Atomic Oxygen-Resistant Polyimide Composite Films Containing Nanocaged Polyhedral Oligomeric Silsesquioxane Components in Matrix and Fillers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
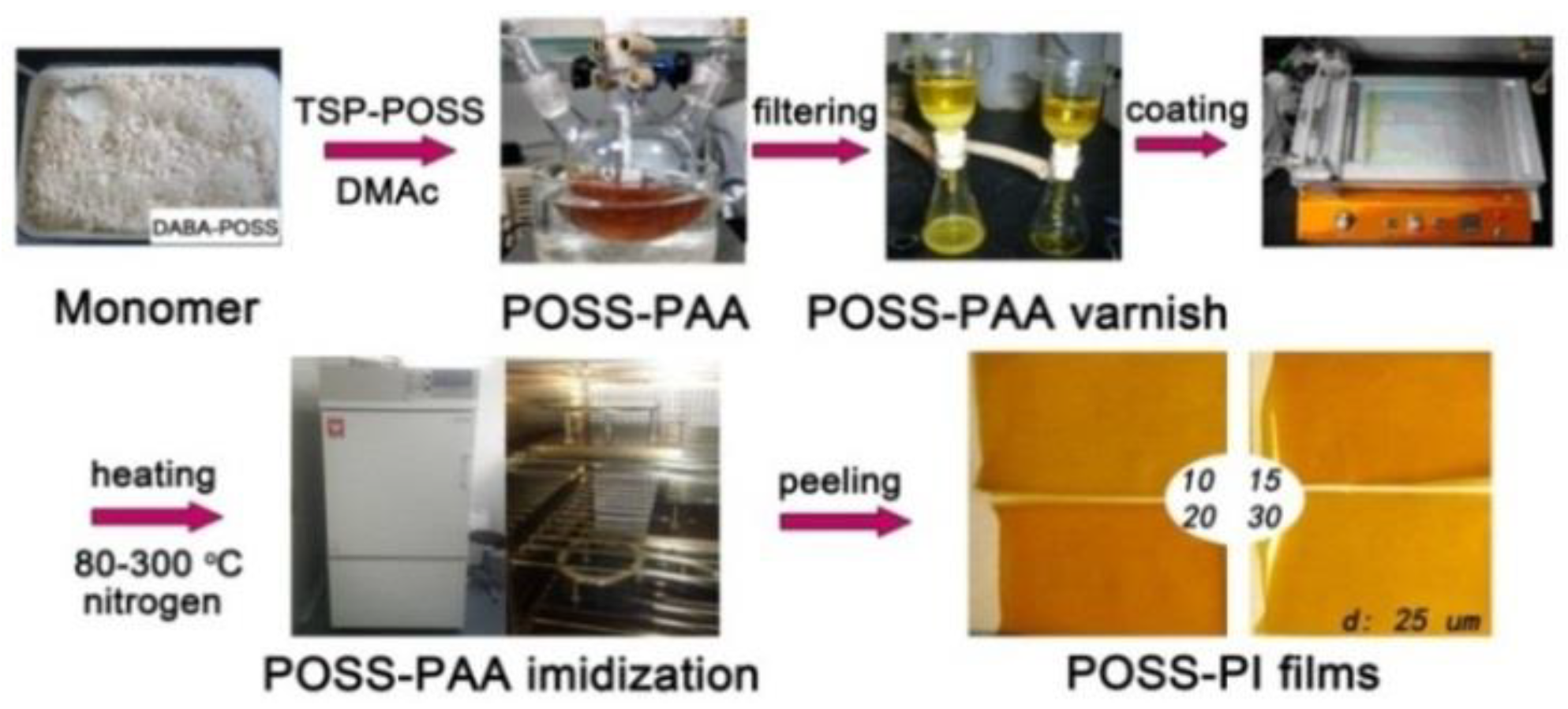
2.3. Synthesis of PAA Varnishes and Preparation of PI Composite Film
3. Results and Discussion
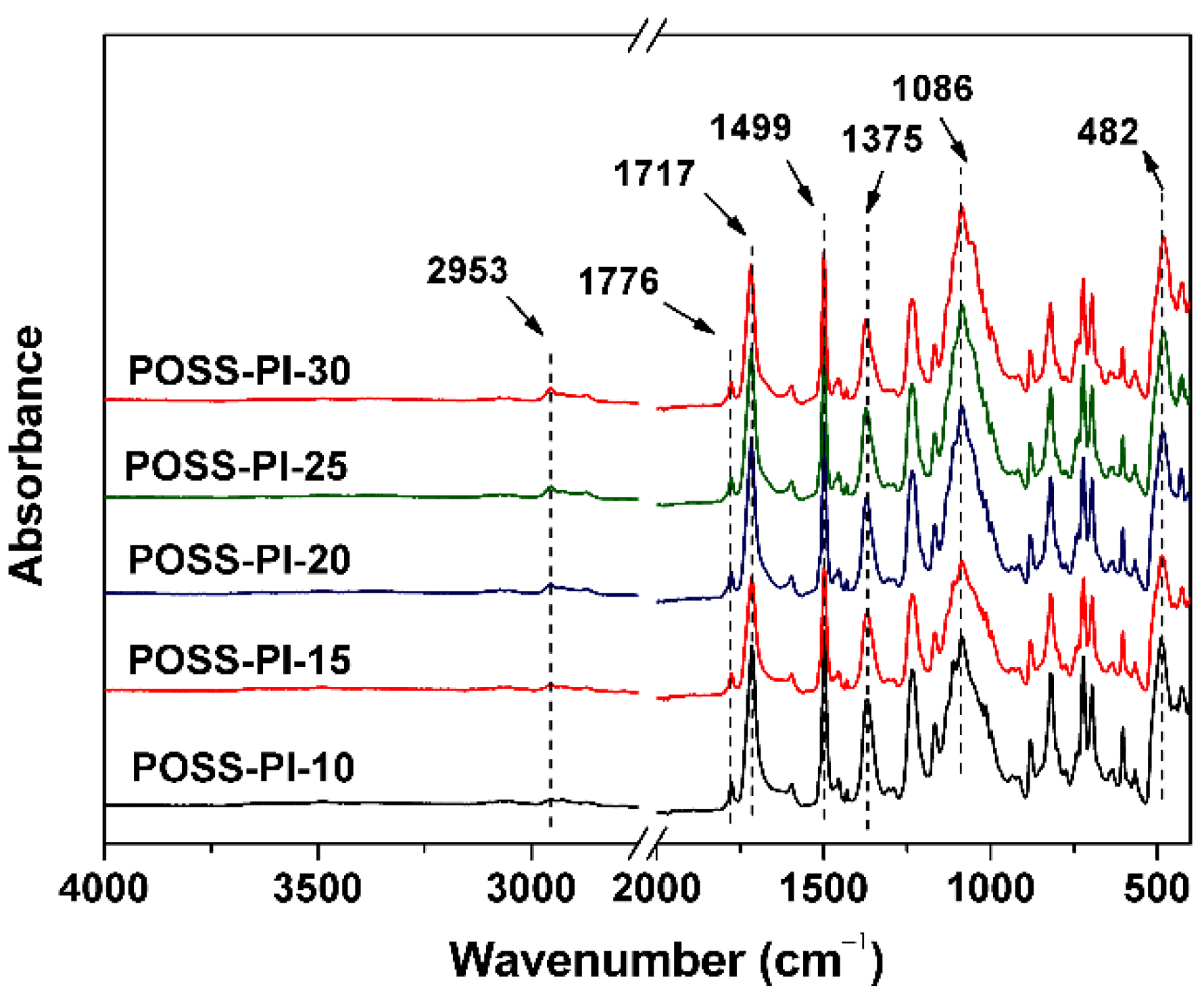
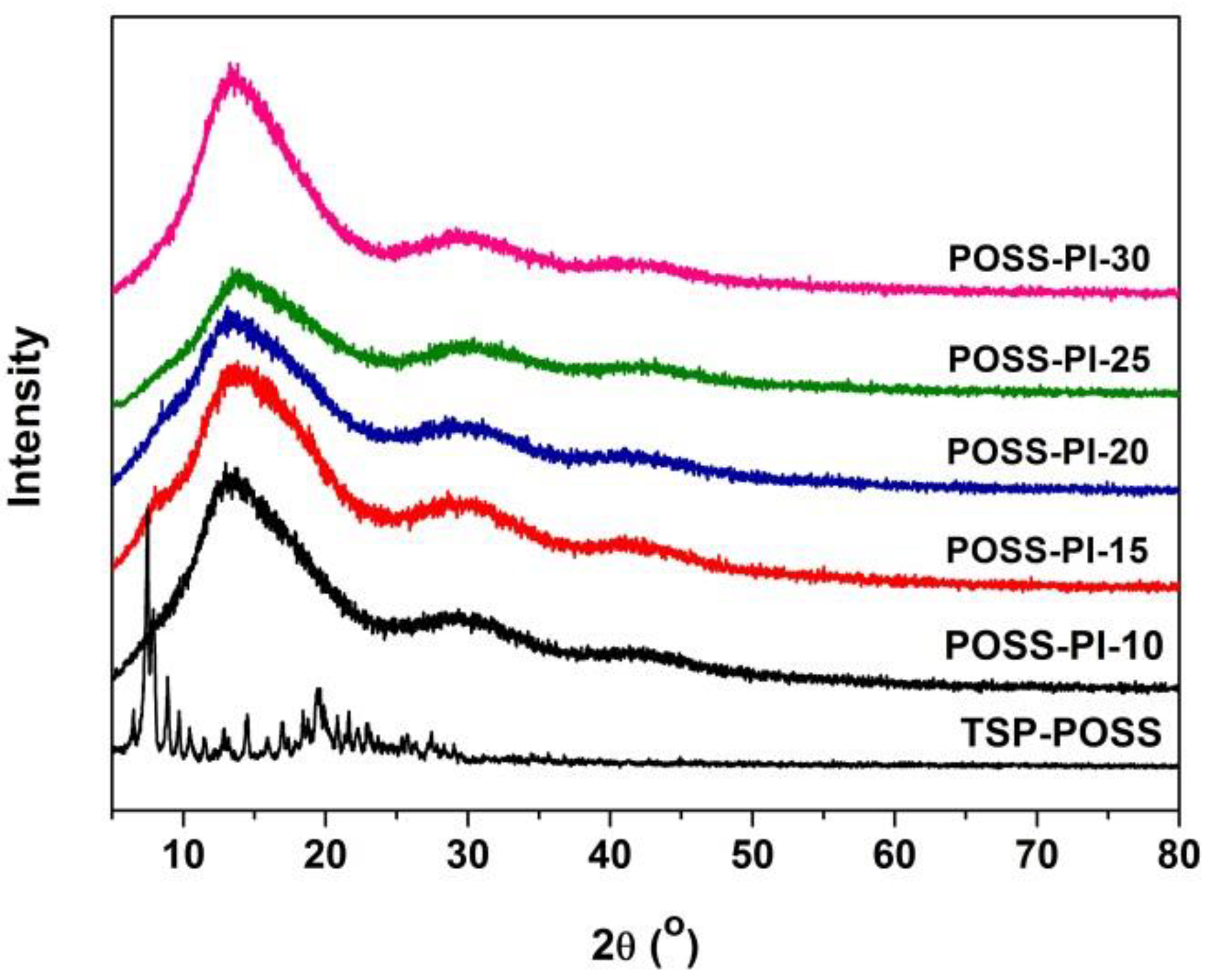
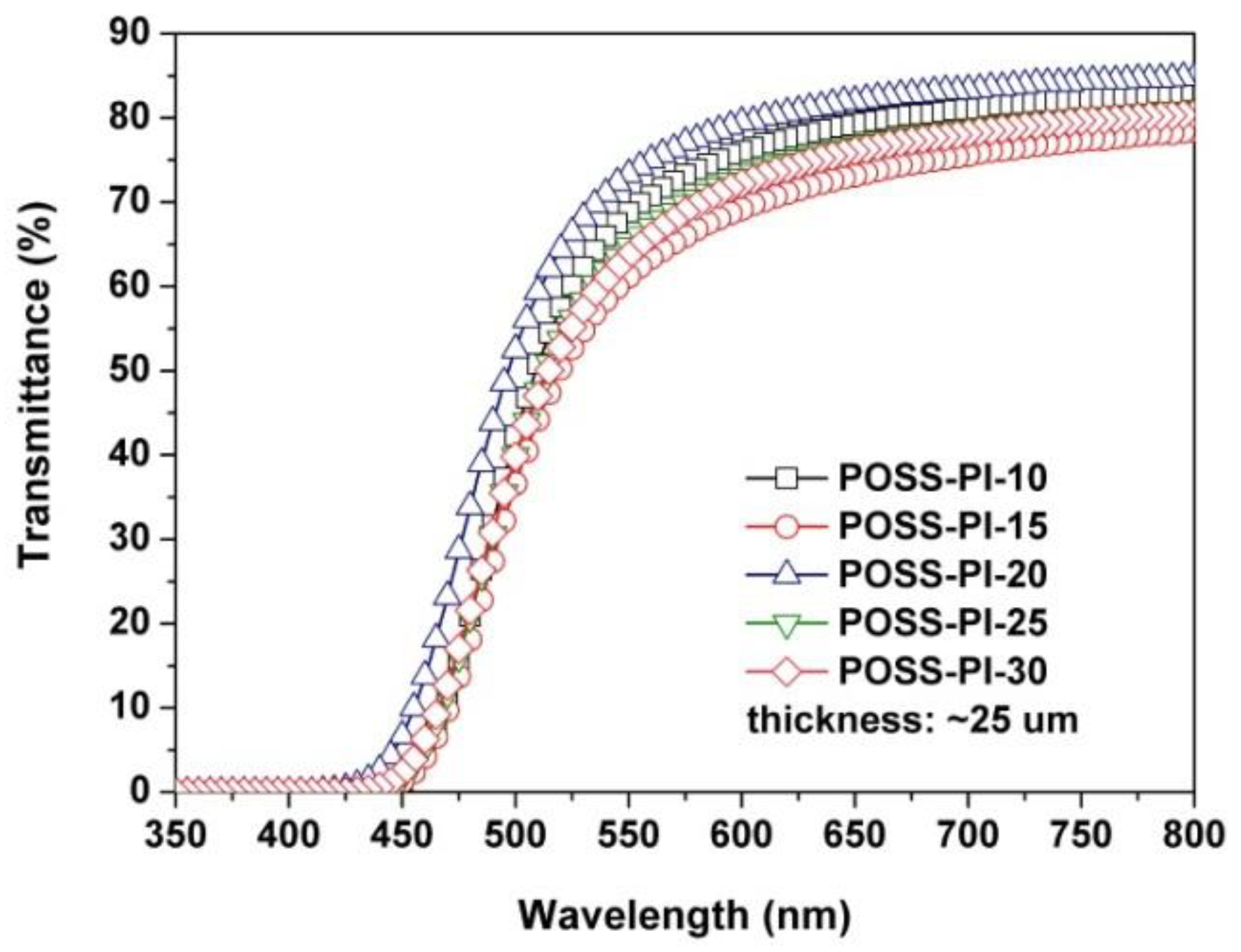
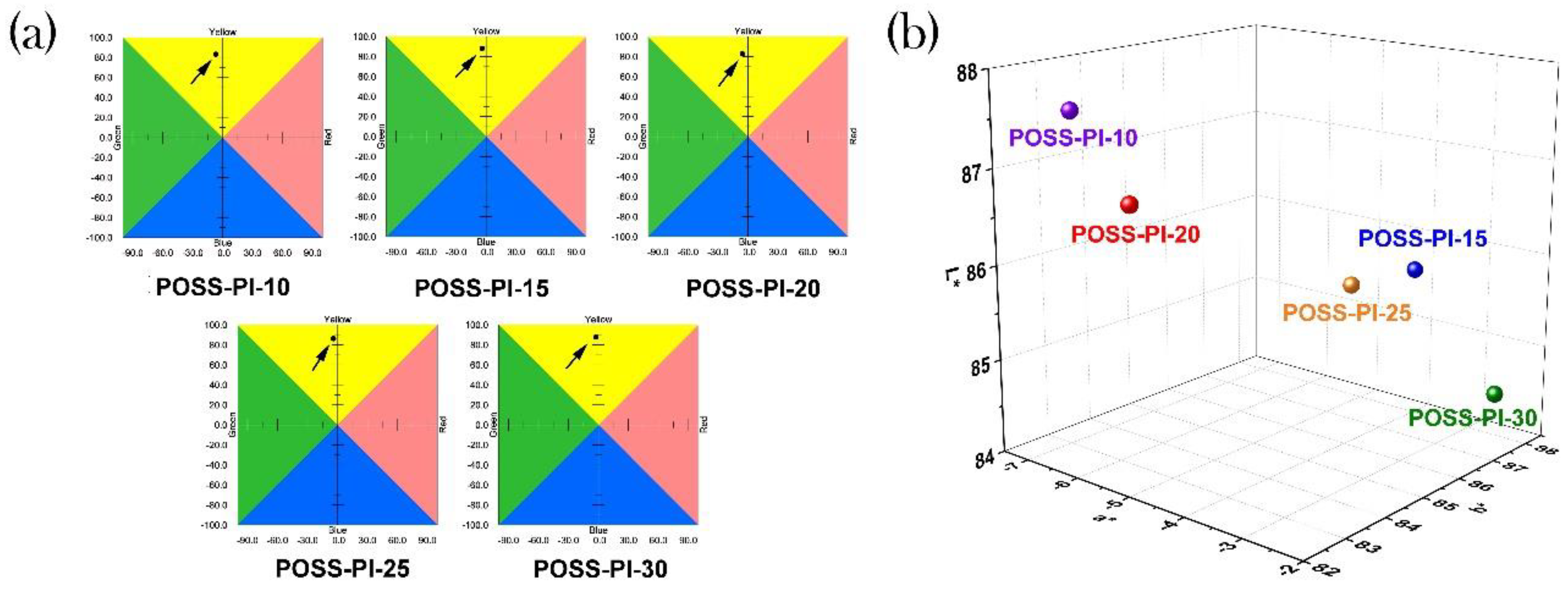
3.1. PI Composite Films Preparation
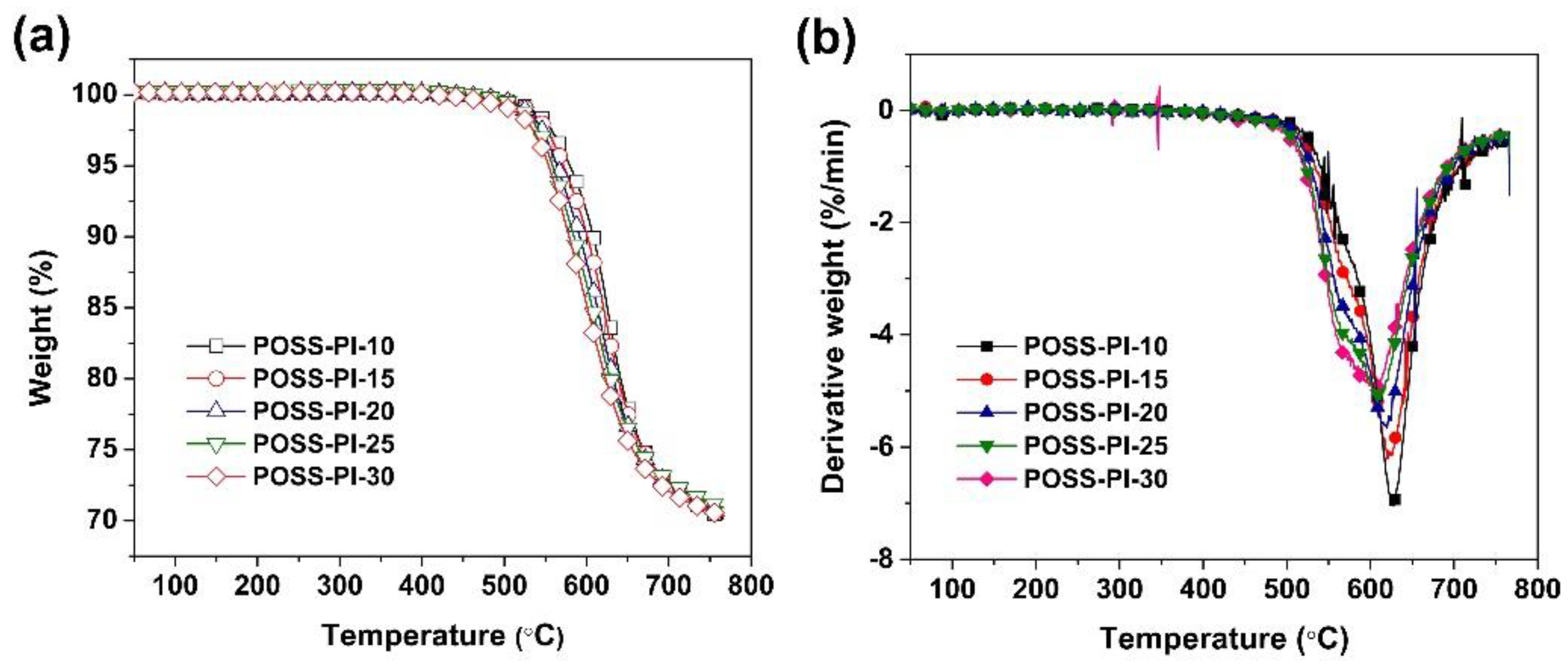
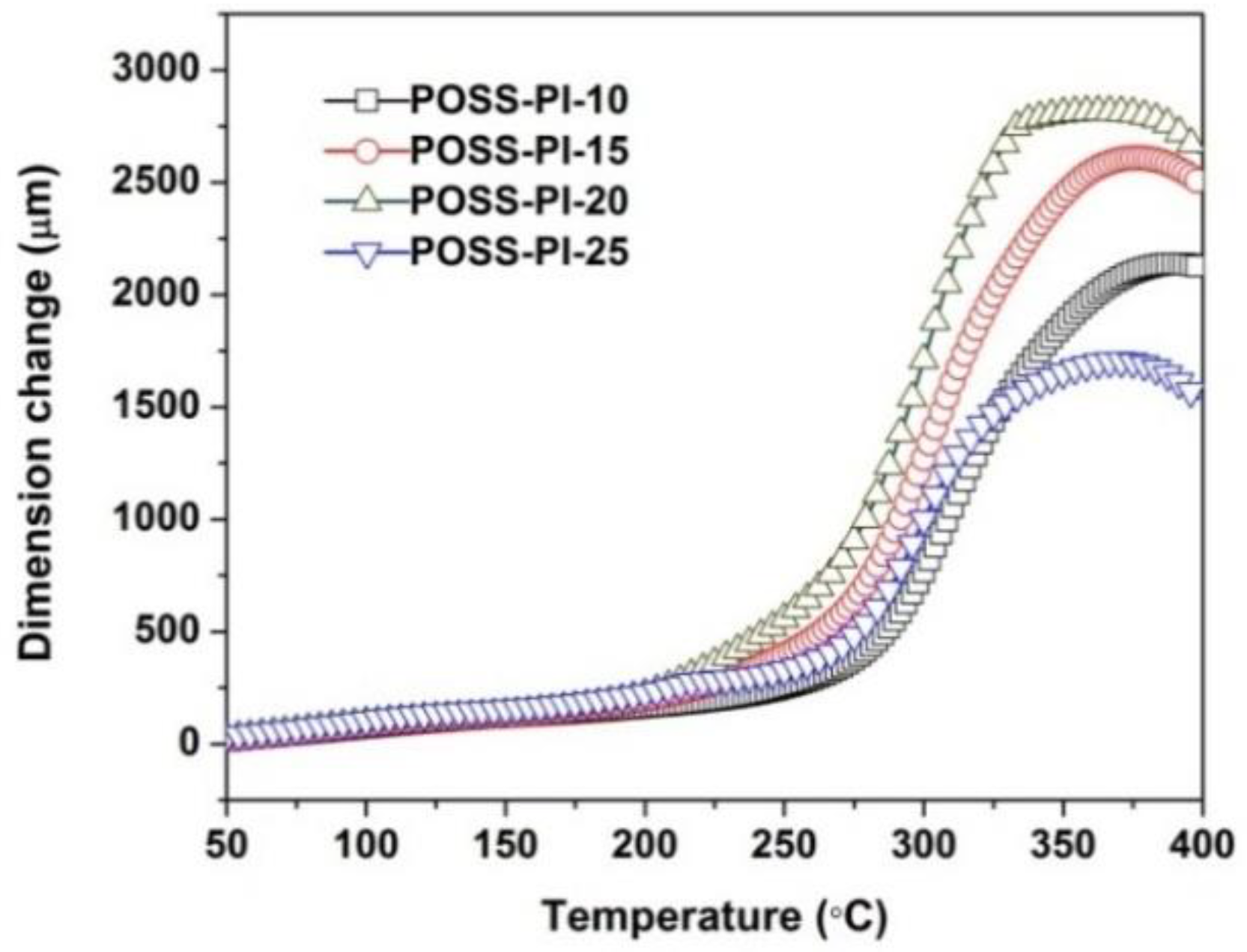
3.2. Thermal Properties
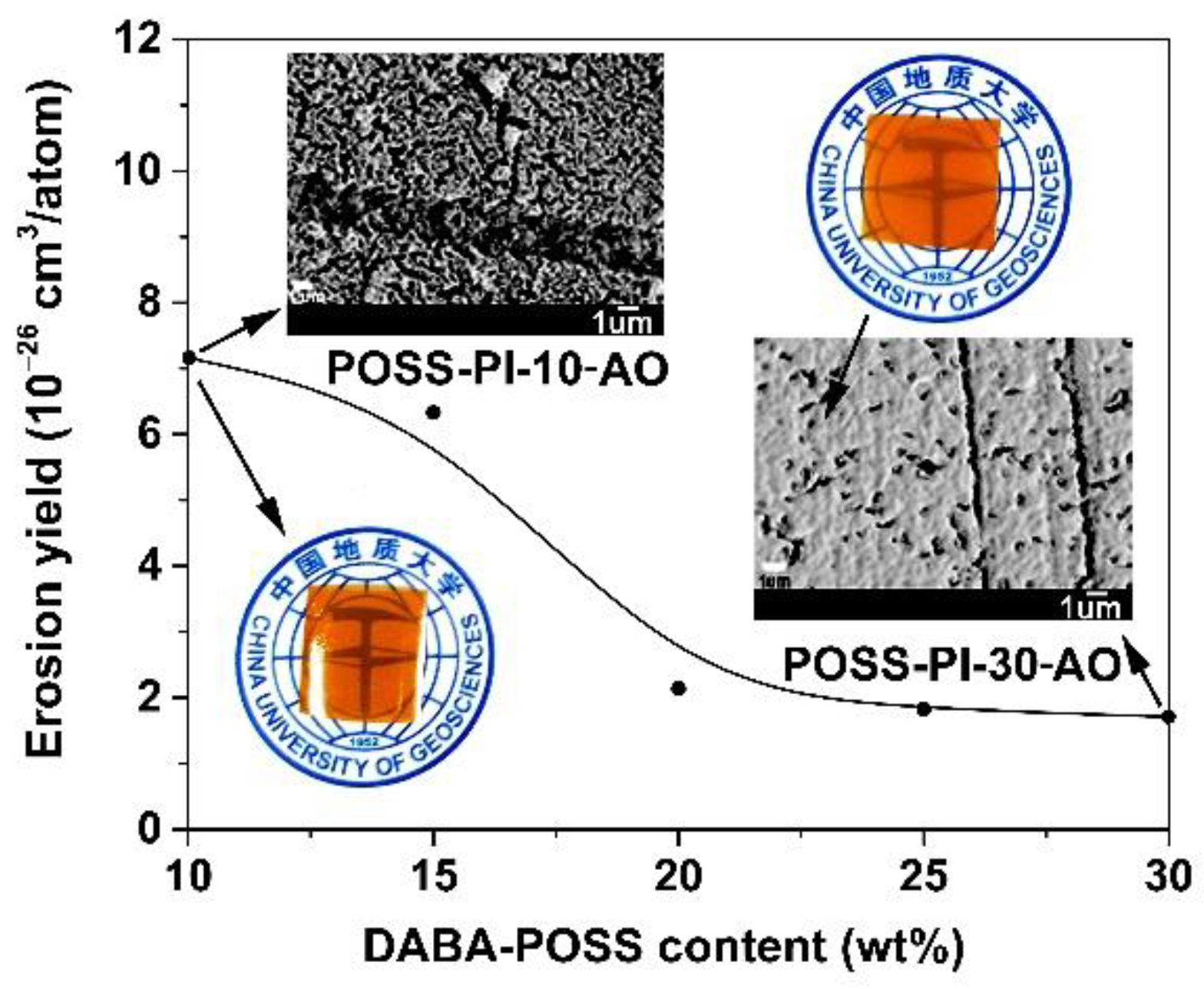
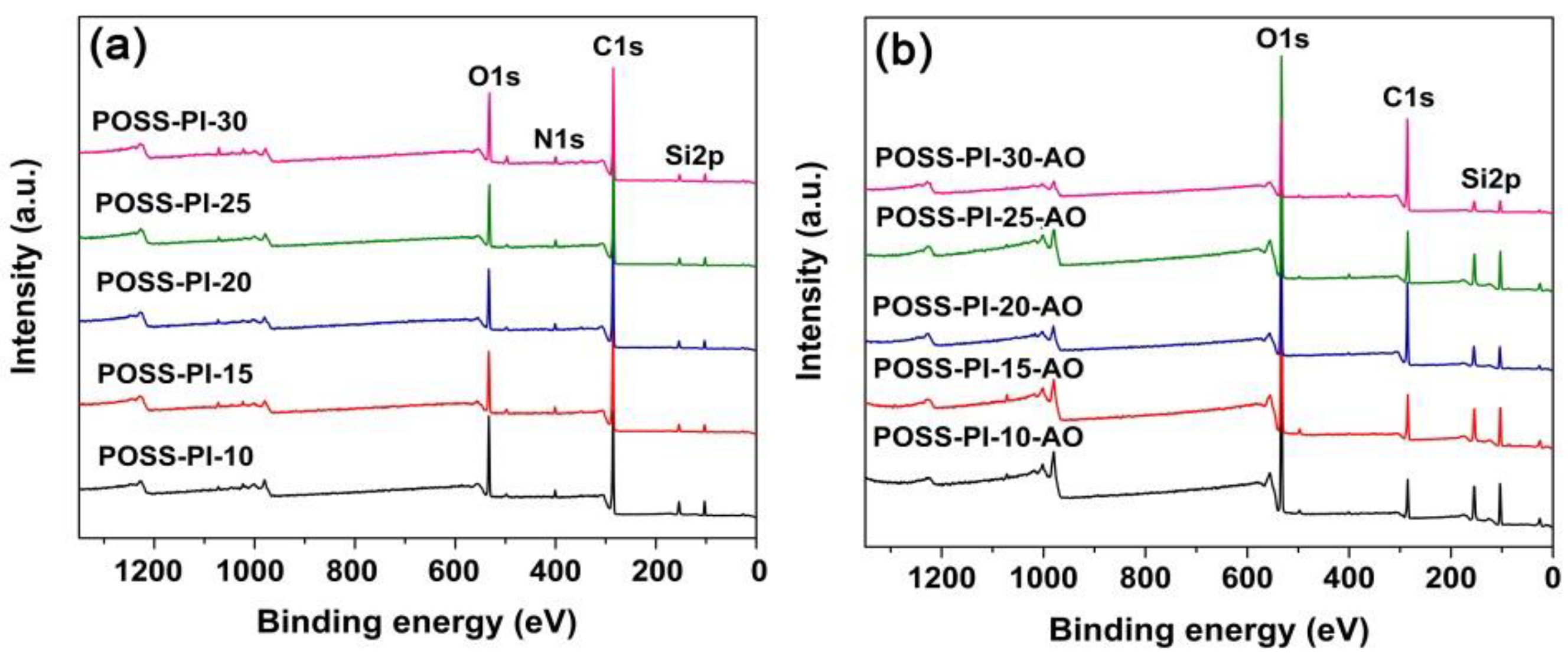
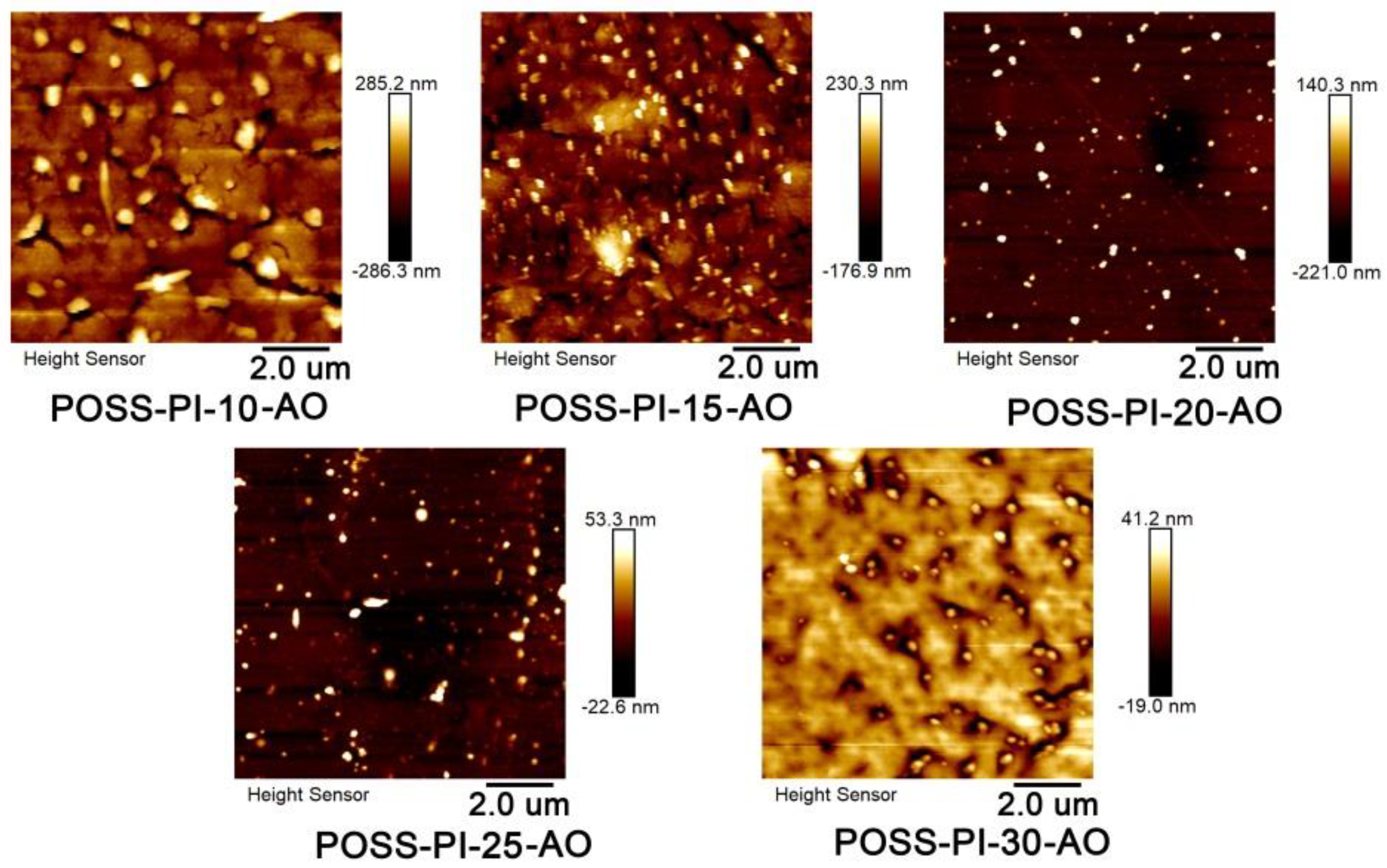
3.3. AO Erosion Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Elia, N. Advances in polyimide—Based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef] [PubMed]
- Samwel, S.W. Low earth orbital atomic oxygen erosion effect on spacecraft material. Space Res. J. 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Minton, T.K.; Wu, B.; Zhang, J.; Lindholm, N.F.; Abdulagatov, A.I.; O’Patchen, J.; George, S.M.; Groner, M.D. Protecting polymers in space with atomic layer deposition coatings. ACS Appl. Mater. Interfaces 2010, 2, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Dever, J.A.; Miller, S.K.; Sechkar, E.A.; Wittberg, T.N. Space environment exposure of polymer films on the materials international space station experiment: Results from MISSE 1 and MISSE 2. High Perform. Polym. 2008, 20, 371–387. [Google Scholar] [CrossRef]
