Abstract
Nano-graphene-based energetic materials, as a new type of composite energetic materials such as desensitizer and combustion catalyst, have attracted extensive attention from energetic researchers. In this paper, the preparation of nano-graphene-based energetic materials, the desensitization effect of nano-graphene-based on energetic compounds, the thermal decomposition and combustion behavior of the materials are reviewed. Meanwhile, the existing problems and future development of nano-graphene-based energetic compounds are discussed.
1. Introduction
In modern war, the increasingly harsh battlefield environment brings unprecedented challenges to the battlefield survivability of combatants and weapon platforms. It is urgent to improve the storage and use of the safety of weapons and ammunition, as well as the ability of anti-attack and anti-explosion, which makes insensitive high-energy materials become one of the research hotspots in the field of energetic materials [1,2]. The traditional high-energy propellants have high sensitivity and weak anti-stimulation ability, so they are easy to ignite accidentally when they encounter unexpected stimulation, and often become the prime factor of ammunition safety accidents.
In recent years, nano-graphene-based energetic materials, as a new type of composite energetic materials such as desensitizer [3,4,5] and combustion catalyst [6,7,8,9], have attracted extensive attention from energetic researchers. In terms of microstructure, graphene is a single or multilayer two-dimensional material composed of six-membered rings of carbon atoms, and this structure gives it extremely strong compressive resistance [10]. Due to the unique conjugated π bond structure in graphene, the electron motion in graphene is very unique, the carrier mobility of graphene is 10 times that of silicon [11]. Therefore, graphene has excellent properties such as high strength [12], electrical conductivity [13], thermal conductivity [14] and specific surface area [15], which have attracted great attention in the fields of propellants and explosives [16,17]. The special properties of graphene make it an excellent additive in significantly increasing the mechanical, thermal and electrical properties of composites materials [18,19].
In this paper, in order to provide a reference for the application research of nano-graphene-based energetic compounds, the preparation, desensitization, thermal decomposition and combustion properties of nano-graphene-based energetic compounds were reviewed.
2. Preparation of Nano-Graphene-Based Energetic Materials
The preparation of nano-graphene-based energetic compounds is the basis of the research of this compound. Researchers have carried out a lot of research on the preparation of nano-graphene-based energetic compounds.
At present, there are various graphene-based materials, and different uses have different requirements for graphene. The typical materials mainly include graphene-based ammonium nitrate compound, graphene-based metallic compound [20], graphene-based high nitrogen compound, et al.
2.1. Graphene-Based Ammonium Perchlorate Compound
As the main component of high-energy composite propellant, Ammonium perchlorate (AP) also needs to be further improved in order to make it safer. Wang [21] et al. prepared an ammonium perchlorate/graphene nanocomposite based on oxidant ammonium perchlorate and graphene aerogel by sol–gel method. The ammonium perchlorate/graphene nanocomposite is similar in structure to graphene, but there are still many gaps inside it. The specific surface area was up to 49.2 m2/g, and a large number of ammonium perchlorate particles (average particle size 69.4 nm) were attached to the graphene skeleton. Figure 1 shows the SEM image of graphene and AP/graphene nanocomposites. From (a), it can be seen that GA has a rich pore structure, and from (b), it can be seen that many AP particles are adsorbed on the graphene framework. The results of the elemental analysis show that the content of ammonium perchlorate in the material can reach 94%, indicating that ammonium perchlorate/graphene is a very promising composite material as the main energy component of the propellant.
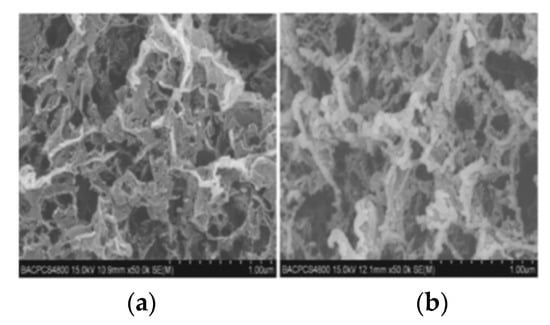
Figure 1.
SEM images of graphene (a) and AP/graphene nanocomposites (b). Reprinted from ref. [21].
Lan et al. [22] prepared a novel graphene/Fe2O3/ammonium perchlorate nanostructured energetic material by sol–gel method and supercritical carbon dioxide drying technology based on ammonium perchlorate/graphene nanocomposites. The experimental results showed that Fe2O3 and ammonium perchlorate were evenly dispersed in the nano-graphene aerogel layer. The porosity (SSA) of graphene and graphene/Fe2O3/ammonium perchlorate nanocomposites are 717 m2 g−1 and 123 m2 g−1, respectively. The total pore volumes (Vtot) of graphene and graphene/Fe2O3/ammonium perchlorate were 3.37 cm2 g−1 and 0.46 cm2 g−1, respectively.
2.2. Graphene-Based CL-20 (Hexanitrohexaazaisowurtzitane) Compound
Ye et al. [23] developed CL-20/graphene nanocomposites with the ball milling method, as shown in Figure 2 and they proposed that the formation of CL-20/graphene composites during ball milling could be divided into two processes: the peeling of graphite material and the refinement of CL-20, and the formation of sandwich composites.
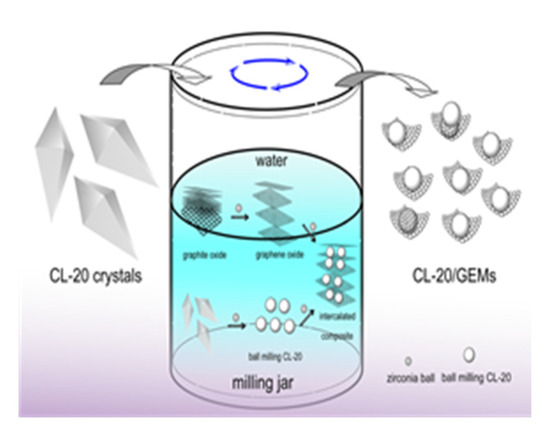
Figure 2.
Schematic diagram of the formation of CL-20/graphene composite material. Reprinted from ref. [23].
The particle size of composites prepared by this method is generally about 7–8 microns. However, the energetic material may generate local hot spots during the ball milling process. Figure 3 shows the crushing and mixing process and the mechanochemical effects of the material during the ball milling process. At the same time, the energetic material may also undergo crystalline changes and other reactions under the influence of high temperature and high pressure, so the safety performance needs to be improved.
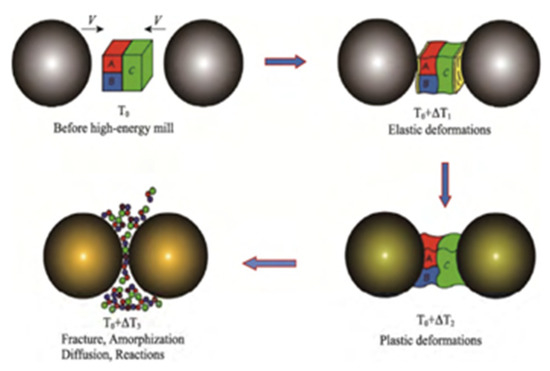
Figure 3.
