Abstract
Toxic industrial chemicals (TICs), when accidentally released into the workplace or environment, often form a gaseous mixture that complicates detection and mitigation measures. However, most of the existing gas sensors are unsuitable for detecting such mixtures. In this study, we demonstrated the detection and identification of gaseous mixtures of TICs using a chemiresistor array of single-walled carbon nanotubes (SWCNTs). The array consists of three SWCNT chemiresistors coated with different molecular/ionic species, achieving a limit of detection (LOD) of 2.2 ppb for ammonia (NH3), 820 ppb for sulfur dioxide (SO2), and 2.4 ppm for ethylene oxide (EtO). By fitting the concentration-dependent sensor responses to an adsorption isotherm, we extracted parameters that characterize each analyte-coating combination, including the proportionality and equilibrium constants for adsorption. Principal component analysis confirmed that the sensor array detected and identified mixtures of two TIC gases: NH3/SO2, NH3/EtO, and SO2/EtO. Exposing the sensor array to three TIC mixtures with various EtO/SO2 ratios at a fixed NH3 concentration showed an excellent correlation between the sensor response and the mixture composition. Additionally, we proposed concentration ranges within which the sensor array can effectively detect the gaseous mixtures. Being highly sensitive and capable of analyzing both individual and mixed TICs, our gas sensor array has great potential for monitoring the safety and environmental effects of industrial chemical processes.
1. Introduction
The detection and identification of toxic industrial chemicals (TICs) are essential for workplace safety, public health, and environmental monitoring [1,2]. Several gas sensors have been developed using various nanomaterials such as carbon nanotubes (CNTs) [3,4], graphene [5,6], nanowire [7], semiconducting materials [8,9,10] and metal–organic frameworks [11] because of their high surface-to-volume ratios and sensitivity to chemical environments. CNTs, in particular, have a one-dimensional electronic structure, where all atoms reside only on the surface and are extremely sensitive to molecular adsorption [12,13]. However, most previous studies have focused on improving the sensitivity and selectivity toward a single gaseous analyte rather than the analysis of multi-analyte mixtures.
To overcome the limitations of detecting and identifying complex gas mixtures, advanced technologies have been developed, such as gas sensor arrays based on nanomaterials [14,15,16,17,18,19,20,21,22,23]. For example, Guerin et al. demonstrated a CNT chemiresistor array with an electrode–CNT interface-derived sensitivity difference for H2, NH3, toluene, and ethanol using various metal electrodes (Pt, Pd, and Au) [24]. Yi et al. reported a gas sensor array using a nanowire-like network film of ZnO, Co3O4, IN2O3, and SnO2 that enables the selective detection and identification of C7H8, NH3, HCHO, and CH3COCH3 [25]. Although previous studies have demonstrated excellent selectivity for single gaseous analytes, the sensing performance for mixture analysis has not been validated yet. Chu et al. recently reported reliable identification of mixtures using sensor arrays [26]. However, complex numerical analysis and the assistance of neural networks are necessary [26,27,28,29,30,31].
In this work, the detection and identification of gaseous mixtures of toxic chemicals were enabled using a single-walled carbon nanotube (SWCNTs) chemiresistor array. We chose ammonia (NH3), sulfur dioxide (SO2), and ethylene oxide (EtO) as target analyte gases. The selection of these gases was based on the fact that each individual analyte is a hazardous gas, and their mixture poses a significant threat to the environment and safety. For example, in a moisturized environment, the reaction between NH3 and SO2 can lead to the formation of acid rain [32,33,34]. Furthermore, the reaction between NH3 and EtO has the potential to result in an explosion [35,36,37]. Different adsorption/desorption properties of gas molecules on SWCNTs were obtained using polymeric and ionic chemical coatings, thus allowing identification of the three TICs—NH3, SO2, and EtO—alone or as mixtures. In addition, by analyzing the concentration-dependent response curves, we estimated parameters that govern the analyte adsorption, such as equilibrium constant and limits of detection (LOD) of each analyte. Our sensor array has the potential to be applied to a wide range of multi-analyte sensing systems for environmental monitoring and alarm systems to prevent accidents and minimize the potential harm caused by exposure to hazardous chemicals.
2. Materials and Methods
2.1. Materials
All chemicals and reagents were commercially available, and we utilized these without additional purification. 1-ethyl-3-methylimidazolium bis(trifluoro-methylsulfonyl)imide (EMIM), polypyrrole (Ppy), dimethyl sulfoxide (DMSO), and phosphate buffered saline (PBS) were purchased from Sigma-Aldrich. The gas cylinders for NH3 (10 ppm), SO2 (76.3 ppm), and EtO (666 ppm) were purchased from RIGAS (Daejeon, Korea). Graphing and PCA analysis were achieved by using Origin software (OriginPro 2020, OriginLab Corp., Northampton, MA, USA)
2.2. Sensor Fabrication
Pristine SWCNTs (AP-SWNT, Carbon Solutions, Inc., Riverside, CA, USA) were dispersed in a 1 wt% aqueous solution of sodium dodecyl sulfate (Sigma-Aldrich, St. Louis, MO, USA) by homogenization (6600 rpm, 1 h), followed by a bath sonication for 1 h. The dispersion was then centrifuged (14,000 rpm for 1 h) to remove large aggregates, and a homogeneous dispersion was obtained by collecting the supernatant. The concentration of the SWCNT dispersion was estimated to be 16.5 mg/L by UV-VIS-NIR absorbance at 632 nm (Cary 5000, Agilent Technologies, Santa Clara, CA, USA) and an extinction coefficient of ε632 = 0.036 (mg/L)−1 cm−1 (Figure S1) [38]. The 25 μL of SWCNT dispersion was vacuum filtered through a polycarbonate membrane with 0.2 μm pores (GTTP02500, MERCK, St. Louis, MO, USA) and transferred onto a silicon substrate with 300 nm thermal oxides (DASOM RMS, Anyang, Korea) as reported previously [39].
