Abstract
Chitosan and alginate are two of the most studied natural polymers that have attracted interest for multiple uses in their nano form. The biomedical field is one of the domains benefiting the most from the development of nanotechnology, as increasing research interest has been oriented to developing chitosan-alginate biocompatible delivery vehicles, antimicrobial agents, and vaccine adjuvants. Moreover, these nanomaterials of natural origin have also become appealing for environmental protection (e.g., water treatment, environmental-friendly fertilizers, herbicides, and pesticides) and the food industry. In this respect, the present paper aims to discuss some of the newest applications of chitosan-alginate-based nanomaterials and serve as an inception point for further research in the field.
1. Introduction
The development of nanotechnology has led to disruptive applications in many fields, including medicine, biology, electronics, energy, agriculture, and the food industry [1,2,3,4,5,6]. Various materials started being reduced to nanoscale dimensions and tailored to meet the exact requirements of specific applications. Particularly, polymers have been noted for their variety, versatility, and tunability, attracting extensive research efforts in their translation to nanomaterial formulations of use in diversified domains [7,8,9].
In biomedicine, of special interest are natural polymeric nanoparticles (NPs) that can be used as vehicles for the controlled and sustained delivery of a broad range of therapeutics without raising toxicity concerns. Thus, numerous polysaccharide and protein-based nanosystems have recently emerged as potential drug delivery platforms. Out of the plethora of possibilities, marine-sourced carbohydrates are among the most researched materials for biomedical purposes, benefiting from wide availability, abundance, simplicity of fabrication, biocompatibility, biodegradability, and ease of functionalization [10,11,12,13]. Chitosan and alginate are two of the most studied polymers of this sort, being the base materials for a multitude of drug carriers, wound dressings, and tissue engineering scaffolds [14,15,16,17,18,19]. Despite their individual uses, chitosan and alginate have been recently investigated together as a strategy to overcome the limitations of each material, produce synergistic outcomes, and expand their range of applications.
This paper aims to briefly present the properties of interest of chitosan, alginate, and chitosan-alginate nanostructures as argumentation of their recent extensive research, further focusing on their novel uses. In this respect, an overview of their biomedical applications has thoroughly gathered the recent advances in chitosan-alginate-based drug delivery systems, antimicrobial nanoparticles, and vaccine adjuvants. Moreover, other applications are considered, including reducing water pollution, developing better solutions for agriculture, and designing innovative food additives.
2. Individual and Synergic Properties
Chitosan is a Food and Drug Administration (FDA)-approved biopolymer with excellent biological properties, including antitumor, antioxidant, antimicrobial, and wound healing activities, that render it suitable for biomedical applications. Its biocompatibility, biodegradability, nontoxicity, mucoadhesivity, and hemocompatibility are ideal characteristics for designing nanocarriers with controlled delivery and sustained release of various therapeutics [10,20,21,22,23,24,25,26,27]. Chitosan has shown great promise in nanodelivery for the treatment of cancer [28,29,30], diabetes [31,32,33], eye diseases [34,35,36], infectious diseases [37,38,39,40,41], and more, being also recently employed in the delivery of vaccines [42,43,44,45].
Moreover, its structure is abundant in modifiable functional groups (i.e., hydroxyl, amino, and carboxyl groups) (Figure 1), allowing chemical modifications towards enhancing water solubility, increasing targeting, and improving stability [10,46,47]. Specifically, the insolubility of chitosan at physiological pH can be solved by additional deacetylation or chemical modification of amino groups leading to soluble derivatives and expanding the applications of this already versatile material [48]. In addition, the stability of this polysaccharide can be enhanced by controlling the environmental factors, launching a proper stabilizing compound, creating blends with other polymers (e.g., alginate, polylysine, poly(γ-glutamic acid), and short-chain amylose) or altering its structure with chemical/ionic agents [25,46].
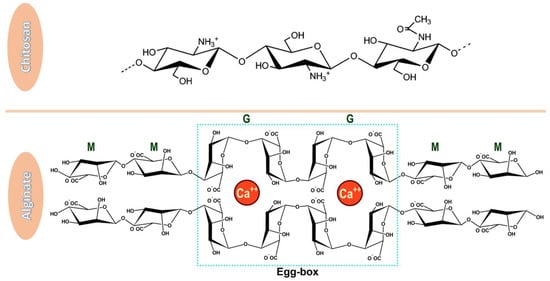
Figure 1.
Chemical structures of chitosan and alginate. Chitosan has a linear structure comprised of β–(1-4) linked 2-acetamide-2-deoxy-d-glucose and 2-amino-2-deoxy-d-glucose [49], while alginate is a copolymer of β-D-mannuronic acid (M) and α-L-guluronic acid (G) which, in the presence of calcium ions form an egg-box structure [50]. Adapted from open-access sources.
One of the most convenient natural polymers for forming blends with chitosan is alginate (Figure 1), especially because its anionic nature complements the cationic backbone of chitosan towards forming a more stable nanomaterial. Alginate is also known for desirable biological and physicochemical characteristics, being a profitable FDA-approved material for the development of drug delivery systems [51]. It is a highly available, low-cost, renewable, non-toxic, biodegradable, and biocompatible material that can be further improved through chemical or physical modifications. Alginate also exhibits mucoadhesive properties and, in contrast to chitosan, it is also water-soluble. In addition, studies have shown that alginate-based substances are pH-sensitive, being a useful tool for controlling the release of encapsulated biomolecules [26,47,52,53,54]. Alginate is a promising candidate material for the oral, ocular, nasal, parenteral, and mucosal drug delivery of various moieties, including nucleic acids, drugs, and vaccine formulations [55,56,57,58,59].
Furthermore, alginate generally shrinks at low pH and dissolves at high pH values. Contrarily, chitosan dissolves at low pH, while at high pH values becomes insoluble. Thus, creating polyelectrolyte complexes between these two materials has become of important research interest for overcoming the limitations of each material [60,61]. Adding chitosan in alginate-based nanoparticulate formulations can prolong the contact time of active ingredients with the epithelium, and the absorption can be enhanced by opening the intracellular tight junctions [25,62]. Moreover, chitosan and alginate work synergistically to protect the loaded biomolecules from oxidation, enzymatic degradation, and hydrolysis, ensuring safe and effective delivery to the desired tissues or organs [63].
3. Preparation Methods
Several techniques have been reported in the literature concerning the synthesis methods of chitosan-alginate nanomaterials. One of the most frequently utilized production methods is ionotropic gelation (IG) (Figure 2). IG is based on the electrostatic interactions between two ionic species that, under certain conditions, can produce nanoparticles [64,65]. Drug-encapsulated beads can also be formed through IG by dropping a drug-loaded polymeric solution into the aqueous solution of polyvalent cations. Similarly, biomolecules can also be entrapped in the polysaccharide beads under mild conditions, preserving their three-dimensional structure [66]. IG is frequently used in nanoparticle production due to many advantages, including high encapsulation efficiency, cost-effectiveness, and bio-safe protocols. Nonetheless, this technique may lead to particle size heterogeneity, requiring controlled stirring and a constant speed of solution dropping [64].
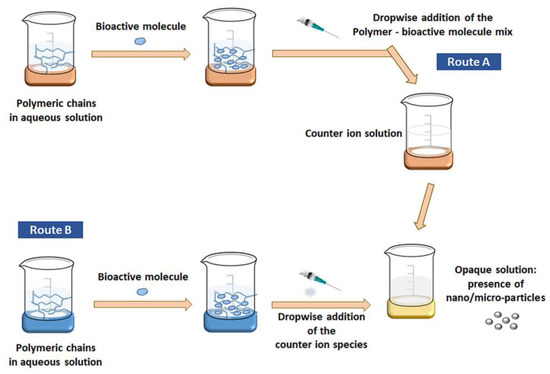
Figure 2.
Schematic representation of nanoparticles production via IG method. Reprinted with permission from [64], © 2020, Society of Chemical Industry, Published by John Wiley and Sons.
The electrostatic interactions between chitosan and alginate lead to the production of chitosan-alginate nanoparticles (CANPs) with a chitosan core and a chitosan-alginate surface. However, studies have reported that the stability of the interaction can be increased by modifying the IG procedure with the addition of a salt. In this manner, there could be employed a pre-gelation phase (between one of the polysaccharides and the salt) and a polyelectrolyte complexation phase (when the second polysaccharide is added to the reaction) [64,66].