Schematic diagram of mechanochemical effects in the ball milling process. Reprinted from ref. [23].
2.3. Graphene-Based HMX (Cyclotetramethylene Tetranitramine) Compound
In a typical preparation process, HMX is dissolved in dimethyl sulfoxide at 40 °C and then graphene (about 2%) is added. Graphene was completely dispersed in a dimethyl sulfoxide solution of HMX by ultrasound, and CH2Cl2 (in which graphene is insoluble) was added to the mixture. After filtration, washing and drying at 40 °C, the HMX/graphene complex was obtained. The specific operation process is shown in Figure 4 [24]. It is found that the addition of graphene does not change the crystal shape of HMX, but the surface morphology of HMX and HMX/graphene is very different. The surface of the unmodified HMX crystal is smooth and clean, while some wrinkles are observed on the surface of the HMX/graphene complex, which can significantly improve the safety of HMX.
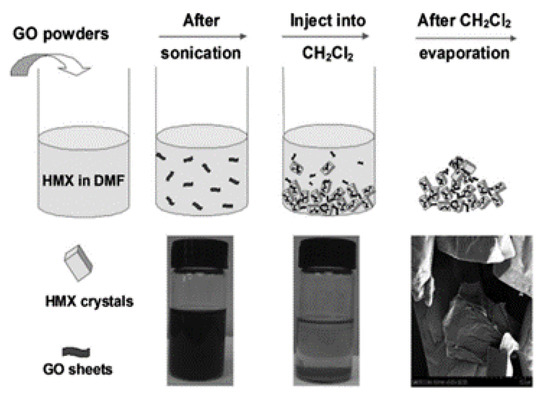
Figure 4.
The production scheme of HMX/graphene composite material. Reprinted with permission from ref. [24]. Copyright 2013 John Wiley and Sons.
2.4. Graphene-Based Metallic Compound
Thiruvengadathan et al. [25] independently developed the self-assembly method of Al and Bi2O3 particles on functionalized graphene sheets (FGS). The self-assembly process and the formation of chemical bonds are shown in Figure 5. The nanocomposite structure was formed in a colloidal suspension and eventually condensed into an ultra-dense macrostructure containing 5% of graphene.
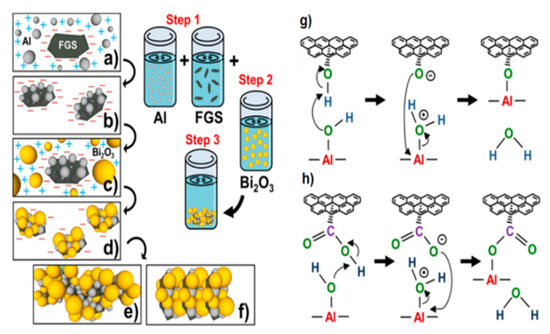
Figure 5.
Schematic of the self-assembly process: (a) electrostatic attraction of Al to graphene, (b) covalent bonding of graphene/Al existing as a stable graphene/Al dispersion, (c) electrostatic attraction of Bi2O3 to graphene/Al nanostructures, (d) noncovalent assembly of Bi2O3 on graphene/Al; instability of graphene/Al/Bi2O3 dispersion continues the self-assembly process to form ultradense macrostructures, (e) The relative ratio of GO to Al and Bi2O3 drives the iterative self-assembly process of the macrostructures into layer by layer, (f) The relative ratio of GO to Al and Bi2O3 drives the iterative self-assembly process of the macrostructures into random orientations, (g) Chemical interactions between hydroxyl groups of GO and surface hydroxyl groups of Al nanoparticles leading to C–O–Al covalent bond, and (h) carboxylic groups of GO and hydroxyl groups of Al nanoparticles leading to O=C–O–Al covalent bond. Reprinted with permission from ref. [25]. Copyright 2014 American Chemical Society.
2.5. Graphene-Based High Nitrogen Compound
Gozin et al. [26] used 5,5′-azo-1,2,3,4-tetrazole (TEZ) and 4,4′-azo-1,2,4-triazole (4,4′-azo-1,2,4-triazole, ATRZ) as ligands, a variety of three-dimensional graphene-based energetic coordination polymers were synthesized by reaction with GO-Cu (II) complexes and additional metal ions. Two typical graphene-based high nitrogen compounds were synthesized, the molecular structure was shown in Figure 6. GO was reacted with Cu(NO3)2 in an aqueous solution to obtain graphene-Cu (II) complex, and if the reaction temperature is controlled above 60 °C, the yield will be higher.
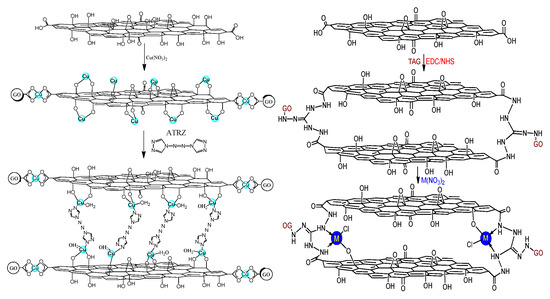
Figure 6.
Graphene-Cu (II)-4,4′-azo 1,2,4-triazole and graphene-triaminoguanidine M were prepared by triaminoguanidine. Reprinted with permission from ref. [26]. Copyright 2016 American Chemical Society.
3. Reduced Sensitivity of Nano-Graphene on Energetic Materials
According to the explosive hot spot theory, the process of explosive reaction causing an explosion is divided into three steps. Firstly, the hot spot is generated under the action of an external stimulus. Then the hot spot as the core causes the reaction to diffuse to the surrounding explosives, and gradually changes to low-speed combustion. After that, the low-speed combustion slowly grows to stable detonation and further produces an explosion. It can be seen that explosive explosion must meet two conditions, hot spot generation and propagation. As long as the two conditions of explosive explosion can be inhibited, that is, reduce the occurrence of a hot spot or cut off its transmission path. In this situation, explosives can be insensitive and prevent the further occurrence of explosions.
With the development of carbon nanomaterials, fullerenes, carbon nanotubes, nanocrystalline diamond and graphene have been used to reduce the sensitivity of explosives. Compared with fullerenes, carbon nanotubes and nano-diamonds, the application advantages of graphene oxide-based materials in the desensitization of energetic materials are more obvious. There are abundant oxygen-containing functional groups such as hydroxyl group, carboxyl group, epoxy group and carbonyl group on the surface and edge of graphene, which weakens the van der Waals force between graphene layers and makes it easy to slide between the layers, thus reducing the probability of hot spots. In addition, graphene has similar properties to polymers, gels and films, with high flexibility. Therefore, when the energetic material is stimulated by external stimuli, graphene can act as a buffer to prevent the formation and development of hot spots.