The sensor array was fabricated by patterning electrodes on the SWCNT networks. First, extra nanotubes that were not required were removed by conventional photolithography and oxygen plasma etching (100 W, 30 sccm O2, 30 s) to reduce the signal interference between the sensors. The density of the SWCNT network was estimated by the amount of SWCNTs (16.5 mg/L × 25 μL) and the area of the SWCNT film (1.95 mm2). Then, interdigitated electrodes (2 nm Cr, 75 nm Au) were patterned on the SWCNT networks via the lift-off process. Finally, a structure of 70 μm-thick SU8-2050 was patterned around each sensor, which served as a well for drop-drying coating materials.
Figure 1a illustrates the design of the SWCNT chemiresistor. The SEM image depicted in Figure 1a shows the SWCNT network on the SiO2 substrate at the density of 0.43 ng/mm2. The sensor array consisted of three SWCNT chemiresistors coated with EMIM, PBS, and Ppy (Figure 1b). For the selection of coating materials, we conducted screening experiments using commercially available chemicals that were reported to exhibit high sensitivity and selectivity to the target analyte gases. Based on the sensor response to NH3, SO2, and EtO (Figure S2), we chose EMIM, PBS, and Ppy as the coating materials. The dimensions of the sensor array used in this study were 2 mm × 6 mm. The sensor coating was performed as follows: For the EMIM and PBS coatings, 0.1 μL of 10 mg/mL EMIM in DMSO solution and 0.1 μL of PBS solution, respectively, was dropped on the sensor and dried in a vacuum desiccator for 4 h. For Ppy coating, 0.1 μL of Ppy was dropped on the sensor and thereafter washed with deionized water and dried with N2. The coated-array sensor was placed in a chip carrier, and thereafter, electrically connected to the Au-pad using a wire bonder.
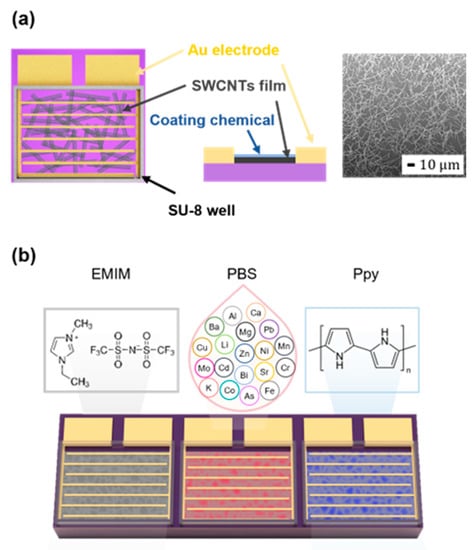
Figure 1.
SWCNT sensor array fabrication. (a) Design of the SWCNT chemiresistor and SEM images for transferred SWCNT film on SiO2 substrate. (b) Chemiresistor array coated with EMIM, PBS, and Ppy, respectively.
2.3. Experimental Setup
Figure 2 shows the experimental setup for the gas sensing performance measurement. The analyte gases (NH3, SO2, EtO) and air cylinders were connected to a mass flow controller (MFC, Kofloc Corp., Kyoto, Japan) to control the on/off status and flow rate of the gas. The total flow rate was fixed at 500 sccm. To control the analyte gas concentration, the carrier gas was balanced using the MFC. A static mixer was used for the uniform mixing of the analyte gases. The resistance of the sensor was measured using a system switch/multimeter (3706A, Keithley, Cleveland, OH, USA). Prior to gas sensing, the sensor array was stabilized in the chamber using carrier gas for 60 s, and the change in resistance was measured by exposing it to the analyte gas for 20 s.

Figure 2.
Experimental setup for measuring the performance of SWCNT chemiresistor array.
3. Results and Discussion
3.1. Gas Sensing Performance
Figure 3 shows the gas sensing performance of the SWCNT chemiresistor array. The sensor response () is defined as , where and represent the resistances of the SWCNT chemiresistor before and after exposure to the analytes, respectively. The measurements of sensor response with increasing gas concentration were performed in one single device. For the purpose of minimizing exposure to hazardous gases and providing rapid alarms, the sensor array was exposed to the analyte gases for only 20 s. Although the resistances of the baseline were not fully recovered, and the deviation was less than 1% (Figure S3), small differences in baseline still existed because of the baseline drift of the SWCNT sensors [40,41].
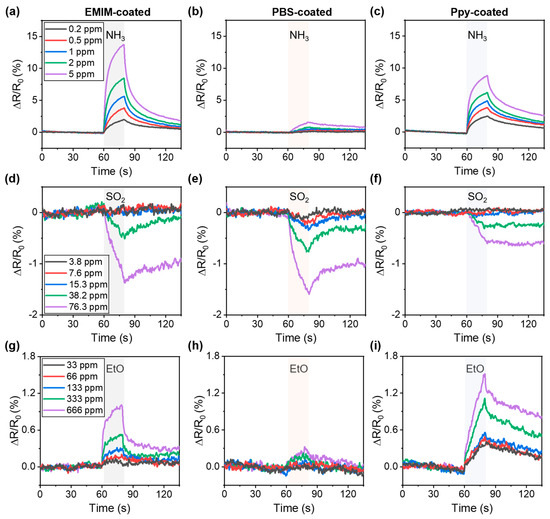
Figure 3.
