Abstract
SnO2 nanoparticles are regarded as attractive, functional materials because of their versatile applications. SnO2 nanoaggregates with single-nanometer-scale lumpy surfaces provide opportunities to enhance hetero-material interfacial areas, leading to the performance improvement of materials and devices. For the first time, we demonstrate that SnO2 nanoaggregates with oxygen vacancies can be produced by a simple, low-temperature sol-gel approach combined with freeze-drying. We characterize the initiation of the low-temperature crystal growth of the obtained SnO2 nanoaggregates using high-resolution transmission electron microscopy (HRTEM). The results indicate that Sn (II) hydroxide precursors are converted into submicrometer-scale nanoaggregates consisting of uniform SnO2 spherical nanocrystals (2~5 nm in size). As the sol-gel reaction time increases, further crystallization is observed through the neighboring particles in a confined part of the aggregates, while the specific surface areas of the SnO2 samples increase concomitantly. In addition, X-ray photoelectron spectroscopy (XPS) measurements suggest that Sn (II) ions exist in the SnO2 samples when the reactions are stopped after a short time or when a relatively high concentration of Sn (II) is involved in the corresponding sol-gel reactions. Understanding this low-temperature growth of 3D SnO2 will provide new avenues for developing and producing high-performance, photofunctional nanomaterials via a cost-effective and scalable method.
1. Introduction
Over the last two decades, the synthesis of self-assembled tin oxide (SnO2) semiconductor nanoparticles has been a fascinating research area because of their potential applications in gas sensing, lithium-ion batteries, solar cells, and catalysts for various organic reactions []. These versatile applications associated with their photophysical, chemical, and electronic properties can be realized by tuning certain factors, such as the size, shape, crystallinity, and electronic states of the SnO2 nanoparticles []. In particular, self-assembled 3D SnO2 nanoaggregates with high surface areas [,] have received considerable attention. A bottom-up, wet-chemical synthesis is one of the major techniques that enables the creation of nanoparticles with various dimensions, ranging from spherical morphologies [] to anisotropic 1D structures [], 2D sheets [], and self-assembled 3D forms [] of low-dimensional motifs. Thus far, such SnO2 production has relied heavily on hydrothermal synthesis, which requires a high temperature and pressure. As an example, to form the target hierarchical nanostructures, a conventional hydrothermal approach has been reported to produce a 3D flower-like SnO2 nano/microstructure material []. From the SnCl4 precursor solution with a poly (acrylic acid) additive, flower-like particles with a dimension of 100 nm consisting of needle-like single-crystal units (ca. 50 nm in size) were obtained under acidic conditions at 150 °C. Several other syntheses of SnO2 particles with flower-like morphology have also been reported to date, using various Sn (II) precursors under hydrothermal conditions [,].
A low-temperature solution process employing a sol-gel method is considered an alternative method for creating oxide nanoparticles with high surface areas. This is because, in this method, nucleation and crystal growth at a single nanometer scale can be controlled by the selection of base solvents, metal-ion sources, and organic additives, compared to the cases in the well-known hydrothermal or solvothermal methods. Among wide-bandgap semiconductor metal oxides, several controlled low-temperature hydrolyses and polycondensations in TiO2 syntheses have been shown to produce TiO2 nanoparticles of different sizes, crystal phases, and morphologies, with dimensions of less than 10 nm [,]. We recently demonstrated that organic-inorganic Ti (IV)-gels prepared at temperatures as low as 40 °C could be used as a precursor to obtain anatase TiO2 nanoaggregates consisting of particles with a grain size of 5 nm []. Freeze drying of the gel precursor accelerates the polycondensation in the Ti-O framework to efficiently produce nanocrystals even at low temperatures.
In contrast to the several low-temperature TiO2 syntheses reported in the literature, the growth mechanism of SnO2 nanoparticles and their 3D structure formation at low temperatures have not been adequately understood to date. SnO2 films processed at relatively low temperatures are regarded as attractive materials with the progress of new-generation solar cells, specifically halide perovskite solar cells [,,,,]. Therefore, fundamental research regarding SnO2 crystallization is essential for developing electron-transport materials that boost device performance.