Polyelectrolyte complexation assumes strong electrostatic interactions between oppositely charged particles, being a convenient alternative to the use of chemical crosslinking agents. More specifically, these physical interactions occur between the negatively charged carboxylic acid groups from alginate and the positively charged amino groups from chitosan [65,67]. In this way, there can be obtained alginate beads with a polyelectrolyte complex on their surface [66].
Alternatively, CANPs can be obtained through water-in-oil emulsification. This is considered a more complex production method, but it is recognized for enhancing control over particle size and particle size distribution. These features are important when uniform particles must be obtained for ensuring repeatable, controlled release behavior [68].
Other preparation techniques include, but are not limited to, sonication [69,70], electrospraying [71], electrostatic gelation [72,73,74], extrusion of polymer dispersions [75], self-assembly of polysaccharides [65], and microfluidic methods [76,77].
4. Biomedical Applications
4.1. Drug Delivery Systems
The unique properties of CANPs make them suitable for delivering a wide range of therapeutics through various administration routes (Figure 3), depending on the treated condition and targeted delivery site.
Figure 3.
Administration routes for chitosan-alginate-based drug delivery systems.
4.1.1. Oral Delivery
Oral drug administration is one of the most common, convenient, and comfortable routes for patients [25]. However, directly administering drugs via the oral route may face several biological or technical obstacles. Specifically, the biological environments through which the active ingredient passes before reaching its target can interact with the drug leading to its denaturation or preventing effective absorption in the desired tissues. On the other hand, technical issues revolve around finding proper methods for avoiding biological barriers [78]. In this context, chitosan-alginate nanoparticles represent a viable solution for protecting orally administered drugs, reducing systemic toxicity, and enhancing cellular uptake at the target site.
CANPs have been reported useful for the encapsulation of chemotherapeutics. Sorasitthiyanukarn et al. have successfully incorporated curcumin diethyl diglutarate [79] and curcumin diglutaric acid [80] in chitosan-alginate-based nanocarriers. The scientists obtained promising results as the encapsulation in polysaccharide particles improved in vitro digestibility and bioaccessibility under simulated gastrointestinal conditions, ensured controlled drug release, increased cellular uptake in cancer cells, and enhanced anticancer activity of the drugs.
Another important application of CANPs is the treatment of diabetes mellitus. Polysaccharides have found uses in oral insulin administration as an alternative to subcutaneous injection, aiming to decrease the associated discomfort and increase patient compliance [81,82,83]. Chitosan-alginate-based delivery systems can also be employed for diabetes treatment when encapsulated with other drugs. For instance, Mukhopadhyay et al. [84] have designed quercetin-succinylated chitosan-alginate core-shell-corona NPs that showed pronounced hypoglycemic effect and efficient glucose homeostasis maintenance. Alternatively, Maity et al. [69] have prepared naringenin-loaded alginate-coated chitosan core-shell NPs (Figure 4) that could effectively deliver the flavonoid and reduce the glycemia of tested animals without raising toxicity concerns.
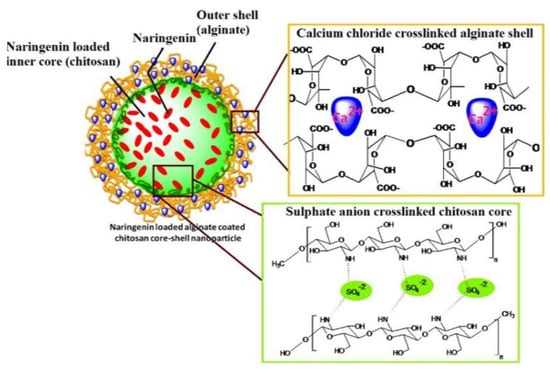
Figure 4.
Schematic representation of the delivery system developed by Maity et al. [69]. Reprinted with permission from [69]. © 2017, Elsevier Ltd.
Other emerging applications of CANPs-based oral delivery systems include improving solubility and therapeutic effects of antilipidemic drugs [85], protecting against oxidative stress [72,73], potentiating the treatment of obesity, cardiovascular diseases [86], and multiple sclerosis [87], and enhancing the bioavailability of active compounds in functional foods, nutraceuticals, and dietary supplements [88].
Several relevant examples of CANPs for oral drug delivery are described in Table 1 from the points of view of their synthesis method, physicochemical properties, and potential biomedical application.

Table 1.
Examples of oral drug delivery systems based on chitosan-alginate nanoparticles.
4.1.2. Ocular Delivery
The eye is a highly sensitive organ, being exposed to both environmental harm and internal damaging factors, such as age, genetics, and diabetes. Nonetheless, the eye is protected by several anatomic and physiological barriers (e.g., blinking, different membraneous layers, blood-retinal barrier, choroidal and conjunctival blood flow, lymphatic clearance, lacrimation) that limit the supply of drugs to affected tissues [71,89,90,91]. Thus, developing nanocarriers for ocular delivery became necessary for improving drugs bioavailability, controlling drug release, decreasing the frequency of administrations, and increasing therapeutic efficiency [71,92,93].
In this context, chitosan and alginate are ideal base materials for ocular drug delivery vehicles due to their favorable properties, including superior biocompatibility, bioadhesion, permeability-enhancing properties, ability to prolong drug’s residency time, and capacity for sustained drug release [94,95]. Consequently, researchers started investigating various chitosan and alginate-based nanosystems, recently focusing on different combinatorial approaches for treating eye diseases (Table 2).

Table 2.
Examples of ocular drug delivery systems based on chitosan-alginate nanoparticles.
4.1.3. Other Drug Delivery Systems
Despite not benefiting yet from the same extensive research efforts as oral and ocular delivery systems, chitosan-alginate nanoparticles have been reported promising for other administration routes as well.
The mucoadhesive properties of these polymers are considered advantageous for nasal drug delivery. For instance, Lee et al. [102] have chosen CANPs for the encapsulation of Apis mellifera bee venom due to these polysaccharides’ slow-releasing properties and bioadhesion. The authors reported that their newly developed system could be applied as a preventive and therapeutic agent against porcine reproductive and respiratory syndrome virus (PRRSV). Its nasal delivery leads to a significant decrease of viral burden in the sera and tissues of the tested pigs and non-specific immune-stimulating actions.
Respiratory infections can also be tackled via intratracheal administration of drugs encapsulated in CANPs. Scolari et al. [103] have approached this strategy to co-deliver rifampicin and ascorbic acid towards attaining enhanced biocide activity against S. aureus. Furthermore, the researchers reported synergic effects between the carrier and the loaded antibiotics, concluding that this delivery platform is suitable for antibiotic lung administration in antimicrobial-resistant infections.
The ability of CANPs to enhance skin penetration of drugs was exploited by Abnoos et al. [104], who have encapsulated pirfenidone (PFD) for transdermal administration. In this way, the scientists avoided the pharmacotherapeutic limitations of orally administered PFD, obtaining 94% efficiency in treating pulmonary fibrosis.
CANPs have also been evaluated for the encapsulation of drugs administered by intravenous injections. Yoncheva et al. [105] have used these carriers for loading doxorubicin as a novel delivery system for melanoma treatment, while Hashemian et al. [70] incorporated curcumin and tested the nanosystem in epilepsy treatment.
Another interesting delivery approach is employing chitosan and alginate-based carriers for intravesical drug administration. As an example, Sahatsapan et al. [106] have used maleimide-bearing chitosan (Mal-CS) and catechol-bearing alginate (Cat-Alg) to create NPs with dual mucoadhesive moieties for doxorubicin encapsulation (Figure 5). The authors reported potent inhibitory effects against mouse bladder carcinoma cell line MB49, with sustained doxorubicin release and enhanced cell uptake, concluding that the designed NPs represent promising delivery vehicles for bladder cancer treatment.
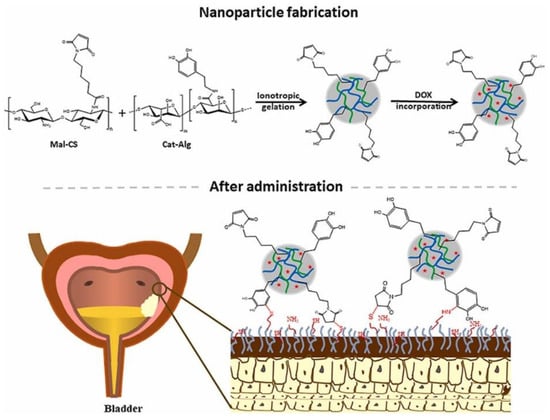
Figure 5.