Gas sensing performance of SWCNT chemiresistor arrays. Sensor responses to 0.2–5 ppm NH3 exposure of (a) EMIM-coated sensor, (b) PBS-coated sensor, and (c) Ppy-coated sensor. Sensor responses to 3.8–76.3 ppm SO2 of (d) EMIM-coated sensor, (e) PBS-coated sensor, (f) Ppy-coated sensor. Sensor responses to 33–666 ppm EtO of (g) EMIM-coated sensor, (h) PBS-coated sensor, (i) Ppy-coated sensor.
First, we investigated the sensor responses when exposed to a concentration range of 0.2–5 ppm NH3, which is lower than the concentration range of NH3 immediately dangerous to life or health (IDLH) (300 ppm) [42]. The resistance of the EMIM-coated sensor rapidly increased when exposed to NH3, owing to the electron-donating characteristics of NH3 on the p-doped SWCNT; the resistance decreased when the exposure to NH3 ceased (Figure 3a) [43,44,45]. The sensor responses increased from 2.18% to 13.1% as the concentration of NH3 increased from 0.2 ppm to 5 ppm. For the PBS- and Ppy-coated sensors, the increase in resistance was 0.13–1.42% (Figure 3b) and 2.27–8.24% (Figure 3c), respectively, as the concentration of NH3 increased from 0.2 ppm to 5 ppm.
In contrast to the sensor responses to NH3, the sensors exhibited a decreased resistance to SO2 exposure, owing to the oxidizing property of SO2 on SWCNTs [46]. Figure 3d–f depict the sensor response of EMIM, PBS, and Ppy-coated sensors, respectively, to 3.8–76.3 ppm of SO2 gas. Note that the IDLH of SO2 was 100 ppm [42]. In the case of EMIM- and Ppy-coated sensors, sensor responses were observed at 38.2 and 76.3 ppm, respectively, whereas no responses were observed at lower concentrations of SO2 (Figure 3d,f). For the PBS-coated sensor, the change in resistance decreased from −0.11% to −1.53% as the concentration of SO2 increased from 3.8 ppm to 76.3 ppm (Figure 3e).
Because the IDLH of EtO is 500 ppm, the sensor responses were obtained in the concentration range of 33–666 ppm for the EtO sensing performance investigation. Since EtO is a reducing gas on SWCNTs [47,48], the EMIM-coated sensor exhibited an increased resistance from 0.09% to 1.0% under EtO exposure (Figure 3g). For the PBS-coated sensor, sensor responses from 0.013% to 0.26% were obtained in the concentration range of 66–666 ppm, whereas no response was observed at a concentration of 33 ppm (Figure 3h). The Ppy-coated sensor exhibited an increase in response from 0.37% to 1.40% over the full concentration range (Figure 3i). A comparison of the characteristics of chemiresistors based on nanomaterials is presented in Table 1. Our sensor array allows the detection of target analytes at concentrations under IDLH even after storage for 6 months in an ambient environment (Figure S4).

Table 1.
A comparison of the characteristics of chemiresistor based on nanomaterials.
Based on the results depicted in Figure 3, we demonstrated that the sensor arrays composed of EMIM, PBS, and Ppy-coated SWCNT networks successfully detected NH3, SO2, and EtO at a lower concentration when compared to the IDLH range, and different sensitivities were obtained depending on the coating chemicals. Thereafter, we performed a principal component analysis (PCA) based on the response patterns of the sensor array under exposure to single species of gaseous analytes. Figure 4 depicts the PCA plot, clearly demonstrating that the sensor array’s response to NH3, SO2, and EtO was distinctly separated without any overlap. Although each sensor in the sensor array showed imperfect specificity toward single target gaseous analytes, the PCA results suggested that our sensor array allows identification of the analyte molecules.

Figure 4.
PCA plot of the sensor array response patterns toward single gaseous analytes (n ≥ 15).
3.2. Adsorption Parameters of SWCNT Sensor
Figure 5 depicts the calibration curve of the response of SWCNT chemiresistor arrays to NH3, SO2, and EtO. In the case of the response to NH3, the sensor responses increased almost linearly as the concentration of NH3 increased for the low concentration range (<1 ppm), whereas the increase in the sensor response tapered off and was saturated for the high concentration range (>1 ppm) (Figure 5a). For the responses to SO2, the resistance decreased linearly with respect to the SO2 concentration range of 3.8–76.3 ppm (Figure 5b). Regarding the responses to EtO, the Ppy-coated sensor exhibited the largest increase in sensor resistance, followed by the EMIM- and PBS-coated sensors. Similarly to the sensor response to NH3 exposure, the resistance of the sensors demonstrated a linear increase for concentrations lower than 200 ppm, whereas saturated responses were obtained for concentrations greater than 200 ppm (Figure 5c).
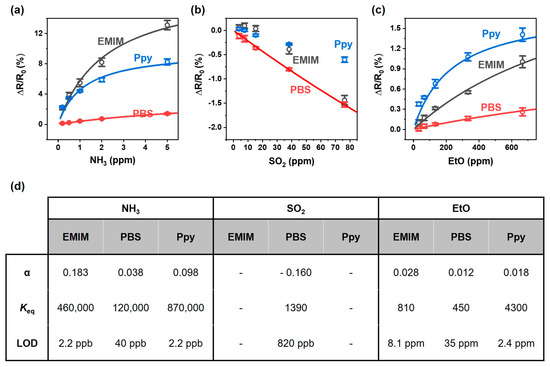
Figure 5.
