Suitability of Mg-Nd and Mg-Zn Alloys to Obtain Biodegradable Structures for Bone Defects
Abstract
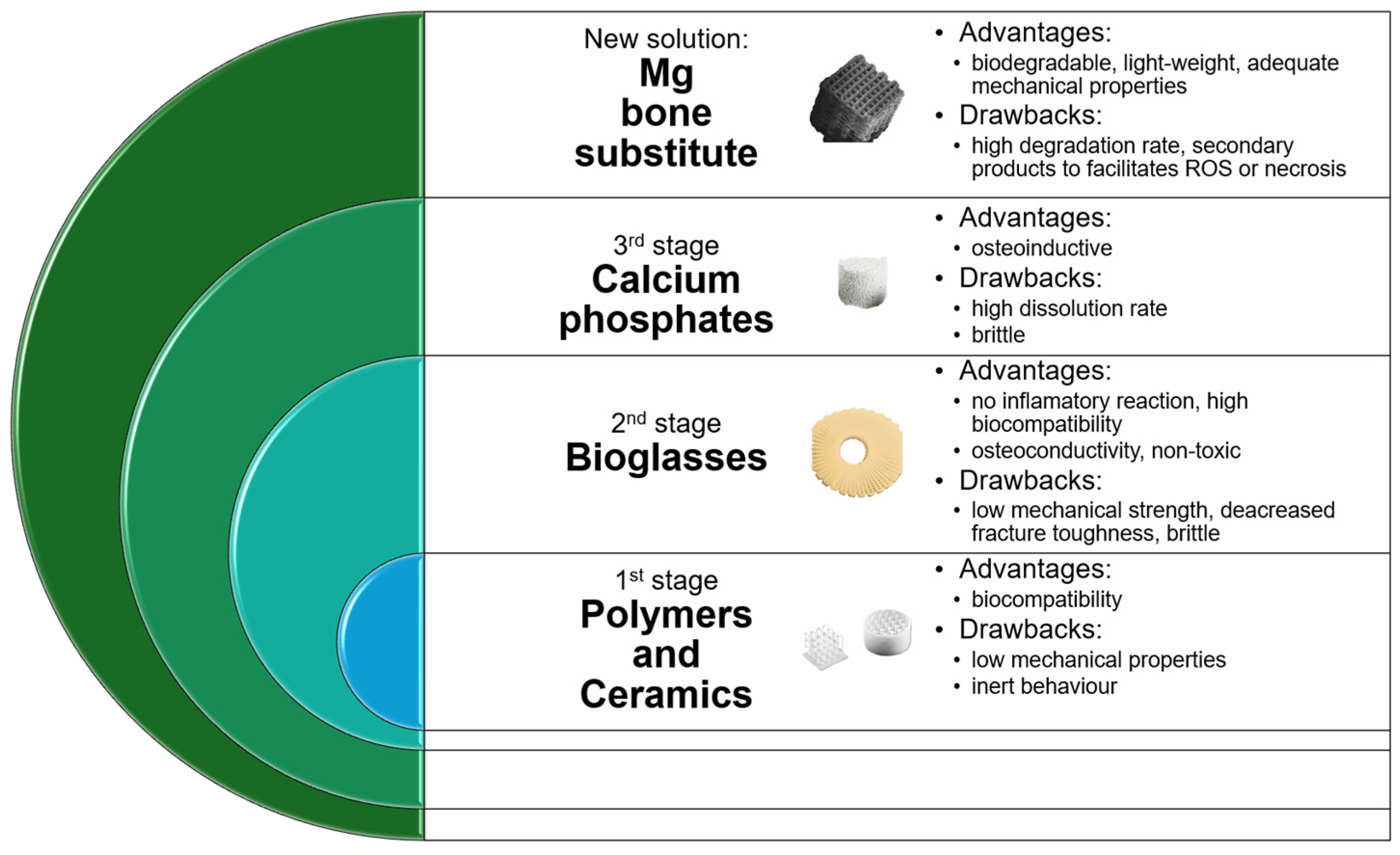
1. Introduction
2. Materials and Methods
2.1. Microstructural Characterization of Experimental Samples
2.2. Mechanical Properties
2.3. In Vitro Corrosion Behavior of the Experimental Samples
2.4. In Vivo Animal Study
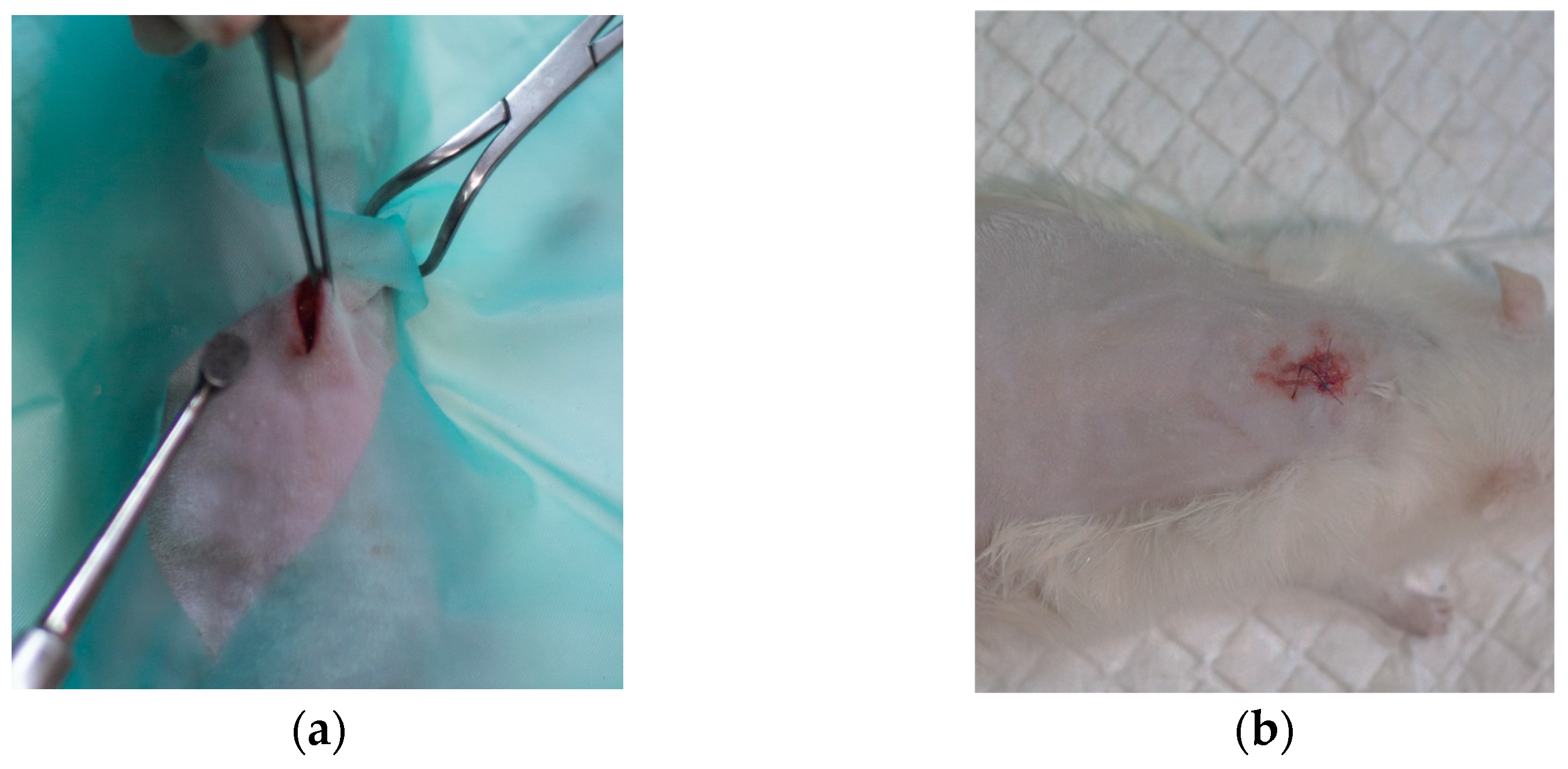
2.4.1. Surgical Procedure and Histopathological Analysis Route
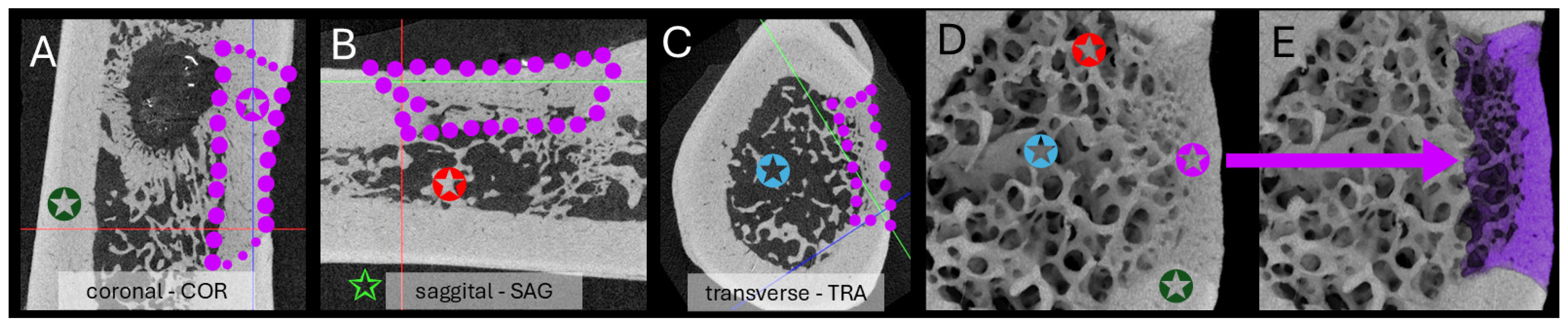
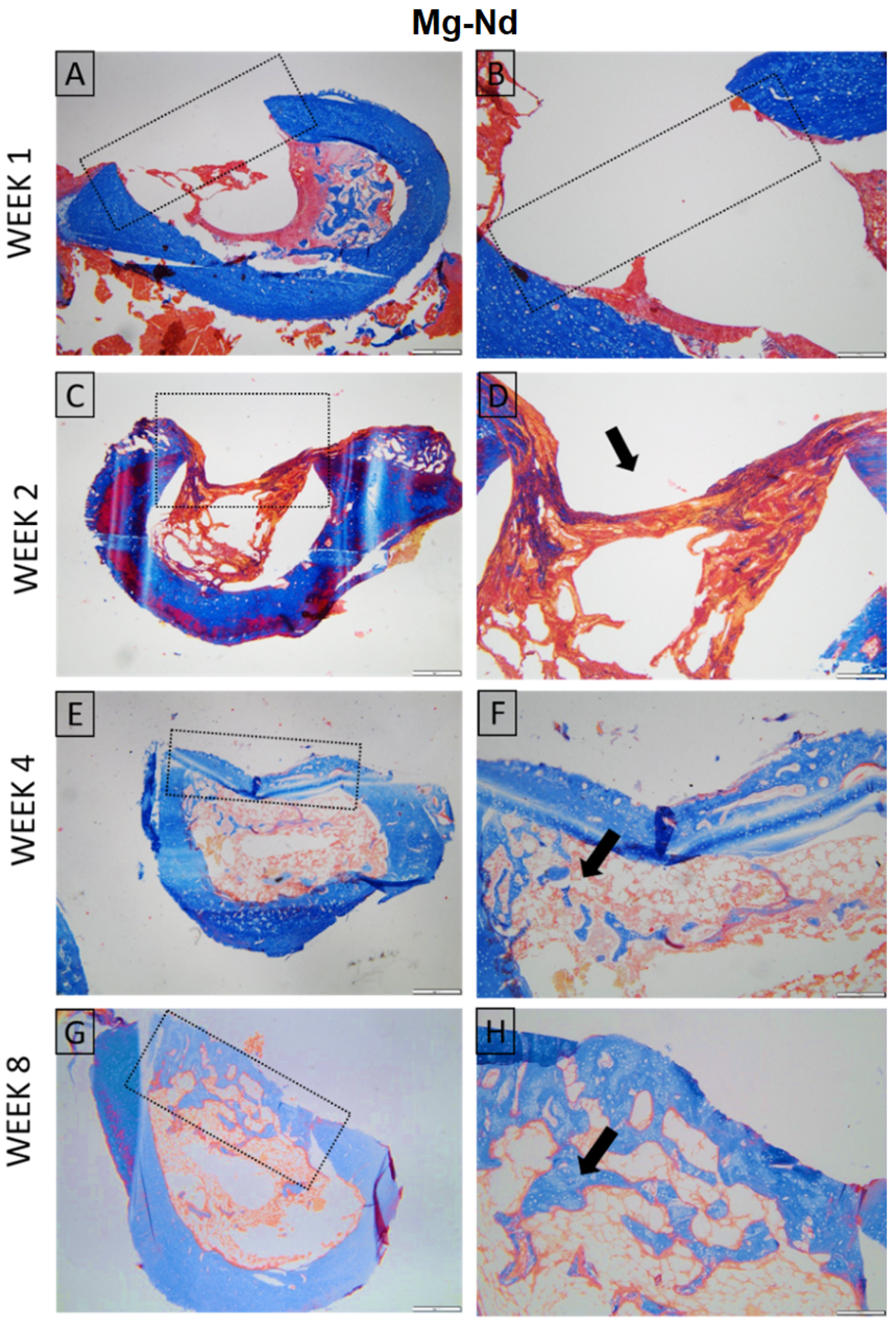
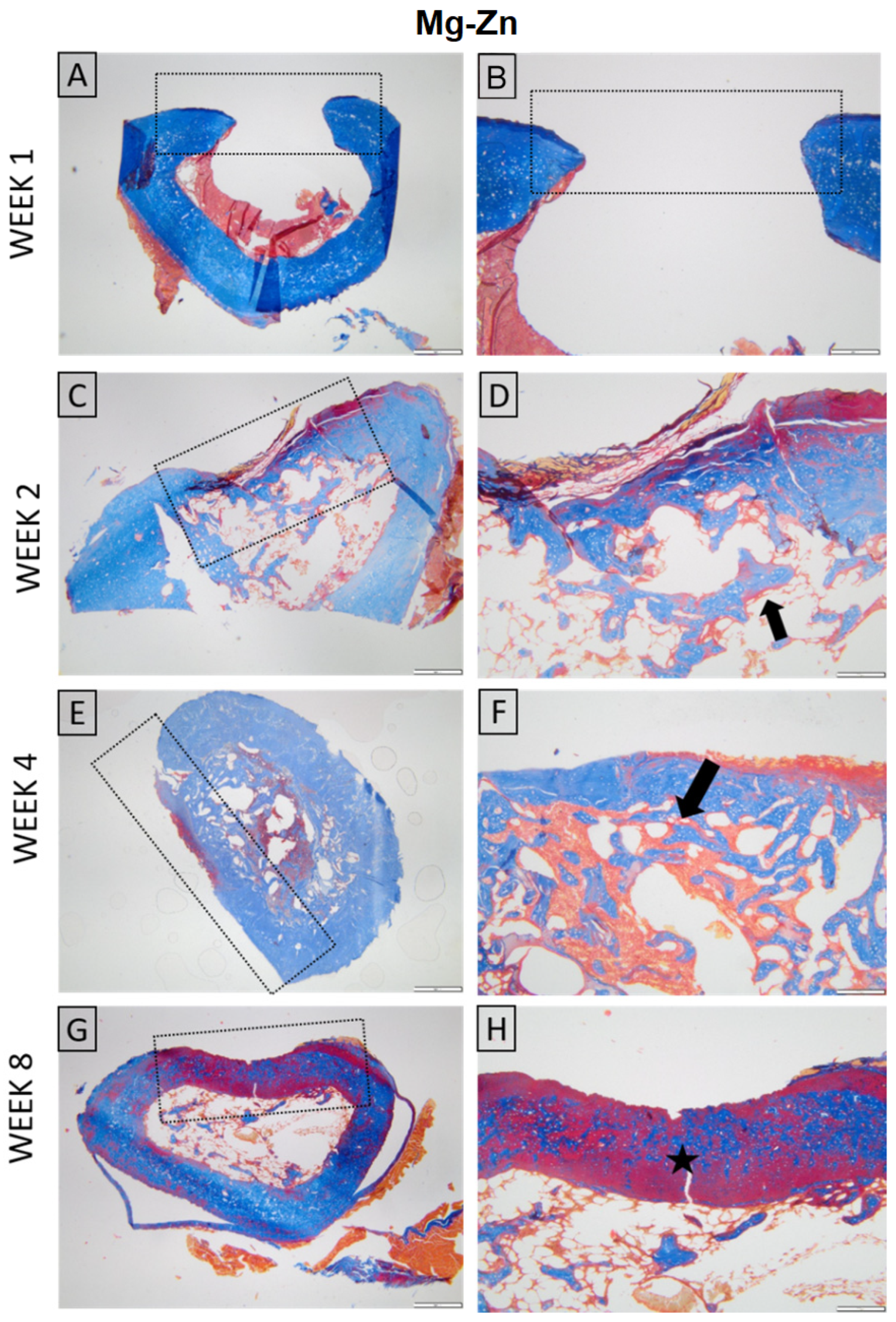
2.4.2. Micro-Computed Tomography (µCT) Acquisition and Analysis
3. Results and Discussion
3.1. Microstructural Characterization of the Mg-Based Alloys
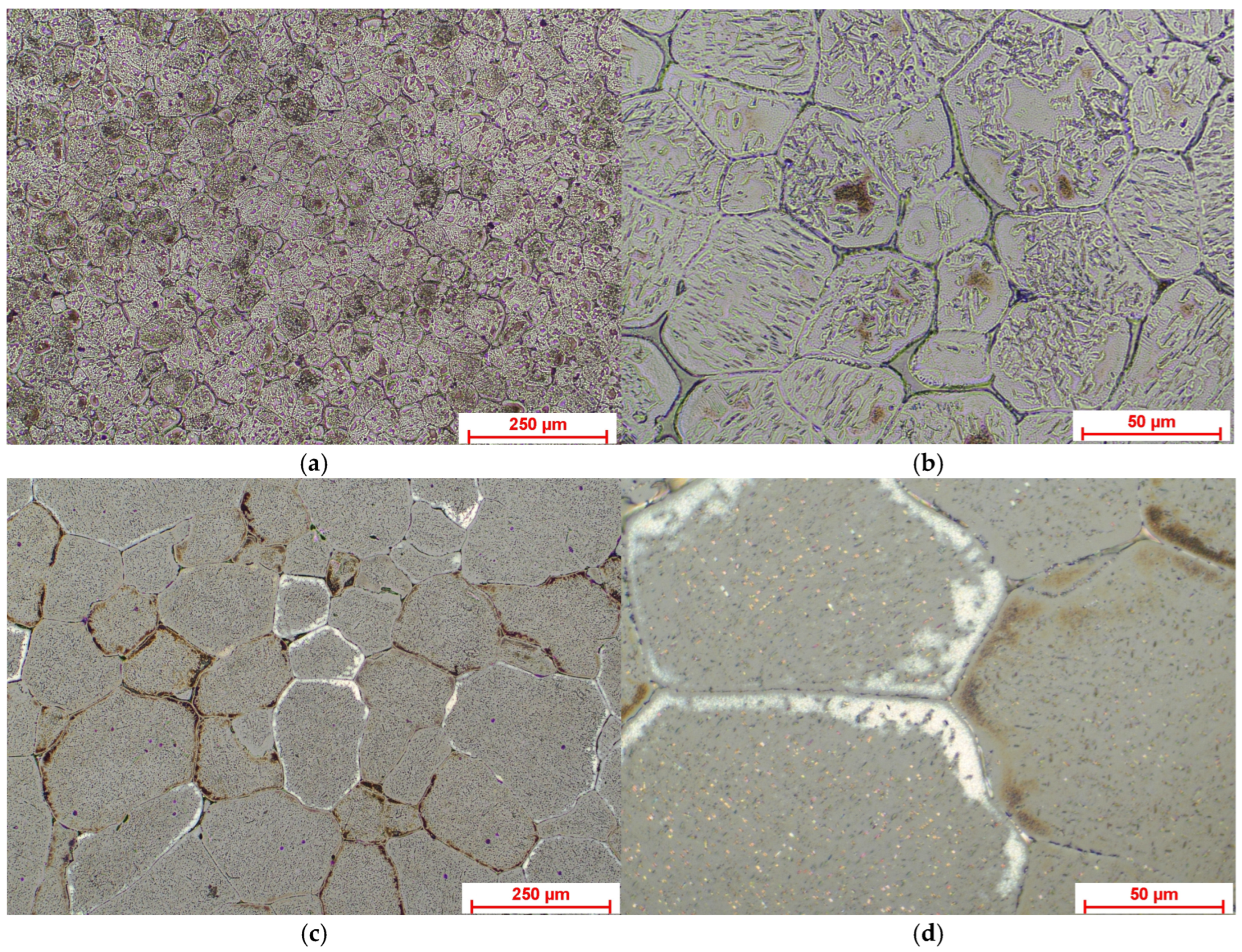
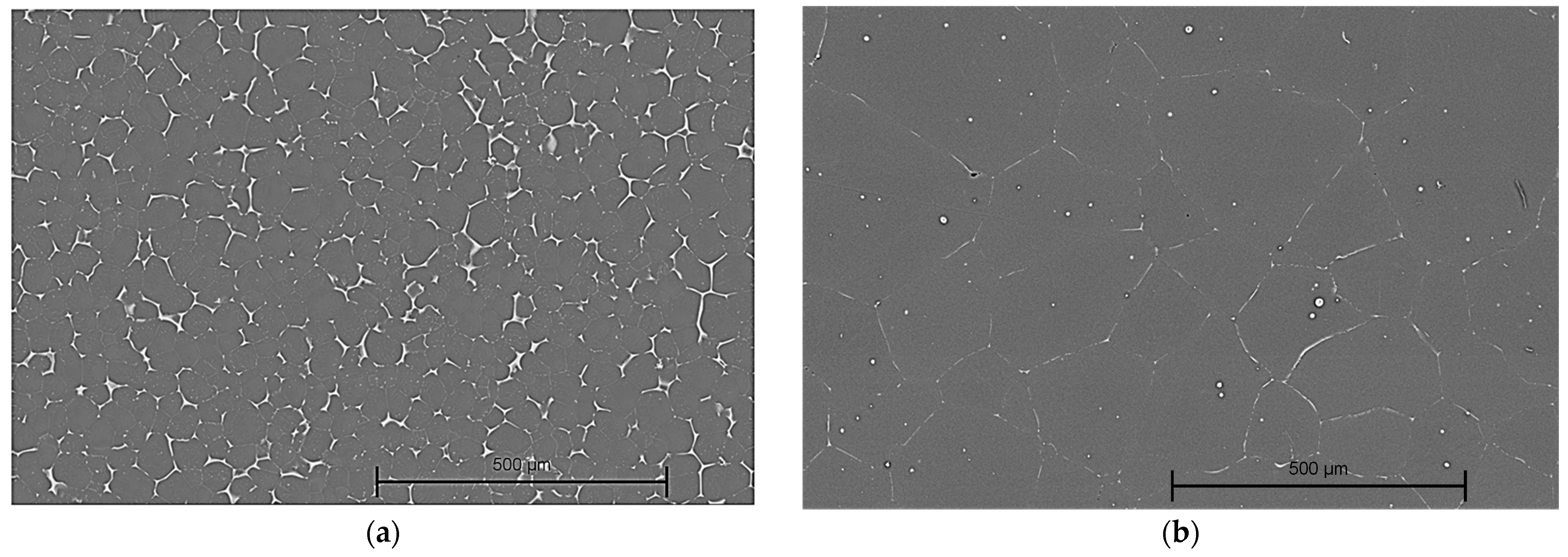
3.1.1. Optical Microscopy
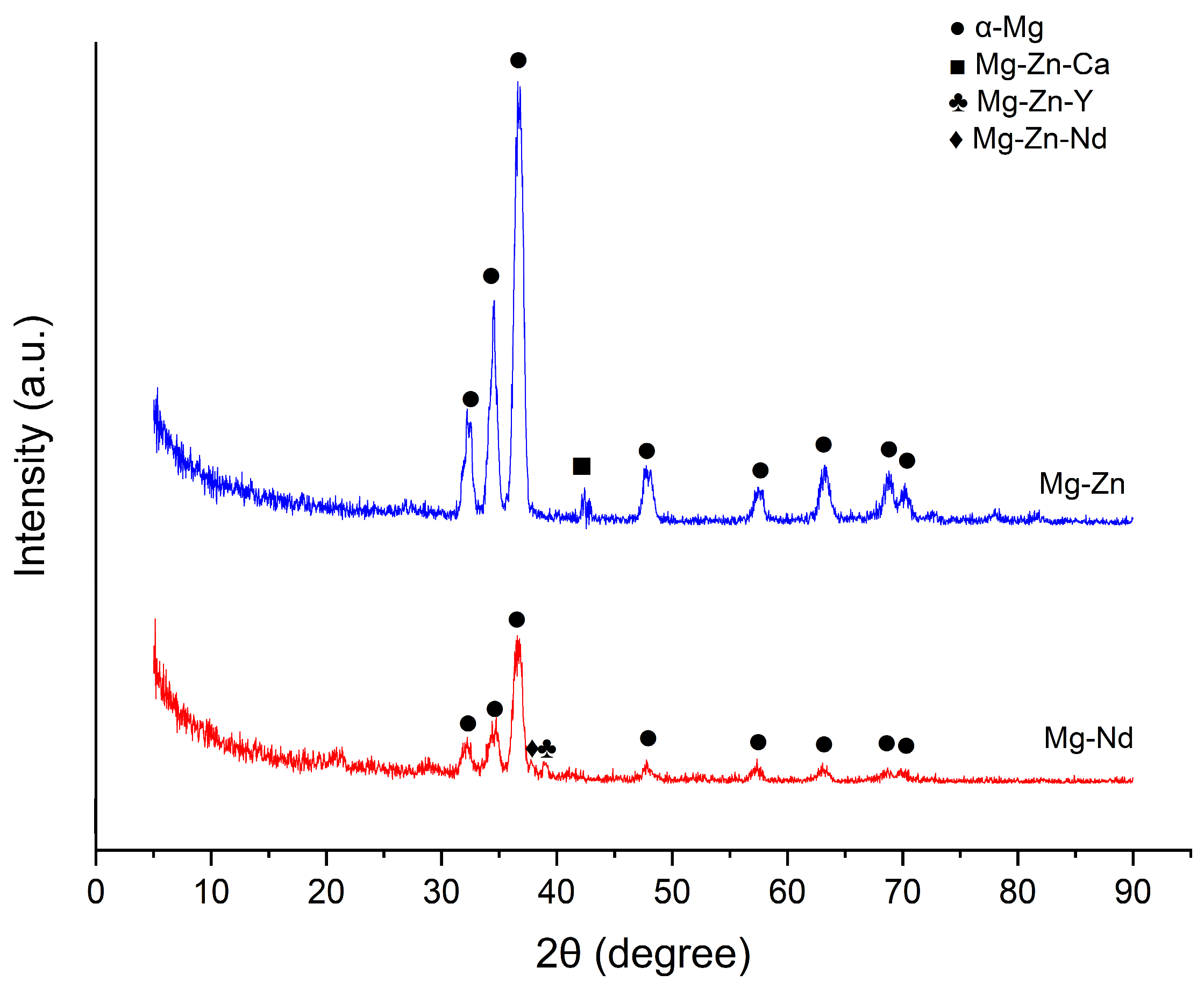
3.1.2. X-Ray Diffraction Analysis (XRD)
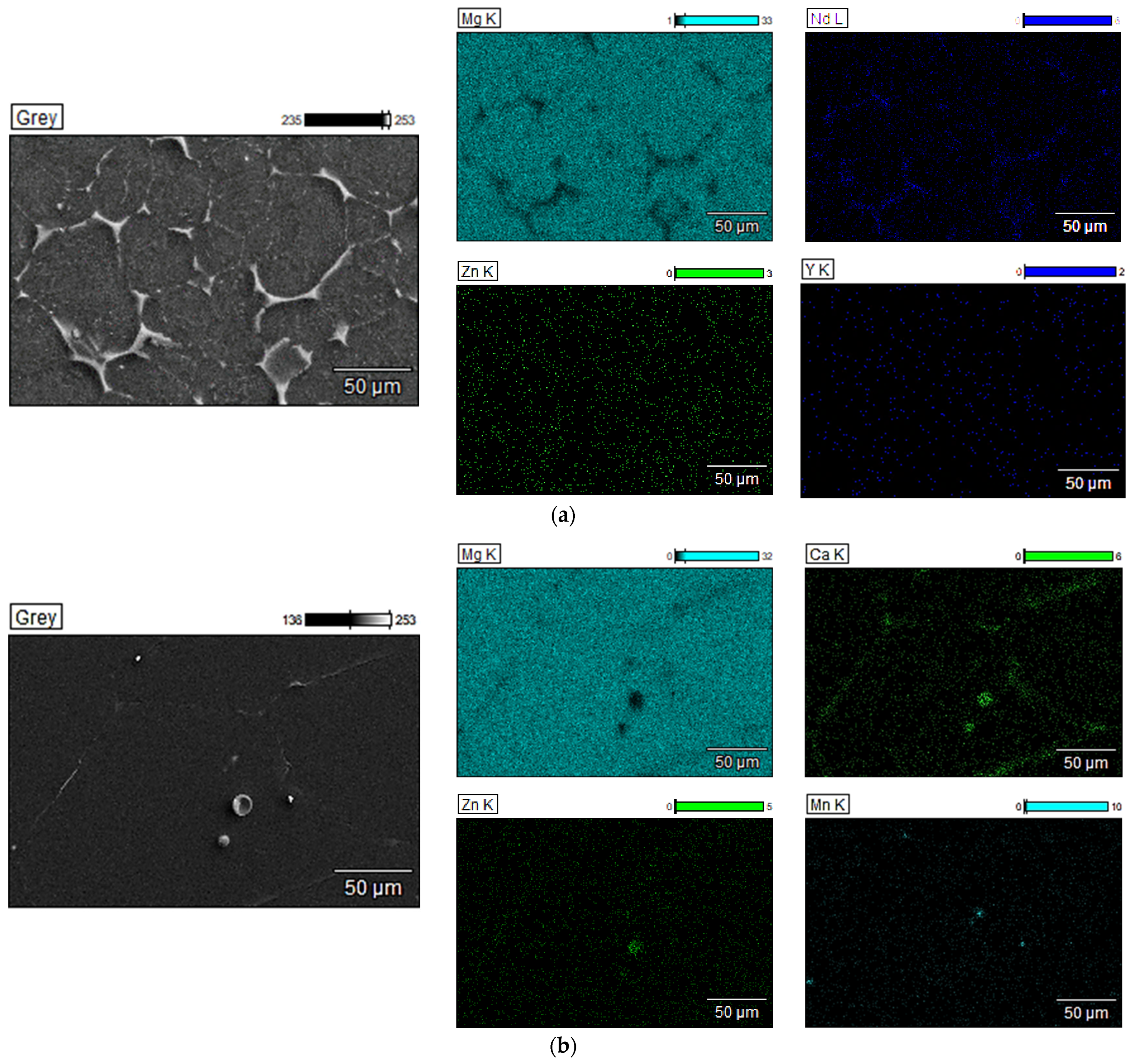
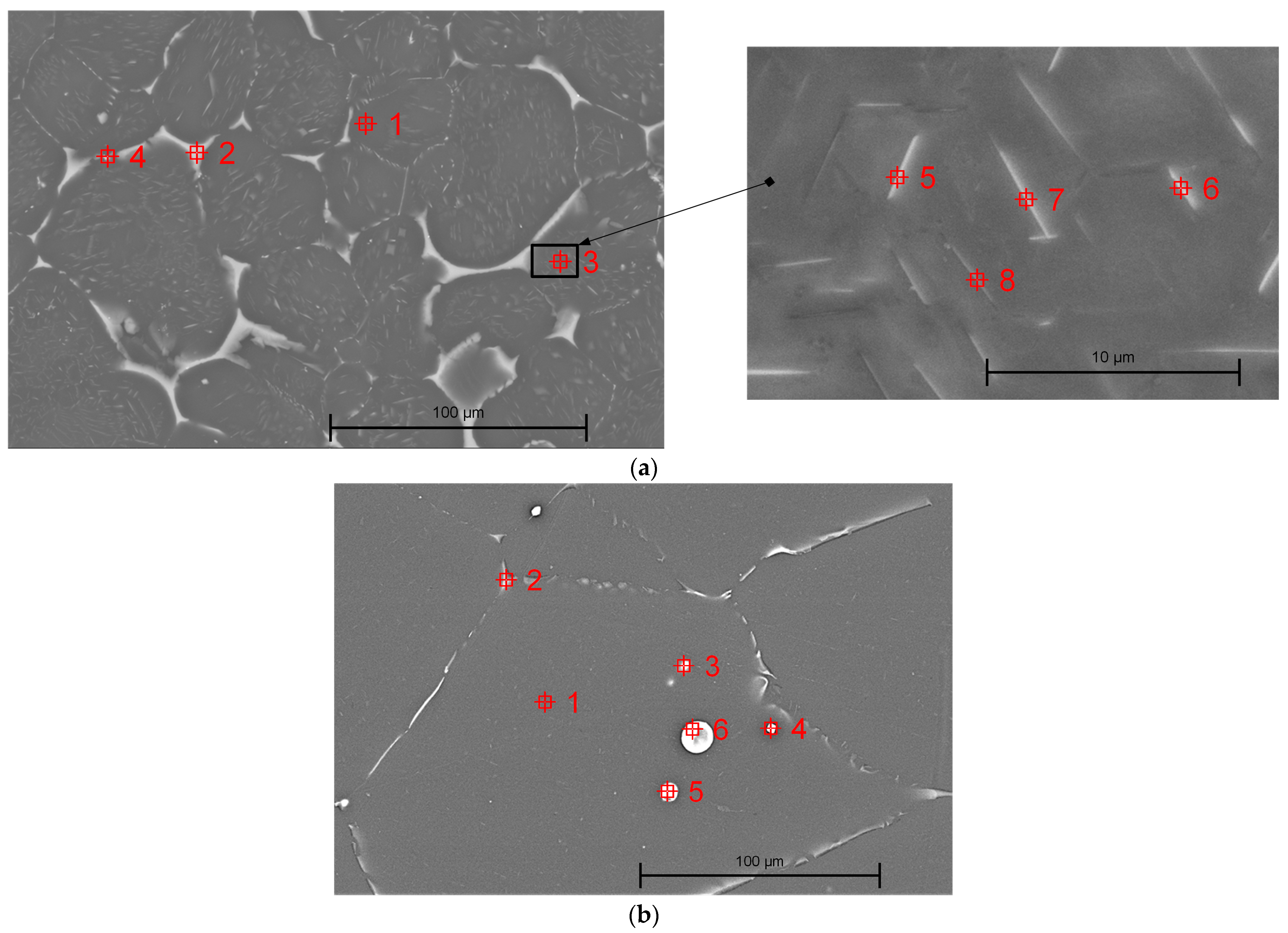
3.1.3. Scanning Electron Microscopy
3.2. Mechanical Characterization of the Mg-Based Alloys
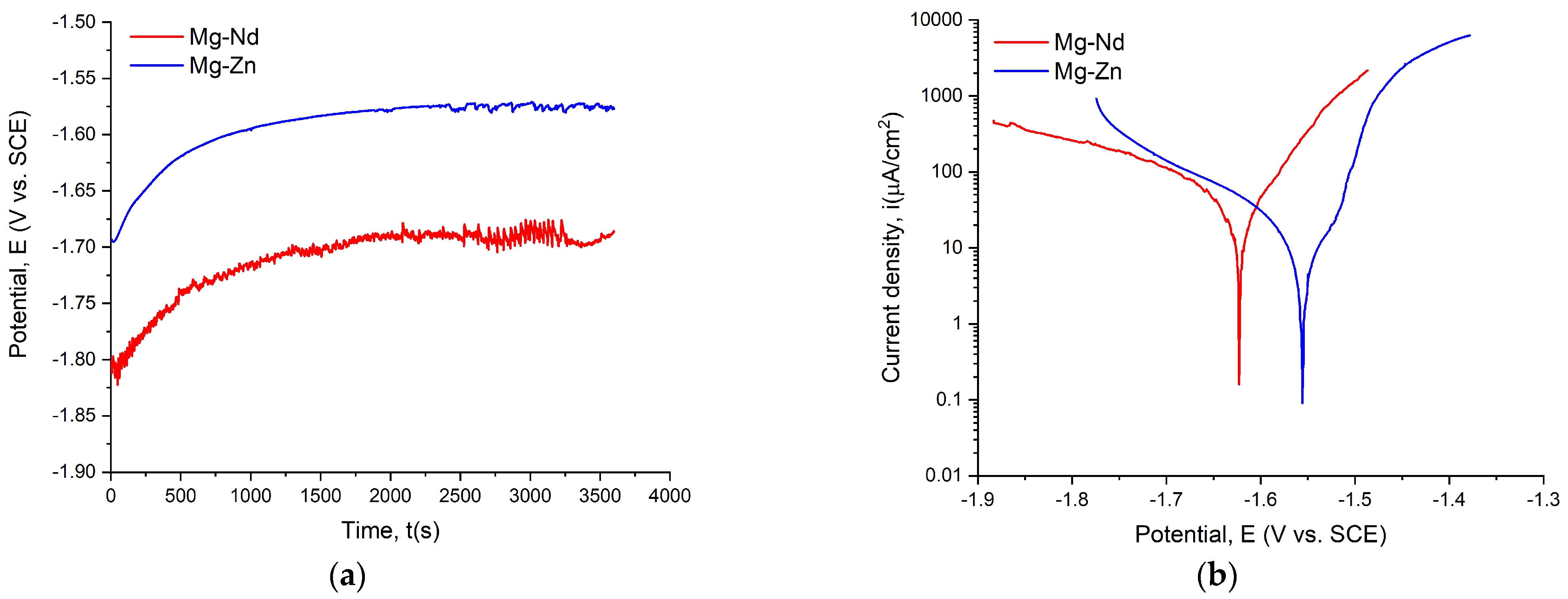
3.3. In Vitro Corrosion Behavior of the Experimental Samples
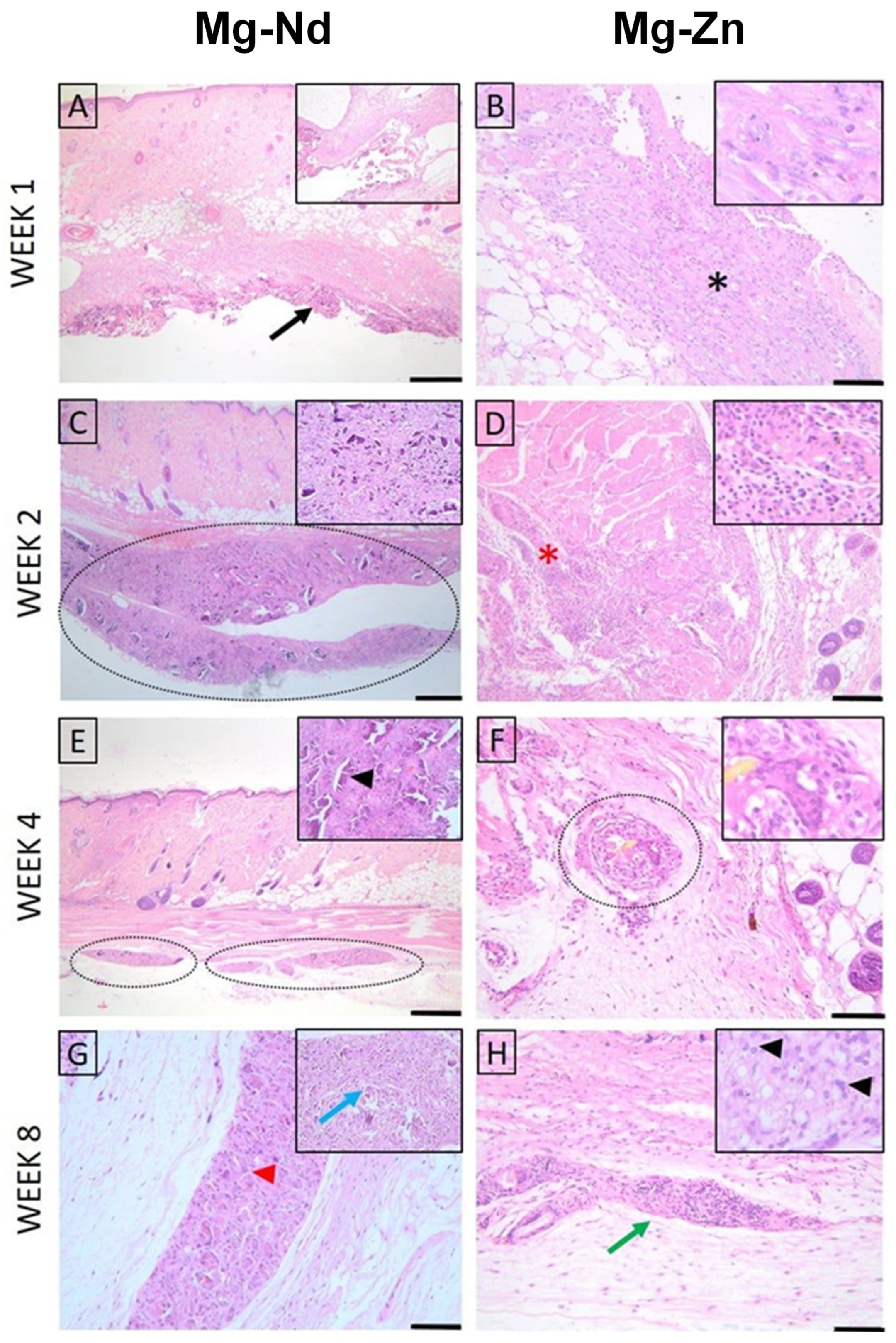
3.4. In Vivo Animal Study
3.4.1. Histopathological Examination
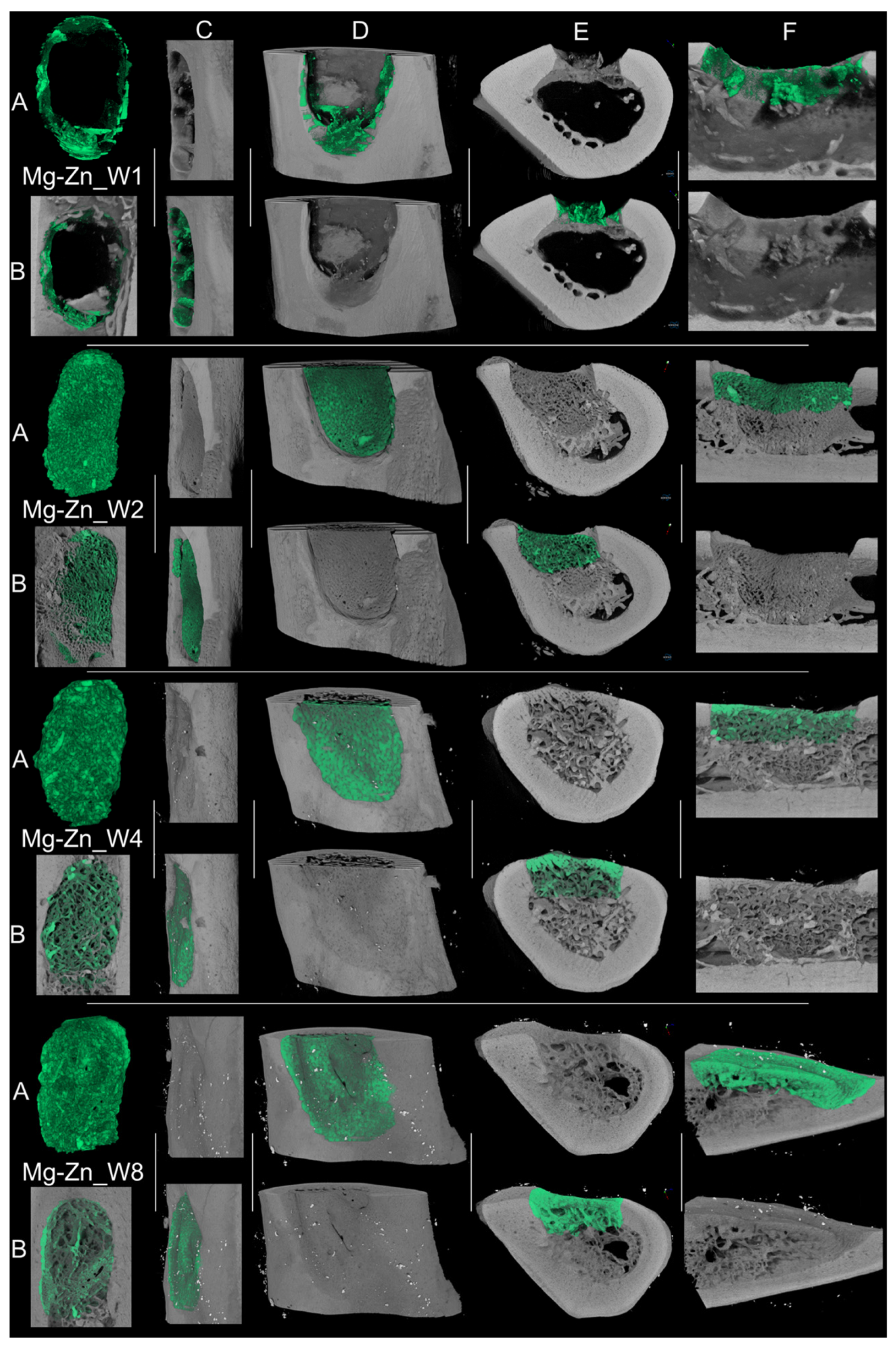
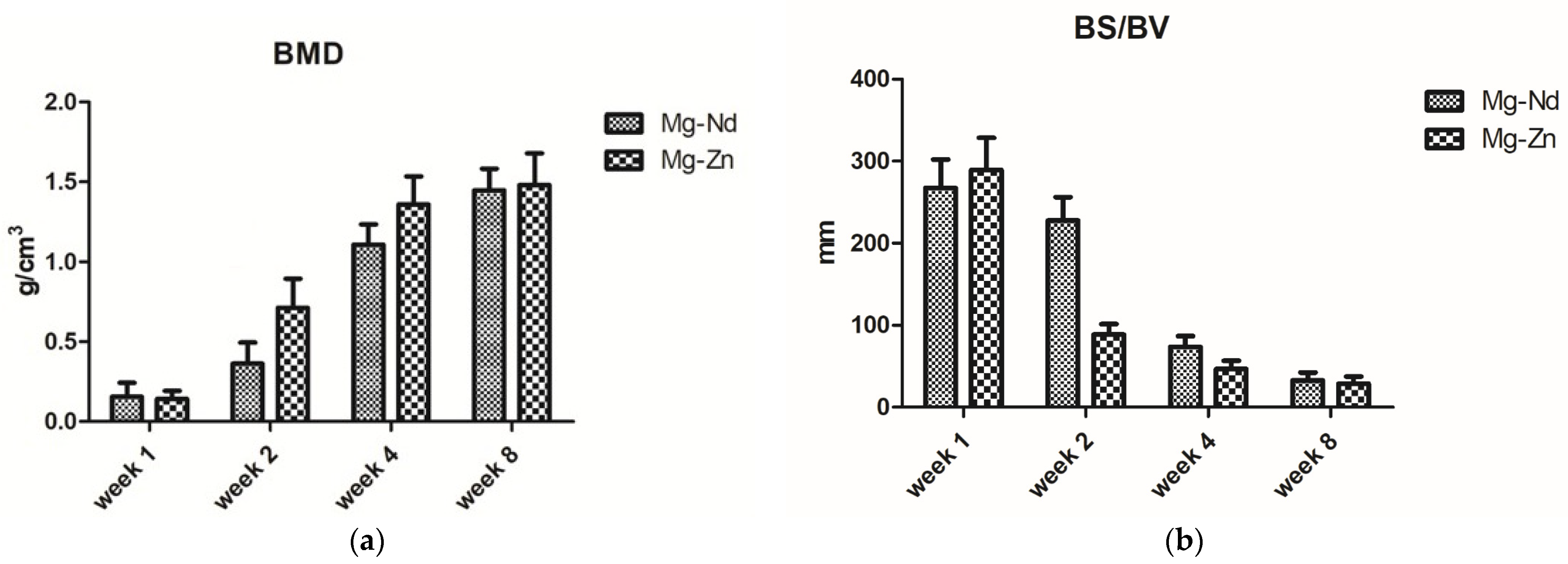
3.4.2. Micro-Computed Tomography Acquisition and Analysis
3.4.3. In Vivo Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current Options and Future Perspectives on Bone Graft and Biomaterials Substitutes for Bone Repair, from Clinical Needs to Advanced Biomaterials Research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Mocanu, A.-C.; Stan, G.E.; Maidaniuc, A.; Miculescu, M.; Antoniac, I.V.; Ciocoiu, R.-C.; Voicu, Ș.I.; Mitran, V.; Cîmpean, A.; Miculescu, F. Naturally-Derived Biphasic Calcium Phosphates through Increased Phosphorus-Based Reagent Amounts for Biomedical Applications. Materials 2019, 12, 381. [Google Scholar] [CrossRef]
- Antoniac, I.; Negrusoiu, M.; Mardare, M.; Socoliuc, C.; Zazgyva, A.; Niculescu, M. Adverse Local Tissue Reaction after 2 Revision Hip Replacements for Ceramic Liner Fracture. Medicine 2017, 96, e6687. [Google Scholar] [CrossRef]
- Botez, P.; Sirbu, P.; Simion, L.; Munteanu, F.l.; Antoniac, I. Application of a Biphasic Macroporous Synthetic Bone Substitutes CERAFORM®: Clinical and Histological Results. Eur. J. Orthop. Surg. Traumatol. 2009, 19, 387–395. [Google Scholar] [CrossRef]
- Allori, A.C.; Sailon, A.M.; Warren, S.M. Biological Basis of Bone Formation, Remodeling, and Repair—Part I: Biochemical Signaling Molecules. Tissue Eng. Part B Rev. 2008, 14, 259–273. [Google Scholar] [CrossRef]
- Antoniac, I.; Manescu, V.; Antoniac, A.; Paltanea, G. Magnesium-Based Alloys with Adapted Interfaces for Bone Implants and Tissue Engineering. Regen. Biomater. 2023, 10, rbad095. [Google Scholar] [CrossRef]
