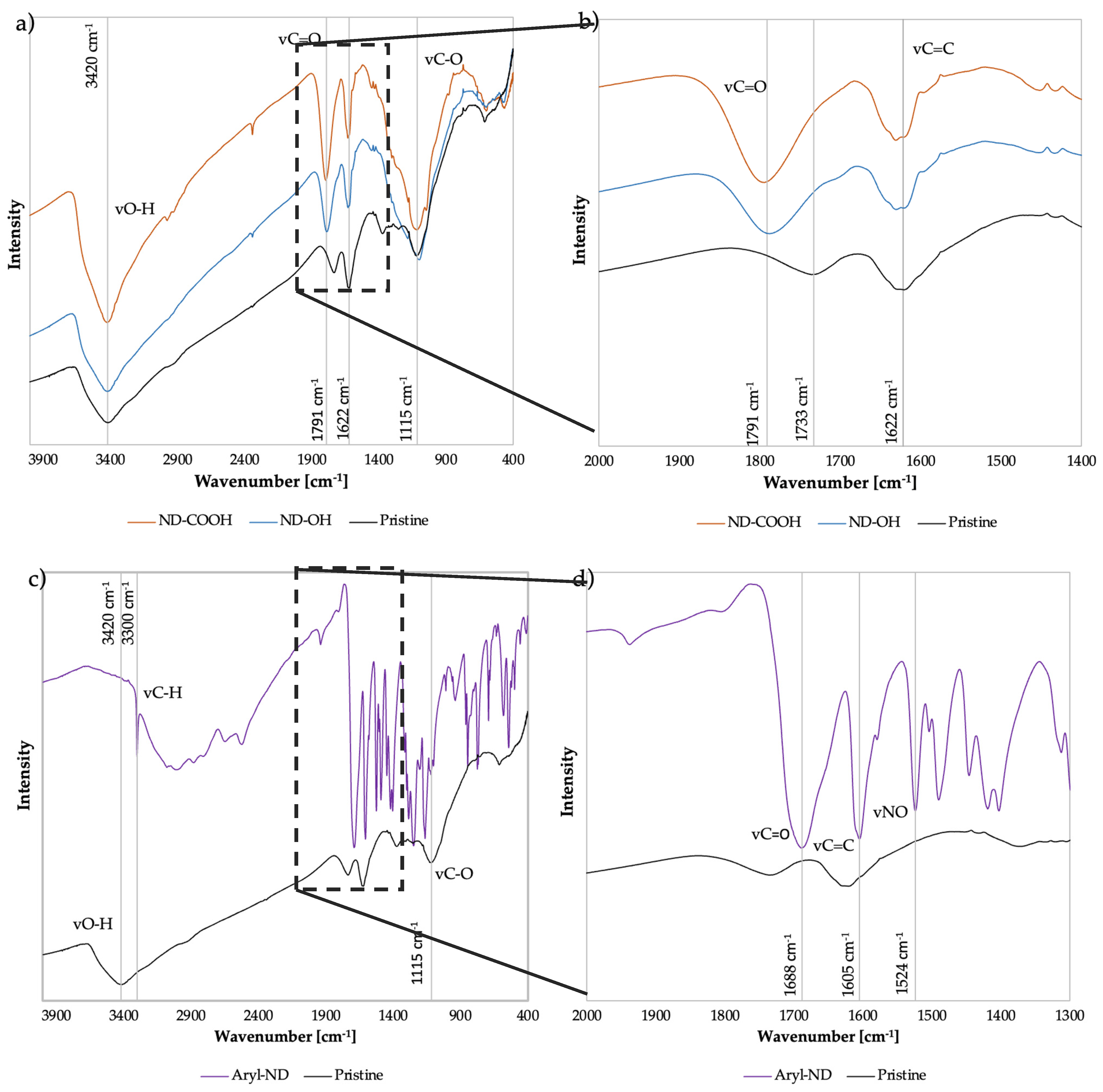
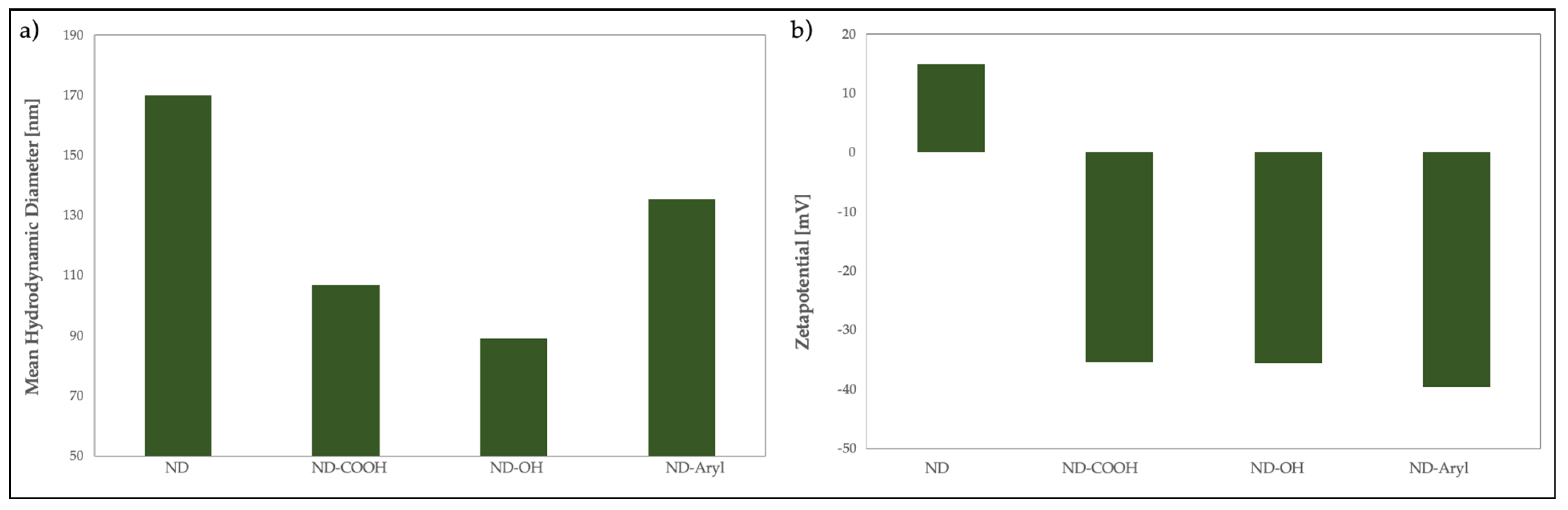
3.1. ND Characterization
3.2. Nitinol PEO Surface Characterization
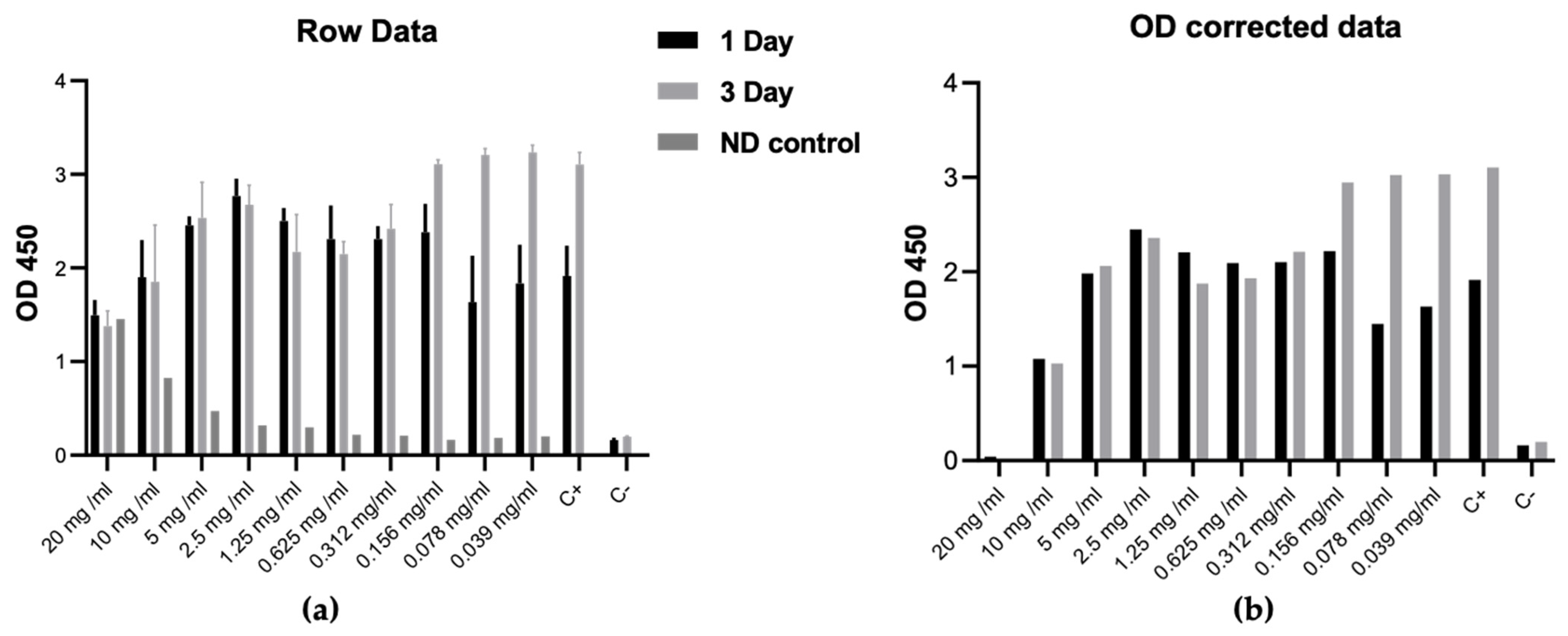
3.3. Biological Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of Cardiovascular Disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Borhani, S.; Hassanajili, S.; Ahmadi Tafti, S.H.; Rabbani, S. Cardiovascular Stents: Overview, Evolution, and next Generation. Prog. Biomater. 2018, 7, 175–205. [Google Scholar] [CrossRef]
- Grabow, N.; Martin, D.P.; Schmitz, K.P.; Sternberg, K. Absorbable Polymer Stent Technologies for Vascular Regeneration. J. Chem. Technol. Biotechnol. 2010, 85, 744–751. [Google Scholar] [CrossRef]
- Fischman, D.L.; Leon, M.B.; Baim, D.S.; Schatz, R.A.; Savage, M.P.; Penn, I.; Detre, K.; Veltri, L.; Ricci, D.; Nobuyoshi, M.; et al. A Randomized Comparison of Coronary-Stent Placement and Balloon Angioplasty in the Treatment of Coronary Artery Disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef]
- Nuhn, H.; Blanco, C.E.; Desai, T.A. Nanoengineered Stent Surface to Reduce In-Stent Restenosis in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 19677–19686. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, Z.; Liu, Y.; Wang, J.; Maitz, M.F.; Wang, Y.; Liu, S.; Chen, J.; Huang, N. Surface Modification with Dopamine and Heparin/Poly-L-Lysine Nanoparticles Provides a Favorable Release Behavior for the Healing of Vascular Stent Lesions. ACS Appl. Mater. Interfaces 2014, 6, 8729–8743. [Google Scholar] [CrossRef]