Calibration curve of the response of SWCNT chemiresistor arrays to (a) NH3, (b) SO2, (c) EtO. (d) The parameters of analyte adsorption on the SWCNTs extracted from calibration curve.
Because the sensor response () originates from the charge transfer between SWCNTs and adsorbed analytes, the response can be described by the following Langmuir adsorption isotherm [57]:
where is the proportionality factor associated with maximum resistance change at high analyte concentration, is the equilibrium constant for adsorption, is the analyte concentration. These parameters can be extracted by fitting the concentration-dependent sensor responses to the isotherm, as indicated by the thick solid lines in Figure 5a–c. The extracted parameters are tabulated in Figure 5d. For sensing NH3, values of 460,000, 120,000, and 870,000 were obtained for the EMIM-, PBS-, and Ppy-coated SWCNT sensors, respectively. In the case of SO2 sensing, a value of 1390 was obtained only from the PBS-coated sensor because the responses of the EMIM- and Ppy-coated sensors could not be obtained for the low concentration range of the analyte (<15.3 ppm). In the case of EtO sensing, values of 810, 450, and 4300 were obtained from the EMIM, PBS, and Ppy- coated SWCNT sensors, respectively. The value was higher for the adsorption of NH3 on SWCNTs when compared to values for the adsorption of SO2 and EtO. In addition, the Ppy-coated sensor exhibited a significantly increased value for the adsorption of EtO when compared to the EMIM- and PBS-coated sensors. It should be noted that the responses shown in Figure 3 did not reach complete equilibrium, meaning that the and values reported in in this work are underestimated.
The limit of detection (LOD) can be estimated by extrapolating the response curves in Figure 5a–c down to three times the noise level, which we define as the standard deviation of ΔR/R0 prior to exposure to the analytes. The LOD values were obtained as follows: 2.2 ppb for NH3 from the EMIM- and Ppy-coated sensors, 820 ppb for SO2 from the PBS-coated sensor, and 2.4 ppm for EtO from the Ppy-coated sensor.
3.3. Sensor Response to Mixtures of Gas Molecules
We investigated whether the chemiresistor array response could be used to analyze a mixture of gaseous chemicals. To understand the response of the sensor to the analyte mixture, which varied depending on the composition of the mixture, the responses to a 250:250 sccm of two species were investigated. Note that the concentration ratio was 5:38.2 ppm for NH3:SO2, 5:333 ppm for NH3:EtO, and 38.2:333 ppm for SO2:EtO mixture.
The sensor array responses to the NH3/SO2 mixture were 3.66%, 0.70%, and 1.80% for the sensors coated with EMIM, PBS, and Ppy, respectively (Figure 6a). In the case of sensor array responses to the mixture of NH3/EtO, the responses were 4.26%, 1.11%, and 2.33% for the sensors coated with EMIM, PBS, and Ppy, respectively (Figure 6b). Because of the significantly larger value of NH3 adsorption on the SWCNTs, both sensor array responses seemed to be similar to the sensor responses to NH3 alone (Figure 3a–c). However, all of the sensor responses to the NH3/EtO mixture were larger than those to the NH3/SO2 mixture. Because SO2 exposure resulted in decreased resistance of the sensors (Figure 3d–f), the resistance increased with respect to the EtO exposure (Figure 3g–i); the difference in sensor response to the mixture was due to a difference in mixture content. As depicted in Figure 6c, in the case of the sensor response to the SO2/EtO mixture, the resistance of the EMIM-coated sensor showed a decreased response (−0.72%) after the gas exposure, and the PBS-coated sensor showed a slightly decreased resistance (−0.38%), while the Ppy-coated sensor showed a slightly increased resistance (0.56%). In contrast to the mixture containing NH3, which had a high (>460,000), the competitive adsorption of the analyte was expected to depend on the value of for SO2 and EtO adsorption on the SWCNTs array. For example, the response of the PBS-coated sensor showed a decreased resistance to the SO2/EtO mixture (red), owing to the larger value of SO2 (1390) when compared to that of EtO (450). On the other hand, the value of SO2 adsorption could not be obtained for the EMIM- and Ppy-coated sensors; however, we could estimate the value of SO2 adsorption. For example, we can assume that the value of SO2 adsorption on EMIM-coated SWCNTs would be larger than 810, which was the value of EtO adsorption, because the resistance of the EMIM-coated sensor decreased with exposure to the SO2/EtO mixture (Figure 6c), even when the resistance of the EMIM-coated sensor increased with exposure to pure EtO (Figure 3g). On the other hand, the value of SO2 adsorption on the Ppy-coated sensor was expected to be smaller than 4300, which was the value of the of EtO adsorption. As indicated by the blue curve in Figure 6c, the resistance increased after exposure to the SO2/EtO mixture; thus, the EtO adsorption was more dominant when compared to SO2 adsorption on the Ppy-coated SWCNTs. To discriminate the analyte mixtures, we performed the PCA analysis based on the response patterns of the sensor array under exposure to the analyte mixture. The PCA plot depicted in Figure 6d indicates that the responses of the sensor array to mixtures of NH3/SO2, NH3/EtO, and SO2/EtO were clearly differentiated into three groups without overlap.
