Abstract
Amine-functionalized metal-organic frameworks (MOFs) are a promising strategy for the high-efficiency capture and separation of CO2. In this work, by tuning the ratio of 1,3,5-benzenetricarboxylic acid (H3BTC) to 5-aminoisophthalic acid (5-NH2-H2IPA), we designed and synthesized a series of amine-functionalized highly stable Ti-based MOFs (named MIP-207-NH2-n, in which n represents 15%, 25%, 50%, 60%, and 100%). The structural analysis shows that the original framework of MIP-207 in the MIP-207-NH2-n (n = 15%, 25%, and 50%) MOFs remains intact when the mole ratio of ligand H3BTC to 5-NH2-H2IPA is less than 1 to 1 in the resulting MOFs. By the introduction of amino groups, MIP-207-NH2-25% demonstrates outstanding CO2 capture performance up to 3.96 and 2.91 mmol g−1, 20.7% and 43.3% higher than those of unmodified MIP-207 at 0 and 25 °C, respectively. Furthermore, the breakthrough experiment indicates that the dynamic CO2 adsorption capacity and CO2/N2 separation factors of MIP-207-NH2-25% are increased by about 25% and 15%, respectively. This work provides an additional strategy to construct amine-functionalized MOFs with the maintenance of the original MOF structure and high performance of CO2 capture and separation.
1. Introduction
More than 85% of the worldwide energy demand is provided by the combustion of fossil fuels [1,2], but at the cost of considerable CO2 (3 × 1013 kg CO2 per year) being emitted into the atmosphere, thus leading to the daunting greenhouse effect [3,4,5]. The carbon capture and storage/sequestration (CCS) technology therefore has been proposed to mitigate emissions of atmospheric CO2. For CCS technology, the breakthrough of novel adsorbents with a large CO2 working capacity as well as a high CO2 selectivity and easy regeneration is the core [6,7,8].
Metal-organic frameworks (MOFs) have been widely used for various applications owing to their ordered crystallinity, high specific surface area, and versatile tunability of chemical environments [9,10,11,12,13,14,15,16,17]. In particular, they can serve as attractive platforms for CO2 adsorption and separation to mitigate the greenhouse effect [18,19,20,21,22,23,24,25]. It is widely acknowledged that amine-functionalized MOFs are one of the most effective ways to capture CO2, because this method has the advantages of a large working CO2 capacity as well as a high CO2 selectivity and a low energy penalty for regeneration [7,26]. Currently, many MOF materials have been functionalized by the direct synthesis or post-synthesis modification method to graft amine [27,28,29,30]. For example, Kim et al. [28] prepared a robust tetraamine-functionalized Mg-MOF by the post-synthesis strategy. The tetraamine-functionalized framework showed an excellent CO2 trapping efficiency under low CO2 partial pressure in the flue stream. Han et al. [31] used a one-step hydrothermal method to synthesize MIL-101(Cr)-NH2 nanoparticles. Compared with MIL-101(Cr), the CO2 adsorption capacity of MIL-101(Cr)-NH2 increased from 1.85 to 2.25 mmol g−1. Moreover, the separation factor of CO2/N2 was enhanced from 7.5 to 11.6 at 1 atm and 35 °C. Recently, Zhong et al. [32] introduced three kinds of organic amine molecules into the channels of MIL-101(Cr) by the post-modification method. The results showed that the CO2/CO selectivity of the Tris(2-aminoethyl) amine-modified MIL-101 was 103 times higher than that of its pure MIL-101 counterpart.
In this work, the MIP-207, fabricated by Ti–O clusters and the H3BTC ligand [21,33], was selected as the porous material, mainly due to its large specific surface area and high chemical stability even in highly acidic media (pH ≤ 0). More importantly, there are uncoordinated and isolated -COOH groups toward the channels of MIP-207 because of the meta-connection mode of the H3BTC ligand, so the chemical environment of MIP-207 cavities can be easily modulated by the mixed linkers strategy [33]. There are quite a few reports on amine-grafted highly stable MIP-207 for highly efficient CO2 capture [33]. Additionally, the accurate grafting of amine molecules without any framework destruction of the host frameworks is less of a concern. Herein, we for the first time prepared a series of amine-functionalized highly stable MIP-207 materials and further tailored the content of -NH2 by the mixed linkers strategy for capturing CO2 from N2. It should be pointed out that amine-functionalized MIP-207 materials cannot be obtained when the mole ratio of H3BTC to 5-NH2-H2IPA exceeds 1 (See Figure 1). The physiochemical properties of the as-prepared materials were systematically characterized and analyzed, and the CO2 adsorption and separation were also investigated. Based on the excellent CO2 capture performance of MIP-207-NH2-25%, the breakthrough experiments further evaluated the dynamic adsorption capacity and separation factors under different gas flow rates.