- Jiang, W.; Tian, Q.; Vuong, T.; Shashaty, M.; Gopez, C.; Sanders, T.; Liu, H. Comparison Study on Four Biodegradable Polymer Coatings for Controlling Magnesium Degradation and Human Endothelial Cell Adhesion and Spreading. ACS Biomater. Sci. Eng. 2017, 3, 936–950. [Google Scholar] [CrossRef]
- Wiebe, J.; Nef, H.M.; Hamm, C.W. Current Status of Bioresorbable Scaffolds in the Treatment of Coronary Artery Disease. J. Am. Coll. Cardiol. 2014, 64, 2541–2551. [Google Scholar] [CrossRef]
- Cicha, I.; Singh, R.; Garlichs, C.D.; Alexiou, C. Nano-Biomaterials for Cardiovascular Applications: Clinical Perspective. J. Control. Release 2016, 229, 23–36. [Google Scholar] [CrossRef]
- Krucoff, M.W.; Kereiakes, D.J.; Petersen, J.L.; Mehran, R.; Hasselblad, V.; Lansky, A.J.; Fitzgerald, P.J.; Garg, J.; Turco, M.A.; Simonton, C.A.; et al. A Novel Bioresorbable Polymer Paclitaxel-Eluting Stent for the Treatment of Single and Multivessel Coronary Disease: Primary Results of the COSTAR (Cobalt Chromium Stent with Antiproliferative for Restenosis) II Study. J. Am. Coll. Cardiol. 2008, 51, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Unverdorben, M.; Sippel, B.; Degenhardt, R.; Sattler, K.; Fries, R.; Abt, B.; Wagner, E.; Koehler, H.; Daemgen, G.; Scholz, M.; et al. Comparison of a Silicon Carbide-Coated Stent versus a Noncoated Stent in Human Beings: The Tenax versus Nir Stent Study’s Long-Term Outcome. Am. Heart J. 2003, 145, E17. [Google Scholar] [CrossRef] [PubMed]