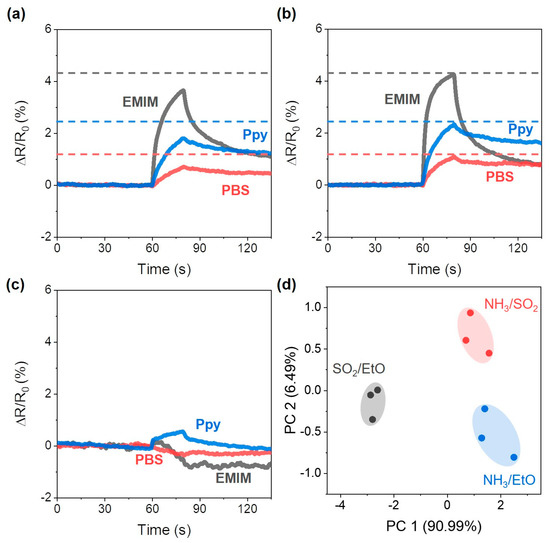
Figure 6.
Sensor array response to (a) NH3/SO2 mixture, (b) NH3/EtO mixture, (c) SO2/EtO mixture. (d) PCA plot of the sensor array response patterns when exposed to the analyte mixture.
To demonstrate the application of our sensor array system for the analysis of complex mixtures, we examined the responses of the sensor array to a three-species mixture of NH3, SO2, and EtO (Figure 7). Because the value of NH3 adsorption is significantly larger than the values of SO2 and EtO adsorption, dominant NH3-derived sensor responses were observed for the mixtures that contained high NH3 content. Figure 7a shows the response of the sensor array to mixtures of NH3, SO2, and EtO with varying contents. It should be noted that the concentration of NH3, [NH3], was fixed to 1 ppm, and the concentration of of SO2, [SO2], increased from 7.63 to 61.04 ppm, whereas the concentration of EtO, [EtO], decreased from 532.8 to 66.6 ppm. In the case of the EMIM-coated sensor (black), a large increase in resistance (2.31%) was observed at [SO2] = 7.63 ppm, and a significant decrease was observed in the increase in resistance when [SO2] increased, until it reached 30.52 ppm (1.06%). The EMIM-coated sensor exhibited a negative response (−0.14%) at [SO2] = 38.2 ppm, and the decrease in resistance increased as [SO2] increased to 61.04 ppm (−2.48%). For the PBS-coated sensor (red), which showed a small increase in resistance upon exposure to NH3 and EtO (Figure 3b,h), a slightly increased resistance was observed (0.30%) when [SO2] = 7.63 ppm. Then, the resistance of the sensor continuously decreased to −2.07% as the [SO2] increased to 61.2 ppm. In the case of the Ppy-coated sensor (blue), which had sensitive responses to NH3 and EtO exposure (Figure 3c,i), the resistance increased to 1.18% at [SO2] = 7.63 ppm. Then, with an increase in [SO2] from 7.63 to 61.04 ppm, the response decreased to 0.28%. We further investigated the responses of the sensor array when the NH3 concentration was higher than 1 ppm (Figure S5). As the PBS-coated sensor is sensitive to SO2 gas, the SO2 and EtO in the mixture seemed to be detected. However, the detection capabilities of our sensor array seemed to be limited due to the dominant sensor responses to NH3 when the concentration of NH3 exceeds 5 ppm. Nevertheless, the expected detectable concentration range for SO2 was 38.2–53.41 ppm, and for EtO, it was 333–466.2 ppm. It is worth noting that these concentration ranges for both SO2 and EtO are still lower than their IDLH concentrations. The sensor array responses were plotted as a function of the gas mixture composition (Figure 7b). As the concentration of SO2 increased, the response of the sensor array decreased linearly. From the linear plot of the sensor array responses, we obtained the relationships between sensor response and concentration of SO2 in our experimental system with high R2 values (R2 = 0.982, 0.986, and 0.941 for the EMIM, PBS, and Ppy-coated sensors, respectively).
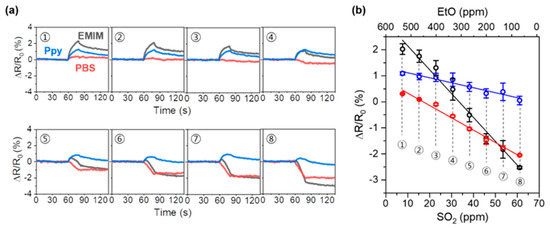
Figure 7.
Detection of three-analyte mixtures. (a) Sensor array response to the mixtures of NH3, SO2, and EtO with content variations, and (b) linear fitting of sensor array response to the concentration of gas mixture.
The relationship can also be described in terms of EtO concentration (R2 = 0.982, 0.986, and 0.941 for the EMIM, PBS, and Ppy-coated sensors, respectively).
As the sensor array response showed a linear correlation with the mixture composition, we expect that our sensor array system has potential for the component analysis of the TIC mixtures.
4. Conclusions
In summary, we developed a highly sensitive SWCNT-based chemiresistor array for TIC gas sensing. The coating of EMIM, PBS, and Ppy on the SWCNT networks successfully tuned the sensitivity to NH3, SO2, and EtO by changing the characteristics of the analyte molecules’ adsorption onto the SWCNT surfaces. Our sensor array not only provided an extremely low limit of detection for a single gaseous analyte, but also allowed the detection and identification of a mixture of gaseous TICs. In addition, the linear correlation of the sensor array responses to component variations in multi-analyte mixtures suggests high potential for the real-time monitoring of TIC gases with qualitative and quantitative component analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano13152199/s1, Figure S1 Coating material screening result compared to pristine SWCNTs sensor. Figure S2 Coating material screening result compared to pristine SWCNTs sensor. Figure S3 Baseline of EMIM-coated sensors after NH3 exposures. Figure S4. Stability of the sensor array. Sensor responses to NH3, SO2, and EtO were obtained after 6 months storage. Figure S5 Sensor array responses toward three species gas mixtures.