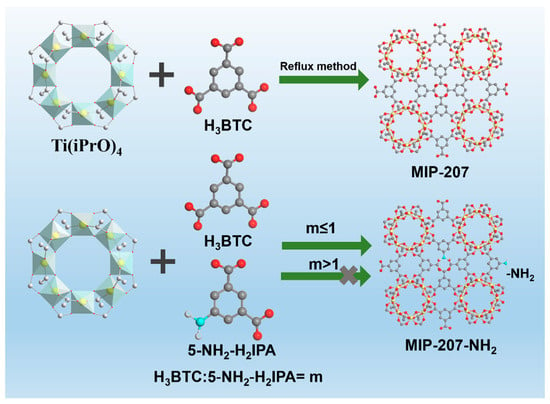
Figure 1.
Schematic Diagram of MIP-207 and amine-functionalized MIP-207; Ti is shown in yellow, C in gray, O in red, N in light blue, and H in white.
2. Experimental Section
2.1. Synthesis of MIP-207
All the reagents used were commercially purchased without further purification. MIP-207 was synthesized in a similar method to the one reported [33]. 1,3,5-benzenetricarboxylic acid (H3BTC), acetic acid, and acetic anhydride were purchased from Maclin, Shanghai, China. Tetraisopropyl titanate was purchased from Sinopharm Chemical Reagent Co., Ltd., Beijing, China. H3BTC (840 mg, 4 mmol), acetic acid (10 mL) and acetic anhydride (10 mL) were added and mixed into a 50 mL round-bottom flask at ambient temperature. Then, tetraisopropyl titanate (800 μL, 2.7 mmol) was added under stirring. The mixture was refluxed at 120 °C for 12 h. After cooling to room temperature, the crude product was separated and washed with boiling anhydrous acetone. Finally, the product was collected by centrifugation and placed in an 80 °C oven for 12 h.
2.2. Synthesis of Amine-Functionalized MIP-207
The amine-functionalized MIP-207 was prepared with a pre-synthesis modification method. Part of the H3BTC ligand was replaced with a certain amount of 5-NH2-H2IPA, which accounted for 15%, 25%, 50%, 60%, and 100% of the total H3BTC, respectively. A series of amine-functionalized MIP-207 materials were synthesized according to the above synthetic steps of MIP-207, and the as-prepared materials were denoted as MIP-207-NH2-n (n = 15%, 25%, 50%, 60%, and 100%), respectively. It must be pointed out that the structure of MIP-207-NH2-100% is completely different from that of MIP-207; it is still named MIP-207-NH2-100% simply for the purpose of comparison.
2.3. Sample Characterization
Powder X-ray diffraction (PXRD) patterns of the samples were recorded on a Rigaku D/max 2400 X-ray diffractometer equipped with Cu Kα radiation operating at 45 kV and 200 mA. Scanning electron microscopy (SEM) tests were conducted on a Hitachi S4800 electron microscope to observe the morphologies of the samples. A N2 physisorption test was carried out on a Quantachrome Autosorb-iQ (Quantachrome Instruments, Boynton Beach, FL, USA) at −196 °C. The elemental analysis (EA) of the samples was performed on an Elementar Analysensysteme GmbH Vario EL (Analytical Instrumentation Department of the Heraeus technology group, Frankfurt, Germany) analyzer to accurately analyze the percentage content of the C, H, and N elements of samples. The specific surface area of the samples was analyzed according to the Brunauer-Emmett-Teller (BET) method and pore size distribution was calculated using the non-local density functional theory (NLDFT) model.
2.4. Gas Adsorption Measurements
All the gases (N2 and CO2) used were of ultrahigh purity (99.999%) in this study. N2 and CO2 adsorption isotherms were measured by a Quantachrome Autosorb-iQ gas adsorption analyzer up to 1 bar, and the temperatures of 0 and 25 °C were both maintained with an ethylene glycol/H2O bath by a cooling and heating system. Before the measurement, about 100 mg of the adsorbent was degassed at 150 °C for 8 h in vacuum condition. The adsorption and desorption of CO2 cyclic stability was carried out on an SDT Q600 analyzer (TA Instruments, New Castle, DE, USA). Firstly, the sample fully absorbed CO2 at 35 °C for 1 h, and then it was injected with N2 gas at 150 °C for 2 h. The breakthrough experiments were performed on a homemade setup to simulate the actual mixture gas (20 vol% CO2, 20 vol% N2, and balanced gas He) separation to evaluate the dynamic CO2/N2 adsorption performance; the setup diagram of the breakthrough experiment can be found in our previous work [34].