- Berlin, T.; Rozenbaum, E.; Arbel, J.; Reges, O.; Erel, J.; Shetboun, I.; Leibovitch, M.; Mosseri, M. Six- and Twelve-Month Clinical Outcomes after Implantation of Prokinetic Bms in Patients with Acute Coronary Syndrome. J. Interv. Cardiol. 2010, 23, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shin, J.H.; Shin, D.H.; Moon, M.W.; Park, K.; Kim, T.H.; Shin, K.M.; Won, Y.H.; Han, D.K.; Lee, K.R. Comparison of Diamond-like Carbon-Coated Nitinol Stents with or without Polyethylene Glycol Grafting and Uncoated Nitinol Stents in a Canine Iliac Artery Model. Br. J. Radiol. 2011, 84, 210–215. [Google Scholar] [CrossRef]
- Schrand, A.M.; Huang, H.; Carlson, C.; Schlager, J.J.; Osawa, E.; Hussain, S.M.; Dai, L. Are Diamond Nanoparticles Cytotoxic? J. Phys. Chem. B 2007, 111, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Mochalin, V.N. Biomedical Applications of Nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef] [PubMed]
- Rifai, A.; Tran, N.; Reineck, P.; Elbourne, A.; Mayes, E.; Sarker, A.; Dekiwadia, C.; Ivanova, E.P.; Crawford, R.J.; Ohshima, T.; et al. Engineering the Interface: Nanodiamond Coating on 3D-Printed Titanium Promotes Mammalian Cell Growth and Inhibits Staphylococcus Aureus Colonization. ACS Appl. Mater. Interfaces 2019, 11, 24588–24597. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Gong, Y.; Zhou, P.; Li, H. Mechanical Properties of Nanodiamond-Reinforced Hydroxyapatite Composite Coatings Deposited by Suspension Plasma Spraying. Appl. Surf. Sci. 2018, 439, 60–65. [Google Scholar] [CrossRef]