Schematic representation of the synthesis mechanism and final structure of the delivery system developed by Sahatsapan et al. [106]. Reprinted with permission from [106], © 2021, Elsevier B.V.
A different strategy reported in the literature is the use of chitosan-alginate-based vehicles for the local delivery of probiotics in treating periodontal disease. For instance, Mirtič et al. [75] have designed probiotic-loaded microcapsules that deliver their cargo within periodontal pockets, promoting their prolonged survival, efficient revival, and successful colonization of the target surface.
For clearance, the above-discussed delivery systems have been summarized in Table 3.

Table 3.
Examples of chitosan-alginate nanoparticles for various administration routes.
In addition to the above-discussed studies, important results have been registered for various other chitosan-alginate-based delivery systems, which, however, have not yet been established for a particular administration route. Such innovative systems are currently in the in vitro testing stage for application in the treatment of different infectious and chronic diseases (Table 4).

Table 4.
Examples of in vitro-tested drug delivery systems based on chitosan-alginate nanoparticles.
Out of the potential applications of chitosan-alginate-based nanomaterials, anticancer formulations have benefited from increased research interest. These polysaccharides have been used due to their biocompatibility, high loading capacity, and appealing biological properties. Particularly, they are considered advantageous due to their ability to ensure controlled and targeted drug release, diminishing the systemic adverse effects associated with carried anticancer drugs. Moreover, the intrinsic antitumor, antioxidant, and pH-responsive character of these polymer nanocomposites recommend them for enhancing treatment outcomes by improving cancer cell uptake and working synergically with the loaded therapeutics [80,109,111].
As it can be noticed from the numerous mentioned studies in the field, CANPs are carriers of choice for encapsulating chemotherapeutic agents to be delivered through various administration routes towards treating different types of cancers. Figure 6 highlights the importance of chitosan-alginate-based nanoconstructs and visually synthesizes the above-presented information.
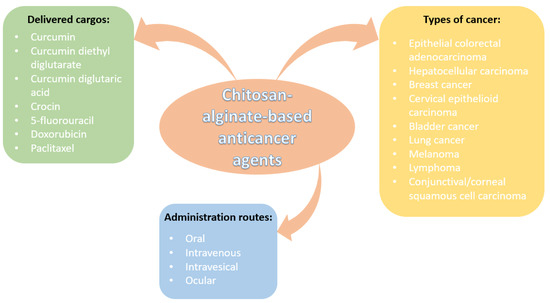
Figure 6.
Overview of chitosan-alginate-based anticancer nanoformulations.
Moreover, recent scientific interest has been drawn to creating smart delivery systems by integrating biodegradable polymers with drugs and metal-based nanoparticles with magnetic properties. In this respect, Song et al. [114] have proposed the encapsulation of curcumin into magnetic alginate-chitosan NPs to improve the bioavailability of this polyphenol and enhance its uptake efficiency and cytotoxicity to breast cancer cells. The researchers deposited alginate and chitosan in a layer-by-layer manner on iron oxide (Fe3O4) magnetic NPs, obtaining final nanoconstructs in the 120–200 nm range. The as-synthesized nanomaterials have demonstrated targeted and sustained delivery of curcumin with the aid of a magnetic field, leading to up to 6-fold higher uptake efficiency than cancer cells treated with free drugs.
Another example is offered by Chen et al. [115], who have developed a self-healing hydrogel encapsulated with magnetic gelatin microspheres (MGMs). The scientists crosslinked carboxyethyl chitosan and oxidized alginate by the Schiff-base reaction, creating a scaffold for MGMs loaded with 5-fluoracil. The as-described delivery platform showed suitable mechanical and biological properties, excellent self-healing ability under physiological conditions, and sustained drug release, being considered promising for drug delivery purposes and soft tissue engineering.
4.2. Antimicrobial Agents
Bacterial infections are a common cause of hospitalization, while nosocomial infections are frequent issues within acute/critical care facilities worldwide [116,117,118]. The majority of these infections could be managed by administering antibiotic drugs, as many antimicrobial agents have been developed over the years. Nonetheless, it was observed that antibiotic misuse and/or overuse resulted in the emergence of antimicrobial resistance, leading to poor compliance to conventional treatments and more virulent infections [119,120,121,122].
In this context, the intrinsic antimicrobial properties of alginate and chitosan have gathered renewed interest for developing innovative antibiotic-free antimicrobial agents. This strategy is considered highly advantageous as it has the potential to fight against drug-resistant pathogens while avoiding antibiotic-associated side effects [123,124,125].
For instance, Yoncheva et al. [74] have incorporated oregano oil into chitosan-alginate NPs and tested them against eight microbial strains. These small-sized (320 nm) and negatively charged (−25 mV) particles have shown promising results against Gram-positive and Gram-negative bacteria, while in vitro cytotoxicity tests on human keratinocytes and in vivo skin irritation tests proved the safety profile of these nanosystems. Hence, the authors concluded that CANPs loaded with oregano oil could serve as a natural topical delivery system for treating microbial infections of the skin and other soft tissues.
Other natural-based cargos were used by Paiva et al. [126]. The authors loaded anacardic acid and cardol into chitosan-alginate core-shell NPs. The particles presented a slow-release rate that contributed to enhancing their inhibitory capacity against dermatophytes; the obtained results are considered promising for developing innovative antimicrobial control applications.
One more example is proposed by Bilal et al. [127], who have synthesized silver nanoparticles loaded in chitosan-alginate nanostructures. Their constructs exhibited a significant reduction in the log values of tested bacterial strains (i.e., Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, Morganella morganii, and Haemophilus influenza). Moreover, cytotoxic activity has been noticed against HeLa cancer cells, suggesting the two-fold biomedical potentiality of the newly designed nanoparticles.
Similarly, Gomez Chabala et al. [128] have tackled the synergistic properties of silver NPs, chitosan, alginate, and Aloe vera. The developed nanosystem demonstrated antibacterial activity against S. aureus and P. aeruginosa, being a potential alternative for antibiotics to be used in wound dressings.
Nanomaterials with enhanced antimicrobial properties have also been obtained by encapsulating different antibiotics into chitosan-alginate-based nanoparticles. This approach was seen to improve the antibacterial activity of the drug while diminishing its associated cytotoxicity to human cells.
For example, Kumar et al. [129] have successfully loaded rifaximin into chitosan-alginate nanoparticles, obtaining nanoparticulate antimicrobial agents with outstanding antibacterial activities against E. coli, P. aeruginosa, and Bacillus haynesii. Similarly, Kadhum and Zaidan [130] have prepared CANPs loaded with doxycycline that exhibited superior effectiveness to the free drug, being suitable for the treatment of Enterobacteriacae infections at low concentrations of antibiotic.
Furthermore, Al-Getahami and Al-Qasmi [131] have obtained important results when loading camptothecin into calcium alginate-chitosan nanocomposites. The authors reported enhanced inhibitory activity against Gram-negative bacteria, which could be explained by the induction of genetic effects. The designed nanoplatform could cause point mutations in treated bacteria, disrupting DNA and protein synthesis biochemical pathways.
For clarity, Figure 7 summarizes the discussion on chitosan-alginate antimicrobial formulations in a clear visual manner.
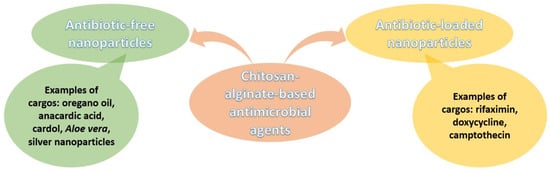
Figure 7.
Overview of chitosan-alginate-based antimicrobial nanoformulations.
4.3. Vaccine Adjuvants
Vaccination is a fundamental method of preventing virus pathogenicity, as it diminishes the burden associated with many infectious diseases. However, most vaccines face limitations in terms of degradation susceptibility, short duration of action, potential side effects, and inflammatory reactions at the administration site [47,132]. To avoid these drawbacks, vaccine formulations can be encapsulated into various nanocarriers, including chitosan-alginate-based vehicles. Moreover, the use of polymer-based nanovaccines brings a series of advantages, including strong cellular immune responses, sustained antigen release, anti-inflammatory responses, the possibility of needle-free dosage, and administration via various routes [133].