3.1. Reduced Sensitivity of Graphene-Based HMX Compound
Li Rui et al. [24] prepared HMX/graphene energy-containing complex by solvent/anti-solvent method. Figure 7 shows SEM images of HMX and HMX/graphene composites, from which it can be seen that the surface of HMX/ graphene composites is smoother. After being combined with graphene, significant folds can be seen on the surface, indicating that graphene is stacked on HMX. It was found that the impact sensitivity of HMX decreased from 100% to 10% and the friction sensitivity decreased from 100% to 32% when 2% of the mass fraction of graphene was added. Graphene can significantly reduce the mechanical sensitivity of HMX compared to carbon nanotubes. Wang Jingyu et al. [27] also studied the effect of graphene on HMX, using viton and graphene as a coating agent and insensitive agent respectively, and using the water suspension method to coat HMX. The impact sensitivity, shock wave sensitivity and thermal decomposition performance of different coated samples were studied. The impact sensitivity of HMX, HMX/Viton, and HMX/Viton/graphene samples was 19.60 cm, 47.66 cm and 48.98 cm, respectively. The shock wave sensitivity of HMX/Viton, HMX/Viton/graphene samples were 9.25 mm, 7.49 mm, respectively. According to the above results, the impact sensitivity and shock wave sensitivity of HMX were significantly reduced after graphene coating, indicating that graphene can effectively reduce the sensitivity of HMX and improve its safety.
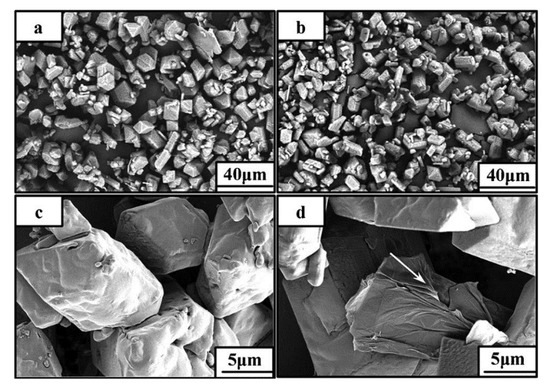
Figure 7.
SEM images of HMX (a,c) and HMX/grapheme (b,d). Reprinted with permission from ref. [24]. Copyright 2013 John Wiley and Sons.
3.2. Reduced Sensitivity of Graphene-Based RDX (Cyclotrimethylenetrinitramine) Compound
Hu Fei et al. [28] selected RDX as the main explosive and graphene as the additive to study the effect of the proportion of additives on the properties of RDX. It can be seen from the experimental results that graphene can reduce the friction sensitivity of RDX within a certain range, and also reduce the explosion heat and explosion pressure of RDX (when the content is 7%, the explosion speed decreases 22.4%). This is because graphene consumes some of its total energy to burn itself during the explosion. Zorainy et al. [29] prepared the graphene-RDX composite using the solvent/anti-solvent method. Compared with three kinds of Polymer-bonded explosives based on RDX (PBXs), the impact sensitivity of the graphene-RDX composite containing 2 wt% graphenes was lower than that of the PBXs containing 9 wt% RDX. The inductance of graphene-RDX composite is similar to that of RDX-PMMA but higher than that of RDX-Viton and RDX-Fluorel. The results showed that graphene had a significant effect on RDX. The activation energy of the graphene-RDX complex is higher than that of RDX-Fluorel and RDX-Viton. The strong interaction between graphene and RDX makes the graphene-RDX complex have high thermal stability. Figure 8 is a schematic diagram of the preparation process of GA/RDX [30].
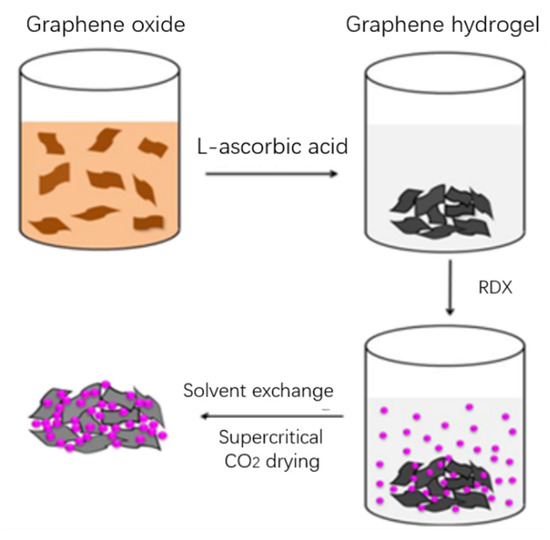
Figure 8.
Schematic diagram of the preparation of GA/RDX nanocomposites [30]. Reprinted with permission from ref. [30]. Copyright 2016 Springer Nature.
3.3. Reduced Sensitivity of Graphene-Based CL-20 Compound
Baoyun Ye et al. [23] peeled graphite into graphene in the aqueous suspension of CL-20 and prepared CL-20/graphene-based composites by ball milling method. Compared to CL-20, CL-20/graphene-based composites have lower impact sensitivity, which is due to the excellent thermal conductivity and lubrication of graphene-based materials, which can effectively reduce the folding, dislocations and hot spots inside CL-20. Li Zhimin et al. [31] studied the effect of graphene foam on Cl-20 and prepared CL-20/graphene foam host-guest composite energetic materials by in situ crystallization method. The synthesis diagram is shown in Figure 9. Due to the induction of graphene foam, Cl-20 crystals are evenly distributed in their 3D skeleton. The addition of 2% graphene foam can reduce the thermal decomposition temperature and apparent activation energy of CL-20, improve the safety of CL-20, and maintain the excellent energy performance of CL-20. The detonation pressure and detonation velocity of the composite system are 44.1 GPa and 9687 m/s, respectively. Graphene-based materials can also reduce the sensitivity of CL-20. Graphene can effectively reduce the impact sensitivity of CL-20. The characteristic drop height (H50) of CL-20 is about 17.3 cm, while the characteristic drop height of composite materials containing 5% GO has increased to more than 150 cm [32]. Jin Zhenhua [33] used the recrystallization method to compound 2% of Gr, rGO and NGO with CL-20 respectively. After analysis, it was found that rGO had the most obvious desensitization effect on CL-20, and the critical drop height H50 was increased from 25.1 cm to 32.5 cm, the friction sensitivity is reduced from 100% to 72%. Song Xiaomin [34] proposed the desensitization mechanism of graphene oxide and polyethyleneimine on CL-20. She believes that the thermal conductivity and lubricity of graphene can slow down external mechanical or thermal stimulation. In addition, the amino groups carried by polyethyleneimine can form hydrogen bonds with nitro groups in CL-20 molecules to improve the charge characteristics of CL-20.
