Abstract
Current dental adhesives lack antibacterial properties. This study aimed to explore the effect of incorporating benzyldimethyldodecyl ammonium chloride (BDMDAC) on the degree of conversion, contact angle, ultimate tensile strength (UTS), microtensile bond strength (µTBS), cytotoxicity, antibacterial and bonding performance after artificial aging. A dental adhesive was doped with BDMDAC in the concentration range of 1–5 wt.%. For antibacterial assays, the BDMDAC compound was subject to planktonic cells of Streptococcus mutans. Then, after incorporation into the dental adhesive, an S. mutans biofilm model was used to grow 48 h-mature biofilms. The biofilms grown over the formulated materials were assessed by colony-forming unit (CFU) counting assay and fluorescence microscopy staining. In addition, the cytotoxicity was evaluated. Samples were subjected to 10,000 thermal cycles for aging and evaluated by UTS, µTBS, and CFU. Incorporating BDMDAC did not increase the cytotoxicity or change the physical properties when the mass fraction of the BDMDAC was 1–5 wt.%. The UTS of BDMDAC-doped adhesives was not impaired immediately or over time. A significant bacterial reduction was obtained for the mass fraction of the BDMDAC greater than 3 wt.%. However, the BDMDAC-doped adhesives did not offer an antibacterial effect after artificial aging. The overall results indicate that the BDMDAC strategy has the potential to control of microbial growth of cariogenic planktonic cells and biofilms. However, other new technological approaches are needed to overcome the deleterious effect of BDMDAC release over time such as those based on the principle of drug delivery systems whereby the BDMDAC is transported on microparticles or core shells, providing tangible benefits to oral health over time.
1. Introduction
Adhesion and subsequent growth of microorganisms on the tooth/material interface are major concerns preventing premature failures. Inside the mouth, tooth restorations are exposed to biofilm acid attacks over time. The degradation of adhesive by bacterial acids present over the interface tooth/restoration contributes to the recurrence of carious lesions around an existing restoration, namely secondary caries [1]. This process occurs mainly because of monomers’ cleavage due to the activity of water, saliva enzymes, and bacterial acids [2,3]. Consequently, improving adhesives’ properties to prevent biofilm formation on the margins of restorations is an exciting approach to assist restoration longevity [4].
The rapid development of biomaterials has forced equally rapid progress in dental materials [5]. As in the dental materials field, doping or incorporation is the intentional introduction of bioactive compounds, mostly antibacterial, into the formulation of the material to convey anti-biofilm properties to the final material [6]. The past decade has seen rapid development in nanotechnology in dentistry, with a notable advance in new dental materials for direct restorations, including dental adhesives [7]. These investigations have driven efforts on the effect of doping dental materials with inorganic metallic nanoparticles due to their potential antibacterial properties [8,9,10,11,12]. However, it should be noted that the nanoparticles have a much larger surface area to volume ratio due to their small size and, thus, highly increased activity [7]. Despite the many advantages, using metal and metal oxide nanoparticles is difficult. The main disadvantages include the increased cost associated with using nanotechnology, loss of antibacterial effect over time, complex synthesis through the multi-stage process, and the possible substantial color change of the final material [13].
In connection with the ongoing process of overcoming the challenges faced by nano-based strategies, great emphasis is put on finding new compounds with the potential to impart antibacterial effects to dental materials [14]. In the past decade, quaternary ammonium compounds have attracted considerable interest from academia and industry owing to their advantages over small molecular biocides, increased stability, low toxicity, facile synthesis, and the potential to reduce the broad spectra of oral pathogens [14]. Quaternary ammonium compounds are cationic surfactants that can disrupt the structure and integrity of cytoplasmic membranes and coagulating matrix constituents, causing enzyme denaturation [15] and inhibiting enzymes related to critical cellular functions such as respiration, energy transfer, ATP synthesis, and substrate oxidation [16]. Benzyldimethyldodecyl ammonium chloride (BDMDAC) is a benzylalkyldimethylethyl ammonium compound that can be bacteriostatic or bactericidal based on the concentration [17]. It is a cationic hydrophilic structure with a long alkyl chain of 12 carbons, which improves its antibacterial capacity. BDMDAC is a benzalkonium chloride component with antiseptic, detergent, and antibacterial properties [17]. It has been used in healthcare products as a preservative and antiseptic in eyewashes, nasal sprays, and injectable solutions [17]. However, there are no studies on doping BDMDAC to dental adhesives.
Therefore, this research aimed to develop the first dental adhesive doped with BDMDAC and study its effects on the degree of conversion, contact angle, ultimate tensile strength (UTS), microtensile bond strength (µTBS), cytotoxicity, and antibacterial and bonding performance after artificial aging. The objectives of the study were to: (1) synthesize BDMDAC-doped dental adhesive by incorporating BDMDAC in a range of 1–5 wt.% and investigate its effect on the mechanical and physicochemical properties before and after aging; (2) explore the antibacterial effect of BDMDAC-doped dental adhesive in a range of 1–5 wt.% before and after aging; (3) explore the cytotoxicity of BDMDAC-doped dental adhesive in a range of 1–5 wt.% on the studied concentrations.