Onuigbo et al. [134] have coated oral fowl typhoid vaccine with chitosan-alginate microparticles, orally administered this formulation to birds, and compared the immune responses with the conventional subcutaneous administration of free-vaccine. Similar results were obtained for both formulations, with 100% protection and no mortality within the tested birds. Nonetheless, the polymeric coating showed protective efficacy of the oral route, preventing vaccine destruction in the gastrointestinal tract.
Giacomello et al. [135] have prepared chitosan-coated alginate microparticles for the oral delivery of immune-prophylactics to finfish. Investigations revealed that the in vivo administration by medicated food protects the microparticles while transiting the stomach, ensuring cargo release in the anterior intestines (i.e., the villum sectum—2 h after feeding, and the basal lamina of epithelial cells—4 h after feeding). The authors concluded that the as-designed immune-prophylaxis vaccines could replace antibiotics use in aquaculture.
Another oral vaccine delivery platform was designed by Biswas et al. [136]. The researchers formulated low molecular weight chitosan nanoparticles entrapped with measles antigen and coated with sodium alginate. Results showed that the engineered formulation induced a strong immune response in tested mice, protected the antigen in the gastric environment, and sustained release kinetics while maintaining low cytotoxicity. The coating strategy was also exploited by Yu et al. [137], who have used alginate-chitosan-coated LDH (layered double hydroxide NPs) nanocomposites as carriers for protein vaccine delivery.
Alternatively, alginate can also be combined with chitosan or trimethyl chitosan (TMC) to modify their immunostimulatory properties and increase their stability. For instance, Mosafer et al. [138] loaded inactivated PR8 influenza virus into chitosan or TMC NPs and coated them with sodium alginate. The authors reported that the PR8-TMC-alginate resulted in a significantly higher IgG2a/IgG1 ratio than the PR8-chitosan-alginate and PR8 free virus, which is an efficient intranasal antigen delivery system.
One more interesting study is proposed by Zhao et al. [139]. The authors developed two nanoplatforms for the nasal delivery of lipopeptide subunit vaccine (LCP-1) against group A streptococcus, namely LCP-1-loaded and alginate/TMC-coated liposomes (Lip-1) and LCP-1/alginate/TMC polyelectrolyte complexes (PEC-1) (Figure 8). It was observed that PEC-1 induced stronger humoral immune responses than Lip-1. Moreover, the liposome-free NPs were easier to prepare and were more cost-effective.
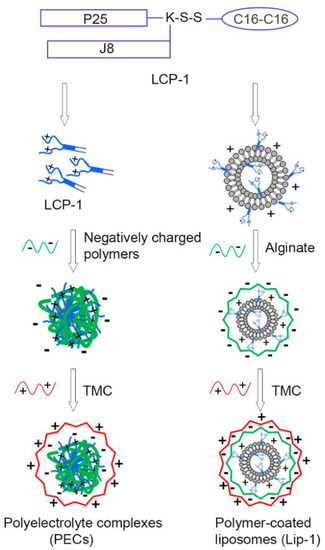
Figure 8.
Schematic representation of the vaccine delivery systems developed by Zhao et al. [139]. Reprinted from an open-access source.
5. Other Applications
In addition to their promising biomedical applications, chitosan-alginate-based nanomaterials can be involved in various other uses. Their loading capacity, encapsulation efficiency, complexation ability, environmental-friendly manufacturing, and safe human use make them ideal candidates for applications in agriculture, the food industry, and water treatment. In this respect, several such applications are discussed in the following subsections.
5.1. Water Treatment
Industrial settings often lead to water pollution by bioaccumulating toxic metals that do not undergo natural biodegradation. Several methods, including ion exchange, chemical precipitation, electrochemical treatment, filtration, and adsorption, have been employed to remove such metals from the aqueous systems. Out of the enumerated possibilities, adsorption to a solid is one of the most economical strategies. Thus, the biodegradability and abundancy of alginate and chitosan have attracted attention for their use in heavy metal ions removal from water effluents [140].
In this regard, Dubey et al. [141] have prepared chitosan-alginate NPs for the effective and economically viable removal of Hg2+ ions. The maximum adsorption capacity of CANPs was noted in the following conditions: 5 pH, 200 mg adsorbent dose, 90 min contact time, 4 g/L initial metal ion concentration, and 30 °C. Moreover, the particles are able to desorb the ions in acidic pH, allowing their regeneration and reuse for further metal removal.
Similarly, Almutairi et al. [142] have studied the removal of hexavalent chromium (Cr6+) from polluted water by the use of negatively charged (−23.2 mV) chitosan-alginate nanocomposites incorporated with iron NPs. High efficiency was reported, the optimum conditions for Cr6+ adsorption being 5.0 pH, 4 g/L adsorbent dose, 210 min contact time, and 75 ppm initial Cr6+ concentration.
In contrast, Ahmed et al. [143] have developed more complex nanocomposites comprising green nano-zerovalent copper, activated carbon, chitosan, and alginate (Figure 9). These nanosystems were able to remove Cr6+ from polluted water in a proportion of up to 97.5%, with optimum adsorption at pH 2, being promising materials for water treatment.
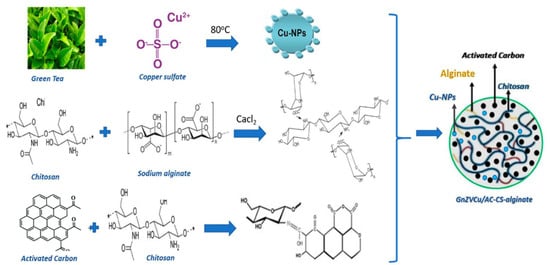
Figure 9.
Schematic representation of synthesis mechanism and final structure of chitosan-alginate-based nanocomposite developed by Ahmed et al. [143]. Reprinted from an open-access source.
5.2. Agricultural Applications
Chitosan-alginate NPs have also found uses in agriculture, as depicted in Figure 10.
Figure 10.
Examples of chitosan-alginate-based nanomaterials applications in agriculture.
To meet the increasing global food demand, scientists have developed novel nanoscale fertilizers. In this respect, Leonardi et al. [144] have designed a shell made by polyelectrolyte complexation of chitosan and sodium alginate into which they incorporated copper oxide NPs. The nanosystem’s efficacy was evaluated on Fortunella margarita Swingle seeds, demonstrating benefits for seedling growth and development of epigean and hypogean parts. Thus, the authors concluded that the tested nanoparticles could serve as a smart delivery nanofertilizer produced by an eco-sustainable method.
Another interesting use of CANPs in agriculture is represented by the encapsulation of insecticides to diminish their adverse effect on the environment. In particular, Kaur et al. [145] have employed ionic gelation and polyelectrolyte complexation techniques to load cartap hydrochloride into chitosan-alginate nanospheres of ~100–170 nm size range. With an encapsulation efficiency higher than 75% and stability maintained for 30 days at ambient temperature, the developed nanosystem is considered promising for reducing field application frequency. Moreover, its slow release to the target organism is also economically relevant and safe for the environment.
Alternatively, Kumar et al. [146] have fabricated alginate-chitosan-based nanocapsules loaded with acetamiprid. The NPs were spherical, stable, with an encapsulation efficiency of 62% and a maximum pesticide release at alkaline pH. The as-described nanoformulation has the potential to reduce pesticides’ frequency of application by controlling the agrochemical release, subsequently reducing the associated side effects.
Herbicides can also be loaded into CANPs. Maruyama et al. [147] have encapsulated imazapic and imazapyr into chitosan-alginate NPs, improving their mode of action, reducing their toxicity to non-targeted organisms, and diminishing the risk of wider contamination. Similarly, Silva et al. [148] have loaded CANPs with paraquat and obtained reduced negative impacts compared to the free herbicide, as the nanocarrier altered the release profile and interaction with the soil.
5.3. Food Additives
The loading capacity of chitosan-alginate nanoparticles can also be exploited for designing novel food additives. For instance, Liu et al. [149] have incorporated ε-polylysine into spherical CANPs within the 200–500 nm size range. The researchers noticed a three times higher bacteriostatic activity of these nanoparticles than free ε-polylysine, concluding that the enhanced antibacterial activity recommends the newly developed nanosystem as a food preservative.
Another example is offered by Yoncheva et al. [74], who have designed chitosan-alginate nanoparticles encapsulated with oregano oil. The scientists reported a considerably enhanced antimetabolic activity than for the pure oil in several microbial strains, recommending this nanoformulation as a food additive with antimicrobial activity. Moreover, the authors suggest that their newly developed system should be tested for its antibacterial effect in vacuum-packed foods, accounting for their compatibility with the oregano oil taste and other organoleptic characteristics.