- Lüscher, T.F.; Landmesser, U.; Von Eckardstein, A.; Fogelman, A.M. High-Density Lipoprotein. Circ. Res. 2014, 114, 171–182. [Google Scholar] [CrossRef]
- Frias, J.C.; Williams, K.J.; Fisher, E.A.; Fayad, Z.A. Recombinant HDL-like Nanoparticles: A Specific Contrast Agent for MRI of Atherosclerotic Plaques. J. Am. Chem. Soc. 2004, 126, 16316–16317. [Google Scholar] [CrossRef] [PubMed]
- Mani, N.; Rifai, A.; Houshyar, S.; Booth, M.A.; Fox, K. Diamond in Medical Devices and Sensors: An Overview of Diamond Surfaces. Med. Devices Sens. 2020, 3, e10127. [Google Scholar] [CrossRef]
- Grausova, L.; Bacakova, L.; Kromka, A.; Potocky, S.; Vanecek, M.; Nesladek, M.; Lisa, V. Nanodiamond as Promising Material for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2009, 9, 3524–3534. [Google Scholar] [CrossRef] [PubMed]
- Grausova, L.; Kromka, A.; Bacakova, L.; Potocky, S.; Vanecek, M.; Lisa, V. Bone and Vascular Endothelial Cells in Cultures on Nanocrystalline Diamond Films. Diam. Relat. Mater. 2008, 17, 1405–1409. [Google Scholar] [CrossRef]
- Chu, Y.C.; Tzeng, Y.; Auciello, O. Microwave Plasma Enhanced Chemical Vapor Deposition of Nanocrystalline Diamond Films by Bias-Enhanced Nucleation and Bias-Enhanced Growth. J. Appl. Phys. 2014, 115, 024308. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional Coatings to Simultaneously Promote Osseointegration and Prevent Infection of Orthopaedic Implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Rifai, A.; Tran, N.; Lau, D.W.; Elbourne, A.; Zhan, H.; Stacey, A.D.; Mayes, E.L.H.; Sarker, A.; Ivanova, E.P.; Crawford, R.J.; et al. Polycrystalline Diamond Coating of Additively Manufactured Titanium for Biomedical Applications. ACS Appl. Mater. Interfaces 2018, 10, 8474–8484. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process-Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef]
- Korniienko, V.; Oleshko, O.; Husak, Y.; Deineka, V.; Holubnycha, V.; Mishchenko, O.; Kazek-Kęsik, A.; Jakóbik-Kolon, A.; Pshenychnyi, R.; Leśniak-Ziółkowska, K.; et al. Formation of a Bacteriostatic Surface on ZrNb Alloy via Anodization in a Solution Containing Cu Nanoparticles. Materials 2020, 13, 3913. [Google Scholar] [CrossRef]
- Oleshko, O.; Husak, Y.; Korniienko, V.; Pshenychnyi, R.; Varava, Y.; Kalinkevich, O.; Pisarek, M.; Grundsteins, K.; Pogorielova, O.; Mishchenko, O.; et al. Biocompatibility and Antibacterial Properties of Zno-Incorporated Anodic Oxide Coatings on TiZrNb Alloy. Nanomaterials 2020, 10, 2401. [Google Scholar] [CrossRef]
- Myakinin, A.; Turlybekuly, A.; Pogrebnjak, A.; Mirek, A.; Bechelany, M.; Liubchak, I.; Oleshko, O.; Husak, Y.; Korniienko, V.; Leśniak-Ziółkowska, K.; et al. In Vitro Evaluation of Electrochemically Bioactivated Ti6Al4V 3D Porous Scaffolds. Mater. Sci. Eng. C 2021, 121, 111870. [Google Scholar] [CrossRef]
- Huan, Z.; Fratila-Apachitei, L.E.; Apachitei, I.; Duszczyk, J. Porous NiTi Surfaces for Biomedical Applications. Appl. Surf. Sci. 2012, 258, 5244–5249. [Google Scholar] [CrossRef]