2. Materials and Methods
2.1. Experimental Design
This is an in vitro experiment with six groups, as illustrated in Table 1.

Table 1.
Description of the dental adhesive formulations investigated in this study.
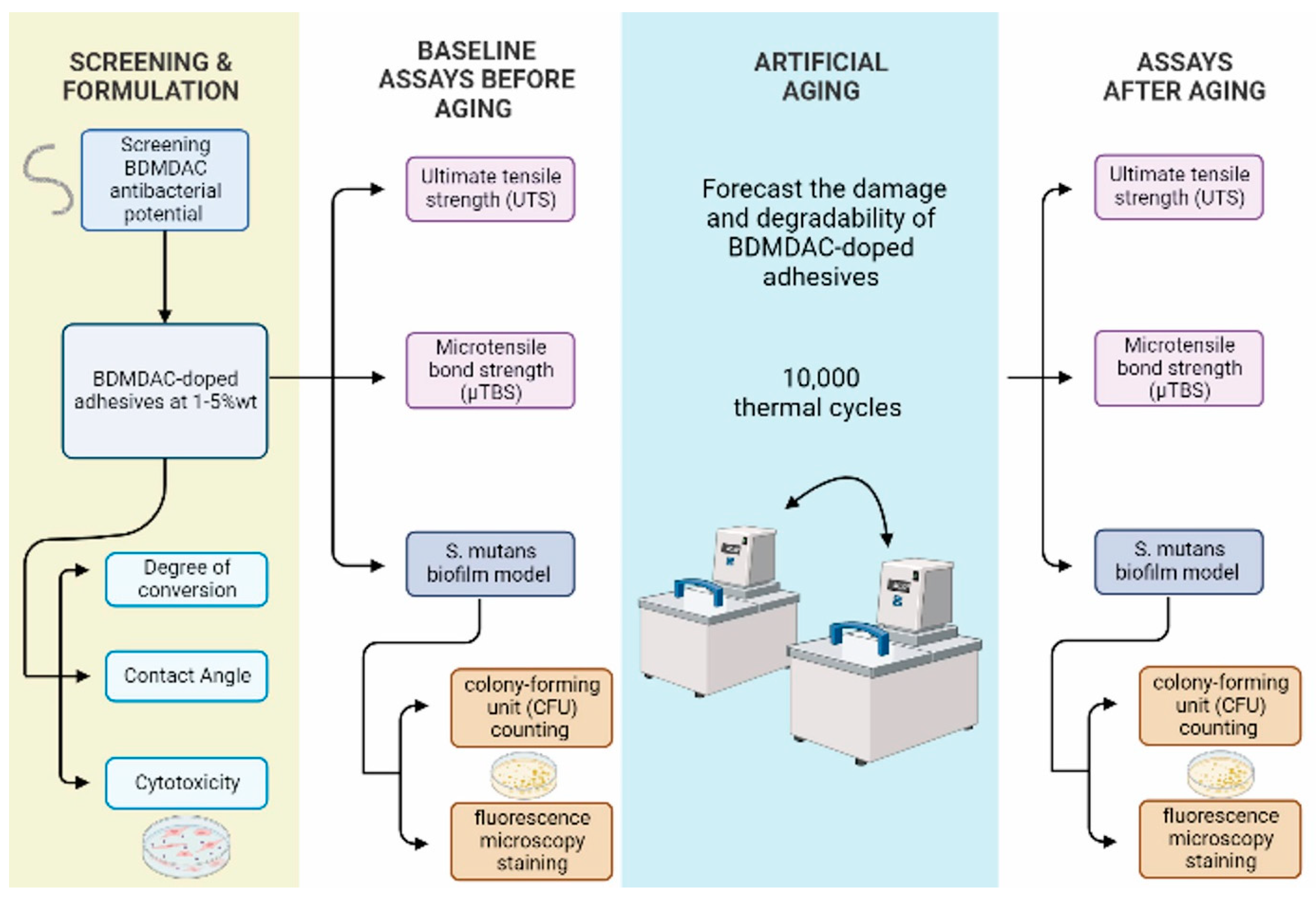
The outcome variables were degree of conversion, contact angle, ultimate tensile strength (UTS), microtensile bond strength (µTBS), cytotoxicity, and antibacterial and bonding performance after artificial aging. Experimental flow chart procedures of this study is presented in Figure 1.
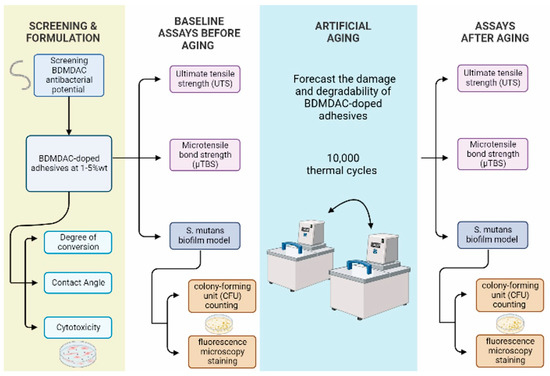
Figure 1.