3. Results and Discussion
3.1. Structural Analysis of Samples
As shown in Figure S1, compared with simulated MIP-207, the characteristic diffraction peak positions and relative intensities of MIP-207 after reflux treatment at 120 °C for 12 h fit very well, illustrating that MIP-207 with a high purity was synthesized. Also, Figure S1 exhibits that the structure of MIP-207 was maintained well after activation at 150 °C. To evaluate the influence of the content of -NH2 on the crystal structure of MIP-207, the PXRD patterns of MIP-207-NH2 were obtained, and the results are shown in Figure 2. The comparison of PXRD patterns among 207-NH2-n (n = 0, 15%, 25%, 50%) supported that they were of the same pure phase. However, the internal crystal structure of the amine-modified MIP-207 composites started to change when the mole ratio of H3BTC to 5-NH2-H2IPA was more than 1. As shown in Figure 2, there were still two characteristic diffraction peaks of 5° and 11.5° in the MIP-207-NH2-60%, but peak relative intensities were significantly reduced, showing that most of the crystal structure of MIP-207 in the composites was changed. When the ligand H3BTC was totally replaced by 5-NH2-H2IPA, the characteristic diffraction peaks (Figure S2) of the sample were totally different from those of the original MIP-207, indicating that another crystalline phase was formed due to the transformation of the coordination mode.
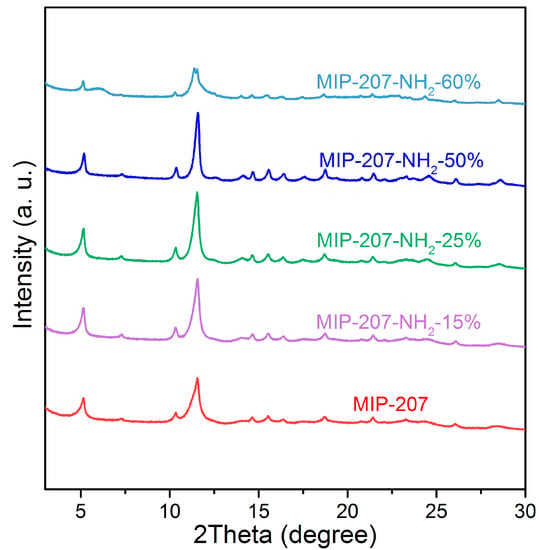
Figure 2.
PXRD pattern of samples.
The SEM images of pristine MIP-207 are presented in Figure S3a,b, and the stacked nanoparticles with a size range of 20–25 nm can be observed. As shown in Figure S3c–e, as the amount of exchange ligand 5-NH2-H2IPA increases, the stacking of nanoparticles becomes loose, and the particle size was also in the range of 20–25 nm in the MIP-207-NH2-n (n = 15%, 25%, 50%) composites, which is consistent with XRD results obtained by the Scherrer equation (Table 1). However, the original morphology of MIP-207 is basically not observed in the MIP-207-NH2-60% (Figure S3f), and particle size sharply reduced to about 15 nm. Overall, based on the above PXRD and SEM analysis, the crystal structure and texture of MIP-207 in the amine-modified MIP-207 composites can be maintained with the amount of added 5-NH2-H2IPA being less than or equal to 50%.

Table 1.
The content of C, H, and N elements of samples and particle size obtained by Scherrer equation.
As shown in Table 1, the N element was not found in the parent MIP-207. The N element was detected and the N content of the amine-modified MIP-207 composites increased with the increase of the added 5-NH2-H2IPA ligand, demonstrating that -NH2 was introduced into the framework of MIP-207 through a mixed linkers strategy. Notably, Table 1 indicates that the N element content in the MIP-207-NH2-n (n = 15%, 25%, 50%) composites was lower than the theoretical value, which is attributed to the electronic effect of the functional group of the ligand. Generally, the electron-donating groups such as -NH2, -OH, and -CH3 are difficult to connect with the second building units of MIP-207 [33]. The theoretical N content of MIP-207-NH2-100% is 3.54%. This value is close to the actual value, while the structure completely changed according to the PXRD results.