- Siu, H.T.; Man, H.C. Fabrication of Bioactive Titania Coating on Nitinol by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2013, 274, 181–187. [Google Scholar] [CrossRef]
- Huan, Z.; Fratila-Apachitei, L.E.; Apachitei, I.; Duszczyk, J. Porous TiO2 Surface Formed on Nickel-Titanium Alloy by Plasma Electrolytic Oxidation: A Prospective Polymer-Free Reservoir for Drug Eluting Stent Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 700–708. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Dudek, Ł.; Malorny, W. SEM And EDS Analysis of Nitinol Surfaces Treated By Plasma Electrolytic Oxidation. Adv. Mater. Sci. 2015, 15, 41–47. [Google Scholar] [CrossRef][Green Version]
- Chung, P.-H.; Perevedentseva, E.; Tu, J.-S.; Chang, C.C.; Cheng, C.-L. Spectroscopic Study of Bio-Functionalized Nanodiamonds. Diam. Relat. Mater. 2006, 15, 622–625. [Google Scholar] [CrossRef]
- Balakin, S.; Missirlis, A.; Klemmed, B.; Lee, J.; Opitz, J.; Yeo, J.-S.; Cuniberti, G. Quantitative Analysis of BMP-2 Derived Peptide Covalently Grafted onto Oxidized Detonation Nanodiamonds. Carbon N. Y. 2019, 152, 740–745. [Google Scholar] [CrossRef]
- Fraczyk, J.; Rosowski, A.; Kolesinska, B.; Koperkiewcz, A.; Sobczyk-Guzenda, A.; Kaminski, Z.J.; Dudek, M. Orthogonal Functionalization of Nanodiamond Particles after Laser Modification and Treatment with Aromatic Amine Derivatives. Nanomaterials 2018, 8, 908. [Google Scholar] [CrossRef]