Author Contributions
S.L. (Seongwoo Lee) and S.P. contributed equally to this study: S.L. (Seongwoo Lee) and C.Y.L. conceived the project. S.L. (Seongwoo Lee) and S.L. (Seongyeop Lim) designed the experiments. S.P., S.L. (Seongwoo Lee) and C.Y.L. wrote the manuscript. S.L. (Seongwoo Lee), S.L. (Seongyeop Lim) and C.L. performed the experiments. S.P., S.L. (Seongwoo Lee) and S.L. (Seongyeop Lim) analyzed the data. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Institute of Civil Military Technology Cooperation Center funded by the Defense Acquisition Program Administration and Ministry of Trade, Industry, and Energy of the Korean government under grant No. 20-CM-BR-05, and by the Technology Innovation Program (20018357, Development of CMF design for user biological and environmental information reactive printed electronic chameleon sheet) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea), and from the National Research Foundation of Korea (NRF, NRF-2022R1C1C2006097).
Data Availability Statement
Data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge funding from the Institute of Civil Military Technology Cooperation Center, Ministry of Trade, Industry and Energy, and National Research Foundation of Korea. This study includes results obtained at the UNIST Central Research Facilities (UCRF).
Conflicts of Interest
The authors declare no competing financial interest.
References
- Korolkoff, N.O. Survey of toxic gas sensors and monitoring systems. Solid State Technol. 1989, 32, 49–63. [Google Scholar]
- Watt, M.M.; Watt, S.J.; Seaton, A. Episode of toxic gas exposure in sewer workers. Occup. Environ. Med. 1997, 54, 277. [Google Scholar] [CrossRef]
- Moon, S.M.; Lee, S.; Min, H.; Park, S.; Yoon, S.; Choi, J.H.; Yoon, S.M.; Jung, B.; Im, T.; Jeong, C.-S.; et al. Design and Integration of a Gas Sensor Module that Indicates the End of Service Life of a Gas Mask Canister. Adv. Mater. Technol. 2022, 7, 2100711. [Google Scholar] [CrossRef]
- Bekyarova, E.; Davis, M.; Burch, T.; Itkis, M.E.; Zhao, B.; Sunshine, S.; Haddon, R.C. Chemically Functionalized Single-Walled Carbon Nanotubes as Ammonia Sensors. J. Phys. Chem. B 2004, 108, 19717–19720. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Lee, S.; Park, S.; Lee, C.Y. Graphene chemiresistors modified with functionalized triphenylene for highly sensitive and selective detection of dimethyl methylphosphonate. RSC Adv. 2019, 9, 33976–33980. [Google Scholar] [CrossRef] [PubMed]
- Freddi, S.; Gonzalez, M.C.R.; Carro, P.; Sangaletti, L.; De Feyter, S. Chemical Defect-Driven Response on Graphene-Based Chemiresistors for Sub-ppm Ammonia Detection. Angew. Chem. Int. Ed. 2022, 61, e202200115. [Google Scholar] [CrossRef]
- Xu, F.; Li, X.; Zhang, Y.; Geng, J.; Hu, J.; Tan, W. Green recycling of spent electrode of binder-free Cu supported Ag nanowires in gas sensor field: Attempt of turning waste into treasure for the rechargeable battery. Appl. Surf. Sci. 2022, 581, 152326. [Google Scholar] [CrossRef]
- Jung, S.; Baik, K.H.; Jang, S. GaN Based Carbon Dioxide Sensor. ECS Trans. 2017, 77, 121–125. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, Y.; Bae, J.; Kim, J.; Baik, K.H.; Jang, S. (100) Plane β-Ga2O3 Flake Based Field Effect Transistor and Its Hydrogen Response. ECS J. Solid State Sci. Technol. 2021, 10, 125004. [Google Scholar] [CrossRef]
- Baik, K.H.; Jang, S. AlGaN/GaN Heterostructure Based Hydrogen Sensor with Temperature Compensation. ECS J. Solid State Sci. Technol. 2020, 9, 045010. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Lee, C.Y.; Strano, M.S. Understanding the Dynamics of Signal Transduction for Adsorption of Gases and Vapors on Carbon Nanotube Sensors. Langmuir 2005, 21, 5192–5196. [Google Scholar] [CrossRef]
- Sumanasekera, G.U.; Adu, C.K.W.; Fang, S.; Eklund, P.C. Effects of gas adsorption and collisions on electrical transport in single-walled carbon nanotubes. Phys. Rev. Lett. 2000, 85, 1096–1099. [Google Scholar] [CrossRef]
- Freddi, S.; Sangaletti, L. Trends in the Development of Electronic Noses Based on Carbon Nanotubes Chemiresistors for Breathomics. Nanomaterials 2022, 12, 2992. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hou, M.; Yang, L.; Gao, J.; Zhang, G.; Guo, R.; Guo, S. Combinatorial Material Strategy: Parallel Synthesis and High-Throughput Screening of WO3 Nanoplates Decorated with Noble Metals for VOCs Sensor. Chemosensors 2023, 11, 239. [Google Scholar] [CrossRef]