In this study, simple template-free reaction conditions employing SnCl2 and Na2CO3 in water were applied to investigate a low-temperature SnO2 crystal growth, along with freeze-drying. The formation of 3D SnO2 nanoaggregates with uniform, single nanometer-scale grains, and their crystallization were clarified, mainly using reaction time-dependent, high-resolution HRTEM analysis. For the first time, we showed the low-temperature crystal growth characteristics of SnO2 nanoaggregates involving the formation of oxygen vacancies. The detailed crystallization of the pre-organized Sn-based gel precursors was assessed to form 3D SnO2 nanoaggregates with controllable microstructure surfaces and electronic properties.
2. Materials and Methods
2.1. Synthesis of SnO2 Nanoaggregates
SnCl2·2H2O and Na2CO3 were purchased from Sigma-Aldrich, St. Luis, Missouri, USA and used as received. Three different synthesis methods were applied to synthesize the semiconductor SnO2 nanoparticles. The mass ratio of ultrapure water (UPW), SnCl2, and Na2CO3 were varied, as shown in Methods 1–3 below.
Method 1: The precursor solution was prepared by mixing SnCl2 (2 g) dissolved in UPW (400 mL). Na2CO3 (400 mg) was dissolved separately in UPW (500 mL) and completely mixed with the SnCl2 solution. The solution was placed in a water bath and maintained at 40 °C for a given time, denoted as Day X (X: 1–7 or 1–8) hereinafter. The SnO2 solution was sampled by transferring 150 mL to a centrifugation container. Thereafter, the container was centrifuged at 4000 rpm for 10 min. The reaction temperature was maintained at 40 °C. After centrifugation, the liquid was discarded. The solid gel was carefully mixed in 5–10 mL of water and sonicated for uniform dispersion. Subsequently, it was added to a vial and capped. The vial was placed in a container filled with liquid nitrogen for 5–10 min to freeze the gel solution. Afterward, the vial was placed into a jar and attached to a vacuum freeze-drying apparatus (BenchTop Pro with Omnitronics, VirTis SP Scientific, USA) at ~200 mTorr. After 24–48 h of freeze-drying to remove the moisture, SnO2 nanoparticles were produced in a powdered form.
Method 2: The precursor solution was prepared using a mixture of SnCl2 (5 g) dissolved in UPW (500 mL). Na2CO3 (500 mg) was dissolved separately in UPW (500 mL) and completely mixed with the SnCl2 solution. Thereafter, the solution was placed in a water bath and maintained at 40 °C for a given period. The SnO2 nanoparticles were isolated using a similar procedure to that in Method 1.
Method 3: The precursor solution was prepared by dissolving SnCl2 (20 g) in UPW (500 mL), in contrast to using 5 g of SnCl2, as in Method 2. The SnO2 nanoparticles were isolated using a similar procedure to that in Method 1.
2.2. Structure Characterization of SnO2 Nanoaggregates
SnO2 powder samples were characterized using X-ray diffraction (XRD; Rigaku RINT Ultima/PC with monochromated Cu–Kα radiation, Tokyo, Japan). The crystallite size of the SnO2 aggregates was estimated using the Scherrer equation (D = Kλ/βcosθ) based on the XRD data, where D, K, λ, and θ indicate the crystallite size, Scherrer constant (0.90), X-ray wavelength (1.54 Å), and Bragg angle, respectively. The SnO2 sample surface was analyzed by XPS (XPS; ULVAC, Quantera SXM, Kanagawa, Japan). Nanostructure analysis was carried out by TEM (JEM-2100, Tokyo, Japan). The Brunauer–Emmett–Teller (BET) surface area was evaluated by N2 physisorption measurement at 77 K, using the Micromeritics TriStar II 3020 (Kyoto, Japan). Photoabsorption spectra were measured using a Hitachi U-4000 spectrophotometer. Ultraviolet–visible (UV–Vis) spectra were obtained from the diffuse reflectance of the dry powder samples.
3. Results
3.1. XRD Patterns and XPS Spectra of the Obtained Powder Samples
We previously reported that anatase TiO2 nanoaggregates can be produced by freeze drying using Ti-gel precursors []. This result stimulated our interest in the growth control of SnO2 nanoaggregates at low temperatures. The XRD patterns of the powder samples obtained using three sets of reactions (Methods 1–3) are shown in Figure 1a–c. All the observed peaks are in agreement with those of tetragonal rutile SnO2 (JCPDS 01-072-1147), except for the Method 1–Day 1 sample. In the early stage of the sol–gel process in Method 1, only the impurity phase of NaCl is detected, and no SnO2 peak is observed. All the peaks corresponding to SnO2 are identified for the rest of the samples obtained via Method 1. It is observed that the intensity of the SnO2 peaks increases with the reaction times for all methods, indicating that long reaction times lead to the enhancement of the SnO2 crystal growth.