The results of the measurement of N2 adsorption and desorption isotherms are shown in Figure 3 and Table 2, and the N2 adsorption–desorption curves of MIP-207 (Figure 3) conform to the typical I-type isotherm characteristics in the low-pressure zone (0–0.6 atm) relating to microporous characteristics [35]. With the increase of pressure, there was a hysteresis loop in the adsorption–desorption curves, indicating the existence of mesopores, which may be caused by the accumulation of materials. The specific surface area of MIP-207 was 563 m2 g−1, where the specific surface area was mainly micropores (534 m2 g−1), which confirms that the mesopores are caused by stacked pores. Figure S4 shows that the average pore size of MIP-207 was mainly distributed at 0.57 and 0.82 nm. Obviously, the BET area and pore volume of the MIP-207-NH2-n (n = 15%, 25%, 50%) materials were higher than that of the unmodified MIP-207 (Table 2), mainly because the mass and volume of the -NH2 group is smaller than the -COOH group, so the BET area of MIP-207-NH2-n (n = 15%, 25%, 50%) increased. Among them, the BET area of MIP-207-NH2-25% was the highest, reaching 735 m2 g−1. On the contrary, the BET area of MIP-207-NH2-60% reduced in comparison with MIP-207. In addition, compared with the MIP-207-NH2-n (n = 15%, 25%, 50%) (Table 2), the pore volume of MIP-207-NH2-60% (0.44 cm3 g−1) decreased. The probable reason is that the original structure of MIP-207 cannot be maintained with the -NH2 increasing to over 60%. The specific surface area of MIP-207-NH2-100% sharply decreased and the micropores almost disappeared (Figure S5 and Table S1), further demonstrating the changes from the MIP-207 framework in MIP-207-NH2-100%.
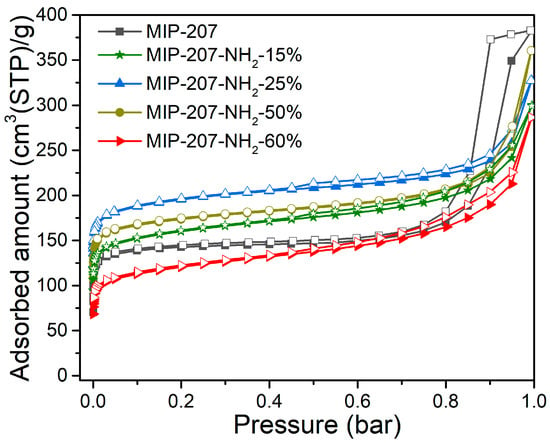
Figure 3.
N2 adsorption and desorption isotherms at −196 °C.

Table 2.
The summary of specific surface area, pore volume, and particle size of samples.
3.2. Gas Adsorption Performance of Materials
The CO2 adsorption data of as-prepared materials over N2 are presented in Figure 4 and Table 3. As can be seen from Table 3, the CO2 adsorption capacity of MIP-207-NH2-25% was up to 3.96 and 2.91 mmol g−1 at 0 and 25 °C, which means an improvement of 20.7% and 43.3% compared with the pure MIP-207, respectively. Moreover, the CO2 capture performance of MIP-207-NH2-25% outperforms most reported amine-modified MOF CO2 adsorbents (Table 3). Similarly, the CO2 adsorption capacity of MIP-207-NH2-50% was higher than that of the unmodified MIP-207. The increase of CO2 adsorption capacity is mainly due to the amine-grafted MIP-207 materials with a high specific area (Figure S6) and many Lewis basic sites (LBS), which greatly enhance their affinity for CO2 [36,37]. Unfortunately, as the added exchange ligand 5-NH2-H2IPA went above 50%, the CO2 working capacity in the MIP-207-NH2-60% adsorbent sharply decreased. One reasonable explanation is that excess 5-NH2-H2IPA slows down the rate of the crystal nucleation formation of MIP-207 and disturbs the self-assembly process. When the ligand reactant is completely 5-NH2-H2IPA, the resulting product cannot even form the original crystal nucleus structure of MIP-207. It can be seen that the adsorption performance is a result of both the adsorption sites and the spatial framework of materials.
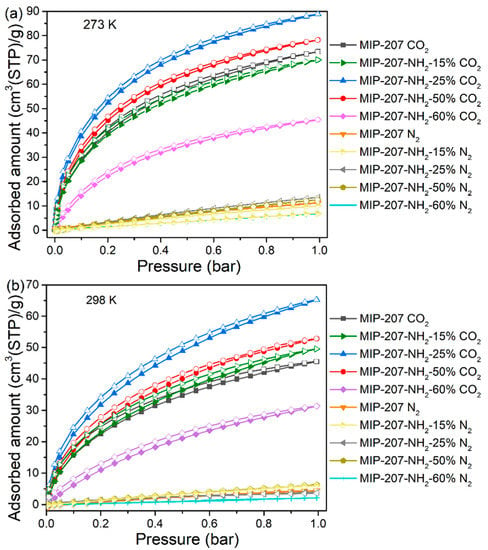
Figure 4.
CO2 and N2 adsorption and desorption isotherms at (a) 0 °C and (b) 25 °C.

Table 3.
The summary of BET area and CO2 adsorption results in this work and reported amine-functionalized MOFs.