One more example for food microbial growth control is proposed by Zimet et al. [150]. The researchers fabricated nisin-loaded CANPs and reported that this nanoformulation was able to sustain its antimicrobial activity against L. monocytogenes for 21 days in vitro at 4 °C, and for up to 24 days in vacuum-sealed, refrigerated beef samples (as compared to 17 days for free nisin). Thus, the authors recommend the use of the designed NPs for their antimicrobial activity and ability to extend the shelf-life of lean beef.
A different application in the food industry has been reported by Ding et al. [151], who have created an innovative fish oil delivery system. The scientists prepared several multilayer emulsions of gelatin particles and chitosan-alginate shells through layer-by-layer electrostatic deposition, concluding that emulsion creaming stability during storage and the emulsion droplet stability against the gastric phase depend on the interfacial layer number. These alginate-chitosan-based delivery systems are considered promising for food products, such as food beverages and cheese, overcoming the fishy taste and poor water solubility of fish oils.
Another interesting use is proposed by Benucci et al. [152]. The researchers employed double-layer calcium alginate-chitosan microcapsules for entrapping yeast cells with application in sparkling wine production. By use of as-such immobilized yeasts, there can be accelerated and simplified riddling and subsequent disgorging procedures, while the sensory properties (i.e., aroma, taste, and body) remain similar to those produced by free yeasts.
6. Conclusions
In summary, chitosan-alginate-based nanoparticles’ unique structures and properties make them suitable for carrying numerous and varied cargos. Considering the recent research progress highlighted in this paper, CANPs hold great promise for creating advanced formulations for the biomedical and pharmaceutical domains. Furthermore, as CANPs-based systems have been developed for various administration routes, including oral, ocular, nasal, transdermal, and intravenous delivery, these nanocarriers can be considered key factors for efficiently treating many infectious and chronic diseases without imparting toxicity to healthy tissues. Nonetheless, additional research is required before translating these emerging nanosystems to the clinic, especially because, to date, most of the studies have been performed in vitro or/and on animal models.
Moreover, interesting possibilities can be envisaged in agriculture, the food industry, or water pollution management. Given that the tested chitosan-alginate nanoparticulate systems showed promising results, it can soon be expected that some of these formulations may enter the market as more environmentally friendly solutions than currently used fertilizers, pesticides, and herbicides or as more performant food additives.
In conclusion, current results should serve as an inception point for further research of novel and improved chitosan-alginate-based nanosystems, paving the way for implementation into the practice of smart nano-enabled products.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rudramurthy, G.R.; Swamy, M.K. Potential applications of engineered nanoparticles in medicine and biology: An update. JBIC J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- Konur, O. Nanotechnology Applications in Diesel Fuels and Related Research Fields: A Review of the Research. In Biodiesel Fuels. Science, Technology, Health, and Environment; Konur, O., Ed.; CRC Press: Boca Raton, FL, USA, 2021; Volume 1, pp. 89–110. [Google Scholar]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Chapter 4-Applications of Nanotechnology in Daily Life. In Interface Science and Technology; Nasrollahzadeh, M., Sajadi, S.M., Sajjadi, M., Issaabadi, Z., Atarod, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 113–143. [Google Scholar]
- Damasco, J.A.; Ravi, S.; Perez, J.D.; Hagaman, D.E.; Melancon, M.P. Understanding Nanoparticle Toxicity to Direct a Safe-by-Design Approach in Cancer Nanomedicine. Nanomaterials 2020, 10, 2186. [Google Scholar] [CrossRef]
- Chee, C.Y. Nanomaterials and nanotechnology for composites: Synthesis, structure, properties and new application opportunities. Biointerface Res. Appl. Chem. 2020, 10, 5634–5635. [Google Scholar] [CrossRef]
- Monfared, V.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Razzaghi, M.; Berto, F. A Brief Review on Additive Manufacturing of Polymeric Composites and Nanocomposites. Micromachines 2021, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.T.; Mohammadinejad, R.; Sillanpaa, M.; et al. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2021, 19, 583–611. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.; Alkhawaldeh, M.; AlKhatib, H.S. Carrageenan-stabilized chitosan alginate nanoparticles loaded with ethionamide for the treatment of tuberculosis. J. Drug Deliv. Sci. Technol. 2017, 39, 442–449. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Wang, G. Review on marine carbohydrate-based gold nanoparticles represented by alginate and chitosan for biomedical application. Carbohydr. Polym. 2020, 244, 116311. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Wu, T.; Li, Y.; Lee, D.S. Chitosan-based composite hydrogels for biomedical applications. Macromol. Res. 2017, 25, 480–488. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. 1-Fundamentals of chitosan for biomedical applications. In Chitosan Based Biomaterials Volume 1; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2017; pp. 3–30. [Google Scholar]
- Injorhor, P.; Ruksakulpiwat, Y.; Ruksakulpiwat, C. Effect of shrimp shell chitosan loading on antimicrobial, absorption and morphological properties of natural rubber composites reinforced with silica-chitosan hybrid filler. Biointerface Res. Appl. Chem. 2020, 10, 5656–5659. [Google Scholar] [CrossRef]
- Xu, K.; Ganapathy, K.; Andl, T.; Wang, Z.; Copland, J.A.; Chakrabarti, R.; Florczyk, S.J. 3D porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials 2019, 217, 119311. [Google Scholar] [CrossRef] [PubMed]
- Idrees, H.; Zaidi, S.Z.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Pramanik, S.; Sali, V. Connecting the dots in drug delivery: A tour d’horizon of chitosan-based nanocarriers system. Int. J. Biol. Macromol. 2021, 169, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, A.M.; Meikhail, M.S.; Hegazy, E.; Badr, S.I.; Agag, D.A. Microbial activity and swelling behavior of chitosan/polyvinyl alcohol/sodium alginate seminatural terpolymer interface containing amoxicillin for wound dressing applications. Biointerface Res. Appl. Chem. 2019, 9, 4368–4373. [Google Scholar]
- Ali, S.F.A.; Gad, E.S. Investigation of an adsorbent based on novel starch/chitosan nanocomposite in extraction of indigo carmine dye from aqueous solutions. Biointerface Res. Appl. Chem. 2020, 10, 5556–5563. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Edison, T.N.J.I.; LewisOscar, F.; Kumar, P.; Shanmugam, S.; Pugazhendhi, A. Chitosan nanopolymers: An overview of drug delivery against cancer. Int. J. Biol. Macromol. 2019, 130, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Delfi, M.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Farahani, M.V.; Sharifzadeh, S.O.; Hamzehlou, S.; et al. Biomedical application of chitosan-based nanoscale delivery systems: Potential usefulness in siRNA delivery for cancer therapy. Carbohydr. Polym. 2021, 260, 117809. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.B.; Manna, P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Seyam, S.; Nordin, N.A.; Alfatama, M. Recent Progress of Chitosan and Chitosan Derivatives-Based Nanoparticles: Pharmaceutical Perspectives of Oral Insulin Delivery. Pharmaceuticals 2020, 13, 307. [Google Scholar] [CrossRef]
- Barbosa, F.C.; Silva, M.C.; Silva, H.N.; Albuquerque, D.; Gomes, A.A.; Silva, S.M.; Fook, M.V. Progress in the Development of Chitosan Based Insulin Delivery Systems: A Systematic Literature Review. Polymers 2020, 12, 2499. [Google Scholar] [CrossRef]