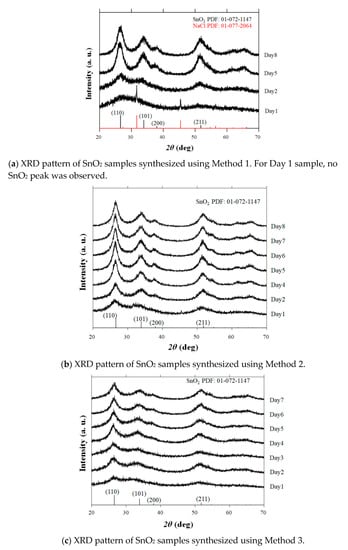
Figure 1.
Powder X-ray diffraction (XRD) patterns of Method 1–3 samples synthesized by varying the reaction times. The database pattern is presented at the bottom of each figure.
The samples obtained via Methods 1 and 2 exhibited a pale-yellow color when the solgel reactions were stopped in a short time (except for the Method 1–Day 1 samples, which exhibited a white color), and became white when the reactions were extended. Conversely, all the samples for Method 3 had a pale-yellow color (Figure 2). To understand the color-change tendency, XPS measurements of Method 1–Day 1, Method 1–Day 8, Method 2–Day 8, and Method 3–Day 7 samples were carried out as illustrated in Figure 3a,b. The binding energies corresponding to the peaks of Sn 3d5/2 for the Day 7 and 8 samples were 486.2, 486.4, and 486.2 eV for Method 3–Day 7, Method 1–Day 8, and Method 2–Day 8, respectively, which are almost consistent with the reported values for Sn(IV) bound to an oxygen atom in SnO2 [,,]. Notably, all three pale-yellow samples showed a marked peak shift compared to that of the Method 1–Day 1 sample. For the Method 1–Day 1 sample, the Sn3d XPS peaks were observed at a lower binding energy (485.2 eV) than those for the other samples, suggesting that the Sn(II) in this sample was not oxidized to Sn(IV). Similarly, a peak shift was observed for the pale-yellow sample (Method 2–Day 1) when compared to that of Method 1–Day 1, as shown in Figure 3c.

Figure 2.
Photographs of the obtained representative powder samples synthesized by a sequential low-temperature sol–gel and freeze-drying approach.
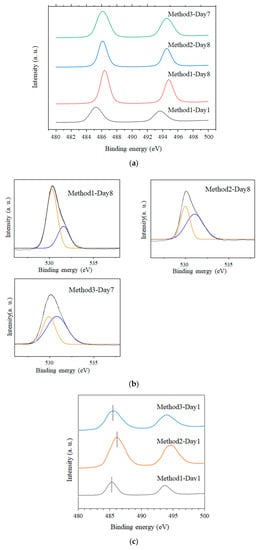
Figure 3.
(a) X-ray photoelectron spectroscopy (XPS) spectra of Method 1–Day 1, Method 1–Day 8, Method 2–Day 8, and Method 3–Day 7 samples corresponding to Sn 3d5/2; (b) XPS spectra of the same samples (except Method 1–Day 1) showing O 1s peaks; (c) XPS spectra of Method 3–Day 1, Method 2–Day 1, and Method 1–Day 1 samples.
The high-resolution O 1s spectra from the same sample could be deconvoluted into two peaks at approximately 530–532 eV, as indicated by the orange and blue lines. It has been reported that the low energy peak is ascribed to the oxygen bound to Sn atoms, whereas the high energy peak corresponds to the formation of oxygen vacancies [,]. Considering that SnO2 peaks were only detected in the XRD patterns, the large amount of Sn(II) source for Method 3 probably enhanced the formation of oxygen-vacancy-induced SnO2, even for long reactions.