To figure out the CO2 adsorption separation performance, the selectivity of CO2/N2 was calculated by the IAST model (Supporting Information). As shown in Figure 5a, compared with the separation factor of MIP-207 (59), the CO2/N2 separation factor of MIP-207-NH2-15% was 69, which was 17% higher than that of MIP-207. Additionally, MIP-207-NH2-25% exhibited the highest CO2/N2 separation factor (77), which was 33% higher than MIP-207, mainly because the introduction of the -NH2 group into the MIP-207 channels could produce more adsorption sites, leading to an enhanced affinity toward CO2. However, the structure of MIP-207-NH2-60% possessed more -NH2 and the separation factor of MIP-207-NH2-60% was only 22, illustrating that the spatial framework of MIP-207 in MIP-207-NH2-60% was destroyed, thus increasing the non-selective uptake. In addition to focusing on the adsorption and separation performance, it is also necessary to take energy consumption into account during the regeneration process in industrial applications [32]. The isosteric heat of CO2 adsorption (Qst) of the materials was obtained from the CO2 adsorption isotherms at 0 °C. As shown in Figure 5b, the MIP-207-NH2-n (n = 15%, 25%, 50%) adsorbents had an isosteric adsorption heat of about 30–35 kJ mol−1, which exhibits a medium-strength interaction with CO2. It can also be found that the Qst of MIP-207-NH2-60% was significantly lower than that of the above four materials, which indicates that the framework structure of MIP-207-NH2-60% has a negative effect on the adsorption capacity of CO2. Considering that the MIP-207-NH2-25% exhibited superior CO2 adsorption performance with a remarkable adsorption heat, a test of the cyclic stability of CO2 was performed and the results are displayed in Figure 6. The CO2 adsorption capacity of MIP-207-NH2-25% after six cycles did not significantly decrease, indicating that MIP-207-NH2-25% has an outstanding CO2 adsorption–desorption stability.
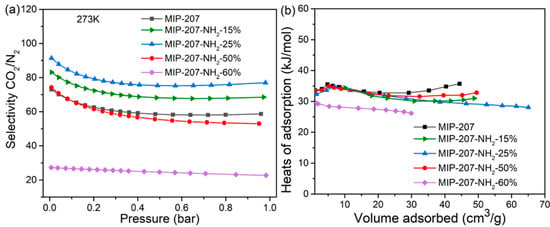
Figure 5.
(a) CO2/N2 selectivity at 0 °C and (b) CO2 adsorption enthalpy curves.
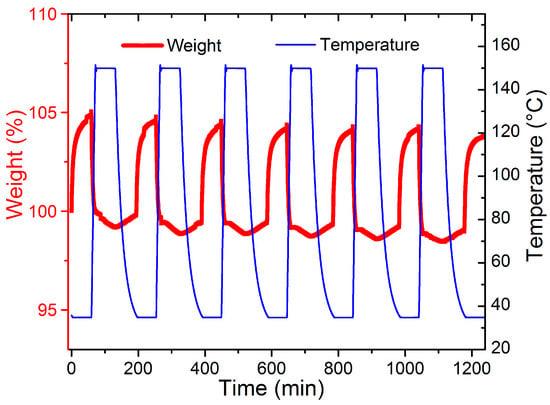
Figure 6.
CO2 adsorption and desorption cycle of MIP-207-NH2-25%.
The results of the dynamic CO2/N2 adsorption of both MIP-207-NH2-25% and MIP-207 (as a comparison) are shown in Figure 7. There was an obvious difference in breakthrough time between CO2 and N2 under different gas flow rates. After an initial period where the N2 and CO2 were fully absorbed, the N2 preferentially penetrated the adsorption bed, followed by the CO2. The outlet concentration of N2 exceeded the inlet concentration because CO2 adsorption equilibrium was not reached. Finally, CO2 began to be eluted and the concentration of N2 and CO2 gradually reached the feed concentration value (c/c0 = 1), indicating that the adsorption bed was saturated. The interval of breakthrough time between CO2 and N2 in MIP-207-NH2-25% was longer than that of MIP-207 at any gas flow rate, especially at 10 sccm, which fully demonstrates that MIP-207-NH2-25% has a better dynamic separation performance. The reason is that the electric field of the MIP-207-NH2-25% framework has the stronger interaction with CO2 due to the presence of LBSs and the hydrogen bond [37,39,40,41]. Moreover, from Figure 7, MIP-207-NH2-25% has a larger slope than MIP-207 under different gas flow rates, indicating that the mass transfer resistance of gas in MIP-207-NH2-25% is smaller, which is more conducive to gas diffusion and spread. The possible reason for this is that MIP-207-NH2-25% has a larger pore volume.