- Freddi, S.; Vergari, M.; Pagliara, S.; Sangaletti, L. A Chemiresistor Sensor Array Based on Graphene Nanostructures: From the Detection of Ammonia and Possible Interfering VOCs to Chemometric Analysis. Sensors 2023, 23, 882. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Haick, H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomedicine 2013, 8, 785–806. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Raya, I.; Kzar, H.H.; Mahmoud, Z.H.; Al Ayub Ahmed, A.; Ibatova, A.Z.; Kianfar, E. A review of gas sensors based on carbon nanomaterial. Carbon Lett. 2022, 32, 339–364. [Google Scholar] [CrossRef]
- Palacín, J.; Martínez, D.; Clotet, E.; Pallejà, T.; Burgués, J.; Fonollosa, J.; Pardo, A.; Marco, S. Application of an Array of Metal-Oxide Semiconductor Gas Sensors in an Assistant Personal Robot for Early Gas Leak Detection. Sensors 2019, 19, 1957. [Google Scholar] [CrossRef]
- Li, D.; Liu, G.; Zhang, Q.; Qu, M.; Fu, Y.Q.; Liu, Q.; Xie, J. Virtual sensor array based on MXene for selective detections of VOCs. Sens. Actuators B Chem. 2021, 331, 129414. [Google Scholar] [CrossRef]
- Song, L.; Yang, L.; Wang, Z.; Liu, D.; Luo, L.; Zhu, X.; Xi, Y.; Yang, Z.; Han, N.; Wang, F.; et al. One-step electrospun SnO2/MOx heterostructured nanomaterials for highly selective gas sensor array integration. Sens. Actuators B Chem. 2019, 283, 793–801. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Sung, S.-H.; Lee, G.; Kim, J.; Seong, J.; Shim, Y.-S.; Jun, S.C.; Jeon, S. High-performance gas sensor array for indoor air quality monitoring: The role of Au nanoparticles on WO3, SnO2, and NiO-based gas sensors. J. Mater. Chem. A 2021, 9, 1159–1167. [Google Scholar] [CrossRef]
- Guerin, H.; Le Poche, H.; Pohle, R.; Buitrago, E.; Fernández-Bolaños Badía, M.; Dijon, J.; Ionescu, A.M. Carbon nanotube gas sensor array for multiplex analyte discrimination. Sens. Actuators B Chem. 2015, 207, 833–842. [Google Scholar] [CrossRef]
- Yi, S.; Tian, S.; Zeng, D.; Xu, K.; Peng, X.; Wang, H.; Zhang, S.; Xie, C. A novel approach to fabricate metal oxide nanowire-like networks based coplanar gas sensors array for enhanced selectivity. Sens. Actuators B Chem. 2014, 204, 351–359. [Google Scholar] [CrossRef]
- Chu, J.; Li, W.; Yang, X.; Wu, Y.; Wang, D.; Yang, A.; Yuan, H.; Wang, X.; Li, Y.; Rong, M. Identification of gas mixtures via sensor array combining with neural networks. Sens. Actuators B Chem. 2021, 329, 129090. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Thomson, B.; Debnath, R.; Motayed, A.; Rao, M.V. Nanowire-Based Sensor Array for Detection of Cross-Sensitive Gases Using PCA and Machine Learning Algorithms. IEEE Sens. J. 2020, 20, 6020–6028. [Google Scholar] [CrossRef]
- Laref, R.; Losson, E.; Sava, A.; Siadat, M. On the optimization of the support vector machine regression hyperparameters setting for gas sensors array applications. Chemom. Intell. Lab. Syst. 2019, 184, 22–27. [Google Scholar] [CrossRef]
- Tang, S.; Chen, W.; Jin, L.; Zhang, H.; Li, Y.; Zhou, Q.; Zen, W. SWCNTs-based MEMS gas sensor array and its pattern recognition based on deep belief networks of gases detection in oil-immersed transformers. Sens. Actuators B Chem. 2020, 312, 127998. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Song, Z.; Ye, W.; Fan, Z. Smart gas sensor arrays powered by artificial intelligence. J. Semicond. 2019, 40, 111601. [Google Scholar] [CrossRef]
- Srivastava, A.K. Detection of volatile organic compounds (VOCs) using SnO2 gas-sensor array and artificial neural network. Sens. Actuators B Chem. 2003, 96, 24–37. [Google Scholar] [CrossRef]
- Yong, Y.; Gao, R.; Wang, X.; Yuan, X.; Hu, S.; Zhao, Z.; Li, X.; Kuang, Y. Highly sensitive and selective room-temperature gas sensors based on B6N6H6 monolayer for sensing SO2 and NH3: A first-principles study. Results Phys. 2022, 33, 105208. [Google Scholar] [CrossRef]
- Liu, J.; Cui, N.; Xu, Q.; Wang, Z.; Gu, L.; Dou, W. High-Performance PANI-Based Ammonia Gas Sensor Promoted by Surface Nanostructuralization. ECS J. Solid State Sci. Technol. 2021, 10, 027007. [Google Scholar] [CrossRef]
- Taha, R.A.; Shalabi, A.S.; Assem, M.M.; Soliman, K.A. DFT study of adsorbing SO2, NO2, and NH3 gases based on pristine and carbon-doped Al24N24 nanocages. J. Mol. Model. 2023, 29, 140. [Google Scholar] [CrossRef]
- Dinh, L.T.T.; Rogers, W.J.; Sam Mannan, M. Reactivity of ethylene oxide in contact with basic contaminants. Thermochim. Acta 2008, 480, 53–60. [Google Scholar] [CrossRef]
- Palassis, J. Preventing Worker Injuries and Deaths from Explosions in Industrial Ethylene Oxide Sterilization Facilities; National Institute for Occupational Safety and Health (NIOSH), the U.S. Environmental Protection Agency (EPA), and the Ethylene Oxide Sterilization Association (EOSA): Washington, DC, USA, 2007. [Google Scholar]