- Quan, P.H.; Antoniac, I.; Miculescu, F.; Antoniac, A.; Manescu, V.; Robu, A.; Bița, A.-I.; Miculescu, M.; Saceleanu, A.; Bodog, A.D.; et al. Fluoride Treatment and In Vitro Corrosion Behavior of Mg-Nd-Y-Zn-Zr Alloys Type. Materials 2022, 15, 566. [Google Scholar] [CrossRef]
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Abbah, S.A.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic Approaches in Bone Tissue Engineering: Integrating Biological and Physicomechanical Strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Burcea, M.; Ionescu, R.D.; Balta, F. IOL’s Opacification: A Complex Analysis Based on the Clinical Aspects, Biomaterials Used and Surface Characterization of Explanted IOL’s. Mater. Plast. 2015, 52, 109–112. [Google Scholar]
- Şerboiu, C.S.; Aliuș, C.; Dumitru, A.; Țăpoi, D.; Costache, M.; Nica, A.E.; Alexandra-Ana, M.; Antoniac, I.; Grădinaru, S. Gallbladder Pancreatic Heterotopia—The Importance of Diagnostic Imaging in Managing Intraoperative Findings. Medicina 2023, 59, 1407. [Google Scholar] [CrossRef]
- Onisâi, M.; Dumitru, A.; Iordan, I.; Aliuș, C.; Teodor, O.; Alexandru, A.; Gheorghiță, D.; Antoniac, I.; Nica, A.; Mihăilescu, A.-A.; et al. Synchronous Multiple Breast Cancers—Do We Need to Reshape Staging? Medicina 2020, 56, 230. [Google Scholar] [CrossRef] [PubMed]
- Grădinaru, E.S.; Stoicea, M.-C.; Mocanu, L.; Antoniac, I.; Gheorghiță, D.; Grigore, A.G.M. Rare Breast Carcinoma with Paradoxical Plasma Cell Immunoprofile: A Case Report. Medicina 2020, 56, 62. [Google Scholar] [CrossRef]
- Bongio, M.; Van Den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Jansen, J.A. Development of Bone Substitute Materials: From ‘Biocompatible’ to ‘Instructive’. J. Mater. Chem. 2010, 20, 8747–8759. [Google Scholar] [CrossRef]
- Paltanea, G.; Manescu, V.; Antoniac, I.; Antoniac, A.; Nemoianu, I.V.; Robu, A.; Dura, H. A Review of Biomimetic and Biodegradable Magnetic Scaffolds for Bone Tissue Engineering and Oncology. Int. J. Mol. Sci. 2023, 24, 4312. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef]
- Cristescu, I.; Antoniac, I.; Branzei, M.; Ghiban, B.; Ciocoiu, R.; Ciuca, I.; Semenescu, A.; Gradinaru, S.; Pop, D.; Raiciu, A.D. Fractography Study of Explanted Intramedullary Nails. Mater. Plast. 2019, 56, 759–773. [Google Scholar] [CrossRef]
- De Aza, P.N.; Luklinska, Z.B.; Santos, C.; Guitian, F.; De Aza, S. Mechanism of Bone-like Formation on a Bioactive Implant in Vivo. Biomaterials 2003, 24, 1437–1445. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive Glass in Tissue Engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Ali, R.H.; Sghaier, Z.; Ageorges, H.; Ben Salem, E.; Hidouri, M. Magnesium-Substituted Zinc-Calcium Hydroxyfluorapatite Bioceramics for Bone Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2025, 166, 106933. [Google Scholar] [CrossRef]
- Sampaio, R.F.; Carvalho, C.N.; Bradaschia-Correa, V.; Gonçalves, B.L.L.; Arana-Chavez, V.; de Carvalho, A.P.L.; Nogueira, A.P.A.; Grazziotin-Soares, R.; Bauer, J.; Gavini, G.; et al. Apical Sealing and Bioactivity of an Experimental Gutta-Percha Containing Niobium Phosphate Bioglass. Polymers 2023, 15, 1679. [Google Scholar] [CrossRef]
