One-Step Electrodeposition Synthesized Aunps/Mxene/ERGO for Selectivity Nitrite Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
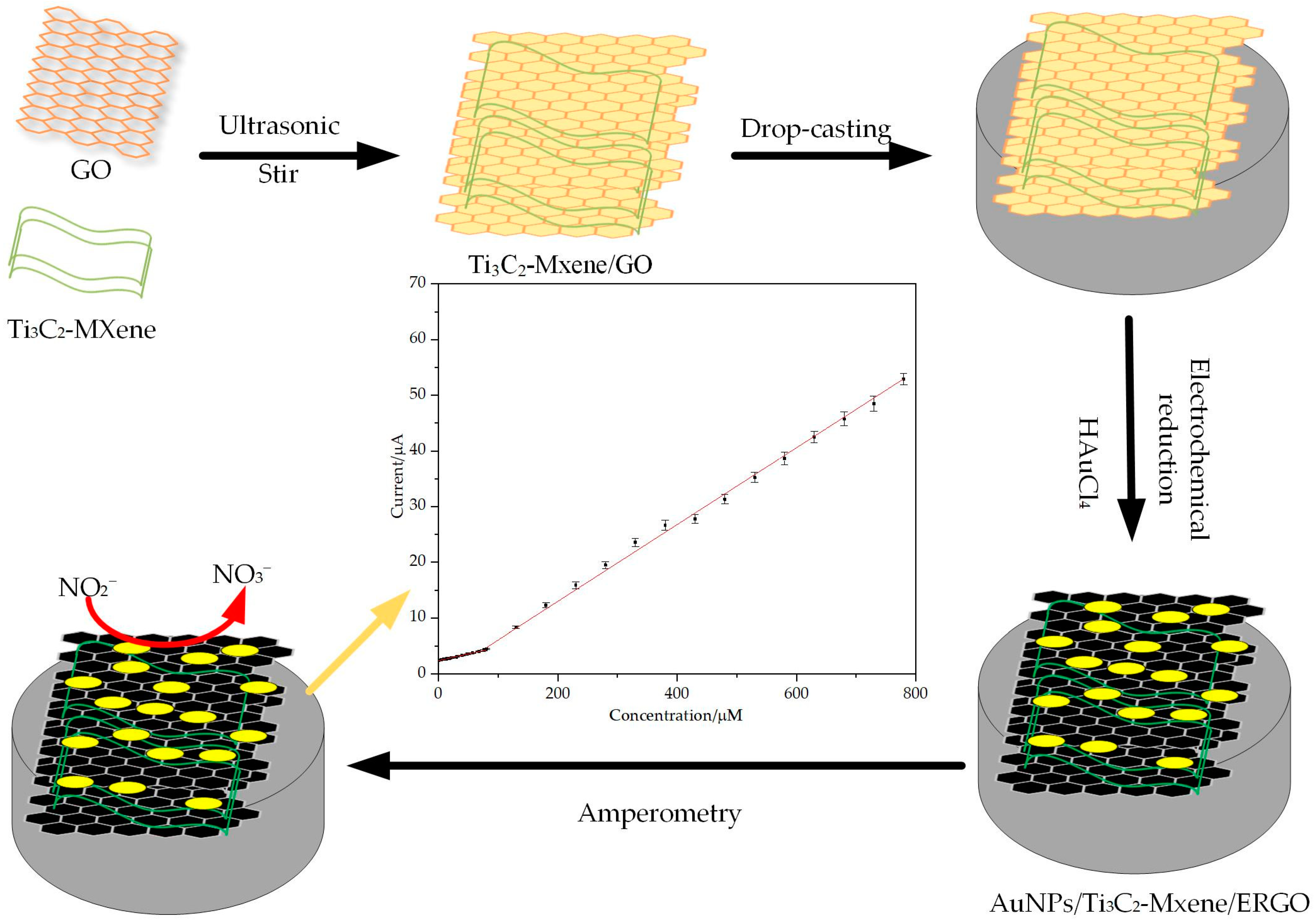
2.2. Preparation of Ti3C2-MXene/GO
2.3. Preparation of AuNPs/MXene/ERGO Modified Glass Carbon Electrode (GCE)
2.4. Electrochemical Tests
3. Results
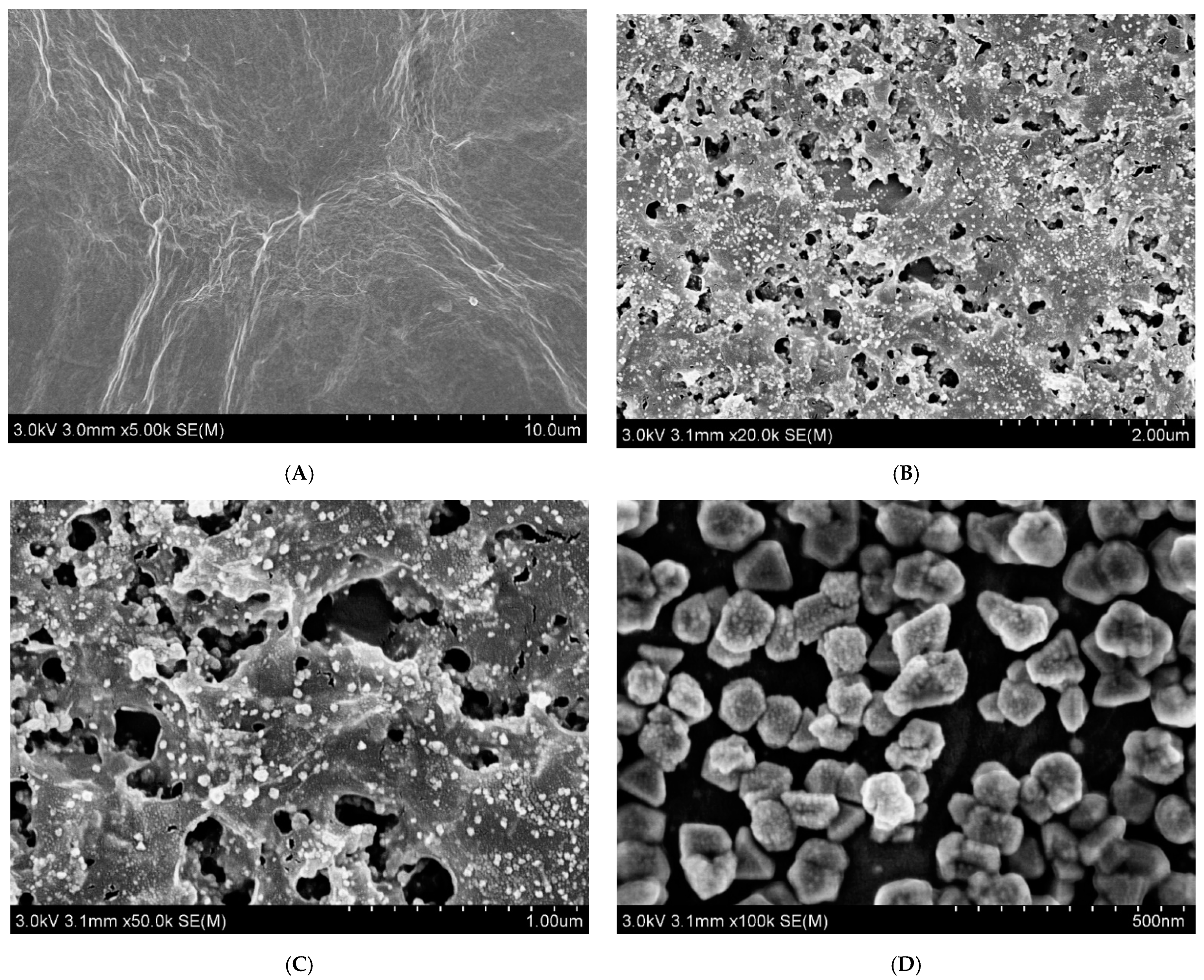
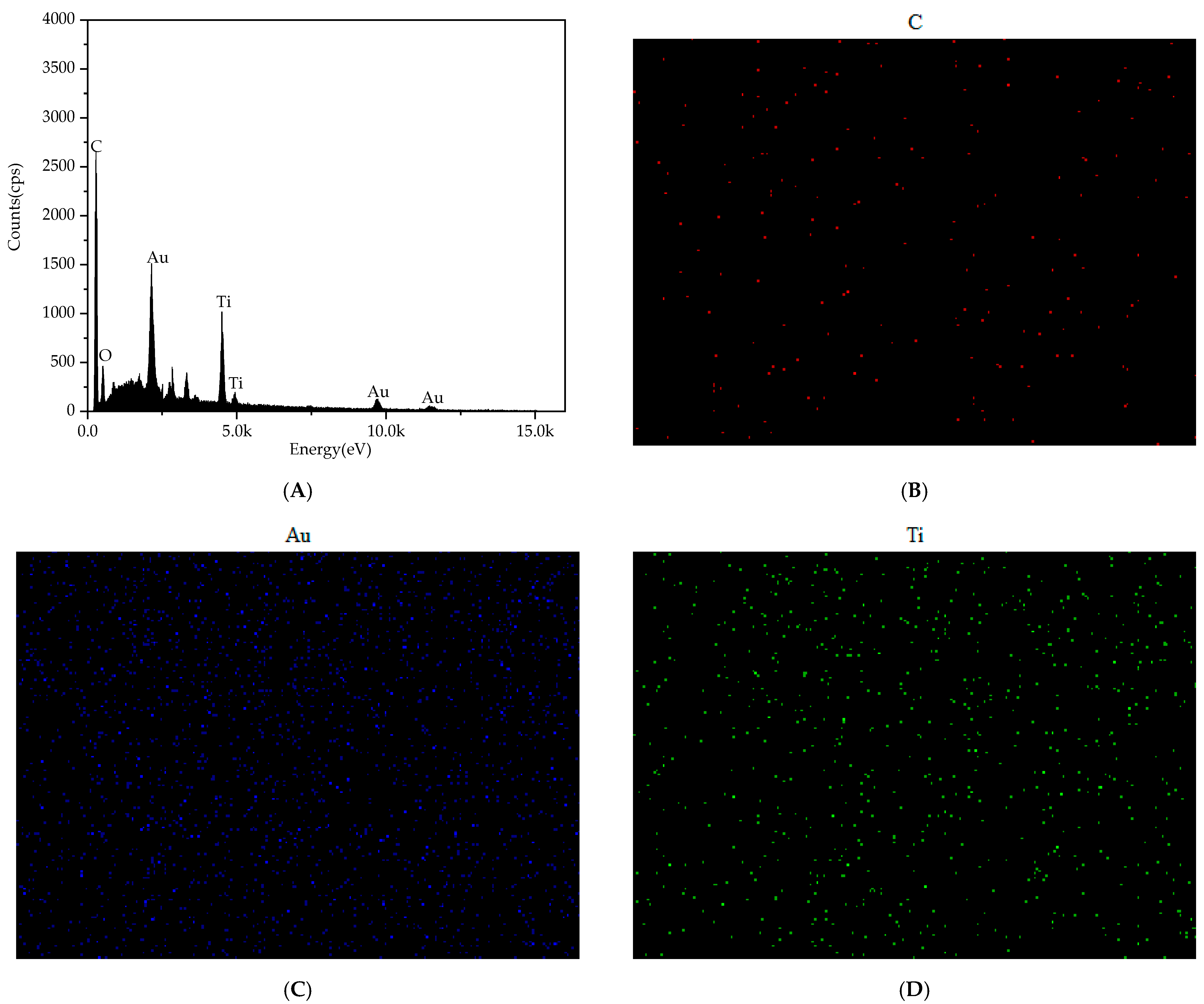
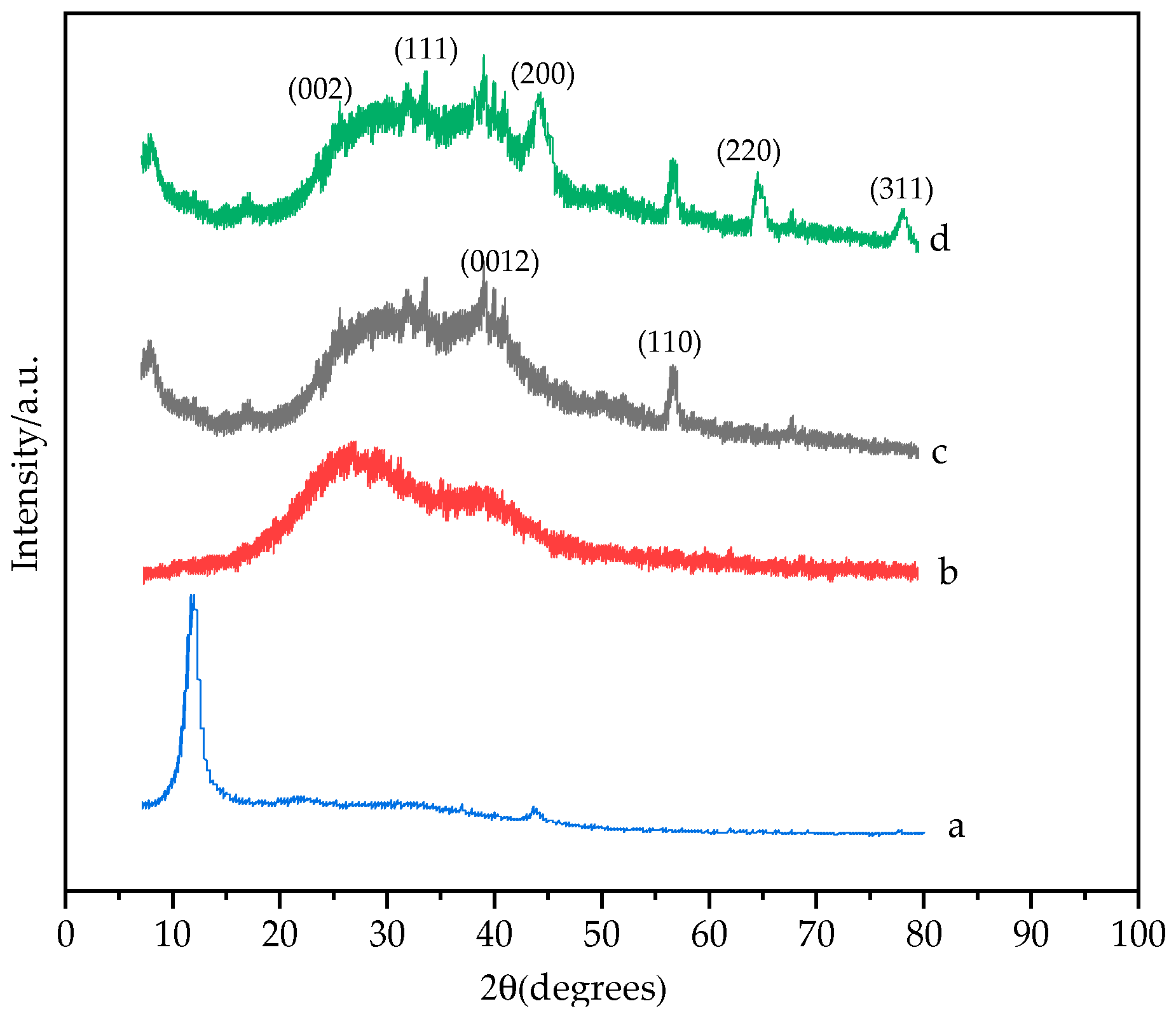
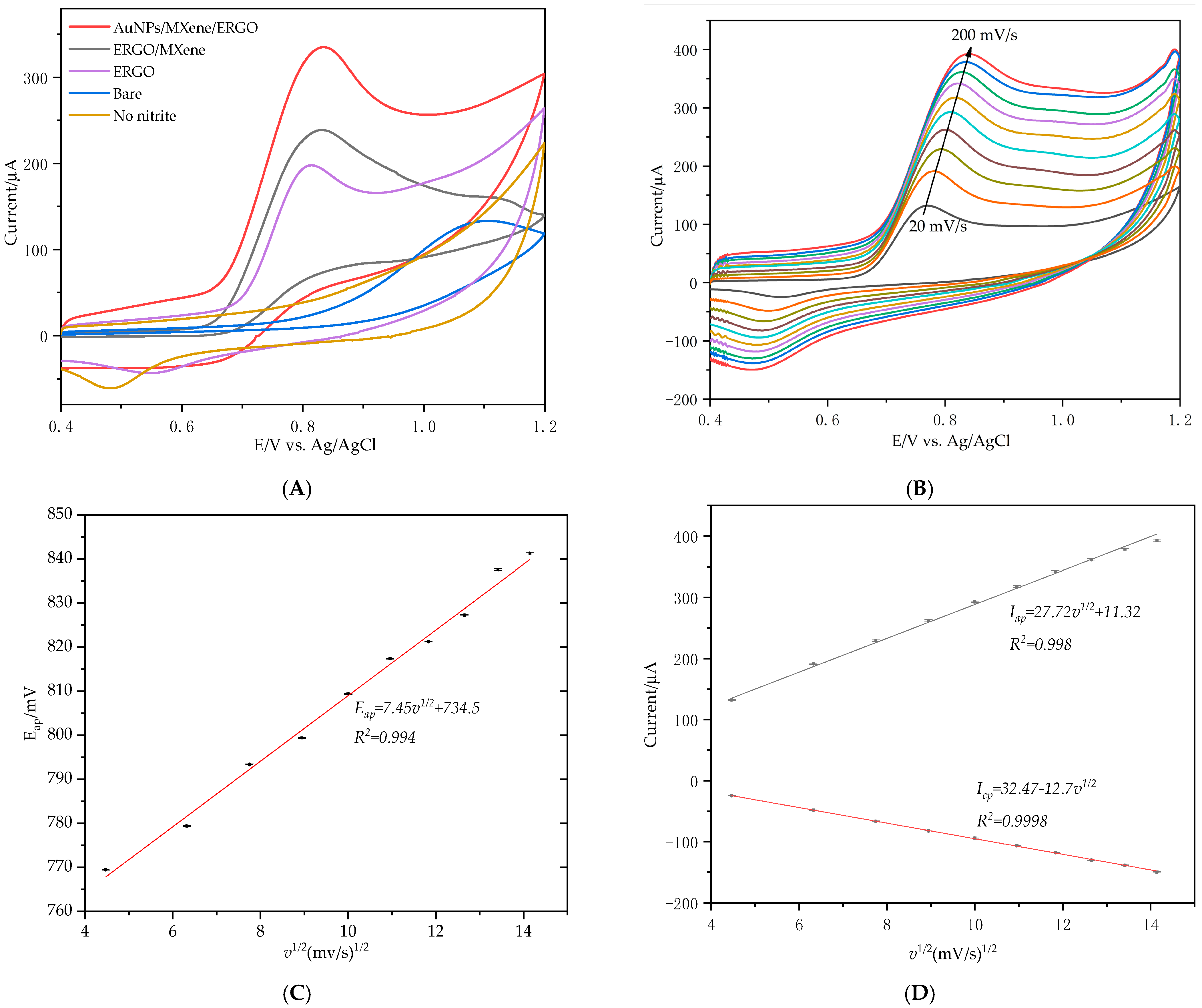
3.1. Characterization of AuNPs/MXene/ERGO Nanocomposite
3.1.1. Structural Characterization
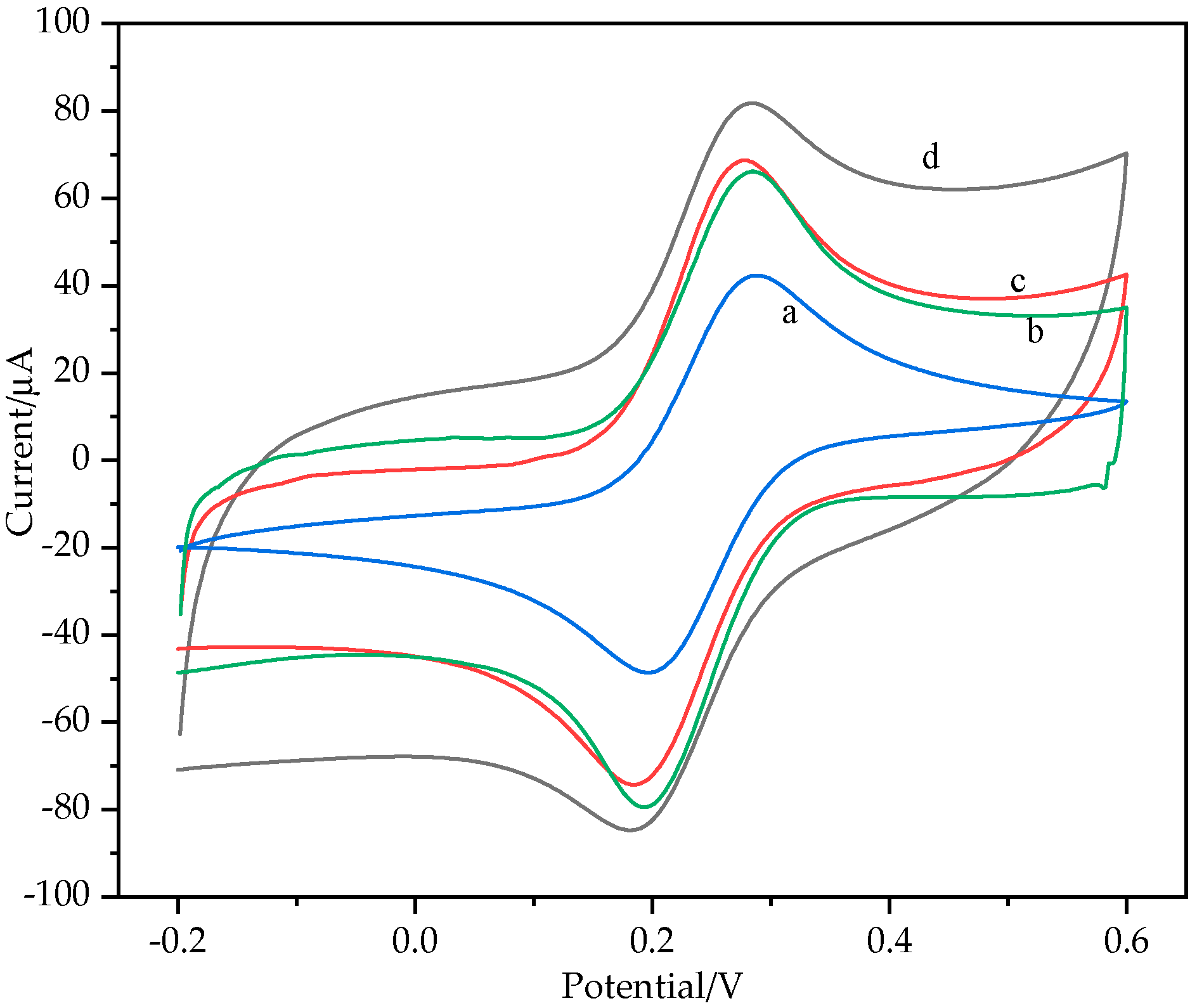
3.1.2. Electrochemical Characterization of AuNPs/MXene/ERGO Nanocomposite
3.1.3. Electrical Behavior of Modified Electrode toward Nitrite
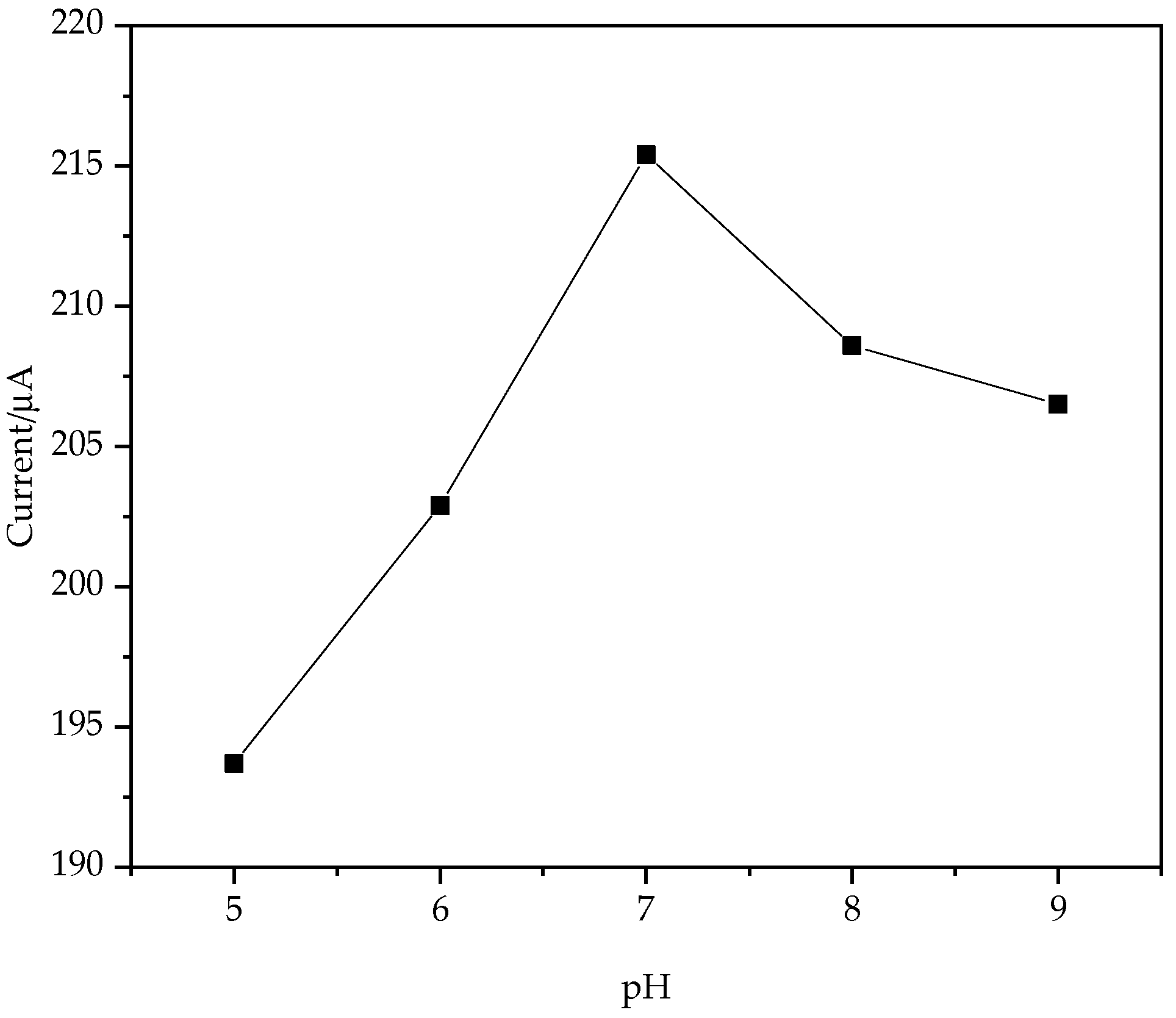
3.2. Effect of Solution pH
3.3. Amperometry
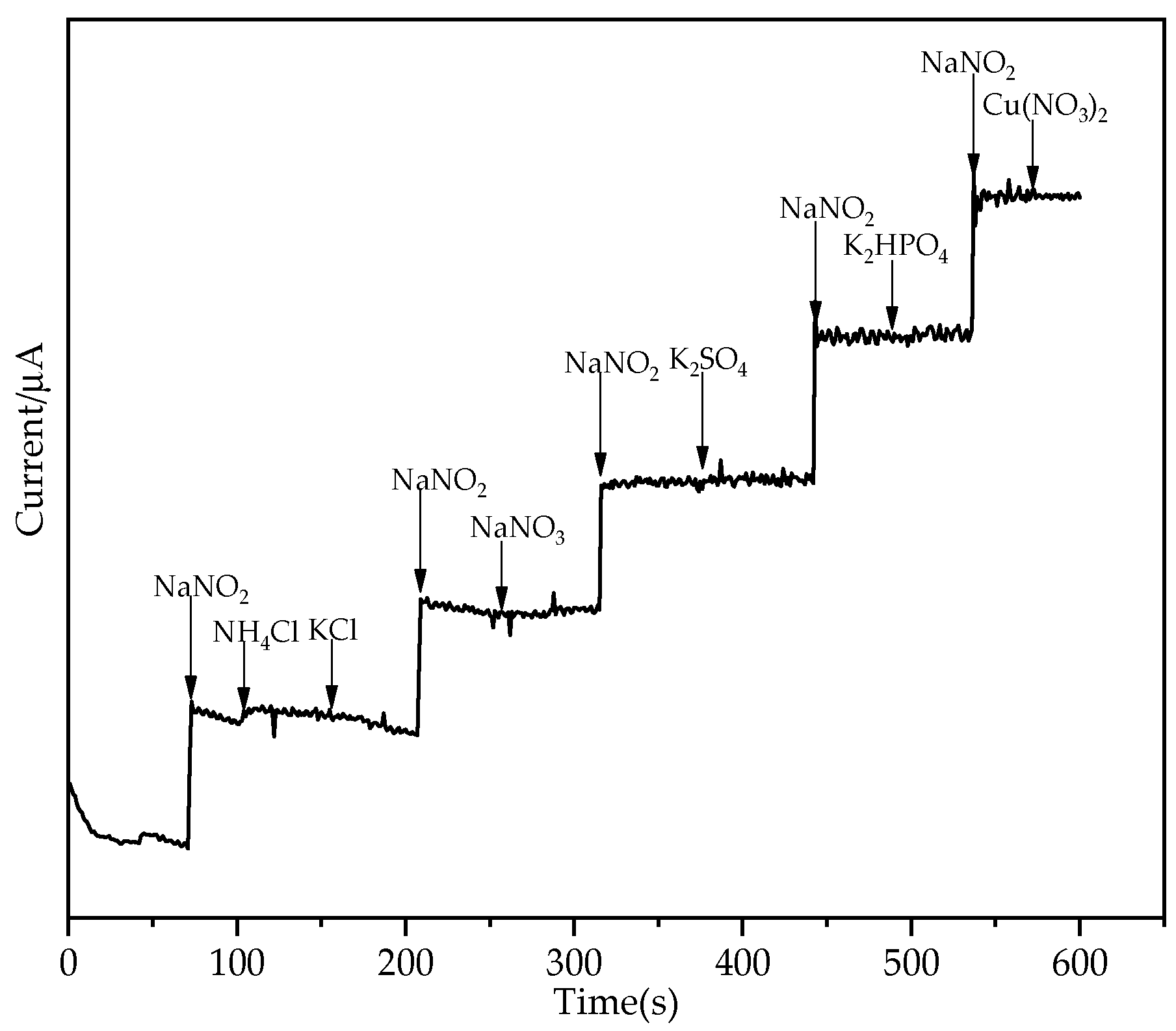
3.4. Selectivity, Reproducibility and Stability of the Modified Electrode
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kocour Kroupová, H.; Valentová, O.; Svobodová, Z.; Šauer, P.; Máchová, J. Toxic effects of nitrite on freshwater organisms: A review. Rev. Aquac. 2018, 10, 525–542. [Google Scholar] [CrossRef]
- Ciji, A.; Akhtar, M.S. Nitrite implications and its management strategies in aquaculture: A review. Rev. Aquac. 2020, 12, 878–908. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Lu, K.; Song, K.; Wang, G.; Zhang, C. Dietary hydroxyl methionine selenium supplementation enhances growth performance, antioxidant ability and nitrite tolerance of Litopenaeus vannamei. Aquaculture 2021, 537, 736513. [Google Scholar] [CrossRef]
- Sun, S.; Ge, X.; Xuan, F.; Zhu, J.; Yu, N. Nitrite-induced hepatotoxicity in Bluntsnout bream (Megalobrama amblycephala): The mechanistic insight from transcriptome to physiology analysis. Environ. Toxicol. Phar. 2014, 37, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.H.; Le Thi, H.G.; Lek, S.; Vu, N.U.; Nguyen, T.P. Effects of nitrite at different temperatures on physiological parameters and growth in clown knifefish (Chitala ornata, Gray 1831). Aquaculture 2020, 521, 735060. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y.S. Impact of nitrite exposure on endocrine, osmoregulatory and cytoprotective functions in the marine teleost Sparus sarba. Aquat. Toxicol. 2007, 82, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Miao, L.; Zhang, W.; Pan, W.; Liang, H.; Ge, X.; Xu, Y.; Liu, B.; Ren, M.; Zhou, Q.; et al. Effect of nitrite exposure on oxygen-carrying capacity and gene expression of NF-kappa B/HIF-1 alpha pathway in gill of bighead carp (Aristichthys nobilis). Aquac. Int. 2018, 26, 899–911. [Google Scholar] [CrossRef]
- Hvas, M.; Damsgaard, C.; Le Thi, H.G.; Do, T.T.H.; Jensen, F.B.; Bayley, M. The effect of environmental hypercapnia and size on nitrite toxicity in the striped catfish (Pangasianodon hypophthalmus). Aquat. Toxicol. 2016, 176, 151–160. [Google Scholar] [CrossRef]
- Stormer, J.; Jensen, F.B.; Rankin, J.C. Uptake of nitrite, nitrate, and bromide in rainbow trout, Oncorhynchus mykiss: Effects on ionic balance. Can. J. Fish Aquat. Sci. 1996, 53, 1943–1950. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Wang, C.; Zhou, X.; Li, J.; Li, D. Highly sensitive and selective method for detection of trace amounts of nitrite in aquaculture water by SERRS coupled with diazo reaction. Sens. Actuators B Chem. 2019, 297, 126757. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Q.; Liu, S.; Li, X.; Li, Y.; Zhang, M. One-step 3D printed flow cells using single transparent material for flow injection spectrophotometry. Talanta 2019, 201, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Duan, T.; Tang, D.; Zheng, Z.; Zhu, J.; Wang, R.; He, B.; Cheng, H.; Feng, L.; Zhu, Q. Review the Application of Chromatography in the Analysis of Nitric Oxide-derived Nitrite and Nitrate Ions in Biological Fluids. Curr. Anal. Chem. 2014, 10, 609–621. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Zhao, J.; Kong, X.; Lu, K.; Yang, M.; Zhang, J.; Sun, Z.; You, J. A facile dual-function fluorescent probe for detection of phosgene and nitrite and its applications in portable chemosensor analysis and food analysis. Talanta 2021, 221, 121477. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Huang, C.; Chen, T.; Wu, J. Ultra-Weak Chemiluminescence Enhanced by Cerium-Doped LaF3 Nanoparticles: A Potential Nitrite Analysis Method. Front. Chem. 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, D.; Li, D. Electrochemical and Other Methods for Detection and Determination of Dissolved Nitrite: A Review. Int. J. Electrochem. Sci. 2015, 10, 1144–1168. [Google Scholar]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of Graphene-Based Materials for Detection of Nitrate and Nitrite in Water—A Review. Sensors 2020, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ping, J.; Ying, Y. Recent developments in carbon nanomaterial-enabled electrochemical sensors for nitrite detection. TrAC Trend Anal. Chem. 2019, 113, 1–12. [Google Scholar] [CrossRef]
- Zhang, D.; Fang, Y.; Miao, Z.; Ma, M.; Du, X.; Takahashi, S.; Anzai, J.; Chen, Q. Direct electrodeposion of reduced graphene oxide and dendritic copper nanoclusters on glassy carbon electrode for electrochemical detection of nitrite. Electrochim. Acta 2013, 107, 656–663. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, J. Non-enzymatic electrochemical sensor for nitrite based on a graphene oxide–polyaniline–Au nanoparticles nanocomposite. Microchem. J. 2021, 164, 106034. [Google Scholar] [CrossRef]
- Dou, B.; Yan, J.; Chen, Q.; Han, X.; Feng, Q.; Miao, X.; Wang, P. Development of an innovative nitrite sensing platform based on the construction of carbon-layer-coated In2O3 porous tubes. Sens. Actuators B Chem. 2021, 328, 129082. [Google Scholar] [CrossRef]