Displays the experimental flow chart procedures of screening and adhesives formulations, aging method, and assays before and after aging used in this study.
2.2. Chemicals and Reagents
Materials purchased from Sigma-Aldrich (Saint Louis, MO, USA) and used without any purification: Camphorquinone; Ethyle 4-dimethylaminobenzoate; Butylated hydroxytoluene; and Benzyldimethldodecylammonium Chloride (BDMDAC). Monomers were purchased from Esstech (Essington, PA, USA): bisphenol A glycerolate dimethacrylate (BisGMA) and 2-hydroxyethyl methacrylate (HEMA); no further purification was made.
2.3. Determination of the Antibacterial Activity of Isolated BDMDAC Compound against S. mutans in Planktonic Cultures
The antibacterial activity of isolated BDMDAC compound against S. mutans in planktonic cultures was assessed to verify the potential antibacterial effect of this compound before incorporating it into the dental adhesive formulation. The bacterial suspension was adjusted with brain-heart infusion (BHI) broth to an initial optical density of 0.1 at 600 nm after being cultured for 24 h. Equal volumes of the diluted S. mutans liquid and BDMDAC (the final concentration is 10% (w/v), corresponding to 10 mg/mL) were mixed into 96-well plates. Then, we performed a dilution to achieve 7.5, 5, 3, 1.5, and 0.75% (w/v) of BDMDAC, followed by mixing solutions. Wells, without the addition of BDMDAC, served as negative growth controls. The 96-well plates were incubated at 37 °C for 24 h in an anaerobic incubator, and the inhibition of visible growth was observed. After, aliquots of each group were plated in BHI agar to observe the lowest concentration of BDMDAC at which there was no bacterial growth on the agar.
2.4. Formulation of Base Dental Adhesive Resins
Two methacrylate monomers were mixed in the following percentages to formulate the adhesive resins: 66.66 wt.% of bisphenol A glycerolate dimethacrylate (BisGMA) and 33.33 wt.% of 2-hyrdoxyethyl methacrylate (HEMA). Camphorquinone and ethyl 4-dimethylaminobenzoate were added at 1 mol% according to BisGMA and HEMA moles as photoinitiator/co-initiator systems. Butylated hydroxytoluene was added at 0.01 wt.% as a polymerization inhibitor [18]. Different concentrations of benzyldimethldodecylammonium chloride (BDMDAC) were selected following the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) results. BDMDAC was added to the adhesive resins’ formulation at 1, 2, 3, 4, and 5 wt.%. A group without BDMDAC was used as a control. The adhesives were mixed using a mechanical mixer (DAC 150 Speed mixer, Flacktec, Landrum, SC, USA) at 2800 rpm for 1 min.
2.5. Degree of Conversion
Prepared adhesives (n = 3) [19] were allotted directly on the ATR of FTIR crystal using a mold of polyvinylsiloxane to standardize sample thickness in 1 mm; each sample was covered by a polyester strip and photoactivated for 20 s using a light-cure unit with 1000 mW/cm2 placing the tip as close as possible to the sample surface [20]. Each sample was analyzed before and after the photoactivation using Opus 6.5 software and Blackman Harris 3-Term, OMNIC series at 4 cm−1 resolution. The degree of conversion was calculated using the peaks high at 1640 cm−1 and 1610 cm−1 following the below equation [20]:
2.6. Ultimate Tensile Strength (UTS)
A metallic hourglass-shaped mold was used to prepare the samples (n = 5) [19] with the dimensions of (8 mm long, 2 mm wide, 1 mm thick, and a cross-sectional area of 1 mm2) [18]. Polyester strips were placed above and below each mold, and each sample was photoactivated for 20 s. After that, samples were measured using a digital caliper, then soaked in distilled water for 24 h at 37 °C. Finally, each sample was fixed in a metallic jig using cyanoacrylate resin, maintaining parallelism between the applied force and the sample’s long axis, in preparation for tension load in a universal testing machine (EZ-SX Series, Shimadzu, Kyoto, Japan) at 1 mm/min of crosshead speed. Later, samples were broken at the concentration area, values were obtained in newton (N) and divided by the concentration area revealing results in megapascal (MPa).
2.7. Microtensile Bond Strength (µTBS)
Extracted human second and third molars were used in this study after the university’s Institutional Board (IRB) approval (HP-00088564). First, each tooth was cut under running water at the cementoenamel junction to separate its roots [19]. Then, silicon carbide sandpaper (600-grit) was used to flatten the dentin and produce the smear layer for 30 s with distilled water irrigation [8,18].