- Liang, Y.; Ozawa, M.; Krueger, A. A General Procedure to Functionalize Agglomerating Nanoparticles Demonstrated on Nanodiamond. ACS Nano 2009, 3, 2288–2296. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L. FTIR Spectroscopy of Nanodiamonds: Methods and Interpretation. Diam. Relat. Mater. 2018, 89, 52–66. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Gogotsi, O.; Baginskiy, I.; Balitskyi, V.; Zahorodna, V.; Husak, Y.; Yanko, I.; Pernakov, M.; Roshchupkin, A.; Lyndin, M.; et al. MXene-Assisted Ablation of Cells with a Pulsed Near-Infrared Laser. ACS Appl. Mater. Interfaces 2022, 14, 28683–28696. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, Y.O.; Garkusha, I.E.; Taran, A.V.; Coy, E.; Iatsunskyi, I.; Diedkova, K.; Roshchupkin, A.; Tymoshenko, O.; Pogorielov, M.; Misiruk, I. Development of Hydrophilic NbCuSi(N) &TiAlNb(N) Coatings as a New Strategy for Medical Implants Modification. Ceram. Int. 2022, 49, 4099–4108. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Warchoł, F.; Oleshko, O.; Husak, Y.; Kazek-Kęsik, A.; Korniienko, V.; Deineka, V.; Sowa, M.; Maciej, A.; Michalska, J.; et al. Effects of the Sources of Calcium and Phosphorus on the Structural and Functional Properties of Ceramic Coatings on Titanium Dental Implants Produced by Plasma Electrolytic Oxidation. Mater. Sci. Eng. C 2021, 119, 111607. [Google Scholar] [CrossRef] [PubMed]
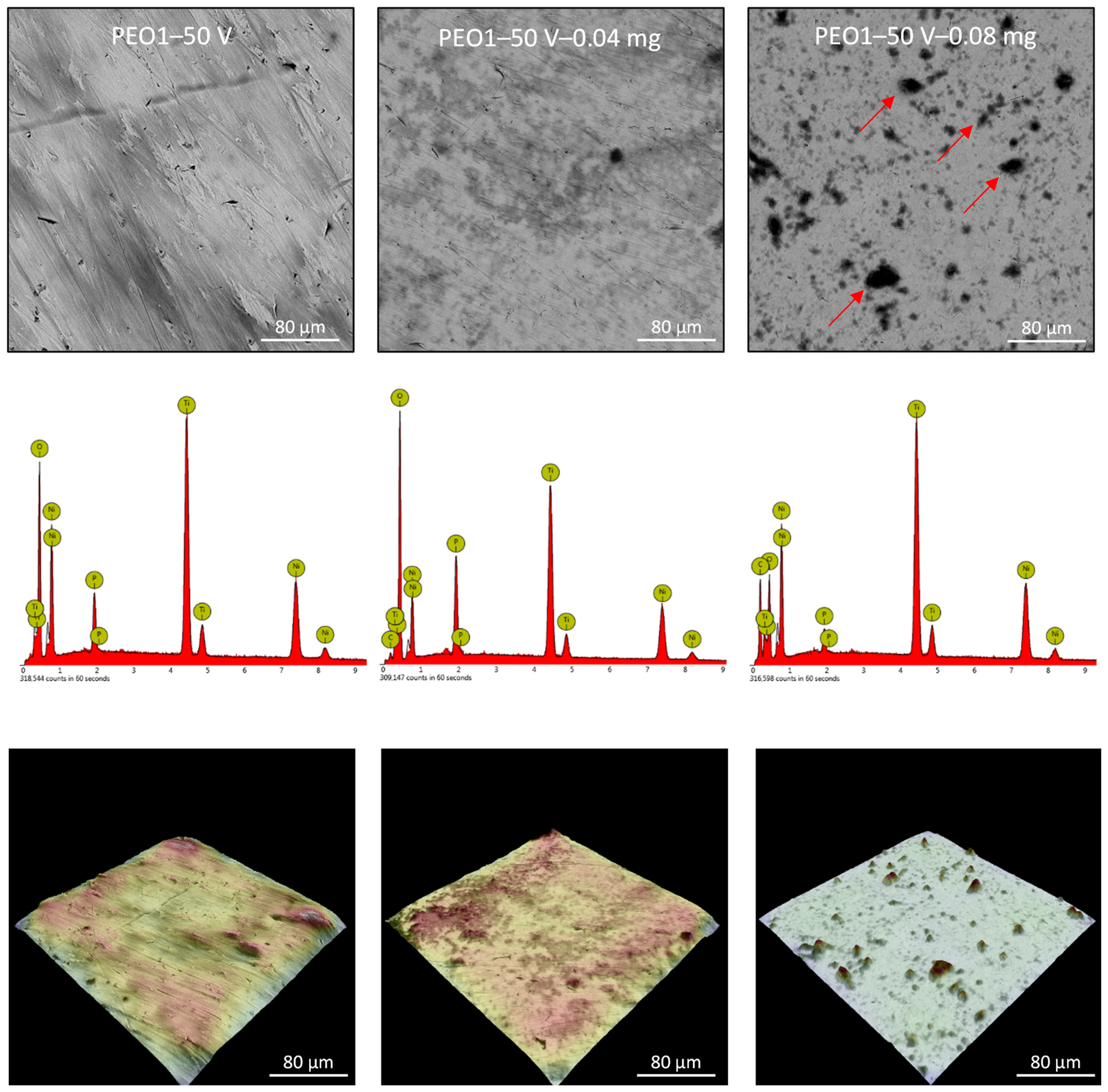
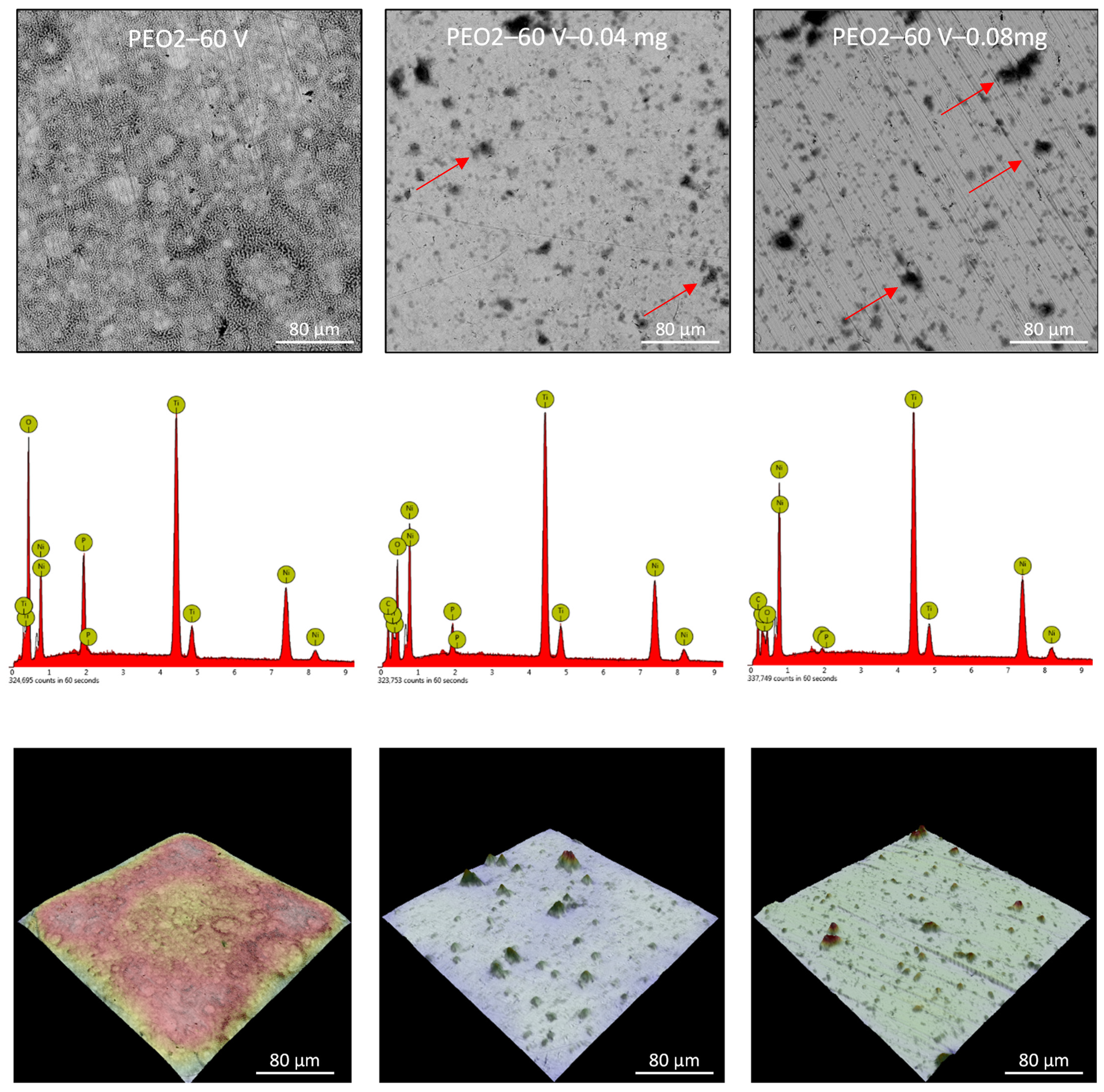

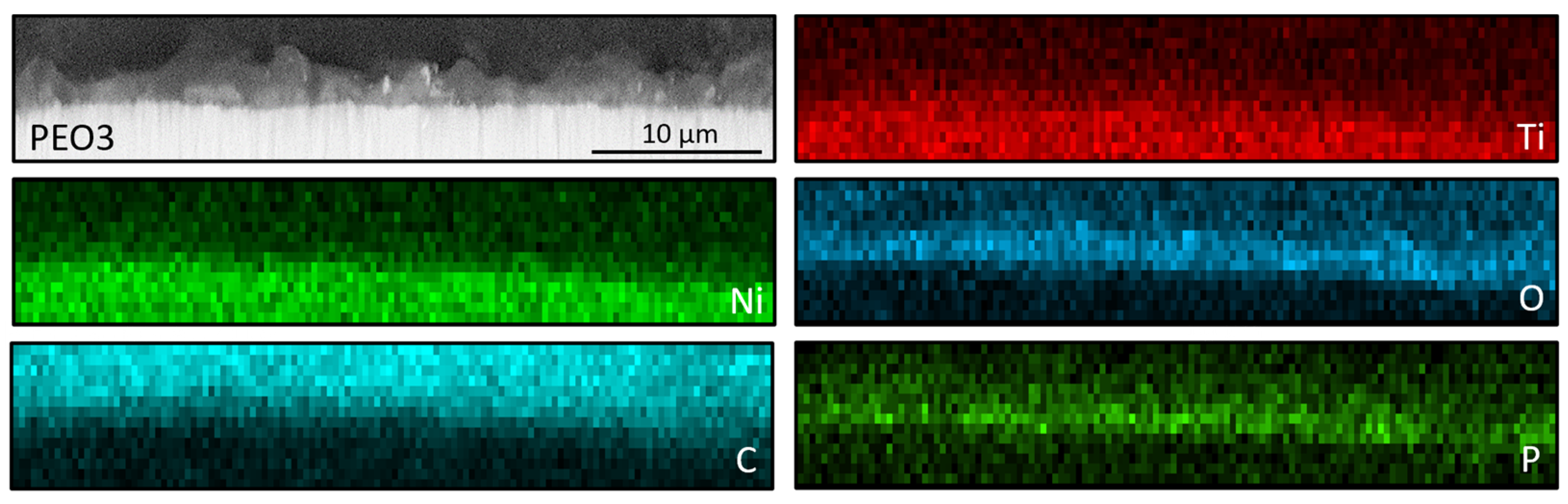

| Sample Label | Contact Angle, ° | Roughness, µm |
|---|---|---|
| PEO1—50 V | 28.01 ± 2.28 | 2.80 |
| PEO2—60 V | 27.95 ± 4.89 | 2.00 |
| PEO3—70 V | 80.85 ± 5.00 | 1.42 |