3.2. Proposed Growth Mechanism of SnO2 Based on TEM Analysis and Its Optical Property
To resolve the SnO2 nanostructures and elucidate their growth mechanism, HRTEM measurements were performed, as shown in Figure 4. The crystallization was not significant for all early-stage samples, such as the Method 1–Day 1 sample, as shown in the selected area diffraction (SAD) pattern (not shown). As the reaction times increase, lattice fringes with d-spacings of 0.33 nm and 0.27 nm corresponding to the (110) and (101) planes, respectively, are clearly observed for all methods. The measurements indicated that the aggregated particles consisting of tiny nanocrystals were formed from precursor Sn(II)-based gels, where the average sizes of the primary nanocrystals were in the range of 2~5 nm (Table 1), which are consistent with the estimated crystallite sizes in Table 2. Notably, the diffraction spots in SAD patterns become more noticeable as the reaction proceeds, although the corresponding measured area showed numerous tiny nanoparticles, as shown in Figure 5. This further indicated the improved crystallinity of the long-reaction-time samples.
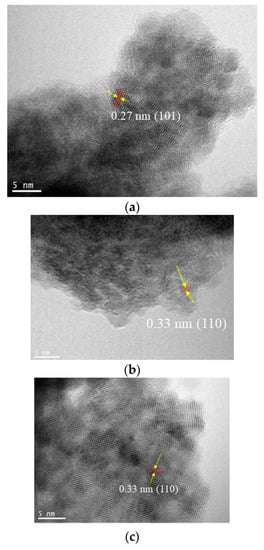
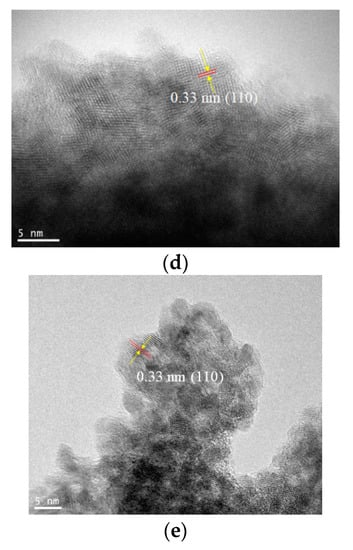
Figure 4.
Transmission electron microscopy (TEM) images of (a) Method 1–Day 5, (b) Method 2–Day 1, (c) Method 2–Day 5, (d) Method 3–Day 1, and (e) Method 3–Day 5 samples.

Table 1.
Sizes of SnO2 particles in the nanoaggregates observed in the TEM images of Figure 4a–e. The maximum length perpendicular to the lattice fringes of SnO2 nanoparticles was measured. The average nanoparticle sizes for the TEM images (a)–(e) were 2.6 nm, 2.7 nm, 4.4 nm, 3.5 nm, and 4.3 nm, respectively.

Table 2.
Crystallite sizes and specific surface areas of the SnO2 samples synthesized using different reaction conditions and times.
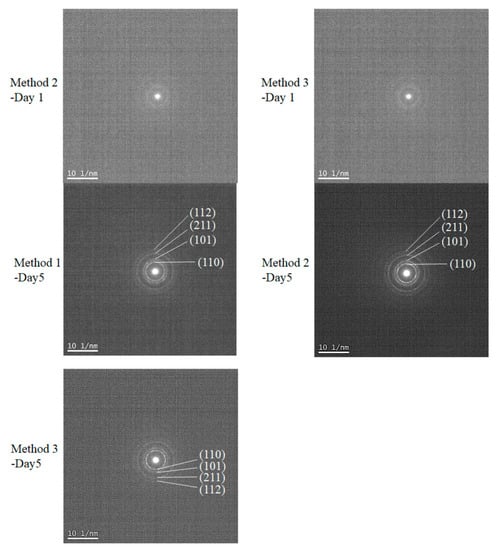
Figure 5.
Selected area diffraction (SAD) pattern of Method 1–Day 5, Method 2–Day 1, Method 2–Day 5, Method 3–Day 1, and Method 3–Day 5 samples. These were measured using the same samples as those in Figure 4, which shows the TEM images.