Samples (n = 20) were then rinsed for 1 min and dried in preparation for bonding. Dentin was conditioned with 37% phosphoric acid for 15 s, then rinsed for 30 s and gently dried with absorbent paper to keep dentin moist. A primer (ScotchBond Multipurpose, 3M ESPE, Saint Paul, MN, USA) was applied actively for 20 s using a micro-brush, then the solvent evaporated via air drying. Next, adhesive resins were applied in two layers using a micro-brush and photoactivated for 20 s. A commercially available composite was used (Amelogen Plus A4, Ultradent Product, South Jordan, UT, USA) in two increments of 2 mm and photoactivated for 20 s in each layer. After that, samples were kept for 24 h in distilled water at 37 °C. Then, beams were cut in the size of 0.7 × 0.7 mm at the bonding area using (Isomet; #15HC blade 127 × 0.04 mm) under distilled water. Beam’s size was confirmed using an electronic caliper, fixed in a metallic jig via cyanoacrylate resin, and through the microtensile tester’s tensile strength (New Research Day, West Chicago, IL, USA) at 1 mm/min till a fracture occurs. Interface fractures’ values were recorded in newton (N), divided by the bonding area, and expressed in MPa [21]. Failures at the composite or dentin were excluded from the final tensile calculation as their values do not represent the dentin–adhesive interface. Fractured beams were classified according to their fracture location using a microscope (50X; VWR® Stereo Zoom Binocular Microscope, Radnor, PA, USA) into adhesive (failure at the adhesive interface), mixed (at the adhesive interface with composite or dentin), cohesive in dentin, and cohesive in resin composite [22].
2.8. Contact Angle Assays
The contact angle was performed with water drops on the surface of the adhesive and with adhesive drops on the dentin surface. For the test with water, the sessile drop method was used to measure the static contact angle of water by dropping 10 µL on the surface of adhesive discs (n = 3) [23]. Three images were captured within 2 s of drop placement; the first image captured the droplet on a flat surface, creating the baseline. Later, the droplet edge and the gradient of the tangent of the droplet edge to the point where it meets the baseline were programmatically marked through goniometer software, and the resulting angle was measured from both sides. A single operator/observer was involved in this experiment [23].
For the test with the adhesives, a flat dentin surface was prepared [24], then the static contact angle was performed using the sessile drop method by placing a 10 µL of liquid adhesive using a micropipette (n = 3) [23]. A set of 3 images was captured within 2 s after placing the liquid adhesive on the dentin specimen; a contact angle goniometer was used for measurements. Again, a single operator was observing the measurements [25].
2.9. Sample Preparation for Microbiological Assays
Disc samples were prepared using a polyethylene mold with a diameter of 8 mm and a thickness of 1 mm [13,26]. Both disc surfaces were photoactivated for 20 s using a light-emitting diode with 1000 mW/cm2 (VALO Cordless, Ultradent Product, South Jordan, UT, USA). Later, samples were kept overnight in distilled water in an incubator at 37 °C, dried with absorbent paper, and sterilized with ethylene oxide gas (AnproleneAN 74i, Andersen, Haw River, NC, USA).
2.10. Streptococcus mutans Biofilm Model
An S. mutans biofilm model was used in this study. For that, the Streptococcus mutans strain (UA159) was cultured in BHI (Sigma-Aldrich) for 18 h in an incubator at 5% CO2 and 37 °C. The inoculum was prepared by adding 2 wt.% sucrose to the bacterial culture. Samples (n = 6) [19] were placed at the bottom of a 24-well plate. Then, 1.5 mL of the inoculum was added, and the plate was incubated for 24 h at 5% CO2 and 37 °C. Next, samples were transferred to a new 24-well plate containing 1.5 mL of fresh BHI broth supplemented with 2 wt.% of sucrose and incubated for 24 h at 5% CO2 and 37 °C [19].
2.11. Colony-Forming Unit (CFU) Counting Assay
CFU assay quantifies the total number of viable bacterial occurring in the 48 h biofilm. Biofilm was moved to a glass vial filled with 1 mL of cysteine peptide water (CPW) and collected by 10 s vortexing (BenchMixer Vortex Mixer, Benchmark Scientific, Inc., Sayreville, NJ, USA) 1 m sonication (CGOLDENWALL Ultrasonic Homogenizer, SPW Industrial, Laguna Hills, CA, USA) and 10 s vortexing. The bacterial suspension of each sample was serially diluted, and three drops of 10 µL from each dilution were plated onto BHI agar plates and incubated for 48 h at 5% CO2 and 37 °C for CFU analysis [27,28,29].
2.12. Fluorescence Microscopy Staining
The 2-day biofilm specimens were rinsed with phosphate-buffered saline (PBS) to eradicate non-adherent bacteria. Next, adhesive samples were stained via a live/dead bacterial kit (Molecular Probes, Eugene, OR, USA). A measurement of 2.5 µM of each SYTO 9 and propidium iodide was mixed and applied to the samples for 15 min. Propidium iodide stained the dead bacteria and emitted red fluorescence, and SYTO 9 stained live bacteria and emitted green fluorescence [30]. An inverted fluorescence microscope (Eclipse TE2000-S, Nikon, Melville, NY, USA) was used to image three stained disks per group, and five randomly picked views were taken for each sample yielding a total of 15 images per group.