- Miller, S.K.R.; Banks, B.A.; Waters, D.L. Investigation into the differences between atomic oxygen erosion yields of materials in ground-based facilities and LEO. High Perform. Polym. 2008, 20, 523–534. [Google Scholar] [CrossRef]
- Waters, D.L.; Banks, B.A.; De Groh, K.K.; Miller, S.K.R.; Thorson, S.D. The atomic oxygen erosion depth and cone height of various materials at hyperthermal energy. High Perform. Polym. 2008, 20, 512–522. [Google Scholar] [CrossRef]
- Zhao, W.; Li, W.; Liu, H.; Zhu, L. Erosion of a polyimide material exposed to simulated atomic oxygen environment. Chin. J. Aeronaut. 2010, 23, 268–273. [Google Scholar]
- Devapal, D.; Packirisamy, S.; Korulla, R.M.; Ninan, K.N. Atomic oxygen resistant coating from poly(tetramethyldisilylene-co-styrene). J. Appl. Polym. Sci. 2004, 94, 2368–2375. [Google Scholar] [CrossRef]
- Lei, X.; Yao, P.; Qiao, M.; Sun, W.; Zhang, H.; Zhang, Q. Atomic oxygen resistance of polyimide/silicon hybrid thin films with different compositions and architectures. High Perform. Polym. 2014, 26, 712–724. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, K.; Zhan, M. Atomic oxygen erosion resistance of titania–polyimide hybrid films derived from titanium tetrabutoxide and polyamic acid. J. Appl. Polym. Sci. 2012, 123, 143–151. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Ohmae, N.; Kinoshita, H. Volume diffusion of atomic oxygen in alpha-SiO2 protective coating. High Perform. Polym. 2000, 12, 53–63. [Google Scholar] [CrossRef]