- Kowalska, K.J.; Czechowska, J.P.; Yousef, E.S.; Zima, A. Novel Phosphate Bioglasses and Bioglass-Ceramics for Bone Regeneration. Ceram. Int. 2024, 50, 45976–45985. [Google Scholar] [CrossRef]
- Safarova, Y.; Nessipbekova, A.; Syzdykova, A.; Olzhayev, F.; Umbayev, B.; Kassenova, A.; Fadeeva, I.V.; Askarova, S.; Rau, J.V. Strontium- and Copper-Doped Ceramic Granules in Bone Regeneration-Associated Cellular Processes. J. Funct. Biomater. 2024, 15, 352. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate (CaPO4) Containing Composites for Biomedical Applications: Formulations, Properties, and Applications. J. Compos. Sci. 2024, 8, 218. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Méndez, Y.P.; Kharisov, B.I.; Nikolaev, A.L.; Dorozhkin, S.V.; Mena, D.N.; García, B.O. Biomineralization of Calcium Phosphates in Nature. Nano-Struct. Nano-Objects 2025, 41, 101425. [Google Scholar] [CrossRef]
- Colilla, M.; Manzano, M.; Vallet-Regí, M. Recent Advances in Ceramic Implants as Drug Delivery Systems for Biomedical Applications. Int. J. Nanomed. 2008, 3, 403–414. [Google Scholar] [CrossRef][Green Version]
- Miu, D.-M.; Pavaloiu, R.D.; Sha’at, F.; Vladu, M.-G.; Neagu, G.; Manoiu, V.-S.; Eremia, M.-C. Preparation and Optimization of a Polyhydroxyoctanoate-Hydroxyapatite Composite Available to Scaffolds in Implantable Devices. Molecules 2025, 30, 730. [Google Scholar] [CrossRef]
- Wang, Z.; Shang, J.; Zhang, Z. Composite or Modified Hydroxyapatite Microspheres as Drug Delivery Carrier for Bone and Tooth Tissue Engineering. Curr. Med. Chem. 2025, 32, 974–981. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, B.; Wan, T.; Zhou, C.; Fan, Y.; Tian, W.; Jing, W. A 3D-Printed Biphasic Calcium Phosphate Scaffold Loaded with Platelet Lysate/Gelatin Methacrylate to Promote Vascularization. J. Mater. Chem. B 2022, 10, 3138–3151. [Google Scholar] [CrossRef]
- Sukul, M.; Nguyen, T.B.L.; Min, Y.-K.; Lee, S.-Y.; Lee, B.-T. Effect of Local Sustainable Release of BMP2-VEGF from Nano-Cellulose Loaded in Sponge Biphasic Calcium Phosphate on Bone Regeneration. Tissue Eng. Part A 2015, 21, 1822–1836. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Nanotechnology for Bone Materials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 336–351. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Y.; Li, B.; Yao, Y.; Xu, J.; Liu, R.; Peng, J.; Chang, Z.; Zhao, R.; Hou, R.; et al. Nanocomposite Hydrogels Optimize the Microenvironment by Exterior/Interior Crosstalk for Reprogramming Osteoporotic Homeostasis in Bone Defect Healing. J. Control. Release 2025, 380, 976–993. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hassanein, K.M.A.; Hussein, S.I.Z.; Semieka, M.M.A.; Elshahawy, A.M. Evaluation of a Chitosan/Polyvinyl Alcohol Hydrogel Loaded with Graphene Oxide and Nano TiO2 for Bone Defect Reconstruction in a Dog Model. J. Mater. Chem. B 2025, 13, 3581–3592. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yao, H.; Chang, L.; Zhu, W.; Zhang, Y.; Li, X.; Yang, B.; Dai, B.; Chen, X.; Lei, L.; et al. Magnesium Nanocomposite Hydrogel Reverses the Pathologies to Enhance Mandible Regeneration. Adv. Mater. 2025, 37, e2312920. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Gao, L.; Zhang, W. Hydrogels in Alveolar Bone Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 7337–7351. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Zou, D.; Zu, H.; Li, W.; Zheng, Y. An Overview of Magnesium-Based Implants in Orthopaedics and a Prospect of Its Application in Spine Fusion. Bioact. Mater. 2024, 39, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Antoniac, I.; Miculescu, M.; Mănescu, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef]
- Prakash, G.; Singh, N.K.; Kumar, D.; Chandel, P. Compression and flexure behaviours of magnesium alloy AZ41 at different strain rates. U.P.B. Sci. Bull. Ser. D 2020, 82, 211–222. [Google Scholar]
- Voicu, M.-E.; Golgovici, F. A BIOINTERFACE GROWTH at IMMERSION of a BIODEGRADABLE MAGNESIUM ALLOY in SIMULATED BODY FLUID. U.P.B. Sci. Bull. Ser. B 2020, 82, 57–68. [Google Scholar]
- Zhen, Z.; Liu, X.; Huang, T.; Xi, T.; Zheng, Y. Hemolysis and Cytotoxicity Mechanisms of Biodegradable Magnesium and Its Alloys. Mater. Sci. Eng. C 2015, 46, 202–206. [Google Scholar] [CrossRef]
- Durlach, J.; Durlach, V.; Bac, P.; Bara, M.; Guiet-Bara, A. Magnesium and Therapeutics. Magnes. Res. 1994, 7, 313–328. [Google Scholar] [PubMed]
- Kim, K.-J.; Choi, S.; Sang Cho, Y.; Yang, S.-J.; Cho, Y.-S.; Kim, K.K. Magnesium Ions Enhance Infiltration of Osteoblasts in Scaffolds via Increasing Cell Motility. J. Mater. Sci. Mater. Med. 2017, 28, 96. [Google Scholar] [CrossRef]
- Tian, L.; Sheng, Y.; Huang, L.; Chow, D.H.-K.; Chau, W.H.; Tang, N.; Ngai, T.; Wu, C.; Lu, J.; Qin, L. An Innovative Mg/Ti Hybrid Fixation System Developed for Fracture Fixation and Healing Enhancement at Load-Bearing Skeletal Site. Biomaterials 2018, 180, 173–183. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Wang, X.; Sheng, L.; Zheng, L.; Song, B.; Wu, G.; Zhang, R.; Yao, H.; Zheng, N.; et al. Magnesium-Pretreated Periosteum for Promoting Bone-Tendon Healing after Anterior Cruciate Ligament Reconstruction. Biomaterials 2021, 268, 120576. [Google Scholar] [CrossRef]
- Bita, A.I.; Antoniac, A.; Cotrut, C.; Vasile, E.; Ciuca, I.; Niculescu, M.; Antoniac, I. In Vitro Degradation and Corrosion Evaluation of Mg-Ca Alloys for Biomedical Applications. J. Optoelectron. Adv. Mater. 2016, 18, 394–398. [Google Scholar]
- Pogorielov, M.; Husak, E.; Solodivnik, A.; Zhdanov, S. Magnesium-Based Biodegradable Alloys: Degradation, Application, and Alloying Elements. Interv. Med. Appl. Sci. 2017, 9, 27–38. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High Concentrations of Magnesium Modulate Vascular Endothelial Cell Behaviour in Vitro. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, D.; Nasulewic, A.; Mazur, A.; Maier, J.A.M. Magnesium and Microvascular Endothelial Cells: A Role in Inflammation and Angiogenesis. Front. Biosci. 2005, 10, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-Ceramic Coated Mg-Ca Alloys for Biomedical Implant Applications. Mater. Sci. Eng. C 2016, 64, 362–369. [Google Scholar] [CrossRef]