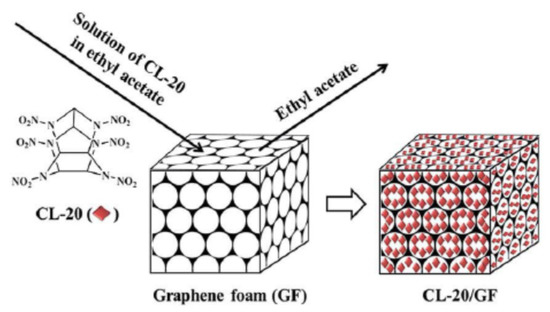
Figure 9.
Schematic diagram of the production of CL-20/GF. Reprinted from ref. [31].
4. Thermal Decomposition and Combustion Behavior of Nano-Graphene-Based Materials
Thermal decomposition and combustion behavior are important characteristics of energetic materials when the materials are used in the propellant. The theoretical calculation, thermal decomposition and combustion behavior are reviewed as follows.
4.1. Molecular Dynamics Simulation of Nano-Graphene-Based Energetic Compounds
Since ReaxFF (a commonly used molecular dynamics simulation method) was used to simulate molecular dynamics (MD) [35,36,37,38], it has been widely used in the study of the thermal decomposition process of energetic materials because it can analyze the molecular changes in the thermal decomposition reaction of energetic materials from the microscopic perspective.
The molecular dynamics method was used to study the improvement of the combustion rate of nitromethane by functionalized graphene sheets [39]. The results showed that the vacancy defect of graphene sheets, namely the functionalized part of oxygen-containing groups, could promote the thermal decomposition of nitromethane and its derivatives, whose final products were H2O, CO2 and N2. The initial decomposition process is shown in Figure 10.
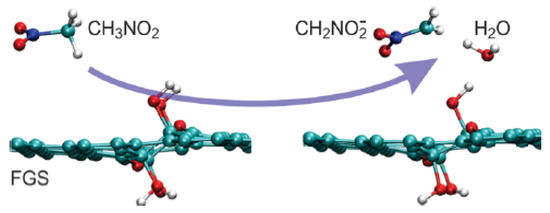
Figure 10.
Initial decomposition of nitromethane on graphene sheets. Reprinted with permission from ref. [39]. Copyright 2012 American Chemical Society.
In order to evaluate the effect of GO on the thermal decomposition of 4,4′-azo-1,2, 4-triazole. Fu Xiaolong et al. [40] studied the thermal decomposition behavior of GO, 4,4′-azo-1,2,4-triazole (ATRZ) and GO-4,4′-azo-1,2,4-triazole complexes using molecular dynamics simulation of the ReaxFF reaction field, as shown in Figure 11. The results showed that the addition of GO increased the decomposition activation energy of 4,4′-azo-1,2,4-triazole. It can be inferred from the increase in activation energy that GO has a passivation effect on the decomposition of 4,4′-azo-1,2,4-triazole. In addition, the activation energy of the reaction to produce hydroxyl radicals in GO was 16.8 kJ·mol−1, indicating that the hydroxyl groups on the GO surface were easily detached.

Figure 11.
Molecule structure of 4,4′-azo 1,2,4-triazole (a), GO (b), and GO-4,4′-azo 1,2,4-triazole (c). Reprinted from ref. [40].
Zhang Chongmin [41] calculated the initial thermal decomposition mechanism and thermal decomposition products of triaminoguanidine (TAG) molecule and GO triaminoguanidine molecule by ReaxFF simulation. The results show that the decomposition products of GO are H2 and H2O at temperatures lower than 2500 K, and the C=O bond at the edge of GO breaks at 3000 K to form CO2 and CO. During the decomposition process, small sheets of GO tend to aggregate into large carbon clusters. The main decomposition products of triaminoguanidine are NH3, N2 and H2. The decomposition process of GO triaminoguanidine is similar to go, forming carbon clusters. The decomposition products are H2O, NH3, N2 and H2. During the decomposition process of triaminoguanidine, HN2, H2N and H radicals are generated. The initial decomposition activation energy of triaminoguanidine was 94.0 kJ∙mol−1, and that of triaminoguanidine in GO triaminoguanidine was 78.6 kJ∙mol−1. In other words, GO reduced the activation energy of triaminoguanidine by 15.4 kJ∙mol−1. By analyzing the initial decomposition process of triaminoguanidine and GO triaminoguanidine, it was found that the initial decomposition of triaminoguanidine was mainly due to intramolecular hydrogen transfer. The catalytic effect of GO on the initial decomposition of triaminoguanidine was mainly reflected in the carbon atoms at the edge of GO, which promoted the decomposition of triaminoguanidine molecules. Figure 12 shows the molecular evolution of Go-triaminoguanidine.
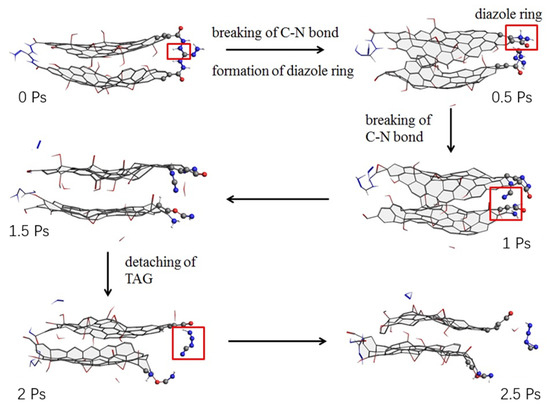
Figure 12.
Molecular evolution of GO triaminoguanidine. Reprinted from ref. [40].
4.2. Thermal Decomposition and Combustion Behavior of Nano-Graphene-Based Energetic Compounds
Graphene has good combustion properties. At the same time, graphene can be evenly dispersed in water and organic solvents, which is conducive to full recombination with energetic materials. Therefore, graphene has a significant advantage in accelerating the reaction rate and energy release of energetic materials compared to other carbon materials. First of all, graphene has good electrical and thermal conductivity, which can effectively catalyze the thermal decomposition of energetic materials. Secondly, the energetic material forms nano-sized particles in the porous structure, which promotes the mass and heat transfer process of the material and greatly improves the energy release rate of the system. Thirdly, graphene, as a carbon material, is able to react with oxidants to contribute more heat without creating residual particles.
Wang [21] studied the effect of graphene on the thermal decomposition of ammonium perchlorate. Compared with ammonium perchlorate, the heat decomposition temperature of ammonium perchlorate was 66.9 °C in advance in the case of ammonium perchlorate. In addition, the total apparent decomposition of ammonium perchlorate was 621 J/g, and the total apparent decomposition of ammonium perchlorate and graphene was 1786 J/g. graphene significantly improved the apparent decomposition of ammonium perchlorate. The results show that graphene can significantly promote the thermal decomposition of AP. In addition, there is a large number of pro-nuclear oxygen groups in the graphene layer and edge of the oxide graphene layer, which is easy to make with the ability to modify and the perfect treatment of the physical properties of graphene and the material. Zhang et al. [42] used a mixture of nitric acid and sulfuric acid to nitrate graphene oxide to prepare nitrated graphene and introduced ammonium perchlorate to study the catalytic effect of ammonium perchlorate. The thermal decomposition temperature of ammonium perchlorate was reduced by 106 °C, and the apparent decomposition of heat from 875 J/g increased to 3236 J/g. The catalytic effect of ammonium oxide on ammonium perchlorate, which can effectively reduce the heat decomposition temperature of ammonium perchlorate, and improve the apparent decomposition of ammonium perchlorate. In addition, the unique band structure can accelerate the electron transfer of the rapid rate control step of high chlorate and high-temperature decomposition process (the rapid control step of the low-temperature decomposition is transferred from ClO4− to NH4+, and the speed control step of the high-temperature decomposition is transferred from O2 to the superoxide O2−), which can improve the rate of reaction rate [43].