- Muthumariappan, A.; Govindasamy, M.; Chen, S.; Sakthivel, K.; Mani, V. Screen-printed electrode modified with a com-posite prepared from graphene oxide nanosheets and Mn3O4 microcubes for ultrasensitive determination of nitrite. Microchim. Acta 2017, 184, 3625–3634. [Google Scholar] [CrossRef]
- Mani, V.; Govindasamy, M.; Chen, S.; Chen, T.; Kumar, A.S.; Huang, S. Core-shell heterostructured multiwalled carbon nanotubes@reduced graphene oxide nanoribbons/chitosan, a robust nanobiocomposite for enzymatic biosensing of hydrogen peroxide and nitrite. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baby, J.N.; Sriram, B.; Wang, S.; George, M.; Govindasamy, M.; Benadict Joseph, X. Deep eutectic solvent-based manga-nese molybdate nanosheets for sensitive and simultaneous detection of human lethal compounds: Comparing the electro-chemical performances of M-molybdate (M = Mg, Fe, and Mn) electrocatalysts. Nanoscale 2020, 12, 19719. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Jena, B.K.; Ghosh, S.; Bera, R.; Dey, R.S.; Das, A.K.; Raj, C.R. Bioanalytical Applications of Au Nanoparticles. Recent Pat. Nanotechnol. 2010, 4, 41–52. [Google Scholar] [CrossRef]
- Pan, H.; Li, D.; Li, J.; Zhu, W.; Fang, L. Enzyme-Mediated Growing Au Nanoparticles for Electrochemical and Optical Immunosensors: Signal-on and Signal-off. Int. J. Electrochem. Sci. 2012, 7, 12883–12894. [Google Scholar]
- Jia, Q.; Zou, G.; Zhang, H.; Wang, W.; Ren, H.; Zhanwen, A.; Deng, Z.; Yan, S.; Shen, D.; Liu, L. Sintering mechanism of Ag-Pd nanoalloy film for power electronic packaging. Appl. Surf. Sci. 2021, 554. [Google Scholar] [CrossRef]
- Kambolis, A.; Lizarraga, L.; Tsampas, M.N.; Burel, L.; Rieu, M.; Viricelle, J.P.; Vernoux, P. Electrochemical promotion of catalysis with highly dispersed Pt nanoparticles. Electrochem. Commun. 2012, 19, 5–8. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Jabbar, K.A.; Mahmoud, K.A. Platinum nanoparticles/Ti3C2Tx (MXene) composite for the effectual electrochemical sensing of Bisphenol A in aqueous media. J. Electroanal. Chem. 2021, 880, 114934. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Dehghani, H. Sodium-dodecyl-sulphate-assisted synthesis of Ni nanoparticles: Electrochemical properties. Bull. Mater. Sci. 2017, 40, 1361–1369. [Google Scholar] [CrossRef][Green Version]
- Hu, S.; Che, F.; Khorasani, B.; Jeon, M.; Yoon, C.W.; McEwen, J.; Scudiero, L.; Ha, S. Improving the electrochemical oxidation of formic acid by tuning the electronic properties of Pd-based bimetallic nanoparticles. Appl. Catal. B Environ. 2019, 254, 685–692. [Google Scholar] [CrossRef]
- Manoj, D.; Saravanan, R.; Santhanalakshmi, J.; Agarwal, S.; Gupta, V.K.; Boukherroub, R. Towards green synthesis of monodisperse Cu nanoparticles: An efficient and high sensitive electrochemical nitrite sensor. Sens. Actuators B Chem. 2018, 266, 873–882. [Google Scholar] [CrossRef]
- Yang, M.; Guo, Z.; Li, L.; Huang, Y.; Liu, J.; Zhou, Q.; Chen, X.; Huang, X. Electrochemical determination of arsenic(III) with ultra-high anti-interference performance using Au-Cu bimetallic nanoparticles. Sens. Actuators B Chem. 2016, 231, 70–78. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, B.; Wang, X.; Shen, J.; Zhao, B. Detection of organophosphorus pesticides by nanogold/ mercaptomethamidophos multi-residue electrochemical biosensor. Food Chem. 2021, 354. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent advances in MXene–based electrochemical sensors and biosensors. TrAC Trends Anal. Chem. 2019, 120, 115643. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Qiao, J.; Dong, S.; Liang, Q.; Shao, S. Ultrasensitive determination of nitrite based on electrochemical platform of AuNPs deposited on PDDA-modified MXene nanosheets. Talanta 2021, 221, 121605. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, F.; Tu, X.; Ma, X.; Xu, Q.; Dai, R.; Huang, X.; Yu, Y.; Lu, L. Facile Synthesis of MXene/Electrochemically Reduced Graphene Oxide Composites and Their Application for Electrochemical Sensing of Carbendazim. J. Electrochem. Soc. 2019, 166, B1673–B1680. [Google Scholar] [CrossRef]