- Antoniac, I.; Manescu, V.; Paltanea, G.; Antoniac, A.; Nemoianu, I.V.; Petrescu, M.I.; Dura, H.; Bodog, A.D. Additive Manufactured Magnesium-Based Scaffolds for Tissue Engineering. Materials 2022, 15, 8693. [Google Scholar] [CrossRef]
- Manescu, V.; Antoniac, I.; Antoniac, A.; Laptoiu, D.; Paltanea, G.; Ciocoiu, R.; Nemoianu, I.V.; Gruionu, L.G.; Dura, H. Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives. Biomimetics 2023, 8, 618. [Google Scholar] [CrossRef]
- Bita, A.-I.; Antoniac, I.; Ciuca, I. Potential Use of Mg-Ca Alloys for Orthopedic Applications. U.P.B. Sci. Bull. Ser. B 2016, 78, 173–184. [Google Scholar]
- Bița, T.; Corneschi, I.; Ungureanu, E.; Bița, A.-I.; Ignat, N.-D.; Dura, H.; Cârstoc, I.D. INFLUENCE of HEAT TREATMENT on MICROSTRUCTURE and CORROSION BEHAVIOR of BIODEGRADABLE Mg-Ca ALLOY. U.P.B. Sci. Bull. Ser. B 2023, 85, 247–260. [Google Scholar]
- Bița, A.-I.; Antoniac, I.; Miculescu, M.; Stan, G.E.; Leonat, L.; Antoniac, A.; Constantin, B.; Forna, N. Electrochemical and In Vitro Biological Evaluation of Bio-Active Coatings Deposited by Magnetron Sputtering onto Biocompatible Mg-0.8Ca Alloy. Materials 2022, 15, 3100. [Google Scholar] [CrossRef]
- Streza, A.; Antoniac, A.; Manescu, V.; Ciocoiu, R.; Cotrut, C.-M.; Miculescu, M.; Miculescu, F.; Antoniac, I.; Fosca, M.; Rau, J.V.; et al. In Vitro Studies Regarding the Effect of Cellulose Acetate-Based Composite Coatings on the Functional Properties of the Biodegradable Mg3Nd Alloys. Biomimetics 2023, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Du, W.; Fu, J.; Liu, K.; Li, S.; Wang, Z.; Liang, H. A Review on Magnesium Alloys for Application of Degradable Fracturing Tools. J. Magnes. Alloys 2022, 10, 2649–2672. [Google Scholar] [CrossRef]
- Wu, A.; Xia, C. Study of the Microstructure and Mechanical Properties of Mg-Rare Earth Alloys. Mater. Des. 2007, 28, 1963–1967. [Google Scholar] [CrossRef]
- Willbold, E.; Gu, X.; Albert, D.; Kalla, K.; Bobe, K.; Brauneis, M.; Janning, C.; Nellesen, J.; Czayka, W.; Tillmann, W.; et al. Effect of the Addition of Low Rare Earth Elements (Lanthanum, Neodymium, Cerium) on the Biodegradation and Biocompatibility of Magnesium. Acta Biomater. 2015, 11, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Hu, H.; Northwood, D.O.; Li, N. Microstructure and Nano-Scale Mechanical Behavior of Mg–Al and Mg–Al–Ca Alloys. Mater. Sci. Eng. A 2008, 473, 16–27. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Staiger, M.P.; Nisbet, D.; Davies, C.H.J.; Birbilis, N. Performance-Driven Design of Biocompatible Mg Alloys. JOM 2011, 63, 28–34. [Google Scholar] [CrossRef]
- ASTM E9-09; Standard Test Methods of Compression Testing of Metallic Materials at Room Temperature. ASTM International: West Conshohocken, PA, USA, 2009.