- Burhan, A.M.; Klahan, B.; Cummins, W.; Andrés-Guerrero, V.; Byrne, M.E.; O’Reilly, N.J.; Chauhan, A.; Fitzhenry, L.; Hughes, H. Posterior Segment Ophthalmic Drug Delivery: Role of Muco-Adhesion with a Special Focus on Chitosan. Pharmaceutics 2021, 13, 1685. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, Y.; Cong, H.; Hu, H.; Xu, F.-J. An overview of chitosan and its application in infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Şenel, S.; Yüksel, S. Chitosan-based particulate systems for drug and vaccine delivery in the treatment and prevention of neglected tropical diseases. Drug Deliv. Transl. Res. 2020, 10, 1644–1674. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M. Chitosan-Based Nanoparticles Against Viral Infections. Front. Cell. Infect. Microbiol. 2021, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.A.; Ahyat, N.; Mohamad, F.; Hamzah, S. Synthesis of silver nanoparticles: Double-green approach of using chitosan and microwave technique towards antimicrobial activity against pathogenic bacteria. Biointerface Res. Appl. Chem. 2020, 10, 5918–5922. [Google Scholar] [CrossRef]
- Gourapura, R.J.; Renu, S. Chitosan nanoparticle based mucosal vaccines delivered against infectious diseases of poultry and pigs. Front. Bioeng. Biotechnol. 2020, 8, 1316. [Google Scholar]
- Robla, S.; Prasanna, M.; Varela-Calvino, R.; Grandjean, C.; Csaba, N. A chitosan-based nanosystem as pneumococcal vaccine delivery platform. Drug Deliv. Transl. Res. 2021, 11, 581–597. [Google Scholar] [CrossRef]
- Parmaksız, S.; Şenel, S. An Overview on Chitosan-Based Adjuvant/Vaccine Delivery Systems. In Chitosan for Biomaterials IV. Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Eds.; Springer: Cham, Switzerland, 2021; Volume 288, pp. 293–379. [Google Scholar]
- Malviya, R. Exploration of neem gum-chitosan and kheri gum-chitosan polyelectrolyte complex based film for transdermal delivery of protein/peptide. Biointerface Res. Appl. Chem. 2020, 10, 5860–5868. [Google Scholar] [CrossRef]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Fu, D.; Utupova, A.; Sun, D.; Zhou, M.; Jin, Z.; Zhao, K. Applications of polymer-based nanoparticles in vaccine field. Nanotechnol. Rev. 2019, 8, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Grego, E.A.; Siddoway, A.C.; Uz, M.; Liu, L.; Christiansen, J.C.; Ross, K.A.; Kelly, S.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Polymeric Nanoparticle-Based Vaccine Adjuvants and Delivery Vehicles. In Nanoparticles for Rational Vaccine Design; Gill, H.S., Compans, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 29–76. [Google Scholar]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-R.; Jung, S.M.; Yoon, S.; Yoon, W.H.; Park, T.H.; Kim, S.; Shin, H.W.; Hwang, D.S.; Jung, S. Immobilization of planktonic algal spores by inkjet printing. Sci. Rep. 2019, 9, 12357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakil, S.N.A.; Kamal, H.; Abdullah, H.Z.; Idris, M.I. Sodium Alginate-Zinc Oxide Nanocomposite Film for Antibacterial Wound Healing Applications. Biointerface Res. Appl. Chem. 2020, 10, 6289–6296. [Google Scholar] [CrossRef]
- Fahmy, A.; Khafagy, R.M.; Elhaes, H.; Ibrahim, M.A. Molecular properties of polyvinyl alcohol/ sodium alginate composite. Biointerface Res. Appl. Chem. 2020, 10, 4734–4739. [Google Scholar]
- Jazayeri, S.D.; Lim, H.X.; Shameli, K.; Yeap, S.K.; Poh, C.L. Nano and Microparticles as Potential Oral Vaccine Carriers and Adjuvants Against Infectious Diseases. Front. Pharmacol. 2021, 12, 1399. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, Z.; Xue, C. Alginate-based delivery systems for food bioactive ingredients: An overview of recent advances and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5345–5369. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K.; Kurakula, M.; Hoda, M.N. Chapter 6-Alginate nanoparticles in drug delivery. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 129–152. [Google Scholar]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef] [PubMed]
- Alallam, B.; Altahhan, S.; Taher, M.; Mohd Nasir, M.H.; Doolaanea, A.A. Electrosprayed Alginate Nanoparticles as CRISPR Plasmid DNA Delivery Carrier: Preparation, Optimization, and Characterization. Pharmaceuticals 2020, 13, 158. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Ayaad, D.M.; Mahmoud, S.M. Antibacterial and Energy Gap Correlation of PVA/SA Biofilms Doped With Selenium Nanoparticles. Biointerface Res. Appl. Chem. 2020, 10, 6280–6288. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Dharupaneedi, S.P. 12-Production of chitosan-based hydrogels for biomedical applications. In Chitosan Based Biomaterials Volume 1; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2017; pp. 295–319. [Google Scholar]
- Gierszewska, M.; Ostrowska-Czubenko, J.; Chrzanowska, E. pH-responsive chitosan/alginate polyelectrolyte complex membranes reinforced by tripolyphosphate. Eur. Polym. J. 2018, 101, 282–290. [Google Scholar] [CrossRef]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar]
- Loquercio, A.; Castell-Perez, E.; Gomes, C.; Moreira, R.G. Preparation of Chitosan-Alginate Nanoparticles for Trans-cinnamaldehyde Entrapment. J. Food Sci. 2015, 80, N2305–N2315. [Google Scholar] [CrossRef] [PubMed]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Giri, T.K. 5-Nanoarchitectured Polysaccharide-Based Drug Carrier for Ocular Therapeutics. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 119–141. [Google Scholar]
- Patil, J.S.; Kamalapur, M.V.; Marapur, S.C.; Kadam, D.V. Ionotropic gelation and polyelectrolyte complexation: The novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A review. Dig. J. Nanomater. Biostructures 2010, 5, 241–248. [Google Scholar]
- Giri, T.K. 20-Alginate Containing Nanoarchitectonics for Improved Cancer Therapy. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 565–588. [Google Scholar]
- Abreu, F.O.M.S.; Forte, M.M.C.; Kist, T.B.L.; Honaiser, L.P. Effect of the preparation method on the drug loading of alginate-chitosan microspheres. Express Polym. Lett. 2010, 4, 456–464. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Hashemian, M.; Anissian, D.; Ghasemi-Kasman, M.; Akbari, A.; Khalili-Fomeshi, M.; Ghasemi, S.; Ahmadi, F.; Moghadamnia, A.A.; Ebrahimpour, A. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 462–471. [Google Scholar] [CrossRef]
- Kianersi, S.; Solouk, A.; Saber-Samandari, S.; Keshel, S.H.; Pasbakhsh, P. Alginate nanoparticles as ocular drug delivery carriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102889. [Google Scholar] [CrossRef]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Tzankova, V.; Aluani, D.; Kondeva-Burdina, M.; Yordanov, Y.; Odzhakov, F.; Apostolov, A.; Yoncheva, K. Hepatoprotective and antioxidant activity of quercetin loaded chitosan/alginate particles in vitro and in vivo in a model of paracetamol-induced toxicity. Biomed. Pharmacother. 2017, 92, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Benbassat, N.; Zaharieva, M.M.; Dimitrova, L.; Kroumov, A.; Spassova, I.; Kovacheva, D.; Najdenski, H.M. Improvement of the Antimicrobial Activity of Oregano Oil by Encapsulation in Chitosan—Alginate Nanoparticles. Molecules 2021, 26, 7017. [Google Scholar] [CrossRef]