2.13. Cytotoxicity Assay Using Human Gingival Fibroblasts
Adhesive disks (n = 3) [8] were prepared using a 4 mm diameter and 1 mm thickness mold, then photoactivated for 20 s on both sides. After that, they were sterilized using ethylene oxide, followed by seven days of degassing. Based on their relevance to dental adhesives, human gingival fibroblast (HGF, ScienCell, San Diego, CA, USA) was cultured in fibroblast medium (FM) supplemented with 2% fetal bovine serum, 100 IU/mL penicillin, and 100 IU/mL streptomycin. HGFs were inoculated into a 96-well plate at a density of 5000 cells/well in FM [31]. Each disk was immersed in a 4 mL medium for 24 h at 37 °C to obtain eluents [31,32]. As a result, the surface area/solution volume ratio was 0.63 cm2/mL, which was within the 0.5–6 cm2/mL range recommended by the International Organization for Standardization (ISO) [33,34]. The original extracts were diluted with fresh medium at 2-, 4-, 8-, 16-, 32-, 64-, and 128-fold [32], then the HGF were incubated in 100 µL of each sample’s original extract and their dilutions for 24 h. The HGFs in culture media without any extracts were used as a negative control. Following the incubation period, a 10 µL of cell counting kit-8 (CCK-8, Dojindo, Rockville, MD, USA) was added to each well and incubated for 2 h at 37 °C with 5% CO2 to evaluate the cell viability. Later, absorbance was measured (SpectraMAx M5) at 450 nm, representing the cellular dehydrogenase activity of live cells in culture media in percentages.
2.14. Artificial Aging by Thermocycling
A thermocycler (Odeme Thermocycling, OMC-250 L, Luzerna, Brazil) with a computer-controlled two-temperature cycler with distilled water baths of 5 and 55 °C was used. Each cycle consisted of 30 s immersion (dwell time) and 5 s transfer time [35,36]. After baseline measurements of antibacterial properties, microtensile, and ultimate tensile bond strength, samples were aged for 10,000 cycles to estimate a year of clinical service [37]. After the artificial aging, adhesive disks were sterilized with ethylene oxide gas, followed by seven days of degassing. Then the antibacterial tests, µTBS and UTS, were measured as previously described, and data were recorded as aged data.