- Duo, S.; Chang, Y.; Liu, T.; Zhang, H. Atomic oxygen erosion resistance of polysiloxane/POSS hybrid coatings on Kapton. Phys. Proc. 2013, 50, 337–342. [Google Scholar] [CrossRef]
- Duo, S.; Song, M.; Liu, T.; Hu, C.; Li, M. Erosion effects of atomic oxygen on polyhedral pligomeric silsesquioxane-polyimide hybrid films in low earth orbit space environment. J. Nanosci. Nanotechnol. 2013, 13, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, K.; Zhan, M. Atomic oxygen resistant phosphorus-containing polyimides for LEO environment. J. Mater. Sci. 2012, 47, 4904–4913. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.; Tian, D.; Qin, W.; Chen, C.; Zhao, X.; Zhou, H.; Wang, D. Atomic oxygen effects on polymers containing silicon or phosphorus: Mass loss, erosion yield, and surface morphology. High Perform. Polym. 2019, 31, 969–976. [Google Scholar]
- Atar, N.; Grossman, E.; Gouzman, I.; Bolker, A.; Murray, V.J.; Marshall, B.C.; Qian, M.; Minton, T.K.; Hanein, Y. Atomic-oxygen-durable and electrically-conductive CNT-POSS-Polyimide flexible films for space applications. ACS Appl. Mater. Interfaces 2015, 7, 12047–12056. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Qian, Y.; Qi, H.; Li, J.; Sun, J. Mechanically robust atomic oxygen-resistant coatings capable of autonomously healing damage in low earth orbit space environment. Adv. Mater. 