In addition, it was observed that the size of the SnO2 nanocrystals decreased slightly as the SnCl2 amount increased (Table 2). In our low-temperature process, the amount of SnCl2 was another key factor in controlling the size of the primary SnO2 nanoparticles, along with their crystallinity and generation rate of oxygen vacancies. As for the growth mechanism of the SnO2 particles, it is most probable that Sn(II) hydroxide precursors are formed by the hydrolysis of SnCl2 and subsequently converted into submicrometer-scale nanoaggregates comprising uniform SnO2 spherical nanocrystals. Figure 6 shows a conceptual drawing of the crystal growth characteristics of the SnO2 nanoaggregates in our low-temperature sol–gel reaction of Sn(II) and the subsequent freeze-drying process. The longer the sol–gel reaction time, the more enhanced the crystallization through its neighboring particles in a confined part of the nanoaggregates, while the specific surface areas of the SnO2 samples increase. In other words, long reactions produce SnO2 nanoaggregates with single-nanometer-scale lumpy surfaces, providing increased specific surface areas.
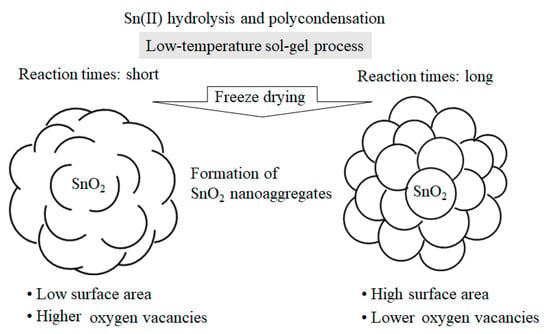
Figure 6.
Plausible crystal growth mechanism of SnO2 nanoaggregates depending on reaction times of the low-temperature sol–gel process.
For Methods 2 and 3, the specific surface areas for Days 2 and 5 were almost comparable, suggesting that both SnO2 nanoaggregates for Day 2 already had lumpy surfaces, as illustrated on the right in Figure 6. Therefore, it is most likely that the SnO2 nanoaggregates after 3–4 days had surfaces similar to the image on the right. For Method 1, it would also be reasonable that, with increasing the reaction times, the SnO2 surface morphology changes from left to right, as shown in Figure 6. However, the surface morphology after 3–4 days cannot be explained solely by the change of the specific surface areas between Day 2 and Day 5. More detailed nanostructure analyses of the assembled SnO2 particles, i.e., 3D grain growth, will benefit from theoretical/computational modeling, in combination with the HRTEM data of SnO2 particles [].
Additionally, we measured the optical absorption of several SnO2 samples of Methods 1–3 using diffuse reflectance spectroscopy, as shown in Figure 7. Notably, the samples of Method 1–Day 2, Method 2–Day 2, Method 3–Day 2, and Method 3–Day 7 showed an absorption tail in the visible wavelength. Their observed bandgaps were estimated to be 3.1, 3.0, 2.9, and 3.1 eV for Method 1–Day 2, Method 2–Day 2, Method 3–Day 2, and Method 3–Day 7, respectively. It was reported that the narrower bandgaps compared to a well-known bandgap of 3.6 eV [,] is due to the elevation of the valence-band maximum of SnO2 induced by the oxygen vacancies [,]. The bandgap of Method 1–Day 8 and Method 2–Day 7 was 3.6 eV.
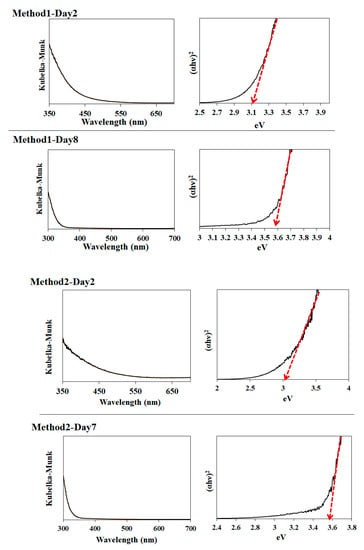
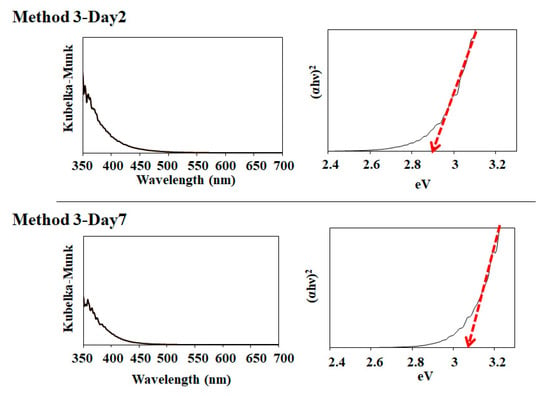
Figure 7.