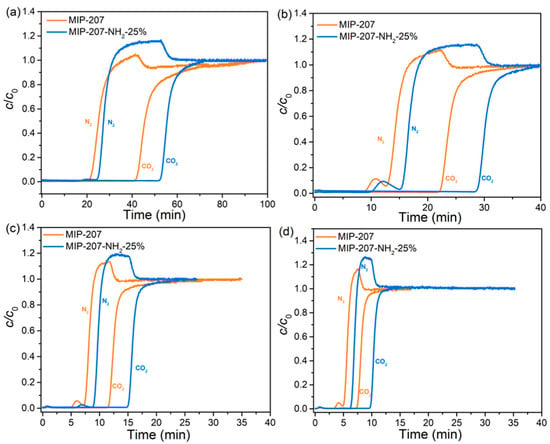
Figure 7.
CO2 and N2 breakthrough curves of MIP-207 and MIP-207-NH2-25% at different gas flow rates: (a) 10 sccm, (b) 20 sccm, (c) 50 sccm, and (d) 100 sccm, respectively.
The dynamic equilibrium adsorption capacity and separation factor of both MIP-207 and MIP-207-NH2-25% were calculated based on previous reports [34,42]. As shown in Figure 8, it can be found that the CO2 equilibrium adsorption capacity of MIP-207-NH2-25% was higher than that of MIP-207 under the four mixed gas flow rates (Figure 8a,b). At 10 and 20 sccm, the CO2/N2 separation factors of MIP-207-NH2-25% were 2.36 and 2.03, which were higher than the 2.01 and 1.93 of MIP-207, respectively. The difference of separation factors between MIP-207 and MIP-207-NH2-25% cannot be clearly observed at the mixture gas flow rates of 50 and 100 sccm (Figure 8a,b). This is because the residence time of gas in the adsorption bed decreases with the increase of flow rate.
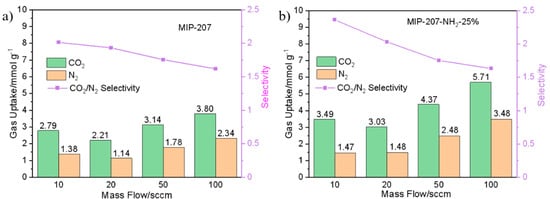
Figure 8.
Dynamic CO2 and N2 adsorption and separation performance of (a) MIP-207 and (b) MIP-207-NH2-25%.
4. Conclusions
In summary, the amine-modified highly stable MIP-207 with different -NH2 content was successfully prepared by the mixed linkers method. The texture and structure framework of the original MIP-207 were maintained in the MIP-207-NH2-n (n = 15%, 25%, 50%) composites. The CO2 adsorption and breakthrough experiments show that MIP-207-NH2-25% demonstrates the superior CO2 capture and separation performance. The highly efficient CO2 uptake is attributed to the introduction of -NH2 into the framework of MIP-207, leading to the increase of specific surface area and more Lewis basic adsorption sites, thereby enhancing the CO2 working capacity and CO2/N2 selectivity. This work provides an additional avenue to prepare highly stable amine-functionalized MOFs for high efficiency CO2 capture.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11123348/s1, Figure S1: PXRD patterns of MIP-207 and MIP-207 after activation at 150 °C. Figure S2: PXRD pattern of MIP-207-NH2-100%. Figure S3: SEM images of (a,b) MIP-207, (c) MIP-207-NH2-15%, (d) MIP-207-NH2-25%, (e) MIP-207-NH2-50%, and (f) MIP-207-NH2-60%. Figure S4: The pore size distribution curves of MIP-207. Figure S5: N2 adsorption and desorption isotherms of MIP-207-NH2-100%. Figure S6: The relationship of between CO2/N2 adsorption and specific surface area of samples. Table S1: The BET data of MIP-207-NH2-100%.
Author Contributions
Data curation, Y.W. (Yinji Wan), Y.M., D.K., Y.W. (Yingxiao Wu) and J.S.; formal analysis, Y.W. (Yinji Wan); funding acquisition, R.Z. (Ruiqin Zhong) and R.Z. (Ruqiang Zou); investigation, T.Q. and Q.Z.; methodology, T.Q.; resources, R.Z. (Ruqiang Zou); supervision, R.Z. (Ruiqin Zhong); writing—original draft, Y.W. (Yinji Wan); writing—review and editing, R.Z. (Ruiqin Zhong) and R.Z. (Ruqiang Zou). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 51772329 and 51972340.
Data Availability Statement
All data are available upon reasonable request.