2018, 30, 1803854. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G. Numerical simulation on atomic oxygen undercutting of Kapton film in low earth orbit. Acta Astronaut. 2010, 67, 388–395. [Google Scholar] [CrossRef]
- Gilman, J.W.; Schlitzer, D.S.; Lichtenhan, J.D. Low earth orbit resistant siloxane copolymers. J. Appl. Polym. Sci. 1996, 60, 591–596. [Google Scholar] [CrossRef]
- Li, Z.; Song, H.; He, M.; Liu, J.; Yang, S. Atomic oxygen-resistant and transparent polyimide coatings from [3,5-bis (3-aminophenoxy) phenyl] diphenylphosphine oxide and aromatic dianhydrides: Preparation and characterization. Prog. Org. Coat. 2012, 75, 49–58. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Y.; Yang, D.; Yang, Y.; Yu, Q.; Che, L.; Liu, J. Self-healing anti-atomic-oxygen phosphorus- containing polyimide film via molecular level incorporation of nanocage trisilanolphenyl POSS: Preparation and characterization. Polymers 2019, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, X.; Jiang, Q.; Mu, J.; Jiang, Z. A novel structural polyimide material with synergistic phosphorus and POSS for atomic oxygen resistance. RSC Adv. 2015, 5, 11980–11988. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional polyimide/polyhedral oligomeric silsesquioxane nanocomposites. Polymers 