LAN Yuanfei et al. [30] prepared graphene aerogel by sol–gel method and supercritical CO2 technology and then immersed RDX in graphene aerogel solution to prepare graphene aerogel/RDX nano-energy-containing composites. Due to the unique nano-porous structure of graphene aerogel, the growth of RDX is limited, which makes the nanoscale RDX evenly dispersed into the graphene aerogel. Graphene aerogels can significantly accelerate the decomposition of RDX, reduce the thermal decomposition temperature and accelerate the decomposition rate, which contributes to the full reaction of RDX.
Zhang et al. [44] used the solvent/anti-solvent method to compound GO and nitrocellulose, transforming NC (nitrocellulose) from fibrous structure to three-dimensional network structure. Nitrocellulose films doped with GO were ignited by a 20 ns laser at low temperatures. It was found that GO could significantly improve the ignition and combustion characteristics of micron-sized Nitrocellulose films. The researchers also investigated micron-sized nitrocellulose films doped with different concentrations of GO (0.1%, 0.5%, 1% and 2%). Figure 13 represents the different morphologies of the Pure NC and GO-doped NC films as observed by SEM. The pure nitrocellulose film is continuous, smooth and homogeneous. When GO was added to nitrocellulose film, the surface morphology changed dramatically and the surface became very rough.
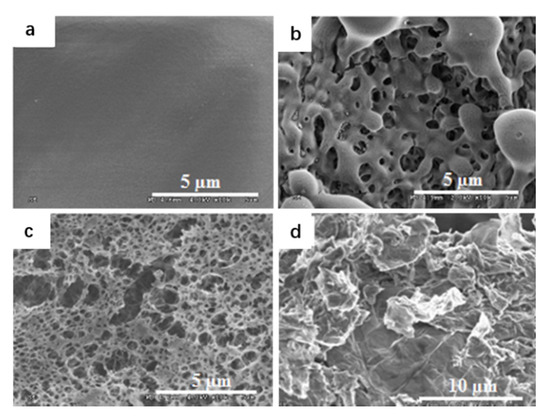
Figure 13.
SEM images of pure and GO doped NC films. SEM image of (a) pure NC film, (b) 0.5% and (c) 2% GO doped NC film; (d) as-produced GO. Reprinted with permission from ref. [44]. Copyright 2013 AIP Publishing.
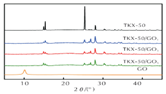
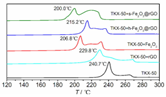
Zhao Fengqi et al. [45] prepared reduced graphene/Fe2O3 composites by hydrothermal and solvothermal methods. Reduced graphene/Fe2O3 had a significant promoting effect on the thermal decomposition of 5,5′-ditetrazole-1,1′-dioxy-dihydroxylammonium (TKX-50), reducing the low-temperature decomposition peak temperature of TKX-50 and increasing the apparent decomposition heat. This is because nano Fe2O3 particles are loaded on graphene, and the dispersibility is significantly improved, which can provide more active sites during the catalytic process and promote the decomposition of TKX-50.
Yuan Shen et al. [46] studied the reaction mechanism of NC catalyzed by nitrographene. He found that an important factor limiting the reaction rate of NC thermal decomposition was the low content of NO2 generated by NC self-decomposition, and the addition of nitrographene could provide NO2 for the reaction, thus increasing the reaction rate and the apparent heat of decomposition greatly.
Zu et al. [47] a composite catalyst containing GO and found that the catalyst could effectively catalyze the thermal decomposition reaction of AP. By analyzing its mechanism, they found that the role of GO was to reduce the decomposition temperature of AP by increasing the transfer rate of electrons in AP from ClO4− to NH4+. Figure 14 shows the catalytic mechanism of GO-coated MgFe2O4 composite.
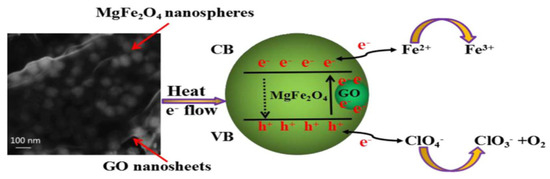
Figure 14.
Schematic diagram of thermal catalytic activity mechanism of GO coated MgFe2O4 composite. Reprinted with permission from ref. [44]. Copyright 2013 AIP Publishing.
In order to investigate the effect of graphene on the reaction mechanism of energetic materials, Liu Yingzhe et al. [48] established a graphene/nitromethane model and explored the effect of graphene on energetic materials using combinational quantum chemistry ONIOM (our Own N-layer Integrated molecular Orbital and molecular Mechanics) method. The results show that graphene can induce the reaction of nitromethane, and nitromethane has hydrogen rearrangement on the surface of graphene, which increases the interaction between them.
5. Applications of Nano-Graphene-Based Materials in Propellants and Explosives
The application of graphene-based compounds in solid propellants is one of the most concerning issues for researchers. The effect of graphene-based compounds on propellant combustion is the key factor for their application [49,50]. At present, countries including the United States and India have carried out research on the application of graphene-based compounds in propellants.
Nasir K. Memon [51] in Purdue University was prepared an energetic composite material based on graphene oxide and ammonium perchlorate. The resulting composite material is found to enhance the thermal decomposition of ammonium perchlorate over just physically mixing. Furthermore, using this material, bimodal AP/hydroxyl-terminated polybutadiene solid propellants were prepared. The combustion behavior of the propellants was investigated, and the graphene-oxide-based solid propellant resulted in a 15% increase in the burning rate at a pressure of 80 atm over the baseline material.
Abhijit Dey in Deemed University [52] synthesis graphene–titanium dioxide nanocomposite (GTNC). The GTNC was identified as an effective burn rate enhancer for an AP-based composite propellant for solid rocket propellants. The results show that the burning rate of the propellant increases by 24% for the TiO2 nanoparticle-based composition compared to the base composition, whereas a significant increase of 50% is achieved in the presence of the GTNC. Hence, the performance is improved significantly for the solid rocket propellant. The specific experimental results of the propellant are shown in Figure 15.
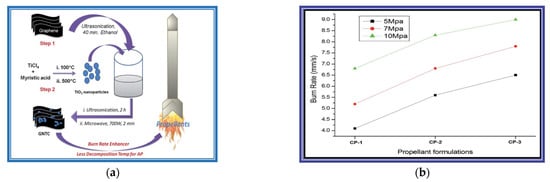
Figure 15.