- Wang, B.; Ruan, T.; Chen, Y.; Jin, F.; Peng, L.; Zhou, Y.; Wang, D.; Dou, S. Graphene-based composites for electrochemical energy storage. Energy Storage Mater. 2020, 24, 22–51. [Google Scholar] [CrossRef]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Rowley-Neale, S.J.; Randviir, E.P.; Dena, A.S.A.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Kubendhiran, S.; Chen, S.M.; Manibalan, K.; Govindasamy, M.; Tamizhdurai, P.; Huang, S.T. Reduced Graphene Oxide Non-covalent Functionalized with Zinc Tetra Phenyl Porphyrin Nanocomposite for Electrochemical Detection of Dopamine in Human Serum and Rat Brain Samples. Electroanalysis 2016, 28, 2126–2135. [Google Scholar] [CrossRef]
- Kathiresan, V.; Thirumalai, D.; Rajarathinam, T.; Yeom, M.; Lee, J.; Kim, S.; Yoon, J.; Chang, S. A simple one-step electrochemical deposition of bioinspired nanocomposite for the non-enzymatic detection of dopamine. J. Anal. Sci. Technol. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Zhe, T.; Sun, X.; Wang, Q.; Liu, Y.; Li, R.; Li, F.; Wang, L. A screen printed carbon electrode modified with a lamellar nanocomposite containing dendritic silver nanostructures, reduced graphene oxide, and β-cyclodextrin for voltammetric sensing of nitrite. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, J.; Hu, S.; Gao, G.; Song, Y. A Novel Electrochemical Sensor Based on Electropolymerized Ion Imprinted PoPD/ERGO Composite for Trace Cd(II) Determination in Water. Sensors 2020, 20, 1004. [Google Scholar] [CrossRef] [PubMed]
- HUMMERS, W.S.; OFFEMAN, R.E. PREPARATION OF GRAPHITIC OXIDE. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Majidi, M.R.; Ghaderi, S. Hydrogen bubble bubble dynamic template fabrication of nanoporous Cu film supported by graphene nanaosheets: A highly sensitive sensor for detection of nitrite. Talanta 2017, 175, 21–29. [Google Scholar] [CrossRef]