| PEO1—50 V − 0.04 mg | 26.00 ± 2.71 | 1.66 |
| PEO2—60 V − 0.04 mg | 31.91 ± 2.96 | 1.36 |
| PEO3—70 V − 0.04 mg | 28.68 ± 3.52 | 1.75 |
| PEO1—50 V − 0.08 mg | 31.66 ± 3.80 | 1.23 |
| PEO2—60 V − 0.08 mg | 24.74 ± 2.31 | 1.27 |
| PEO3—70 V − 0.08 mg | 37.37 ± 1.62 | 1.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grundsteins, K.; Diedkova, K.; Korniienko, V.; Stoppel, A.; Balakin, S.; Jekabsons, K.; Riekstina, U.; Waloszczyk, N.; Kołkowska, A.; Varava, Y.; et al. Nanodiamond Decorated PEO Oxide Coatings on NiTi Alloy. Nanomaterials 2023, 13, 2601. https://doi.org/10.3390/nano13182601
Grundsteins K, Diedkova K, Korniienko V, Stoppel A, Balakin S, Jekabsons K, Riekstina U, Waloszczyk N, Kołkowska A, Varava Y, et al. Nanodiamond Decorated PEO Oxide Coatings on NiTi Alloy. Nanomaterials. 2023; 13(18):2601. https://doi.org/10.3390/nano13182601
Chicago/Turabian StyleGrundsteins, Karlis, Kateryna Diedkova, Viktoriia Korniienko, Anita Stoppel, Sascha Balakin, Kaspars Jekabsons, Una Riekstina, Natalia Waloszczyk, Agata Kołkowska, Yuliia Varava, and et al. 2023. "Nanodiamond Decorated PEO Oxide Coatings on NiTi Alloy" Nanomaterials 13, no. 18: 2601. https://doi.org/10.3390/nano13182601
APA StyleGrundsteins, K., Diedkova, K., Korniienko, V., Stoppel, A., Balakin, S., Jekabsons, K., Riekstina, U., Waloszczyk, N., Kołkowska, A., Varava, Y., Opitz, J., Simka, W., Beshchasna, N., & Pogorielov, M. (2023). Nanodiamond Decorated PEO Oxide Coatings on NiTi Alloy. Nanomaterials, 13(18), 2601. https://doi.org/10.3390/nano13182601