2.15. Statistical Analysis
Data normality was analyzed using the Shapiro–Wilk test; one-way ANOVA and Tukey’s were conducted for multiple comparison tests. To compare data before and after aging, a t-test was performed. All tests were conducted at a significance level of 0.05 using SigmaPlot software version 12.0 (Systat Software, Inc., San Jose, CA, USA).
3. Results
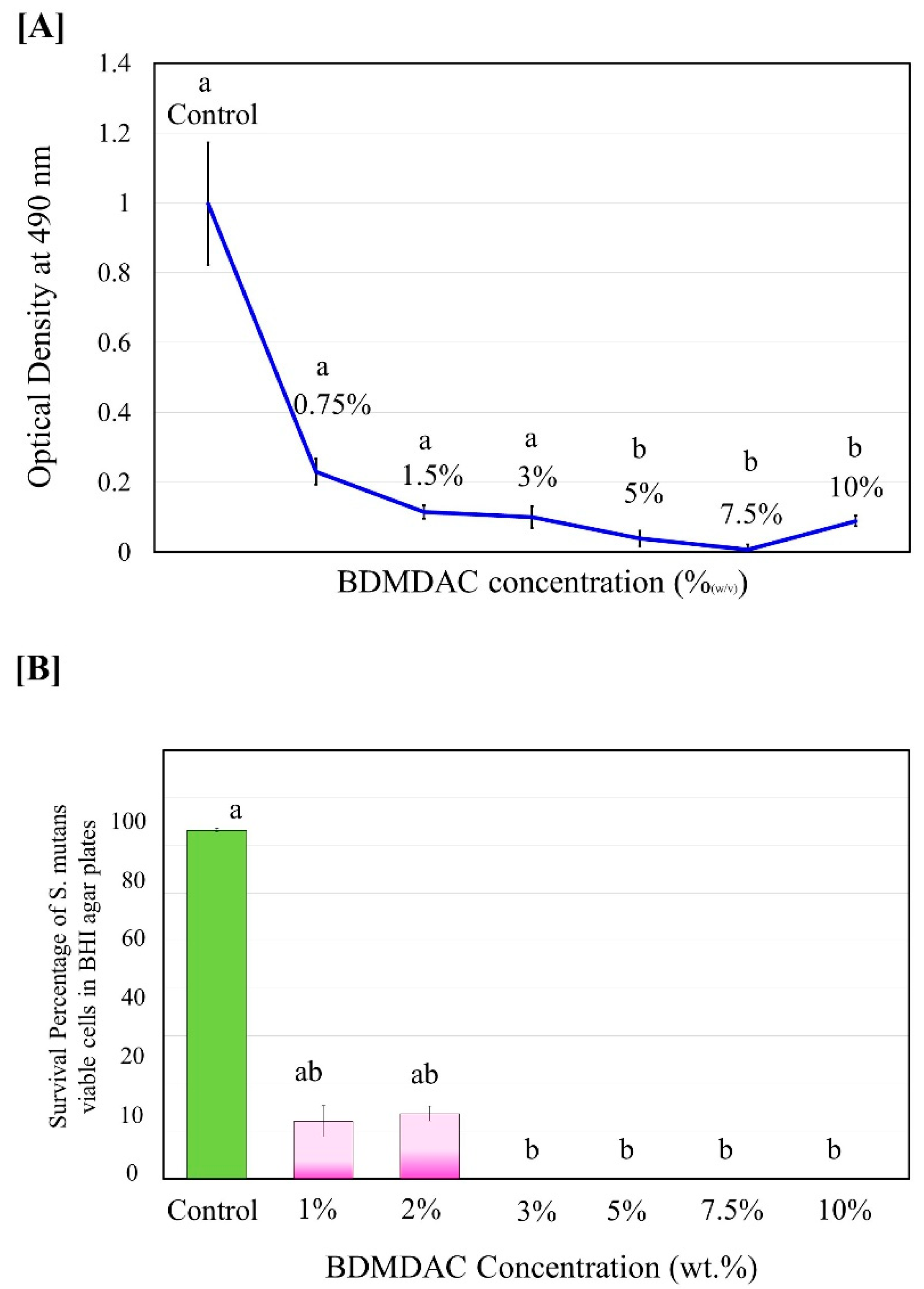
The antibacterial activity of isolated BDMDAC compound against S. mutans in planktonic cultures was determined before being added to the dental adhesive formulation. Figure 2 shows the experimental results of the antibacterial activity assay expressed as the inhibition rate of the BDMDAC compound after incubation for 48 h on S. mutans. The complete death of S. mutans bacteria was observed when the concentration of BDMDAC compound was higher than 7.5 wt.% (Figure 2A), but the bacteriostatic rate was similar to that of 5 and 10 wt.%; the difference was not statistically significant (p ≥ 0.05). In addition, no bacterial growth in agar plates was observed for BDMDAC concentrations greater than 3 wt.% after 24 h (Figure 2B). There is a difference statistically significant for the concentrations at 1 and 2 wt.% (p ≤ 0.05).
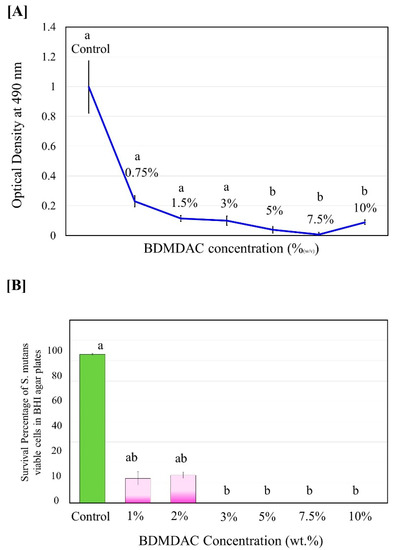
Figure 2.
Antimicrobial potentiality of BDMDAC compound against S. mutans in planktonic cultures before being added to the dental adhesive formulation. (A) Growth curve of planktonic S. mutans subjected to contact with the BDMDAC concentrations of 0–10% (w/v). (B) Survival percentage of S.mutans viable planktonic cells growth in agar. Data are presented as the mean ± SD of three independent tests performed in triplicate. Means denoted by a different letter indicate significant differences between groups (p < 0.05).
The values of the degree of conversion are plotted in Figure 3. The results ranged from 60.84 (±1.62)% for BDMDAC 1 wt.% to 64.79 (±4.24)% for BDMDAC 5 wt.%, without statistically significant difference among them (p > 0.05). All groups reached values higher than 50% of conversion.
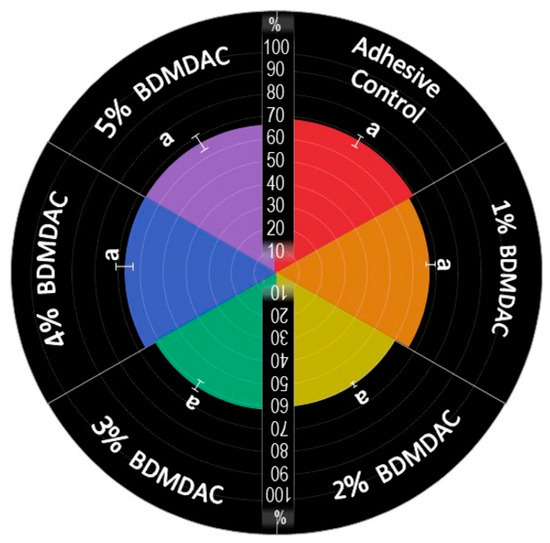
Figure 3.