- Bretherick, L. Bretherick’s Handbook of Reactive Chemical Hazards; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Salem, D.P.; Gong, X.; Liu, A.T.; Koman, V.B.; Dong, J.; Strano, M.S. Ionic Strength-Mediated Phase Transitions of Surface-Adsorbed DNA on Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2017, 139, 16791–16802. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, Conductive Carbon Nanotube Films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef]
- Fennell, J.F., Jr.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbæk, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. 2016, 55, 1266–1281. [Google Scholar] [CrossRef]
- Toxic Industrial Chemicals. J. R. Army Med. Corps 2002, 148, 371. [CrossRef][Green Version]
- Kim, S.; Kwak, D.H.; Choi, I.; Hwang, J.; Kwon, B.; Lee, E.; Ye, J.; Lim, H.; Cho, K.; Chung, H.-J.; et al. Enhanced Gas Sensing Properties of Graphene Transistor by Reduced Doping with Hydrophobic Polymer Brush as a Surface Modification Layer. ACS Appl. Mater. Interfaces 2020, 12, 55493–55500. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Zhou, T.; Xia, H.; Zhang, T. Flexible Room-Temperature Ammonia Gas Sensors Based on PANI-MWCNTs/PDMS Film for Breathing Analysis and Food Safety. Nanomaterials 2023, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Baik, K.H.; Ren, F.; Pearton, S.J.; Jang, S. AlGaN/GaN Heterostructure Based Schottky Diode Sensors with ZnO Nanorods for Environmental Ammonia Monitoring Applications. ECS J. Solid State Sci. Technol. 2018, 7, Q3020. [Google Scholar] [CrossRef]
- Kwon, B.; Bae, H.; Lee, H.; Kim, S.; Hwang, J.; Lim, H.; Lee, J.H.; Cho, K.; Ye, J.; Lee, S.; et al. Ultrasensitive N-Channel Graphene Gas Sensors by Nondestructive Molecular Doping. ACS Nano 2022, 16, 2176–2187. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.B. Patty’s Industrial Hygiene and Toxicology. Volume 3, Part B Lewis J. Cralley, Lester V. Cralley, and James S. Bus (Dow Chemical Company). John Wiley: New York. 1995. x + 765 pp. $195.00. ISBN 0-471-53065-4. J. Am. Chem. Soc. 1996, 118, 1580. [Google Scholar] [CrossRef]
- Inyawilert, K.; Sukee, A.; Siriwalai, M.; Wisitsoraat, A.; Sukunta, J.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Effect of Er doping on flame-made SnO2 nanoparticles to ethylene oxide sensing. Sens. Actuators B Chem. 2021, 328, 129022. [Google Scholar] [CrossRef]
- Abbas, S.; Yi, W.; Yoo, S.; Khalid, A.; Bhalli, Z.; Si, J.; Hou, X. Highly Efficient Response of Ammonia Gas Sensor Based on Surfactant-Free Sorted-Semiconducting Single-Walled Carbon Nanotubes at Room Temperature. Phys. Status Solidi 2022, 219, 2100529. [Google Scholar] [CrossRef]
- Ansari, N.; Lone, M.Y.; Ali, J.; Husain, M.; Husain, S. Enhancement of gas sensor response characteristics of functionalized SWCNTs. AIP Conf. Proc. 2020, 2276, 020033. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Ding, X.; Yu, X.; Yu, X. Enhanced ammonia sensitive properties and mechanism research of PANI modified with hydroxylated single-walled nanotubes. Mater. Chem. Phys. 2019, 226, 378–386. [Google Scholar] [CrossRef]
- Santra, S.; Sinha, A.K.; Ray, S.K. A Flexible Room Temperature Ammonia Sensor Based on Large Area, Transparent Single Wall Carbon Nanotube Thin Film. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar]
- Chaudhary, V.; Channegowda, M.; Ansari, S.A.; Rajan, H.K.; Kaushik, A.; Khanna, V.; Zhao, Z.; Furukawa, H.; Khosla, A. Low-trace monitoring of airborne sulphur dioxide employing SnO2-CNT hybrids-based energy-efficient chemiresistor. J. Mater. Res. Technol. 2022, 20, 2468–2478. [Google Scholar] [CrossRef]
- Jha, R.K.; Nanda, A.; Bhat, N. Sub-ppm sulfur dioxide detection using MoS2 modified multi-wall carbon nanotubes at room temperature. Nano Sel. 2022, 3, 98–107. [Google Scholar] [CrossRef]
- Ingle, N.; Sayyad, P.; Deshmukh, M.; Bodkhe, G.; Mahadik, M.; Al-Gahouari, T.; Shirsat, S.; Shirsat, M.D. A chemiresistive gas sensor for sensitive detection of SO2 employing Ni-MOF modified –OH-SWNTs and –OH-MWNTs. Appl. Phys. A 2021, 127, 157. [Google Scholar] [CrossRef]
- Singkammo, S.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Yodsri, V.; Liewhiran, C. Catalytic roles of Sm2O3 dopants on ethylene oxide sensing mechanisms of flame-made SnO2 nanoparticles. Appl. Surf. Sci. 2018, 454, 30–45. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption Of Gases On Plane Surfaces Of Glass, Mica And Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).