- Pietrzyńska, M.; Voelkel, A. Stability of Simulated Body Fluids Such as Blood Plasma, Artificial Urine and Artificial Saliva. Microchem. J. 2017, 134, 197–201. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting In Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- ASTM G5-94; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2011.
- Dragomir, L.; Antoniac, A.; Manescu, V.; Robu, A.; Dinu, M.; Pana, I.; Cotrut, C.M.; Kamel, E.; Antoniac, I.; Rau, J.V.; et al. Preparation and Characterization of Hydroxyapatite Coating by Magnetron Sputtering on Mg–Zn–Ag Alloys for Orthopaedic Trauma Implants. Ceram. Int. 2023, 49, 26274–26288. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, F.; Cotrut, C.; Ficai, A.; Rau, J.V.; Grosu, E.; Antoniac, A.; Tecu, C.; Cristescu, I. Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating. Materials 2020, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-6; Biological Evaluation of Medical Devices. Part 6: Tests for local effects after implantation. International Organization for Standardization: Geneva, Switzerland, 2021.
- Destrez, A.; Colin, E.; Testelin, S.; Devauchelle, B.; Dakpé, S.; Naudot, M. Evaluation of a Granular Bone Substitute for Bone Regeneration Using an Optimized In Vivo Alveolar Cleft Model. Bioengineering 2023, 10, 1035. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.K.; Kar, A.K.; Singh, A.; Jagdale, P.; Satija, N.K.; Ghosh, D.; Patnaik, S. Control Release of Adenosine Potentiate Osteogenic Differentiation within a Bone Integrative EGCG-g-NOCC/Collagen Composite Scaffold toward Guided Bone Regeneration in a Critical-Sized Calvarial Defect. Biomacromolecules 2021, 22, 3069–3083. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Bae, S.-R.; Suh, J.-Y.; Lee, D.-H.; Kim, S.H.; Kim, H.; Lee, C.S. Evaluation of Bone Healing with Eggshell-Derived Bone Graft Substitutes in Rat Calvaria: A Pilot Study. J. Biomed. Mater. Res. Part A 2008, 87A, 203–214. [Google Scholar] [CrossRef]
- Nowak, B.; Matuszewska, A.; Nikodem, A.; Filipiak, J.; Landwójtowicz, M.; Sadanowicz, E.; Jędrzejuk, D.; Rzeszutko, M.; Zduniak, K.; Piasecki, T.; et al. Oral Administration of Kaempferol Inhibits Bone Loss in Rat Model of Ovariectomy-Induced Osteopenia. Pharmacol. Rep. 2017, 69, 1113–1119. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-Derived Magnesium Induces Local Neuronal Production of CGRP to Improve Bone-Fracture Healing in Rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Song, D.; Fu, Y.; Zhou, Q.; Fu, M.; Wu, X.; Sun, Y.; Bi, W.; Sun, J.; Yang, F.; Guo, H.; et al. Acceleration of Calvarial Bone Regeneration by Stem Cell Recruitment with a Multifunctional Hydrogel. Adv. Healthc. Mater. 2025, 14, 2501452. [Google Scholar] [CrossRef] [PubMed]
- Standard Guide for Metallurgical Characterization of Absorbable Metallic Materials for Medical Implants. Available online: https://store.astm.org/f3160-16.html (accessed on 11 October 2025).