Acknowledgments
The authors extend much thanks to the reviewers for their valuable suggestions that have helped improve our paper substantially.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.G.; Chidambaram, A.; Garcia-Diez, E.; Ireland, C.P.; Daff, T.D.; Bounds, R.; Gladysiak, A.; Schouwink, P.; Moosavi, S.M.; Maroto-Valer, M.M.; et al. Data-driven design of metal-organic frameworks for wet flue gas CO2 capture. Nature 2019, 576, 253–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sovacool, B.K.; Griffiths, S.; Kim, J.; Bazilian, M. Climate change and industrial F-gases: A critical and systematic review of developments, sociotechnical systems and policy options for reducing synthetic greenhouse gas emissions. Renew. Sustain. Energy Rev. 2021, 141, 110759. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.M.; Dewil, R.; Baeyens, J.; Fan, X.F. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 19. [Google Scholar] [CrossRef]
- Zeng, Y.; Zou, R.; Zhao, Y. Covalent Organic Frameworks for CO2 Capture. Adv. Mater. 2016, 28, 2855–2873. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Inamuddin; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-organic frameworks (MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.S.; Zhang, M.; Zou, R.; Xu, Q. Metal-Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef]
- Zou, R.; Li, P.-Z.; Zeng, Y.-F.; Liu, J.; Zhao, R.; Duan, H.; Luo, Z.; Wang, J.-G.; Zou, R.; Zhao, Y. Bimetallic Metal-Organic Frameworks: Probing the Lewis Acid Site for CO2 Conversion. Small 2016, 12, 2334–2343. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal-organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal-Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Bai, L.; Ganguly, R.; Zou, R.; Zhao, Y. Rational Design and Synthesis of a Highly Porous Copper-Based Interpenetrated Metal-Organic Framework for High CO2 and H2 Adsorption. ChemPlusChem 2015, 80, 1259–1266. [Google Scholar] [CrossRef]
- Zheng, B.; Bai, J.; Duan, J.; Wojtas, L.; Zaworotko, M.J. Enhanced CO2 Binding Affinity of a High-Uptake rht-Type Metal-Organic Framework Decorated with Acylamide Groups. J. Am. Chem. Soc. 2011, 133, 748–751. [Google Scholar] [CrossRef]
- Hong, D.-Y.; Hwang, Y.K.; Serre, C.; Ferey, G.; Chang, J.-S. Porous Chromium Terephthalate MIL-101 with Coordinatively Unsaturated Sites: Surface Functionalization, Encapsulation, Sorption and Catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Martinez, F.; Sanz, R.; Orcajo, G.; Briones, D.; Yangueez, V. Amino-impregnated MOF materials for CO2 capture at post-combustion conditions. Chem. Eng. Sci. 2016, 142, 55–61. [Google Scholar] [CrossRef]
- Kong, L.; Zou, R.; Bi, W.; Zhong, R.; Mu, W.; Liu, J.; Han, R.P.S.; Zou, R. Selective adsorption of CO2/CH4 and CO2/N2 within a charged metal–organic framework. J. Mater. Chem. A 2014, 2, 17771–17778. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P.-Z.; Guo, W.; Zhao, Y.; Zou, R. Titanium-based metal-organic frameworks for photocatalytic applications. Coord. Chem. Rev. 2018, 359, 80–101. [Google Scholar] [CrossRef]
- Wang, L.; Zou, R.; Guo, W.; Gao, S.; Meng, W.; Yang, J.; Chen, X.; Zou, R. A new microporous metal-organic framework with a novel trinuclear nickel cluster for selective CO2 adsorption. Inorg. Chem. Commun. 2019, 104, 78–82. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, W.; Guo, W.; An, L.; Xia, D.; Zou, R. Functional Zeolitic-Imidazolate-Framework-Templated Porous Carbon Materials for CO2 Capture and Enhanced Capacitors. Chem. Eur. J. 2013, 8, 1879–1885. [Google Scholar] [CrossRef]
- Xiang, Z.; Hu, Z.; Cao, D.; Yang, W.; Lu, J.; Han, B.; Wang, W. Metal-Organic Frameworks with Incorporated Carbon Nanotubes: Improving Carbon Dioxide and Methane Storage Capacities by Lithium Doping. Angew. Chem. Int. Ed. 2011, 50, 491–494. [Google Scholar] [CrossRef]