(a) Schematic representation of the synthesis and application of the graphene–titanium dioxide nanocomposite (GTNC) (b) Variation of the burn rate with pressure for different propellant compositions. Reprinted from ref. [52].
Abhijit Dey in Deemed University [53] also synthesized nano size iron oxide (Fe2O3) decorated graphene (GINC) hybrid materials, and further extend this application as a burn rate enhancer in the composite propellant. Figure 16a shows the synthesis, processing and application of GINC as a missile system combustion rate regulator. The results show that the burn rate of propellant increases from micron size Fe2O3 (30% increases) to nano Fe2O3 (37% increase). In the presence of GINC, a significant increase (52%) in burn rate is achieved. In GINC, 20 effective iron content is about 50% as compared to nano and micron size Fe2O3. Hence GINC was found to be an excellent burn rate modifier for advanced AP-based propellant systems. (CP-1: Binder (15%) + Al(17%) + AP(68%) + Burn rate enhancer(Fe2O3)-Nil, CP-2: Binder (15%) + Al(17%) + AP(68%) + Burn rate enhancer(GINC)-1 wt.% over the batch, CP-3: Binder (15%) + Al(17%) + AP(68%) + Burn rate enhancer(nano Fe2O3)-1 wt.% over the batch, CP-4: Binder (15%) + Al(17%) + AP(68%) + Burn rate enhancer(Micron size Fe2O3)-1 wt.% over the batch). The burning rates of the four propellants are shown in Figure 16b.
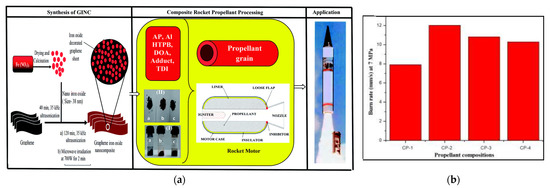
Figure 16.
(a) Synthesis, processing and application of GINC as burn rate modifier in missile system; (b) burn rate of propellant. Reprinted from ref. [53].
S. Isert [54] in Purdue University compared propellants with decorated graphene and propellants with undecorated graphene catalysts. SEM images of graphene and functionalized graphene are shown in Figure 17. Graphene decorated with iron oxide did cause a change in the burning rate. Both the encapsulated and physically mixed cases resulted in a large (>25%) increase in burning rate over the baseline propellant, and in some cases, the increase was almost 100%. Figure 18 shows that when using decorative graphene, there is a difference in the burning rate of the physically mixed propellant and the encapsulated particle propellant.

Figure 17.
SEM images of (a) blank graphene, (b) iron oxide, (c) NH2−functionalized decorated graphene, and (d) SO3H−functionalized decorated graphene. Reprinted with permission from ref. [54]. Copyright 2017 Elsevier.
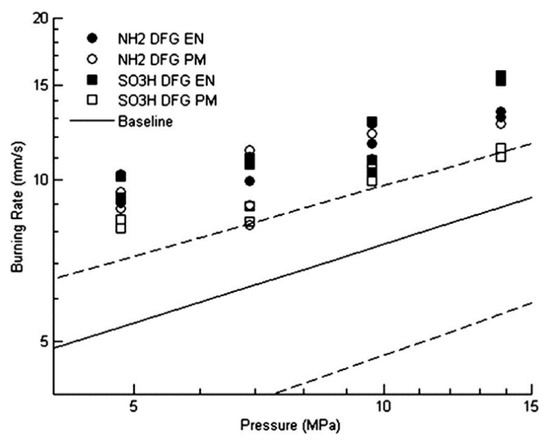
Figure 18.
Burning rates of propellants with decorated graphene. Open symbols represent physical mixes and closed symbols encapsulated catalysts. Solid line is the baseline propellant burning rate, and dashed lines are the 95% confidence interval for the baseline propellant burning rate. Reprinted with permission from ref. [54]. Copyright 2017 Elsevier.
6. Characterization of Nano-Graphene-Based Energetic Materials
In this paper, we also summarized some commonly used characterization methods of graphene-based energetic compounds in Table 1.

Table 1.
Characterization of graphene-based energetic compounds.
7. Conclusions
Nano-graphene-based energetic compounds are a kind of multifunctional component of propellants and explosives with excellent comprehensive properties. At present, researchers have carried out a large number of studies on nano-graphene-based energetic compounds, but the research on nano-graphene-based energetic compounds still has the following problems:
- (1)
- The research on the reaction mechanism and structure design of the preparation of nano-graphene-based energetic compounds is still unclear.
- (2)
- The sensitization mechanism of nano-graphene on energetic compounds is still unclear.
- (3)
- The engineering application of nano-graphene-based energetic compounds still needs long-term exploration.
In conclusion, the molecular structure of nano-graphene-based energetic compounds is complex and elucidating the relationship between their structure and thermal stability will help to further understand the main effects and mechanisms of graphene-based compounds in propellants. Therefore, the research on the hot point inhibition principle, thermal decomposition characteristics and thermal decomposition mechanism of nano-graphene-based energetic compounds are of great practical significance in guiding the design, preparation and application evaluation of new graphene functional materials.