- Lee, D.-H.; Kim, I.-K.; Cho, H.-Y.; Seo, J.-H.; Jang, J.-M.; Kim, J. Effect of Herbal Extracts on Bone Regeneration in a Rat Calvaria Defect Model and Screening System. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 79–85. [Google Scholar] [CrossRef]
- Zhao, N.; Qin, L.; Liu, Y.; Zhai, M.; Li, D. Improved New Bone Formation Capacity of Hyaluronic Acid-Bone Substitute Compound in Rat Calvarial Critical Size Defect. BMC Oral Health 2024, 24, 994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Kaiser, F.; Schröter, L.; Stein, S.; Krüger, B.; Weichhold, J.; Stahlhut, P.; Ignatius, A.; Gbureck, U. Accelerated Bone Regeneration through Rational Design of Magnesium Phosphate Cements. Acta Biomater. 2022, 145, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Effect of Chemistry on Osteogenesis and Angiogenesis towards Bone Tissue Engineering Using 3D Printed Scaffolds. Ann. Biomed. Eng. 2017, 45, 261–272. [Google Scholar] [CrossRef]
- Zemková, M.; Minárik, P.; Dittrich, J.; Bohlen, J.; Král, R. Individual Effect of Y and Nd on the Microstructure Formation of Mg-Y-Nd Alloys Processed by Severe Plastic Deformation and Their Effect on the Subsequent Mechanical and Corrosion Properties. J. Magnes. Alloys 2023, 11, 509–521. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Dong, X.; Yang, Y. Microstructure and Mechanical Properties of Mg-Zn-Y-Nd-Zr Alloys. J. Rare Earths 2013, 31, 616–621. [Google Scholar] [CrossRef]
- Zhou, H.T.; Zhang, Z.D.; Liu, C.M.; Wang, Q.W. Effect of Nd and Y on the Microstructure and Mechanical Properties of ZK60 Alloy. Mater. Sci. Eng. A 2007, 445–446, 1–6. [Google Scholar] [CrossRef]
- Khan, S.A.; Miyashita, Y.; Mutoh, Y.; Sajuri, Z.B. Influence of Mn Content on Mechanical Properties and Fatigue Behavior of Extruded Mg Alloys. Mater. Sci. Eng. A 2006, 420, 315–321. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The Role and Significance of Magnesium in Modern Day Research-A Review. J. Magnes. Alloys 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and Bio-Corrosion Properties of Quaternary Mg–Ca–Mn–Zn Alloys Compared with Binary Mg–Ca Alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, L. Microstructure, Mechanical Properties and Bio-Corrosion Properties of Mg–Zn–Mn–Ca Alloy for Biomedical Application. Mater. Sci. Eng. A 2008, 497, 111–118. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Wang, L.; Wu, Y.; Wang, L.; Zhang, H. Microstructure and Mechanical Properties of Mg–4.5Zn–xNd (x = 0, 1 and 2, wt%) Alloys. Mater. Sci. Eng. A 2008, 479, 339–344. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Wang, Y.; Zeng, X.; Ding, W. Effect of Nd and Y Addition on Microstructure and Mechanical Properties of As-Cast Mg–Zn–Zr Alloy. J. Alloys Compd. 2007, 427, 115–123. [Google Scholar] [CrossRef]
- Yan, K.; Bai, J.; Liu, H.; Jin, Z.-Y. The Precipitation Behavior of MgZn2 and Mg4Zn7 Phase in Mg-6Zn (wt.%) Alloy during Equal-Channel Angular Pressing. J. Magnes. Alloys 2017, 5, 336–339. [Google Scholar] [CrossRef]
- Wei, L.Y.; Dunlop, G.L.; Westengen, H. Precipitation Hardening of Mg-Zn and Mg-Zn-RE Alloys. Metall. Mater. Trans. A 1995, 26, 1705–1716. [Google Scholar] [CrossRef]
- Zengin, H.; Turen, Y. Effect of Y Addition on Microstructure and Corrosion Behavior of Extruded Mg–Zn–Nd–Zr Alloy. J. Magnes. Alloys 2020, 8, 640–653. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Koltygin, A.V.; Sung, M.C.; Park, S.H.; Tselovalnik, Y.V.; Stepashkin, A.A.; Rizhsky, A.A.; Belov, M.V.; Belov, V.D.; Malyutin, K.V. Development of Mg–Zn–Y–Zr Casting Magnesium Alloy with High Thermal Conductivity. J. Magnes. Alloys 2021, 9, 1567–1577. [Google Scholar] [CrossRef]
- Xu, D.K.; Tang, W.N.; Liu, L.; Xu, Y.B.; Han, E.H. Effect of W-Phase on the Mechanical Properties of as-Cast Mg–Zn–Y–Zr Alloys. J. Alloys Compd. 2008, 461, 248–252. [Google Scholar] [CrossRef]
- Luo, Z.P.; Zhang, S.Q. High-Resolution Electron Microscopy on the X-Mg12ZnY Phase in a High Strength Mg-Zn-Zr-Y Magnesium Alloy. J. Mater. Sci. Lett. 2000, 19, 813–815. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, D.H.; Lim, H.K.; Kim, D.H. Effects of Zn/Y Ratio on Microstructure and Mechanical Properties of Mg-Zn-Y Alloys. Mater. Lett. 2005, 59, 3801–3805. [Google Scholar] [CrossRef]
- Luo, S.; Tang, A.; Pan, F.; Song, K.; Wang, W. Effect of Mole Ratio of Y to Zn on Phase Constituent of Mg-Zn-Zr-Y Alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 795–800. [Google Scholar] [CrossRef]
- Farahany, S.; Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul Kadir, M.R.; Lotfabadi, A.F.; Ourdjini, A. In-Situ Thermal Analysis and Macroscopical Characterization of Mg–xCa and Mg–0.5Ca–xZn Alloy Systems. Thermochim. Acta 2012, 527, 180–189. [Google Scholar] [CrossRef]
- Jiang, M.G.; Xu, C.; Nakata, T.; Yan, H.; Chen, R.S.; Kamado, S. Development of Dilute Mg–Zn–Ca–Mn Alloy with High Performance via Extrusion. J. Alloys Compd. 2016, 668, 13–21. [Google Scholar] [CrossRef]
- Schäublin, R.E.; Becker, M.; Cihova, M.; Gerstl, S.S.A.; Deiana, D.; Hébert, C.; Pogatscher, S.; Uggowitzer, P.J.; Löffler, J.F. Precipitation in Lean Mg–Zn–Ca Alloys. Acta Mater. 2022, 239, 118223. [Google Scholar] [CrossRef]
- Du, Y.Z.; Zheng, M.Y.; Qiao, X.G.; Wu, K.; Liu, X.D.; Wang, G.J.; Lv, X.Y. Microstructure and Mechanical Properties of Mg–Zn–Ca–Ce Alloy Processed by Semi-Continuous Casting. Mater. Sci. Eng. A 2013, 582, 134–139. [Google Scholar] [CrossRef]
- Du, Y.Z.; Zheng, M.Y.; Xu, C.; Qiao, X.G.; Wu, K.; Liu, X.D.; Wang, G.J.; Lv, X.Y. Microstructures and Mechanical Properties of As-Cast and as-Extruded Mg-4.50Zn-1.13Ca (wt%) Alloys. Mater. Sci. Eng. A 2013, 576, 6–13. [Google Scholar] [CrossRef]
- Nakata, T.; Xu, C.; Ito, Y.; Kamado, S. Role of Homogenization on Tensile Properties and Microstructures in a Dilute Mg–Zn–Ca–Mn Alloy Sheet. Mater. Sci. Eng. A 2022, 833, 142541. [Google Scholar] [CrossRef]
- Kavyani, M.; Ebrahimi, G.R.; Ezatpour, H.R.; Jahazi, M. Microstructure Refinement, Mechanical and Biocorrosion Properties of Mg–Zn–Ca–Mn Alloy Improved by a New Severe Plastic Deformation Process. J. Magnes. Alloys 2022, 10, 1640–16652. [Google Scholar] [CrossRef]
- Bazhenov, V.E.; Li, A.V.; Komissarov, A.A.; Koltygin, A.V.; Tavolzhanskii, S.A.; Bautin, V.A.; Voropaeva, O.O.; Mukhametshina, A.M.; Tokar, A.A. Microstructure and Mechanical and Corrosion Properties of Hot-Extruded Mg–Zn–Ca–(Mn) Biodegradable Alloys. J. Magnes. Alloys 2021, 9, 1428–1442. [Google Scholar] [CrossRef]
- Cho, D.H.; Nam, J.H.; Lee, B.W.; Cho, K.M.; Park, I.M. Effect of Mn Addition on Grain Refinement of Biodegradable Mg4Zn0.5Ca Alloy. J. Alloys Compd. 2016, 676, 461–468. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Feyerabend, F.; Blawert, C.; Gan, W.; Maawad, E.; You, S.; Gavras, S.; Scharnagl, N.; Bode, J.; et al. Influence of the Amount of Intermetallics on the Degradation of Mg-Nd Alloys under Physiological Conditions. Acta Biomater. 2021, 121, 695–712. [Google Scholar] [CrossRef]
- Gorsse, S.; Hutchinson, C.R.; Chevalier, B.; Nie, J.-F. A Thermodynamic Assessment of the Mg–Nd Binary System Using Random Solution and Associate Models for the Liquid Phase. J. Alloys Compd. 2005, 392, 253–262. [Google Scholar] [CrossRef]
- Thaha, Y.N.; Siswayanti, B.; Elvira, B.R.; Syahid, A.N.; Rachmawati, N.; Erryani, A.; Malau, D.P.; Annur, D.; Lestari, F.P.; Asmaria, T.; et al. Effect of Neodymium on Microstructure, Mechanical Properties, and Corrosion Behavior of Mg-2Si-xNd Alloys Fabricated by Powder Metallurgy. J. Rare Earths 2024, 43, 2569–2578. [Google Scholar] [CrossRef]
- Elkaiam, L.; Hakimi, O.; Goldman, J.; Aghion, E. The Effect of Nd on Mechanical Properties and Corrosion Performance of Biodegradable Mg-5%Zn Alloy. Metals 2018, 8, 438. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, J.; Sun, Y.; Zhu, S.; Wang, L.; Guan, S. Microstructure and Mechanical Properties of the Sub-Rapidly Solidified Mg―Zn―Y―Nd Alloy Prepared by Step-Copper Mold Casting. Mater. Today Commun. 2021, 27, 102308. [Google Scholar] [CrossRef]
- Meng, F.; Lv, S.; Yang, Q.; Qiu, X.; Yan, Z.; Duan, Q.; Meng, J. Multiplex Intermetallic Phases in a Gravity Die-Cast Mg−6.0Zn−1.5Nd−0.5Zr (wt%) Alloy. J. Magnes. Alloys 2022, 10, 209–223. [Google Scholar] [CrossRef]
- Joshi, P.; Rao, R.N.; Golla, C.B.; Özcan, M.; Prasad, P.S. Comprehensive Review of Mg-Based Alloys: Mechanical, Chemical and Biological Properties for Prosthetic and Orthopedic Applications. J. Mater. Res. Technol. 2025, 35, 2487–2502. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.; Su, G.; Tang, D.; Rokhlin, L.L.; Elkin, F.M.; Meng, J. Influence of Zn Content on the Microstructure and Mechanical Properties of Extruded Mg–5Y–4Gd–0.4Zr Alloy. J. Alloys Compd. 2009, 481, 811–818. [Google Scholar] [CrossRef]
- Wang, S.; Pan, H.; Xie, D.; Zhang, D.; Li, J.; Xie, H.; Ren, Y.; Qin, G. Grain Refinement and Strength Enhancement in Mg Wrought Alloys: A Review. J. Magnes. Alloys 2023, 11, 4128–4145. [Google Scholar] [CrossRef]
- Ma, C.; Hou, C.; Zhang, X.; Liu, T.; Zhou, N.; Zheng, K. Study on the Microstructure and Mechanical Properties of Mg–Al–Li–Zn–Ti Multi-Component Alloy. J. Mater. Res. Technol. 2024, 29, 4781–4795. [Google Scholar] [CrossRef]
- Azadi, A.; Ebel, T.; Wolff, M.; O’Cearbhaill, E.; Celikin, M. Additive Manufacturing of Magnesium Alloys for Biomedical Applications: Critical Review on Sinterability and Alloy Development. J. Mater. Res. Technol. 2025, 35, 6986–7007. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, X.; Qiao, Y.; Wang, X.; Lin, Q. Preparation of Medical Mg–Zn Alloys and the Effect of Different Zinc Contents on the Alloy. J. Mater. Sci. Mater. Med. 2022, 33, 9. [Google Scholar] [CrossRef] [PubMed]
- ASTM E111; Standard Test Method for Young’s Modulus, Tangent Modulus, and Chord Modulus. ASTM International: West Conshohocken, PA, USA, 2017.
- Havaldar, R.; Pilli, S.C.; Putti, B.B. Insights into the Effects of Tensile and Compressive Loadings on Human Femur Bone. Adv. Biomed. Res. 2014, 3, 101. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s Modulus of Trabecular and Cortical Bone Material: Ultrasonic and Microtensile Measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Kurniawan, K.A.; Wicaksono, S.T.; Rasyida, A.; Purniawan, A. Improvement of Compressive Strength of Mg-Fe-Ca Alloy by Heat Treatment as Biodegradable Implant. In Proceedings of the International Conference on Science and Applied Science (ICSAS) 2019, Surakarta, Indonesia, 20 July 2019; AIP Publishing: Melville, NY, USA, 2019. [Google Scholar]
- Teslia, S.; Kovalenko, M.; Teslia, M.; Vterkovskiy, M.; Solodkyi, I.; Loboda, P.; Soloviova, T. The Activation of Magnesium Sintering by Zinc Addition. Alloys 2024, 3, 178–189. [Google Scholar] [CrossRef]
- Su, Y.; Lin, J.; Su, Y.; Zai, W.; Li, G.; Wen, C. Investigation on Composition, Mechanical Properties, and Corrosion Resistance of Mg-0.5Ca-X(Sr, Zr, Sn) Biological Alloy. Scanning 2018, 2018, 6519310. [Google Scholar] [CrossRef]
- Sumitomo, T.; Cáceres, C.H.; Veidt, M. The Elastic Modulus of Cast Mg–Al–Zn Alloys. J. Light Met. 2002, 2, 49–56. [Google Scholar] [CrossRef]
- Packham, D.E. Surface Energy, Surface Topography and Adhesion. Int. J. Adhes. Adhes. 2003, 23, 437–448. [Google Scholar] [CrossRef]
- Gambaro, S.; Nascimento, M.L.; Shekargoftar, M.; Ravanbakhsh, S.; Sales, V.; Paternoster, C.; Bartosch, M.; Witte, F.; Mantovani, D. Characterization of a Magnesium Fluoride Conversion Coating on Mg-2Y-1Mn-1Zn Screws for Biomedical Applications. Materials 2022, 15, 8245. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, Z.; Chen, L.; Zhang, L.; Li, M.; Hou, H.; Zhao, Y. Electrochemical Corrosion Behavior of AZ91D Magnesium Alloy-Graphene Nanoplatelets Composites in Simulated Body Fluids. J. Mater. Res. Technol. 2023, 24, 449–462. [Google Scholar] [CrossRef]
- Sasikumar, Y.; Solomon, M.M.; Olasunkanmi, L.O.; Ebenso, E.E. Effect of Surface Treatment on the Bioactivity and Electrochemical Behavior of Magnesium Alloys in Simulated Body Fluid. Mater. Corros. 2017, 68, 776–790. [Google Scholar] [CrossRef]
- ASTM G59-97; Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. ASTM International: West Conshohocken, PA, USA, 2020.