- Kung, C.; Li, Y.; Lee, M.; Wang, S.; Chiang, W.; Ho, K. In situ growth of porphyrinic metal-organic framework nanocrystals on graphene nanoribbons for the electrocatalytic oxidation of nitrite. J. Mater. Chem. A 2016, 4, 10673–10682. [Google Scholar] [CrossRef]
- Ozturk, A.; Alanyalioglu, M. Electrochemical fabrication and amperometric sensor application of graphene sheets. Superlattices Microstruct. 2016, 95, 56–64. [Google Scholar] [CrossRef]
- Ikhsan, N.I.; Rameshkumar, P.; Pandikumar, A.; Shahid, M.M.; Huang, N.M.; Kumar, S.V.; Lim, H.N. Facile synthesis of graphene oxide-silver nanocomposite and its modified electrode for enhanced electrochemical detection of nitrite ions. Talanta 2015, 144, 908–914. [Google Scholar] [CrossRef]
- Mo, R.; Wang, X.; Yuan, Q.; Yan, X.; Su, T.; Feng, Y.; Lv, L.; Zhou, C.; Hong, P.; Sun, S.; et al. Electrochemical Determination of Nitrite by Au Nanoparticle/Graphene-Chitosan Modified Electrode. Sensors 2018, 18, 1986. [Google Scholar] [CrossRef]
- Mani, V.; Periasamy, A.P.; Chen, S. Highly selective amperometric nitrite sensor based on chemically reduced graphene oxide modified electrode. Electrochem. Commun. 2012, 17, 75–78. [Google Scholar] [CrossRef]
- Kesavan, S.; Kumar, D.R.; Baynosa, M.L.; Shim, J. Potentiodynamic formation of diaminobenzene films on an electrochemically reduced graphene oxide surface: Determination of nitrite in water samples. Mater. Sci. Eng. C 2018, 85, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Nie, F.; Sheng, Q.; Zheng, J. An electrochemical sensor for sensitive determination of nitrites based on Ag-Fe3O4-graphene oxide magnetic nanocomposites. Chem. Pap. 2015, 69, 911–920. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Pandikumar, A.; Yusoff, N.; Huang, N.M.; Lim, H.N. Electrochemical sensing of nitrite using a glassy carbon electrode modified with reduced functionalized graphene oxide decorated with flower-like zinc oxide. Microchim. Acta 2015, 182, 1113–1122. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]