2018, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Minton, T.K.; Wright, M.E.; Tomczak, S.J.; Marquez, S.A.; Shen, L.; Brunsvold, A.L.; Cooper, R.; Zhang, J.; Vij, V.; Guenthner, A.J.; et al. Atomic oxygen effects on POSS polyimides in low earth orbit. ACS Appl. Mater. Interfaces 2012, 4, 492–502. [Google Scholar] [CrossRef]
- Lei, X.F.; Chen, Y.; Zhang, H.P.; Li, X.J.; Yao, P.; Zhang, Q.Y. Space survivable polyimides with excellent optical transparency and self-healing properties derived from hyperbranched polysiloxane. ACS Appl. Mater. Interfaces 2013, 5, 10207–10220. [Google Scholar] [CrossRef]
- Li, X.; Al-Ostaz, A.; Jaradat, M.; Rahmani, F.; Nouranian, S.; Rushing, G.; Manasrah, A.; Alkhateb, H.; Finckenor, M.; Lichtenhan, J. Substantially enhanced durability of polyhedral oligomeric silsequioxane-polyimide nanocomposites against atomic oxygen erosion. Eur. Polym. J. 2017, 92, 233–249. [Google Scholar] [CrossRef]
- Qian, M.; Murray, V.J.; Wei, W.; Marshall, B.C.; Minton, T.K. Resistance of POSS polyimide blends to hyperthermal atomic oxygen attack. ACS Appl. Mater. Interfaces 2016, 8, 33982–33992. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tang, Y.; Yu, Z.; Gu, J.; Kong, J. Advanced aromatic polymers with excellent antiatomic oxygen performance derived from molecular precursor strategy and copolymerization of polyhedral oligomeric silsesquioxane. ACS Appl. Mater. Interfaces 2015, 7, 20144–20155. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Qiao, M.; Tian, L.; Chen, Y.; Zhang, Q. Evolution of surface chemistry and morphology of hyperbranched polysiloxane polyimides in simulated atomic oxygen environment. Corros. Sci. 2015, 98, 560–572. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Guo, Y.D.; Qi, H.R.; An, Y.C.; Jia, Y.J.; Tan, Y.Y.; Liu, J.G.; Wu, B.H. Preparation and properties of intrinsically atomic-oxygen resistant polyimide films containing polyhedral oligomeric silsesquioxane (POSS) in the side chains. Polymers 2020, 12, 2865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.G.; Wu, X.; Guo, C.Y.; Qu, L.Q.; Zhang, X.M. Trisilanolphenyl-POSS nano-hybrid poly(phenyl dianhydride-p-phenylenediamine) polyimide composite films: Miscibility and structure-property relationship. J. Polym. Res. 2018, 25, 139. [Google Scholar] [CrossRef]
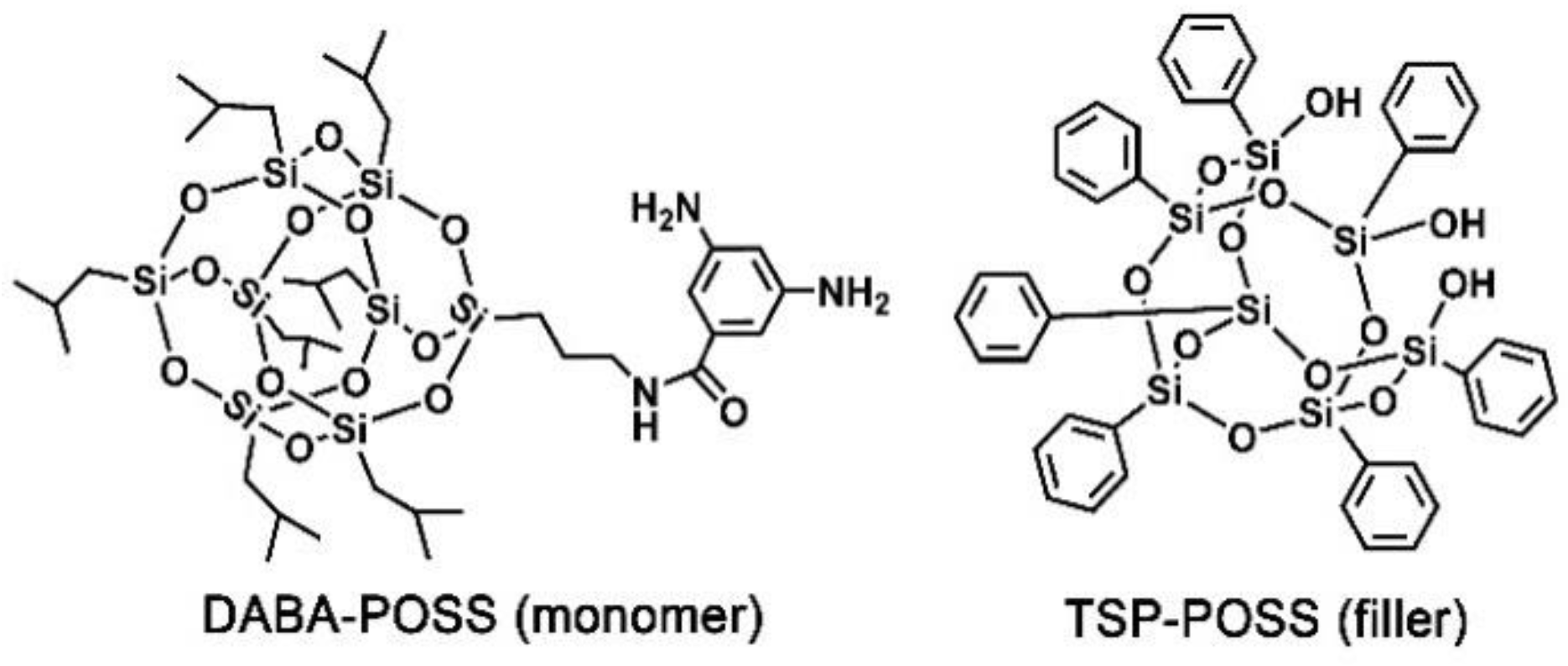
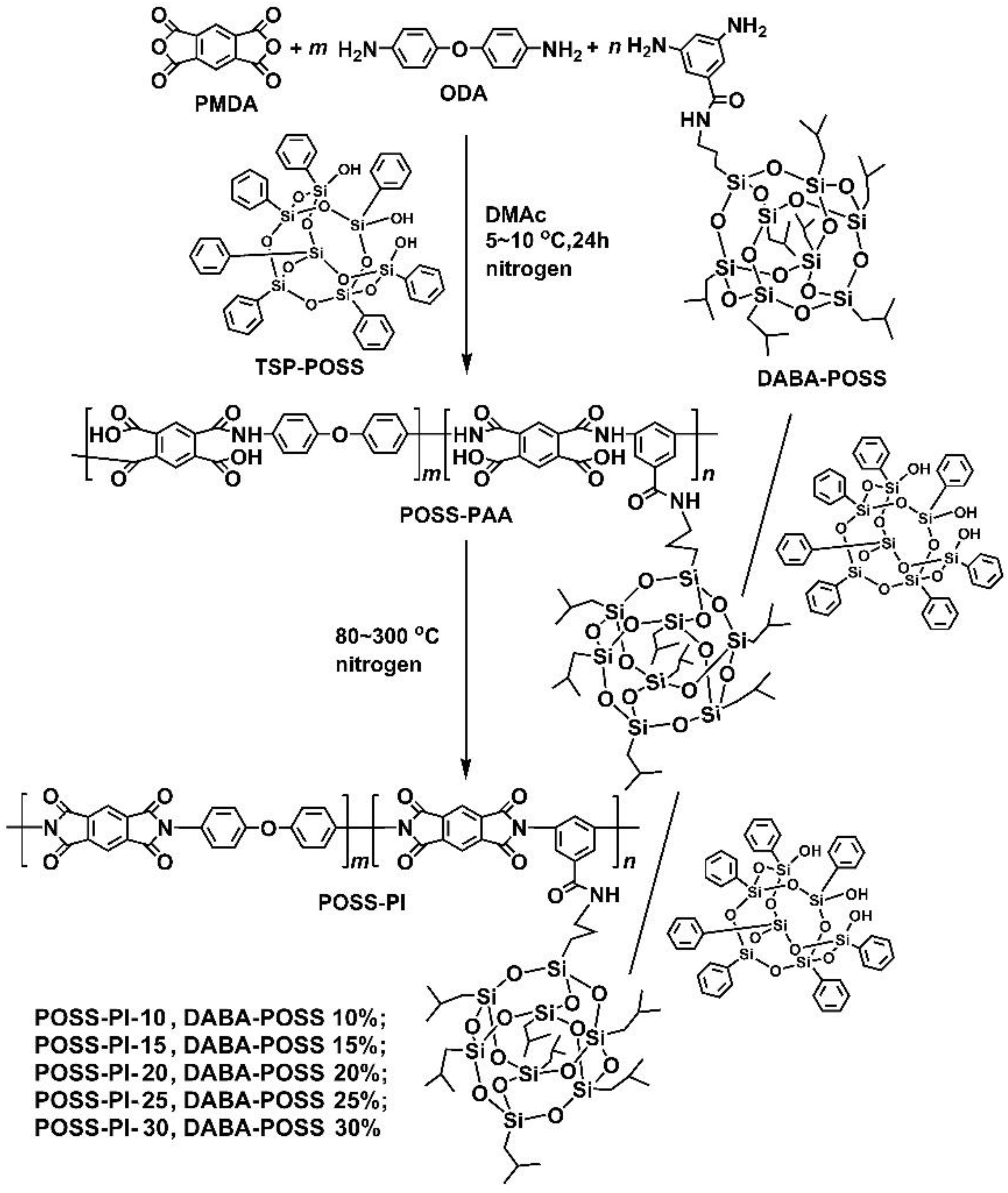
| PAA | PMDA (M = 218.12 g/mol) | ODA (M = 200.24 g/mol) | DABA-POSS (M = 1008.71 g/mol) | TSP-POSS (M = 931.34 g/mol) | DMAc (g) |
|---|---|---|---|---|---|
| POSS-PAA-10 | 20.00 g (91.69 mmol) | 17.53 g (87.56 mmol) | 4.17 g (4.13 mmol) | 10.43 g | 208.5 g |
| POSS-PAA-15 | 20.00 g (91.69 mmol) | 17.06 g (85.21 mmol) | 6.54 g (6.48 mmol) | 10.90 g | 218.0 g |
| POSS-PAA-20 | 20.00 g (91.69 mmol) | 16.55 g (82.64 mmol) | 9.14 g (9.06 mmol) | 11.42 g | 228.4 g |
| POSS-PAA-25 | 20.00 g (91.69 mmol) | 15.98 g (79.80 mmol) | 11.99 g (11.89 mmol) | 11.99 g | 239.9 g |
| POSS-PAA-30 | 20.00 g (91.69 mmol) | 15.35 g (76.67 mmol) | 15.15 g (15.02 mmol) | 12.63 g | 252.5 g |