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Daroonparvar, M.; Kasiri-Asgarani, M.; Medraj, M. Synthesis and Biodegradation Evaluation of Nano-Si and Nano-Si/TiO2 Coatings on Biodegradable Mg–Ca Alloy in Simulated Body Fluid. Ceram. Int. 2014, 40, 14009–14018. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Zhang, K.; Wang, C.; Li, H.; Ma, M.; Zhang, B. Corrosion and Electrochemical Behavior of Mg–Y Alloys in 3.5% NaCl Solution. Trans. Nonferrous Met. Soc. China 2013, 23, 1226–1236. [Google Scholar] [CrossRef]
- Sudholz, A.D.; Gusieva, K.; Chen, X.B.; Muddle, B.C.; Gibson, M.A.; Birbilis, N. Electrochemical Behaviour and Corrosion of Mg–Y Alloys. Corros. Sci. 2011, 53, 2277–2282. [Google Scholar] [CrossRef]
- Davenport, A.J.; Padovani, C.; Connolly, B.J.; Stevens, N.P.C.; Beale, T.A.W.; Groso, A.; Stampanoni, M. Synchrotron X-Ray Microtomography Study of the Role of Y in Corrosion of Magnesium Alloy WE43. Electrochem. Solid-State Lett. 2006, 10, C5. [Google Scholar] [CrossRef]
- Rogova, V.-V.; Peev, S.; Yotsova, R.; Gerova-Vatsova, T.; Parushev, I. Histomorphometric Assessment of Non-Decalcified Plastic-Embedded Specimens for Evaluation of Bone Regeneration Using Bone Substitute Materials—A Systematic Review. Materials 2024, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Pesode, P.; Barve, S. Additive Manufacturing of Magnesium Alloys and Its Biocompatibility. Bioprinting 2023, 36, e00318. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, M.; Cai, Y.; Yao, J.; Wang, P.; Atrens, A. Recent Advances in Bio-Functional Ta-Based Bone Materials: Materials Design and Bioactivity. Int. J. Extreme Manuf. 2024, 6, 062010. [Google Scholar] [CrossRef]
- Taguchi, T.; Lopez, M.J. An Overview of de Novo Bone Generation in Animal Models. J. Orthop. Res. 2021, 39, 7–21. [Google Scholar] [CrossRef]
- Thudium, C.S.; Nielsen, S.H.; Sardar, S.; Mobasheri, A.; van Spil, W.E.; Lories, R.; Henriksen, K.; Bay-Jensen, A.-C.; Karsdal, M.A. Bone Phenotypes in Rheumatology—There Is More to Bone than Just Bone. BMC Musculoskelet. Disord. 2020, 21, 789. [Google Scholar] [CrossRef]
- Niu, Y.; Du, T.; Liu, Y. Biomechanical Characteristics and Analysis Approaches of Bone and Bone Substitute Materials. J. Funct. Biomater. 2023, 14, 212. [Google Scholar] [CrossRef] [PubMed]



| Alloy | Composition (Mass Percents %) | |||||
|---|---|---|---|---|---|---|
| Zn | Y | Nd | Ca | Mn | Mg | |
| Mg-Nd | 0.4 | 0.3 | 2.6 | - | - | Bal. |
| Mg-Zn | 1.4 | - | - | 0.3 | 0.6 | Bal. |
| Element | Mg-Nd | |||||
| O | Zn | Y | Nd | Mg | Total | |
| Weight (%) | 1.56 ± 0.15 | 0.38 ± 0.04 | 0.32 ± 0.03 | 2.58 ± 0.12 | 95.16 ± 0.34 | 100.00 |
| Atom (%) | 2.08 ± 0.23 | 0.14 ± 0.02 | 0.09 ± 0.02 | 0.41 ± 0.02 | 97.28 ± 0.35 | 100.00 |
| Element | Mg-Zn | |||||
| O | Zn | Ca | Mn | Mg | Total | |
| Weight (%) | 0.69 ± 0.09 | 1.20 ± 0.13 | 0.35 ± 0.06 | 0.54 ± 0.07 | 97.22 ± 0.33 | 100.00 |
| Atom (%) | 1.02 ± 0.13 | 0.51 ± 0.05 | 0.31 ± 0.02 | 0.23 ± 0.03 | 97.93 ± 0.32 | 100.00 |
| Point | Mg-Nd | ||||
| O | Zn | Y | Nd | Mg | |
| 1 | 1.69 ± 0.16 | 0.21 ± 0.05 | 0.18 ± 0.05 | 1.20 ± 0.10 | 96.72 ± 0.31 |
| 2 | 2.17 ± 0.16 | 1.51 ± 0.13 | 0.00 | 16.45 ± 0.32 | 79.87 ± 0.26 |
| 3 | 1.70 ± 0.15 | 0.53 ± 0.05 | 0.00 | 2.25 ± 0.15 | 95.52 ± 0.28 |
| 4 | 1.35 ± 0.14 | 0.70 ± 0.06 | 3.02 ± 0.30 | 0.00 | 94.93 ± 0.30 |
| 5 | 1.87 ± 0.16 | 0.55 ± 0.05 | 0.00 | 2.55 ± 0.11 | 95.03 ± 0.29 |
| 6 | 1.25 ± 0.17 | 0.61 ± 0.10 | 0.00 | 3.94 ± 0.11 | 94.20 ± 0.30 |
| 7 | 1.28 ± 0.10 | 0.46 ± 0.05 | 0.00 | 2.41 ± 0.11 | 95.85 ± 0.30 |
| 8 | 1.44 ± 0.16 | 0.41 ± 0.05 | 0.00 | 2.26 ± 0.10 | 95.89 ± 0.30 |
| Point | Mg-Zn | ||||
| O | Zn | Ca | Mn | Mg | |
| 1 | 0.67 ± 0.09 | 1.2 ± 0.11 | 0.22 ± 0.02 | 0.35 ±0.07 | 98.23 ± 0.31 |
| 2 | 0.82 ± 0.10 | 12.71 ± 0.18 | 11.05 ± 0.11 | 0.00 | 75.42 ± 0.27 |
| 3 | - | 0.61 ± 0.05 | 0.12 ± 0.02 | 29.90 ± 0.15 | 69.37 ± 0.22 |
| 4 | - | 0.76 ± 0.09 | 0.19 ± 0.04 | 38.97 ± 0.16 | 60.08 ± 0.24 |
| 5 | 0.81± 0.09 | 13.73 ± 0.18 | 12.72 ± 0.10 | 0.00 | 72.74 ± 0.25 |
| 6 | 0.96 ± 0.11 | 15.16 ± 0.18 | 10.04 ± 0.09 | 0.00 | 73.84 ± 0.26 |
| Sample ID | Eoc (V) | Ecorr (mV) | icorr (μA/cm2) | βc (mV) | βa (mV) | Rp (kΩcm2) | CR (mm/Year) |
|---|---|---|---|---|---|---|---|
| Mg-Nd | −1.675 | −1.620 | 55.700 | 268.73 | 87.28 | 0.505 | 1.215 |
| Mg-Zn | −1.567 | −1.566 | 6.859 | 158.24 | 381.75 | 7.110 | 0.154 |
| Samples | Time | Inflammation | Neovascularization | Fibrosis | Fatty Infiltration | Subtotal | TOTAL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute | Chronic | Subtotal | |||||||||||
| Necrosis | Polymorphonuclear Neutrophil | Lymphocytes | Plasma Cells | Macrophage | Giant Cells | ||||||||
| Mg-Nd | Week 1 | 1 | 1.5 | 1.5 | 0.5 | 2 | 1 | 7.5 | 1.5 | 2.5 | 0.5 | 4.5 | 12 |
| Week 2 | 0.5 | 1 | 1.5 | 0 | 1.5 | 0.5 | 5 | 2 | 2 | 0 | 4 | 9 | |
| Week 4 | 1 | 1 | 2.25 | 0.75 | 2.25 | 0.75 | 8 | 1.75 | 1.75 | 0 | 3.5 | 11.5 | |
| Week 8 | 0 | 0.25 | 1.5 | 0 | 2.5 | 1.75 | 6 | 1 | 1.25 | 0 | 2.25 | 8.25 | |
| Mg-Zn | Week 1 | 1.5 | 2.5 | 1 | 0 | 2 | 1 | 8 | 1 | 1 | 0 | 2 | 10 |
| Week 2 | 0 | 0 | 2 | 0.5 | 1.5 | 1.5 | 5.5 | 1 | 1.5 | 0 | 2.5 | 8 | |
| Week 4 | 0 | 0 | 0.5 | 0 | 0.5 | 1.5 | 2.5 | 0 | 1 | 0 | 1 | 3.5 | |
| Week 8 | 0 | 0 | 2 | 0.5 | 1.5 | 0.5 | 4.5 | 0 | 1 | 0 | 1 | 5.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manescu, V.; Antoniac, A.; Moraru, M.C.; Antoniac, I.; Cotrut, C.M.; Gradinaru, S.; Dreanca, A.I.; Sevastre, B.; Pop, R.; Tabaran, F.A.; et al. Suitability of Mg-Nd and Mg-Zn Alloys to Obtain Biodegradable Structures for Bone Defects. J. Funct. Biomater. 2025, 16, 423. https://doi.org/10.3390/jfb16110423
Manescu V, Antoniac A, Moraru MC, Antoniac I, Cotrut CM, Gradinaru S, Dreanca AI, Sevastre B, Pop R, Tabaran FA, et al. Suitability of Mg-Nd and Mg-Zn Alloys to Obtain Biodegradable Structures for Bone Defects. Journal of Functional Biomaterials. 2025; 16(11):423. https://doi.org/10.3390/jfb16110423
Chicago/Turabian StyleManescu (Paltanea), Veronica, Aurora Antoniac, Maria Cristina Moraru, Iulian Antoniac, Cosmin Mihai Cotrut, Sebastian Gradinaru, Alexandra Iulia Dreanca, Bogdan Sevastre, Romelia Pop, Flaviu Alexandru Tabaran, and et al. 2025. "Suitability of Mg-Nd and Mg-Zn Alloys to Obtain Biodegradable Structures for Bone Defects" Journal of Functional Biomaterials 16, no. 11: 423. https://doi.org/10.3390/jfb16110423
APA StyleManescu, V., Antoniac, A., Moraru, M. C., Antoniac, I., Cotrut, C. M., Gradinaru, S., Dreanca, A. I., Sevastre, B., Pop, R., Tabaran, F. A., Vlasceanu, G. M., Ionita, M., & Manole, M. (2025). Suitability of Mg-Nd and Mg-Zn Alloys to Obtain Biodegradable Structures for Bone Defects. Journal of Functional Biomaterials, 16(11), 423. https://doi.org/10.3390/jfb16110423



.png)