Author Contributions
Conceptualization, X.F. (Xiaolong Fu) and X.F. (Xuezhong Fan); writing—original draft preparation, X.F. (Xiaolong Fu); investigation, J.L. and L.J. and X.Z.; writing—review and editing, Y.Z.; funding acquisition, X.F. (Xiaolong Fu). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China with grant number (21975150).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pamela, J.; Kaste, B. Novel energetic materials for the future force: The army pursues the next generation of propellants and explosives. Amptiac Newslett. 2004, 8, 85–89. [Google Scholar]
- Mo, H.J.; Zhao, F.Q. The concept and practice of energetic nanomaterials. Chin. J. Explos. Propellants 2005, 28, 85–89. [Google Scholar]
- Li, Y. Studies on Lead Styphnate Modified by the New Nano-carbon Materials in Thermal Analysis. Master’s Thesis, Beijing Institute of Technology, Beijing, China, 2016. [Google Scholar]
- Li, Y.; Zhao, W.Y.; Mi, Z.H.; Yang, L.; Zhou, Z.N.; Zhang, T.L. Graphene-modified explosive lead styphnate composites. J. Therm. Anal. Calorim. 2016, 124, 683–691. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.J. Chemically modified grapheme: Flame retardant or fuel for combustion? J. Mater. Chem. 2011, 21, 3277–3279. [Google Scholar] [CrossRef]
- Li, N.; Geng, Z.; Cao, M.; Ren, L.; Zhao, X.; Liu, B.; Tian, Y.; Hu, C. Well-dispersed ultrafine Mn3O4, nanoparticles on graphene as a promising catalyst for the thermal decomposition of ammonium perchlorate. Carbon 2013, 54, 124–132. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, J.; Wang, Y.; Zhao, F.; Shen, P.; Gao, H.; Li, F.; Jiang, F.; Li, P. Hydrothermal preparation of Fe2O3/graphene nanocomposite and its enhanced catalytic activity on the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2014, 303, 354–359. [Google Scholar] [CrossRef]
- Lan, Y.F.; Li, X.Y.; Li, G.P.; Luo, Y.J. Sol-gel method to prepare graphene/Fe2O3, aerogel and its catalytic application for the thermal decomposition of ammonium perchlorate. J. Nanopart. Res. 2015, 17, 1–9. [Google Scholar] [CrossRef]
- Fertassi, M.A.; Alali, K.T.; Liu, Q.; Li, R.Z.; Liu, P.A.; Liu, J.Y.; Liu, L.H.; Wang, J. Catalytic effect of CuO nanoplates, a graphene(G)/CuO nanocomposite and an Al/G/CuO composite on the thermal decomposition of ammonium perchlorate. Rsc Adv. 2016, 6, 74155–74161. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Politowicz, A.L.; Chen, E.; Huang, H.X.; Turng, L. Highly compressible ultra-light anisotropic cellulose/graphene aerogel fabricated by bidirectional freeze drying for selective oil absorption. Carbon 2018, 132, 199–209. [Google Scholar] [CrossRef]
- Wang, D.Q.; Liu, X.F.; He, L.; Yin, Y.D.; Wu, D.; Shi, J. Manipulating graphene mobility and charge neutral point with ligand-bound nanoparticles as charge reservoir. Nano Lett. 2010, 10, 4989–4993. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended grapheme. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.J.; Zhu, Y.W.; An, J.H.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Shen, J.P.; Liu, Z.T.; Xu, B.; Chen, F.Y.; Zhu, Y.; Fu, Y.; Kline, D.J.; Liao, X.; Wang, Z.S. Tuning the thermal, mechanical, and combustion properties of NC-TEGDN-RDX propellants via incorporation of graphene nanoplates. J. Energ. Mater. 2020, 38, 326–335. [Google Scholar] [CrossRef]
- Wu, S.X.; Zhou, C.Y.; Hu, Q.W.; Chen, L.; Zhang, T. Study on combustion characteristics of graphene-coated al particles in HTPB propellant. In Proceedings of the International Annual Conference of the Fraunhofer ICT, Karlsruhe, Germany, 25–28 June 2019; pp. 1–5. [Google Scholar]
- Sharma, S.; Rawal, J.; Dhakate, S.R.; Singh, B.P. Synergistic bridging effects of graphene oxide and carbon nanotube on mechanical properties of aramid fiber reinforced polycarbonate composite tape. Compos. Sci. Technol. 2020, 199, 108370. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Z.Q.; Luo, Q.P.; Duan, X.H.; Pei, C.H. Preparation and thermal decomposition properties of nitrated graphene oxide (NGO)/RDX nano-energetic composites. J. Therm. Anal. Calorim. 2020, 139, 1671–1679. [Google Scholar] [CrossRef]
- Pattananuwat, P.; Khampuanbut, A.; Haromae, H. Novel electrode composites of mixed bismuth-iron oxide / graphene utilizing for photo assisted supercapacitors. Electrochim. Acta 2021, 370, 137741. [Google Scholar] [CrossRef]
- Kamat, P.V. Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J. Phys. Chem. Lett. 2009, 1, 520–527. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, J.Q.; Luo, Y.J. Preparation and thermal decomposition behaviour of Ammonium Perchlorate/Graphene aerogel nanocomposites. Chin. J. Explos. Propellants 2012, 35, 76–80. [Google Scholar]
- Lan, Y.F.; Jin, M.M.; Luo, Y.J. Preparation and characterization of Graphene aerogel/Fe2O3/ammonium perchlorate nanostructured energetic composite. J. Sol.-Gel. Sci. Technol. 2015, 74, 161–167. [Google Scholar] [CrossRef]
- Ye, B.; An, C.; Zhang, Y.; Song, C.; Geng, X.; Wang, J. One-Step Ball Milling Preparation of Nanoscale CL-20/Graphene Oxide for Significantly Reduced Particle Size and Sensitivity. Nanoscale Res. Lett. 2018, 13, 42. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Shen, J.P.; Hua, C.; Yang, G.C. Preparation and characterization of insensitive HMX/Graphene Oxide composites. Propellants Explos. Pyrotech. 2013, 38, 798–804. [Google Scholar] [CrossRef]
- Thiruvengadathan, R.; Chung, S.W.; Basuray, S.; Balasubramanian, B.; Staley, C.S.; Gangopadhyay, K.; Gangopadhyay, S. A versatile self-assembly approach toward high performance nanoenergeticcomposite using functionalized Graphene. Langmuir 2014, 30, 6556–6564. [Google Scholar] [CrossRef]
- Cohen, A.; Yang, Y.Z.; Yan, Q.L.; Shlomovich, A.; Petrutik, N.; Burstein, L.; Pang, S.P.; Gozin, M. Highly thermostable and insensitive energetic hybrid coordination polymers based on Graphene Oxide−Cu(II) complex. Chem. Mater. 2016, 28, 6118–6126. [Google Scholar] [CrossRef]
- Wang, J.Y.; Ye, B.Y.; An, C.W.; Wu, B.D.; Li, H.Q.; Wei, Y.J. Preparation and properties of surface-coated HMX with viton and graphene oxide. J. Energ. Mater. 2016, 34, 235–245. [Google Scholar] [CrossRef]
- Hu, F. Study on the Influence of Addition on RDX Sensitivity and Performance; North University of China: Tai Yuan, China, 2014. [Google Scholar]
- Zorainy, M.Y.; Aly, W.F.; Elbeih, A.; Gobara, M. Preparation and Sensitivity Measurements of Graphene Oxide-RDX Composite. In Proceedings of the MATEC Web of Conferences, Hanyang, Korea, 22–25 August 2017; Volume 136, p. 01009. [Google Scholar]