Percentage of the degree of conversion of experimental dental adhesives. The same letters indicate no statistically significant differences among the groups (p > 0.05).
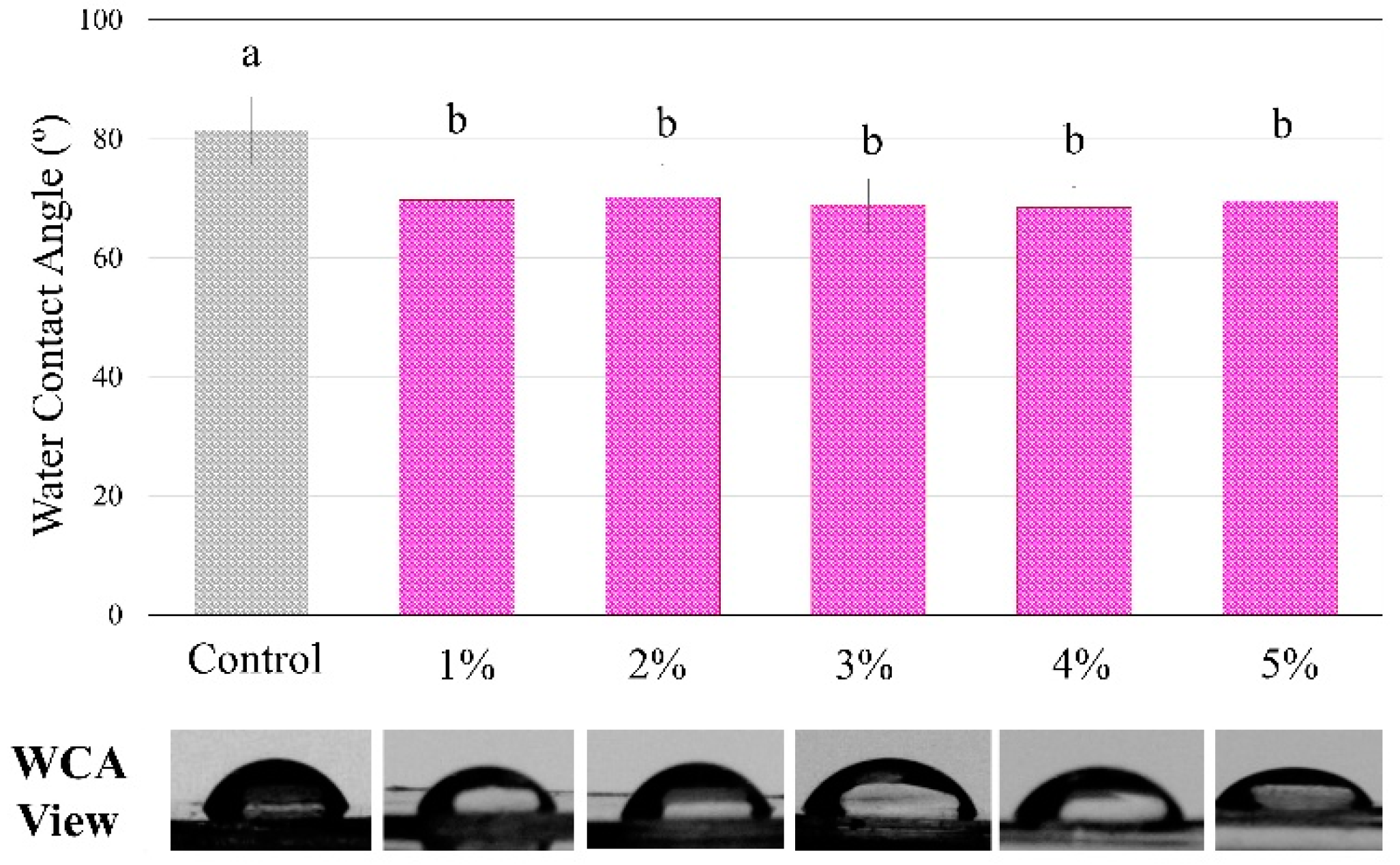
Figure 4 shows the analysis of the contact angle using water drops on the polymers showed statistically significant differences among groups, with a higher angle for the control compared to all BDMDAC groups (p < 0.05). However, there were no differences from 1 to 5 wt.% of BDMDAC (p > 0.05).
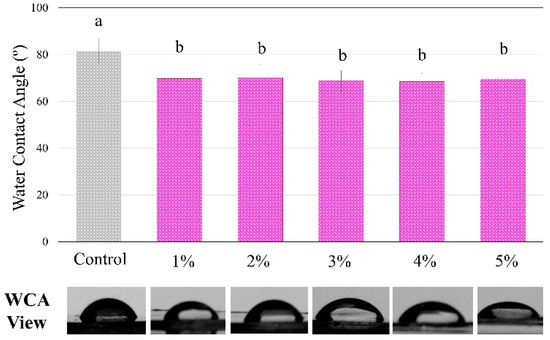
Figure 4.