- Ma, S.; Sun, D.; Simmons, J.M.; Collier, C.D.; Yuan, D.; Zhou, H.-C. Metal-organic framework from an anthracene derivative containing nanoscopic cages exhibiting high methane uptake. J. Am. Chem. Soc. 2008, 130, 1012–1016. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Cao, L.Y.; Lin, Z.K.; Peng, F.; Wang, W.W.; Huang, R.Y.; Wang, C.; Yan, J.W.; Liang, J.; Zhang, Z.M.; Zhang, T.; et al. Self-Supporting Metal-Organic Layers as Single-Site Solid Catalysts. Angew. Chem. Int. Ed. 2016, 55, 4962–4966. [Google Scholar] [CrossRef]
- Kim, E.J.; Siegelman, R.L.; Jiang, H.Z.H.; Forse, A.C.; Lee, J.-H.; Martell, J.D.; Milner, P.J.; Falkowski, J.M.; Neaton, J.B.; Reimer, J.A.; et al. Cooperative carbon capture and steam regeneration with tetraamine-appended metal-organic frameworks. Science 2020, 369, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Yu, X.; Meng, W.; Han, S.; Liu, J.; Ye, Y.; Sun, C.; Chen, G.; Zou, R. A solvent ‘squeezing’ strategy to graft ethylenediamine on Cu3(BTC)2 for highly efficient CO2/CO separation. Chem. Eng. Sci. 2018, 184, 85–92. [Google Scholar] [CrossRef]
- Bae, Y.S.; Farha, O.K.; Hupp, J.T.; Snurr, R.Q. Enhancement of CO2/N2 selectivity in a metal-organic framework by cavity modification. J. Mater. Chem. 2009, 19, 2131–2134. [Google Scholar] [CrossRef]
- Han, G.; Qian, Q.H.; Rodriguez, K.M.; Smith, Z.P. Hydrothermal Synthesis of Sub-20 nm Amine-Functionalized MIL-101(Cr) Nanoparticles with High Surface Area and Enhanced CO2 Uptake. Ind. Eng. Chem. Res. 2020, 59, 7888–7900. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Yu, X.; Meng, W.; Liu, J.; Zhi, C.; Zou, R. Amine-Grafted MIL-101(Cr) via Double-Solvent Incorporation for Synergistic Enhancement of CO2 Uptake and Selectivity. ACS Sustain. Chem. Eng. 2018, 6, 16493–16502. [Google Scholar] [CrossRef]
- Wang, S.; Reinsch, H.; Heymans, N.; Wahiduzzaman, M.; Martineau-Corcos, C.; De Weireld, G.; Maurin, G.; Serre, C. Toward a Rational Design of Titanium Metal-Organic Frameworks. Matter 2020, 2, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Zhong, R.; Liu, J.; Huang, X.; Yu, X.; Sun, C.; Chen, G.; Zou, R. Experimental and theoretical investigation of a stable zinc-based metal-organic framework for CO2 removal from syngas. CrystEngComm 2015, 17, 8221–8225. [Google Scholar] [CrossRef]
- Gandara, F.; Furukawa, H.; Lee, S.; Yaghi, O.M. High methane storage capacity in aluminum metal-organic frameworks. J. Am. Chem. Soc. 2014, 136, 5271–5274. [Google Scholar] [CrossRef] [PubMed]
- Darunte, L.A.; Oetomo, A.D.; Walton, K.S.; Sholl, D.S.; Jones, C.W. Direct Air Capture of CO2 Using Amine Functionalized MIL-101(Cr). ACS Sustain. Chem. Eng. 2016, 4, 5761–5768. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, H.; Wang, H.; Suo, Y.; Li, B.; Kong, C.; Chen, L. Enhanced selective CO2 adsorption on polyamine/MIL-101(Cr) composites. J. Mater. Chem. A 2014, 2, 14658–14665. [Google Scholar] [CrossRef]
- Liao, P.-Q.; Zhou, D.-D.; Zhu, A.-X.; Jiang, L.; Lin, R.-B.; Zhang, J.-P.; Chen, X.-M. Strong and Dynamic CO2 Sorption in a Flexible Porous Framework Possessing Guest Chelating Claws. J. Am. Chem. Soc. 2012, 134, 17380–17383. [Google Scholar] [CrossRef]
- Yoo, D.K.; Yoon, T.U.; Bae, Y.S.; Jhung, S.H. Metal-organic framework MIL-101 loaded with polymethacrylamide with or without further reduction: Effective and selective CO2 adsorption with amino or amide functionality. Chem. Eng. J. 2020, 380, 122496. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, W.; Yang, K.; Dai, Y.; Ruan, X.; Yan, X.; He, G. Amino-functional ZIF-8 nanocrystals by microemulsion based mixed linker strategy and the enhanced CO2/N2 separation. Sep. Purif. Technol. 2020, 236, 116209. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, Y. Rate based modeling of absorption and regeneration for CO2 capture by aqueous ammonia solution. Appl. Energy 2013, 111, 142–152. [Google Scholar] [CrossRef]
- Xiang, S.; He, Y.; Zhang, Z.; Wu, H.; Zhou, W.; Krishna, R.; Chen, B. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions. Nat. Commun. 2012, 3, 954. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).