- Mirtič, J.; Rijavec, T.; Zupančič, Š.; Zvonar Pobirk, A.; Lapanje, A.; Kristl, J. Development of probiotic-loaded microcapsules for local delivery: Physical properties, cell release and growth. Eur. J. Pharm. Sci. 2018, 121, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, Q.; Hui, Y.; Seth, A.; Petrovsky, N.; Zhao, C.-X. Microfluidic formation of core-shell alginate microparticles for protein encapsulation and controlled release. J. Colloid Interface Sci. 2019, 539, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Ma, Q.; Cao, J.; Wang, Y.; Yang, X.; Xu, Q.; Liang, Q.; Sun, Y. Recent advances in microfluidic-aided chitosan-based multifunctional materials for biomedical applications. Int. J. Pharm. 2021, 600, 120465. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Sorasitthiyanukarn, F.N.; Bhuket, P.R.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising carrier of novel curcumin diethyl diglutarate. Int. J. Biol. Macromol. 2019, 131, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Bhuket, P.R.N.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/alginate nanoparticles as a promising approach for oral delivery of curcumin diglutaric acid for cancer treatment. Mater. Sci. Eng. C 2018, 93, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, Y. Recent advances of polysaccharide-based nanoparticles for oral insulin delivery. Int. J. Biol. Macromol. 2018, 120, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, M.H.M.; Hamid, K.A. Chitosan-Coated Alginate Nanoparticles Enhanced Absorption Profile of Insulin Via Oral Administration. Curr. Drug Deliv. 2019, 16, 672–686. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Zhu, W.; Liang, Z.; Zeng, Q. Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin. J. Microencapsul. 2019, 36, 96–107. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Maity, S.; Mandal, S.; Chakraborti, A.S.; Prajapati, A.K.; Kundu, P.P. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym. 2018, 182, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.A.; Alotaibi, N.M. Chitosan-sodium alginate nanoparticle as a promising approach for oral delivery of rosuvastatin calcium: Formulation, optimization and in vitro characterization. J. Pharm. Res. Int. 2020, 32, 50–56. [Google Scholar] [CrossRef]
- Thai, H.; Nguyen, C.T.; Thach, L.T.; Tran, M.T.; Mai, H.D.; Nguyen, T.T.T.; Le, G.D.; Van Can, M.; Dai Tran, L.; Bach, G.L. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Sinha, S.; Garg, V.; Sonali; Singh, R.P.; Dutt, R. Chitosan-alginate core-shell-corona shaped nanoparticles of dimethyl fumarate in orodispersible film to improve bioavailability in treatment of multiple sclerosis: Preparation, characterization and biodistribution in rats. J. Drug Deliv. Sci. Technol. 2021, 64, 102645. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2022, 124, 107246. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Naik, J.B.; Pardeshi, S.R.; Patil, R.P.; Patil, P.B.; Mujumdar, A. Mucoadhesive Micro-/Nano Carriers in Ophthalmic Drug Delivery: An Overview. BioNanoScience 2020, 10, 564–582. [Google Scholar] [CrossRef]
- Cassano, R.; Di Gioia, M.L.; Trombino, S. Gel-Based Materials for Ophthalmic Drug Delivery. Gels 2021, 7, 130. [Google Scholar] [CrossRef]
- Kesavan, K.; Mohan, P.; Gautam, N.; Sheffield, V.C. Topical Ocular Delivery of Nanocarriers: A Feasible Choice for Glaucoma Management. Curr. Pharm. Des. 2020, 26, 5518–5532. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Alonso, M.J.; Sánchez, A. The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 2003, 55, 1451–1463. [Google Scholar] [CrossRef]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2015, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Nagarwal, R.C.; Kumar, R.; Pandit, J.K. Chitosan coated sodium alginate-chitosan nanoparticles loaded with 5-FU for ocular delivery: In vitro characterization and in vivo study in rabbit eye. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2012, 47, 678–685. [Google Scholar] [CrossRef]
- Toragall, V.; Jayapala, N.; Vallikannan, B. Chitosan-oleic acid-sodium alginate a hybrid nanocarrier as an efficient delivery system for enhancement of lutein stability and bioavailability. Int. J. Biol. Macromol. 2020, 150, 578–594. [Google Scholar] [CrossRef]
- Shinde, U.A.; Shete, J.N.; Nair, H.A.; Singh, K.H. Design and characterization of chitosan-alginate microspheres for ocular delivery of azelastine. Pharm. Dev. Technol. 2014, 19, 813–823. [Google Scholar] [CrossRef]
- Taghe, S.; Mirzaeei, S. Preparation and characterization of novel, mucoadhesive ofloxacin nanoparticles for ocular drug delivery. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Su, M.; Tang, S.; Wang, L.; Liang, X.; Meng, F.; Hong, Y.; Xu, Z. Synthesis of thiolated chitosan and preparation nanoparticles with sodium alginate for ocular drug delivery. Mol. Vis. 2012, 18, 1973–1982. [Google Scholar]
- Lee, J.; Kim, Y.-M.; Kim, J.-H.; Cho, C.-W.; Jeon, J.-W.; Park, J.-K.; Lee, S.-H.; Jung, B.-G.; Lee, B.-J. Nasal delivery of chitosan/alginate nanoparticle encapsulated bee (Apis mellifera) venom promotes antibody production and viral clearance during porcine reproductive and respiratory syndrome virus infection by modulating T cell related responses. Vet. Immunol. Immunopathol. 2018, 200, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Scolari, I.R.; Páez, P.L.; Musri, M.M.; Petiti, J.P.; Torres, A.; Granero, G.E. Rifampicin loaded in alginate/chitosan nanoparticles as a promising pulmonary carrier against Staphylococcus aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Abnoos, M.; Mohseni, M.; Mousavi, S.A.J.; Ashtari, K.; Ilka, R.; Mehravi, B. Chitosan-alginate nano-carrier for transdermal delivery of pirfenidone in idiopathic pulmonary fibrosis. Int. J. Biol. Macromol. 2018, 118, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Merino, M.; Shenol, A.; Daskalov, N.T.; Petkov, P.S.; Vayssilov, G.N.; Garrido, M.J. Optimization and in-vitro/in-vivo evaluation of doxorubicin-loaded chitosan-alginate nanoparticles using a melanoma mouse model. Int. J. Pharm. 2019, 556, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sahatsapan, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P.; Patrojanasophon, P. Doxorubicin-loaded chitosan-alginate nanoparticles with dual mucoadhesive functionalities for intravesical chemotherapy. J. Drug Deliv. Sci. Technol. 2021, 63, 102481. [Google Scholar] [CrossRef]
- Kaur, J.; Kour, A.; Panda, J.J.; Harjai, K.; Chhibber, S. Exploring Endolysin-Loaded Alginate-Chitosan Nanoparticles as Future Remedy for Staphylococcal Infections. AAPS PharmSciTech 2020, 21, 233. [Google Scholar] [CrossRef]
- Gomez, C.; Muangnoi, C.; Sorasitthiyanukarn, F.N.; Wongpiyabovorn, J.; Rojsitthisak, P.; Rojsitthisak, P. Synergistic effects of photo-irradiation and curcumin-chitosan/alginate nanoparticles on tumor necrosis factor-alpha-induced psoriasis-like proliferation of keratinocytes. Molecules 2019, 24, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahaiee, S.; Hashemi, M.; Shojaosadati, S.A.; Moini, S.; Razavi, S.H. Nanoparticles based on crocin loaded chitosan-alginate biopolymers: Antioxidant activities, bioavailability and anticancer properties. Int. J. Biol. Macromol. 2017, 99, 401–408. [Google Scholar] [CrossRef]
- Ahmadi, F.; Ghasemi-Kasman, M.; Ghasemi, S.; Tabari, M.G.; Pourbagher, R.; Kazemi, S.; Alinejad-Mir, A. Induction of apoptosis in hela cancer cells by an ultrasonic-mediated synthesis of curcumin-loaded chitosan–alginate–sTPP nanoparticles. Int. J. Nanomed. 2017, 12, 8545. [Google Scholar] [CrossRef] [Green Version]
- Yoncheva, K.; Tzankov, B.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Frosini, M.; Valoti, M.; Tzankova, V. Encapsulation of doxorubicin in chitosan-alginate nanoparticles improves its stability and cytotoxicity in resistant lymphoma L5178 MDR cells. J. Drug Deliv. Sci. Technol. 2020, 59, 101870. [Google Scholar] [CrossRef]
- Tao, L.; Jiang, J.; Gao, Y.; Wu, C.; Liu, Y. Biodegradable Alginate-Chitosan Hollow Nanospheres for Codelivery of Doxorubicin and Paclitaxel for the Effect of Human Lung Cancer A549 Cells. BioMed Res. Int. 2018, 2018, 4607945. [Google Scholar] [CrossRef]
- Nalini, T.; Basha, S.K.; Sadiq, A.M.M.; Kumari, V.S.; Kaviyarasu, K. Development and characterization of alginate/chitosan nanoparticulate system for hydrophobic drug encapsulation. J. Drug Deliv. Sci. Technol. 2019, 52, 65–72. [Google Scholar] [CrossRef]