Photoabsorption spectra of Method 1–Day 2, Method 1–Day 8, Method 2–Day 2, Method 2–Day 7, Method 3–Day 2, Method 3–Day 7 samples and their corresponding bandgap estimations.
As has been demonstrated in solar cells, such as perovskite solar cells, sintering SnO2 nanoparticles can enhance electron transport properties []. Additional studies to evaluate the sintering effects of the obtained SnO2 particles on solar cell performance are in progress.
4. Conclusions
A low-temperature growth mechanism of SnO2 nanoaggregates has been presented for the first time, based on HRTEM, XRD, and XPS analyses of a series of SnO2 samples. The changes in the SnCl2 and Na2CO3 concentrations in the reaction mixture enabled the control of the lumpy surface of SnO2 at the single-nanometer scale, as well as the Sn (II) formation in the SnO2 aggregates. As the sol-gel reaction times increased, the TEM data suggested that the crystallization of tiny nanoparticles was enhanced to form SnO2 nanoaggregates with high specific surface areas. In addition, oxygen-vacancy-induced SnO2 nanoaggregates could be obtained by adjusting certain factors, including the reaction time and starting material concentrations of SnCl2 and Na2CO3. Such a low-temperature synthesis of 3D SnO2 will provide new avenues for developing and producing high-performance functional nanomaterials cost-effectively. Our findings will provide insights for the further investigation of 3D SnO2 and its related functions for versatile applications.
Author Contributions
Conceptualization, S.V. and K.M.; methodology, S.V. and K.M.; formal analysis, N.H., K.M. and T.S.; synthesis, S.V., K.M., A.W., C.E. and U.S. writing—original draft preparation, S.V. and K.M.; writing—review and editing, S.V., K.M., A.W.; project administration, S.V.; funding acquisition, S.V. and K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Illinois Space Grant Consortium, USA, the Research Excellence Program and the Caterpillar Fellowship, Bradley University, USA. This work is also supported by the OGAWA Science and Technology Foundation, Japan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, H.; Rogach, A.-L. Hierarchical SnO2 nanostructures: Recent advances in design, synthesis, and applications. Chem. Mater. 2014, 26, 123–133. [Google Scholar] [CrossRef]
- Periyasamy, M.; Kar, A. Modulating the properties of SnO2 nanocrystals: Morphological effects on structural, photoluminescence, photocatalytic, electrochemical and gas sensing properties. J. Mater. Chem. C. 2020, 8, 4604–4635. [Google Scholar] [CrossRef]
- Wang, B.-J.; Ma, S.-Y.; Pei, S.-T.; Xu, X.-L.; Cao, P.-F.; Zhang, J.-L.; Zhang, R.; Xu, X.-H.; Han, T. High specific surface area SnO2 prepared by calcining Sn-MOFs and their formaldehyde-sensing characteristics. Sens. Actuators B Chem. 2020, 321, 128560. [Google Scholar] [CrossRef]
- Zhao, Q.; Ma, L.; Zhang, Q.; Wang, C.; Xu, X. SnO2-Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries and Supercapacitors. J. Nanomater. 2015, 850147. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, M.-C.; Kim, S.-B.; Kim, Y.-S.; Choi, J.-H.; Park, K.-W. Porous SnO2 nanostructure with a high specific surface area for improved electrochemical performance. RSC Adv. 2020, 10, 10519–10525. [Google Scholar] [CrossRef] [Green Version]
- Matysiak, W.; Tański, T.; Smok, W.; Polishchuk, O. Synthesis of hybrid amorphous/crystalline SnO2 1D nanostructures: Investigation of morphology, structure and optical properties. Sci. Rep. 2020, 10, 14802. [Google Scholar] [CrossRef]
- Wan, W.; Li, Y.; Ren, X.; Zhao, Y.; Gao, F.; Zhao, H. 2D SnO2 Nanosheets: Synthesis, Characterization, Structures, and Excellent Sensing Performance to Ethylene Glycol. Nanomaterials 2018, 8, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, H.; Shirai, Y.; Kozuka, H. Hydrothermal synthesis of flower-like SnO2 particles consisting of single crystalline nano rods through crystal growth in the presence of poly(acrylic acid). RSC Adv. 2012, 2, 4839–4843. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, Z.; Yang, L.; Wang, S.; Liu, H.; Feng, W.; Zhao, Y.; Feng, F. A simple method for synthesis of highly efficient flower-like SnO2 photocatalyst nanocomposites. J. Mater. Sci. Mater. Electron. 2019, 30, 50–55. [Google Scholar] [CrossRef]