| PI | λcut1 (nm) | T5502 (%) | L* 3 | a* 3 | b* 3 | Haze 4 (%) |
|---|---|---|---|---|---|---|
| POSS-PI-10 | 431 | 68.8 | 87.55 | −6.88 | 83.04 | 7.19 (4.78) |
| POSS-PI-15 | 431 | 61.2 | 85.63 | −4.02 | 87.99 | 16.63 (7.65) |
| POSS-PI-20 | 411 | 73.3 | 86.78 | −5.52 | 82.75 | 11.79 (9.55) |
| POSS-PI-25 | 428 | 64.8 | 85.69 | −4.08 | 86.26 | 14.15 (14.53) |
| POSS-PI-30 | 425 | 63.7 | 84.53 | −2.41 | 87.73 | 15.82 (31.70) |
| Samples | T5%1 (nm) | T10%1 (%) | Rw7501 (%) | Tmax1 (°C) | CTE (×10−6/K) |
|---|---|---|---|---|---|
| POSS-PI-10 | 580 (534 3) | 608 (568) | 70.6 (59.1) | 624.6 | 61.0 (45.6 2) |
| POSS-PI-15 | 572 (528) | 601 (528) | 70.7 (58.5) | 620.7 | 74.7 (50.4) |
| POSS-PI-20 | 564 (524) | 592 (548) | 71.0 (59.6) | 620.4 | 81.0 (56.1) |
| POSS-PI-25 | 559 (519) | 585 (541) | 71.3 (59.9) | 611.9 | 79.6 (55.0) |
| POSS-PI-30 | 554 (512) | 579 (531) | 70.7 (59.3) | 602.2 | ND 3 |
| Samples | Es1 (10−26 cm3/atom) | Relative Atomic Concentration (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unexposed Samples | AO Exposed Samples | ||||||||
| Si2p | C1s | O1s | N1s | Si2p | C1s | O1s | N1s | ||
| POSS-PI-10 | 9.21 (26.0 2) | 8.96 | 68.07 | 19.62 | 2.72 | 24.33 | 23.01 | 51.22 | 0.95 |
| POSS-PI-15 | 6.06 (21.0) | 4.90 | 74.65 | 16.63 | 2.94 | 23.36 | 27.56 | 47.78 | 0.70 |
| POSS-PI-20 | 2.15 (16.9) | 4.73 | 75.56 | 16.75 | 2.42 | 15.27 | 53.00 | 31.11 | 0.62 |