- Song, W.; Su, X.; Gregory, D.A.; Li, W.; Cai, Z.; Zhao, X. Magnetic Alginate/Chitosan Nanoparticles for Targeted Delivery of Curcumin into Human Breast Cancer Cells. Nanomaterials 2018, 8, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J.; Xiong, D.; Xing, X.; Niu, X.; et al. Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery. Mater. Sci. Eng. C 2019, 101, 619–629. [Google Scholar] [CrossRef] [PubMed]
- MacVane, S.H. Antimicrobial resistance in the intensive care unit: A focus on gram-negative bacterial infections. J. Intensive Care Med. 2017, 32, 25–37. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Bereket, W.; Hemalatha, K.; Getenet, B.; Wondwossen, T.; Solomon, A.; Zeynudin, A.; Kannan, S. Update on bacterial nosocomial infections. Eur. Rev. Med. Pharm. Sci. 2012, 16, 1039–1044. [Google Scholar]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, K.; Casimeer, S.C.; Ghidan, A.Y.; Al Antary, T.M.; Singaravelu, A. Exploration of Antioxidant, Antibacterial Activities of Green Synthesized Hesperidin Loaded PLGA Nanoparticles. Biointerface Res. Appl. Chem. 2021, 11, 14520–14528. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. Funct. Chitosan 2020, 457–489. [Google Scholar] [CrossRef]
- Asadpoor, M.; Ithakisiou, G.-N.; Van Putten, J.P.M.; Pieters, R.J.; Folkerts, G.; Braber, S. Antimicrobial Activities of Alginate and Chitosan Oligosaccharides Against Staphylococcus aureus and Group B Streptococcus. Front. Microbiol. 2021, 12, 700605. [Google Scholar] [CrossRef] [PubMed]
- Paiva, J.C.; Morais, S.M.D.; Nogueira, A.C.; Cavalcante, G.S.; Silva, N.A.D.; Abreu, F.O.M.D.S. Design of chitosan-alginate core-shell nanoparticules loaded with anacardic acid and cardol for drug delivery. Polímeros 2020, 29. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Li, C.; Hu, H.; Zhang, X. Development of silver nanoparticles loaded chitosan-alginate constructs with biomedical potentialities. Int. J. Biol. Macromol. 2017, 105, 393–400. [Google Scholar] [CrossRef]
- Gómez Chabala, L.F.; Cuartas, C.E.E.; López, M.E.L. Release behavior and antibacterial activity of chitosan/alginate blends with aloe vera and silver nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Kumar, S.; Kumar, S.; Rohatgi, S.; Kundu, P.P. Synthesis of rifaximin loaded chitosan-alginate core-shell nanoparticles (Rif@CS/Alg-NPs) for antibacterial applications. Int. J. Biol. Macromol. 2021, 183, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, W.N.; Zaidan, I.A. The synergistic effects of chitosan-alginate nanoparticles loaded with doxycycline antibiotic against multidrug resistant proteus mirabilis, Escherichia coli and enterococcus faecalis. Iraqi J. Sci. 2020, 61, 3187–3199. [Google Scholar]
- Al-Gethami, W.; Al-Qasmi, N. Antimicrobial Activity of Ca-Alginate/Chitosan Nanocomposite Loaded with Camptothecin. Polymers 2021, 13, 3559. [Google Scholar] [CrossRef]
- Chan, Y.; Ng, S.W.; Singh, S.K.; Gulati, M.; Gupta, G.; Chaudhary, S.K.; Hing, G.B.; Collet, T.; MacLoughlin, R.; Löbenberg, R.; et al. Revolutionizing polymer-based nanoparticle-linked vaccines for targeting respiratory viruses: A perspective. Life Sci. 2021, 280, 119744. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials 2021, 14, 6812. [Google Scholar] [CrossRef] [PubMed]
- Onuigbo, E.; Iseghohimhen, J.; Chah, K.; Gyang, M.; Attama, A. Chitosan/alginate microparticles for the oral delivery of fowl typhoid vaccine: Innate and acquired immunity. Vaccine 2018, 36, 4973–4978. [Google Scholar] [CrossRef]
- Giacomello, E.; Sava, G.; Vita, F.; Delhom, N.; Mahl, P.; Bergamo, A. Chitosan-coated alginate micro-particles delivery of active principles through conventional pelleted food-A study in Tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2020, 165, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Chattopadhyay, M.; Sen, K.K.; Saha, M.K. Development and characterization of alginate coated low molecular weight chitosan nanoparticles as new carriers for oral vaccine delivery in mice. Carbohydr. Polym. 2015, 121, 403–410. [Google Scholar] [CrossRef]
- Yu, X.; Wen, T.; Cao, P.; Shan, L.; Li, L. Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J. Colloid Interface Sci. 2019, 556, 258–265. [Google Scholar] [CrossRef]
- Mosafer, J.; Sabbaghi, A.-H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 2019, 14, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, W.; Cruz, J.G.; Marasini, N.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Skwarczynski, M.; Toth, I. Development of Polyelectrolyte Complexes for the Delivery of Peptide-Based Subunit Vaccines against Group A Streptococcus. Nanomaterials 2020, 10, 823. [Google Scholar] [CrossRef]
- Facchi, D.P.; Cazetta, A.L.; Canesin, E.A.; Almeida, V.C.; Bonafé, E.G.; Kipper, M.J.; Martins, A.F. New magnetic chitosan/alginate/Fe3O4@ SiO2 hydrogel composites applied for removal of Pb (II) ions from aqueous systems. Chem. Eng. J. 2018, 337, 595–608. [Google Scholar] [CrossRef]
- Dubey, R.; Bajpai, J.; Bajpai, A.K. Chitosan-alginate nanoparticles (CANPs) as potential nanosorbent for removal of Hg (II) ions. Environ. Nanotechnol. Monit. Manag. 2016, 6, 32–44. [Google Scholar] [CrossRef]
- Almutairi, F.M.; El Rabey, H.A.; Alalawy, A.I.; Salama, A.A.M.; Tayel, A.A.; Mohammed, G.M.; Aljohani, M.M.; Keshk, A.A.; Abbas, N.H.; Zayed, M.M. Application of Chitosan/Alginate Nanocomposite Incorporated with Phycosynthesized Iron Nanoparticles for Efficient Remediation of Chromium. Polymers 2021, 13, 2481. [Google Scholar] [CrossRef]
- Ahmed, I.A.; Hussein, H.S.; Ragab, A.H.; AlMasoud, N.; Ghfar, A.A. Investigation the Effects of Green-Synthesized Copper Nanoparticles on the Performance of Activated Carbon-Chitosan-Alginate for the Removal of Cr(VI) from Aqueous Solution. Molecules 2021, 26, 2617. [Google Scholar] [CrossRef]
- Leonardi, M.; Caruso, G.M.; Carroccio, S.C.; Boninelli, S.; Curcuruto, G.; Zimbone, M.; Allegra, M.; Torrisi, B.; Ferlito, F.; Miritello, M. Smart nanocomposites of chitosan/alginate nanoparticles loaded with copper oxide as alternative nanofertilizers. Environ. Sci. Nano 2021, 8, 174–187. [Google Scholar] [CrossRef]
- Kaur, I.; Agnihotri, S.; Goyal, D. Fabrication of chitosan–alginate nanospheres for controlled release of cartap hydrochloride. Nanotechnology 2021, 33, 025701. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, N.; Gopal, M.; Kumar, R.; Dilbaghi, N. Development and evaluation of alginate–chitosan nanocapsules for controlled release of acetamiprid. Int. J. Biol. Macromol. 2015, 81, 631–637. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-José, N.; Abhilash, P.C.; Fraceto, L.F.; de Lima, R. Nanoparticles Based on Chitosan as Carriers for the Combined Herbicides Imazapic and Imazapyr. Sci. Rep. 2016, 6, 19768. [Google Scholar] [CrossRef]
- Silva, M.d.S.; Cocenza, D.S.; Grillo, R.; Melo, N.F.S.d.; Tonello, P.S.; Oliveira, L.C.d.; Cassimiro, D.L.; Rosa, A.H.; Fraceto, L.F. Paraquat-loaded alginate/chitosan nanoparticles: Preparation, characterization and soil sorption studies. J. Hazard. Mater. 2011, 190, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, J.; Li, F.; Shi, Y.; Li, D.; Huang, Q. Chitosan-sodium alginate nanoparticle as a delivery system for ε-polylysine: Preparation, characterization and antimicrobial activity. Food Control 2018, 91, 302–310. [Google Scholar] [CrossRef]
- Zimet, P.; Mombrú, Á.W.; Faccio, R.; Brugnini, G.; Miraballes, I.; Rufo, C.; Pardo, H.J.L.-F.S. Optimization and characterization of nisin-loaded alginate-chitosan nanoparticles with antimicrobial activity in lean beef. Technology 2018, 91, 107–116. [Google Scholar] [CrossRef]
- Ding, M.; Liu, L.; Zhang, T.; Tao, N.; Wang, X.; Zhong, J. Effect of interfacial layer number on the storage stability and in vitro digestion of fish oil-loaded multilayer emulsions consisting of gelatin particle and polysaccharides. Food Chem. 2021, 336, 127686. [Google Scholar] [CrossRef] [PubMed]
- Benucci, I.; Cerreti, M.; Maresca, D.; Mauriello, G.; Esti, M. Yeast cells in double layer calcium alginate–chitosan microcapsules for sparkling wine production. Food Chem. 2019, 300, 125174. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).