- Vuong, D.-D.; Hien, V.-X.; Trung, K.-Q.; Chien, N.-D. Synthesis of SnO2 micro-spheres, nano-rods and nano-flowers via simple hydrothermal route. Phys. E 2011, 44, 345–349. [Google Scholar] [CrossRef]
- Sang, L.; Zhao, Y.; Burda, C. TiO2 Nanoparticles as Functional Building Blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef]
- Manseki, K.; Saka, K.; Matsui, M.; Vafaei, S.; Sugiura, T. Structure identification of Ti(iv) clusters in low-temperature TiO2 crystallization: Creating high-surface area brush-shaped rutile TiO2. CrystEngComm 2017, 19, 5844–5848. [Google Scholar] [CrossRef]
- Vafaei, S.; Springaire, L.; Schnupf, U.; Hisae, K.; Hasegawa, D.; Sugiura, T.; Manseki, K. Low temperature synthesis of anatase TiO2 nanocrystals using an organic-inorganic gel precursor. Powder Technol. 2020, 368, 237–244. [Google Scholar] [CrossRef]
- Jena, A.-K.; Kulkarni, A.; Miyasaka, T. Halide perovskite photovoltaics: Background, status, and future prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-J.; Seo, G.; Chua, M.R.; Park, T.-G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.-S.; Jeon, N.-J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Yun, A.-J.; Kim, J.; Hwang, T.; Park, B. Origins of Efficient Perovskite Solar Cells with Low-Temperature Processed SnO2 Electron Transport Layer. ACS Appl. Energy Mater. 2019, 2, 3554–3560. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Wang, K.; Wu, C.; Zhu, X.; Feng, J.; Ren, X.; Fang, G.; Priya, S.; Liu, S.-F. High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2. Nat. Commun. 2018, 9, 3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-Y.; Chueh, C.-C.; Zhu, Z.; Chen, W.-C.; Jen, A.-K.-Y. Low-temperature electrodeposited crystalline SnO2 as an efficient electron-transporting layer for conventional perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 164, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, M.; Ramaprabhu, S. Solar synthesized tin oxide nanoparticles dispersed on graphene wrapped carbon nanotubes as a Li ion battery anode material with improved stability. RSC Adv. 2017, 7, 13789–13797. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-E.; Katayanagi, Y.; Kishi, T.; Yano, T.; Matsushita, N. A solution-processed tin dioxide film applicable as a transparent and flexible humidity sensor. RSC Adv. 2018, 8, 30310–30319. [Google Scholar] [CrossRef] [Green Version]
- Kwoka, M.; Lyson-Sypien, B.; Comini, E.; Krzywiecki, M.; Waczynski, K.; Szuber, J. Surface properties of SnO2 nanolayers prepared by spin-coating and thermal oxidation. Nanotechnology 2020, 31, 315714. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wan, Y.; Yin, S. Oxygen vacancies confined in SnO2 nanoparticles for desirable electronic structure and enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 420, 399–406. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Liu, X.; Zhang, J. Oxygen Vacancies Enabled Porous SnO2 Thin Films for Highly Sensitive Detection of Triethylamine at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 20704–20713. [Google Scholar] [CrossRef]
- Jamshidian, M.; Thamburaja, P.; Rabczuk, T. A multiscale coupled finite-element and phase-field framework to modeling stressed grain growth in polycrystalline thin films. J. Comput. Phys. 2016, 327, 779–798. [Google Scholar] [CrossRef]
- Entradas, T.; Cabrita, J.-F.; Dalui, S.; Nunes, M.-R.; Monteiro, O.-C.; Silvestre, A.-J. Synthesis of sub-5 nm Co-doped SnO2 nanoparticles and their structural, microstructural, optical and photocatalytic properties. Mater. Chem. Phys. 2014, 147, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Kamble, V.-B.; Umarji, A.-M. Defect induced optical bandgap narrowing in undoped SnO2 nanocrystals. AIP Adv. 2013, 3, 082120. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).