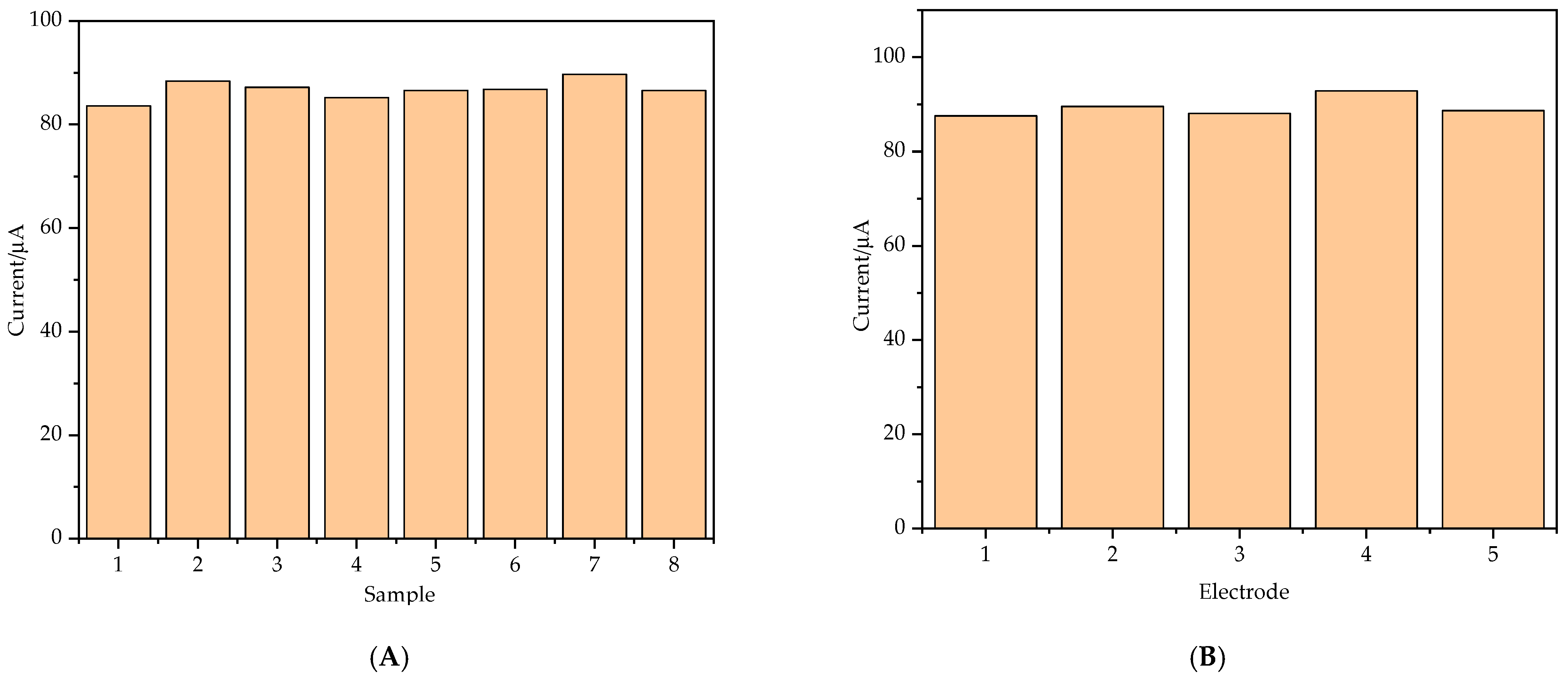
| Modified Electrodes | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|
| NPCF-GNs/GCE | 0.1–100 | 0.088 | [47] |
| MOF-525/GNR/ITOE | 10–2500 | 0.75 | [48] |
| Graphene/GCE | 1–250 | 0.24 | [49] |
| GO-Ag/GCE | 10–180 | 2.1 | [50] |
| GO-CS-AuNPs/GCE | 0.9–18.9 | 0.3 | [51] |
| CR-GO/GCE | 8.9–167 | 1 | [52] |
| PDAB/ERGO/GCE | 7–20,000 | 0.03 | [53] |
| CoHCF/RGO/GCE | 1–100 | 0.27 | [54] |
| fZnO/rFGO/GCE | 10–8000 | 33 | [55] |
| Fe3O4/r-GO/GCE | 1–92 | 0.3 | [56] |
| AuNPs/MXene/ERGO/GCE | 0.5–80 | 0.15 | This work |
| 80–780 | 0.051 |
| Real Sample | Added (μM) | Found (μM) | Recovery (%) |
|---|---|---|---|
| Tap water | 5 | 4.89 | 97.8% |
| 10 | 9.57 | 95.7% | |
| 15 | 15.78 | 105.2% | |
| River water | 5 | 4.86 | 97.2% |
| 10 | 10.5 | 95% | |
| 15 | 15.48 | 103.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, C.; Xu, X.; Li, Z.; Li, D. One-Step Electrodeposition Synthesized Aunps/Mxene/ERGO for Selectivity Nitrite Sensing. Nanomaterials 2021, 11, 1892. https://doi.org/10.3390/nano11081892
Wang T, Wang C, Xu X, Li Z, Li D. One-Step Electrodeposition Synthesized Aunps/Mxene/ERGO for Selectivity Nitrite Sensing. Nanomaterials. 2021; 11(8):1892. https://doi.org/10.3390/nano11081892
Chicago/Turabian StyleWang, Tan, Cong Wang, Xianbao Xu, Zhen Li, and Daoliang Li. 2021. "One-Step Electrodeposition Synthesized Aunps/Mxene/ERGO for Selectivity Nitrite Sensing" Nanomaterials 11, no. 8: 1892. https://doi.org/10.3390/nano11081892
APA StyleWang, T., Wang, C., Xu, X., Li, Z., & Li, D. (2021). One-Step Electrodeposition Synthesized Aunps/Mxene/ERGO for Selectivity Nitrite Sensing. Nanomaterials, 11(8), 1892. https://doi.org/10.3390/nano11081892