| POSS-PI-25 | 1.82 (12.8) | 4.94 | 74.93 | 16.81 | 2.87 | 21.33 | 30.77 | 46.20 | 1.45 |
| POSS-PI-30 | 1.64 (11.1) | 4.61 | 72.89 | 18.60 | 2.76 | 9.55 | 64.29 | 23.94 | 1.99 |
| PI-ref 3 | 300 | ND 4 | ND | ND | ND | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, H.; Guo, Y.-d.; Yang, Y.-b.; Yu, Q.; Liu, J.-g.; Wu, B.-h.; Lv, F.-z. Atomic Oxygen-Resistant Polyimide Composite Films Containing Nanocaged Polyhedral Oligomeric Silsesquioxane Components in Matrix and Fillers. Nanomaterials 2021, 11, 141. https://doi.org/10.3390/nano11010141
Zhang Y, Wu H, Guo Y-d, Yang Y-b, Yu Q, Liu J-g, Wu B-h, Lv F-z. Atomic Oxygen-Resistant Polyimide Composite Films Containing Nanocaged Polyhedral Oligomeric Silsesquioxane Components in Matrix and Fillers. Nanomaterials. 2021; 11(1):141. https://doi.org/10.3390/nano11010141
Chicago/Turabian StyleZhang, Yan, Hao Wu, Yi-dan Guo, Yan-bin Yang, Qiang Yu, Jin-gang Liu, Bo-han Wu, and Feng-zhu Lv. 2021. "Atomic Oxygen-Resistant Polyimide Composite Films Containing Nanocaged Polyhedral Oligomeric Silsesquioxane Components in Matrix and Fillers" Nanomaterials 11, no. 1: 141. https://doi.org/10.3390/nano11010141
APA StyleZhang, Y., Wu, H., Guo, Y.-d., Yang, Y.-b., Yu, Q., Liu, J.-g., Wu, B.-h., & Lv, F.-z. (2021). Atomic Oxygen-Resistant Polyimide Composite Films Containing Nanocaged Polyhedral Oligomeric Silsesquioxane Components in Matrix and Fillers. Nanomaterials, 11(1), 141. https://doi.org/10.3390/nano11010141