- Lan, Y.F.; Wang, X.B.; Luo, Y.J. Preparation and characterization of GA/RDX nanostructured energetic composites. Bull. Mater. Sci. 2016, 39, 1701–1707. [Google Scholar] [CrossRef][Green Version]
- Li, Z.M.; Wang, Y.; Zhang, Y.Q.; Liu, L.; Zhang, S.J. CL-20 hosted in graphene foam as a high energy material with low sensitivity. RSC Adv. 2015, 5, 98925–98928. [Google Scholar] [CrossRef]
- Ye, B.Y. Study on the Construction and Basic Application of Graphene Materials Modified Energetic Composites. Ph.D. Thesis, North University of China, Taiyuan, China, 2018. [Google Scholar]
- Jin, Z.H. Comparative Research on Properties of the HNIW Modified by Nano-Carbon Materials. Master’s Thesis, Beijing Institute of Technology, Beijing, China, 2016. [Google Scholar]
- Song, X. Study on the Desensitization Effect and Mechanism of GO and g-C3N4 on CL-20 and HMX. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2021. [Google Scholar]
- Kuklja, M.M.; Kunz, A.B. Ab initio simulation of defects in energetic materials: Hydrostatic compression of cyclotrimethylene trinitramine. J. Appl. Phys. 1999, 86, 4428–4434. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Zybin, S.V.; van Duin, A.C.T.; Dasgupta, S.; Goddard, W.A.; Kober, E.M. Carbon cluster formation during thermal decomposition of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine and 1,3,5-triamino-2,4,6-trinitrobenzene high explosives from ReaxFF reactive molecular dynamics simulations. J. Phys. Chem. A 2009, 113, 10619–10640. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Strachan, A.; Kober, E.M.; van Duin, A.C.T.; Oxgaard, J.; Goddard, W.A. Thermal decomposition of RDX from reactive molecular dynamics. J. Chem. Phys. 2005, 122, 054502. [Google Scholar] [CrossRef]
- Liu, L.M.; Car, R.; Selloni, A.; Dabbs, D.M.; Aksay, I.A.; Yetter, R.A. Enhanced thermal decomposition of Nitromethane on functionalized Graphene sheets: Ab initio molecular dynamics simulations. J. Am. Chem. Soc. 2012, 134, 19011–19016. [Google Scholar] [CrossRef]
- Zhang, C.M.; Fu, X.L.; Yan, Q.L.; Li, J.Z.; Fan, X.Z.; Zhang, G.F. Study on the thermal decomposition mechanism of graphene oxide functionalized with triaminoguanidine (GO-TAG) by molecular reactive dynamics and experiments. RSC Adv. 2019, 9, 33268–33281. [Google Scholar] [CrossRef]
- Zhang, C.M.; Fu, X.L.; Li, J.Z.; Fan, X.Z.; Zhang, G.F. Desensitizing Effect of Graphene Oxide on thermolysismechanisms of 4,4′-Azo-1,2,4-triazole studied by reactive molecular dynamics simulations. J. Phys. Chem. A 2019, 123, 1285–1294. [Google Scholar] [CrossRef]
- Zhang, W.W.; Luo, Q.P.; Duan, X.H.; Zhou, Y.; Pei, C.H. Nitrated graphene oxide and its catalytic activity in thermal decomposition of ammonium perchlorate. Mater. Res. Bull. 2014, 50, 73–78. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, J.Q.; Luo, Y.J.; Huang, M.H. A novel ammonium perchlorate/graphene aerogel nanostructured energetic composite: Preparation and thermal decomposition. Sci. Adv. Mater. 2014, 6, 530–537. [Google Scholar] [CrossRef]
- Zhang, X.; Hikal, W.M.; Zhang, Y.; Bhattacharia, S.K.; Li, L.; Panditrao, S.; Wang, S.R.; Weeks, B.L. Direct laser initiation and improved thermal stability of nitrocellulose/graphene oxide nanocomposites. Appl. Phys. Lett. 2013, 102, 141905/1–141905/4. [Google Scholar] [CrossRef]
- Zhang, J.K.; Zhao, F.Q.; Xu, S.Y.; Yang, Y.J.; Qu, W.G. Preparation of Fe2O3@rGO nanocomposites and their effect on the thermal decomposition of TKX-50. Chin. J. Energ. Mater. 2017, 25, 564–569. [Google Scholar]
- Yuan, S.; Li, Z.Q.; Duan, X.H.; Luo, Q.P.; Liu, X.; Pei, C.H. Preparation and thermal decomposition properties of NGO/NC composite energetic materials. Chin. J. Energ. Mater. 2017, 25, 203–208. [Google Scholar]
- Zu, Y.; Zhao, Y.; Xu, K.; Yao, T.; Zhao, F. Preparation and Comparison of Catalytic Performance for Nano MgFe2O4, GO-Loaded MgFe2O4 and GO-Coated MgFe2O4 Nanocomposites. Ceram. Int. 2016, 42, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, Y.; Lai, W.; Yu, T.; Ge, Z. Reaction Mechanism of Nitromethane on the Graphene Surface: A Theoretical Study. Chin. J. Energ. Mater. 2015, 23, 871–876. [Google Scholar]
- Wang, H.; Zhao, F.Q.; Li, S.W.; Gao, W.X. Functional of carbon materials used in solid propellants and their action mechanism. Chin. J. Explos. Propellants 2006, 29, 32–35. [Google Scholar]
- Wang, Y.L.; Zhao, F.Q.; Yi, J.H. New progress of study on combustion catalysts used for solid rocket propellants. Chin. J. Explos. Propellants 2012, 35, 1–8. [Google Scholar]
- Memon, N.K.; McBain, A.W.; Son, S.F. Graphene oxide/ammonium perchlorate composite material for use in solid propellants. J. Propuls. Power 2016, 32, 1–5. [Google Scholar] [CrossRef]
- Dey, A.; Nangare, V.; More, P.V.; Khan, M.A.S.; Khanna, P.K.; Sikder, A.K.; Chattopadhyay, S. A graphene titanium dioxide nanocomposite (GTNC): One pot green synthesis and its application in a solid rocket propellant. RSC Adv. 2015, 5, 63777. [Google Scholar] [CrossRef]
- Dey, A.; Athar, J.; Varma, P.; Prasant, H.; Sikder, A.K.; Chattopadhyay, S. Graphene-iron oxide nano composite (GINC): An efficient catalyst for ammonium perchlorate (AP) decomposition and burn rate enhancer for AP based composite propellant. RSC Adv. 2014. [Google Scholar] [CrossRef]
- Isert, S.; Xin, L.; Xie, J.; Son, S.F. The effect of decorated graphene addition on the burning rate of ammonium perchlorate composite propellants. Combust. Flame 2017, 183, 322–329. [Google Scholar] [CrossRef]
- Yu, J.Y.; Wang, J.H.; Liu, Y.C.; Yu, Y.W.; Yuan, J.M.; Jing, S.M. Preparation and thermal properties of CL-20/GO nano-composite energetic materials. Sci. Technol. Eng. 2017, 17, 93–96. [Google Scholar]
- Ye, P.; Lu, Y.F.; Xu, P.F.; Hu, X.; He, J.X.; Wang, Q.; Guo, C.P. Preraration of CoFe2O4@C nano-composites and their catalytic performance for the thermal decomposition of ammonium perchlorate. Chin. J. Explos. Propellants 2019, 42, 358–362. [Google Scholar]
- Wang, X.B.; Li, J.Q.; Luo, Y.J. Effect of Drying Methods on the Structure and Thermal Decomposition Behavior of Ammonium Perchlorate/Graphene Composites. Acta Phys. -Chim. Sin. 2013, 29, 2079–2086. [Google Scholar]
- Wang, H.Y.; Cao, X.; Wo, J.L.; Xu, Y.B.; Shang, Y.P. Preparetion and thermal decomposition characteristics of TKX-50/GO composit energetic materials. Chin. J. Explos. Propellants 2020, 43, 631–635. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).