The degree of water contact angle and respective views of the control and BDMDAC- doped adhesive groups. Groups with the same letters are not statistically different (p > 0.05).
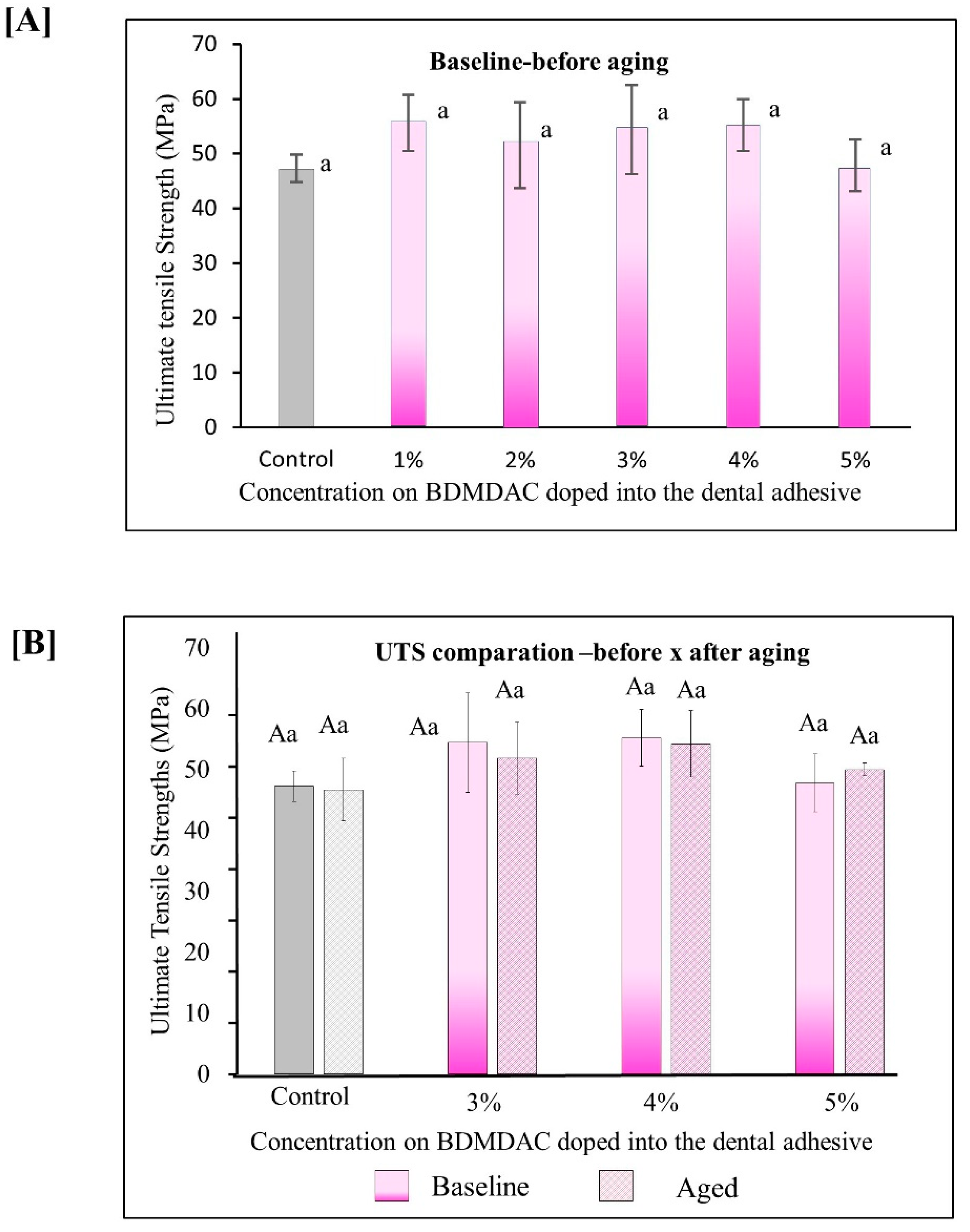
Figure 5 describes the results for ultimate tensile strength (UTS). Figure 5A shows the immediate results of ultimate tensile strength for all the initial concentrated evaluated from 0 to 5 wt.% BDMDAC. The values ranged from 47.27 (±2.51) MPa for the control adhesive to 55.23 (±4.70) MPa for the group with 5 wt.% (p < 0.05). Figure 5B shows the results for the adhesive formulations containing BDMDAC greater than 3 wt.%. Please note that the formulations doped with BDMDAC at concentrations less than 3 wt.% were not subjected to artificial aging due to the lack of antibacterial potential of these formulations expressed in the antibacterial assays. After aging, the UTS of the adhesive formulations containing BDMDAC greater than 3 wt.% was not impaired. The values ranged from 46.70 (±5.14) MPa for control to 54.23 (±5.46) MPa for 5 wt.% (p < 0.05).
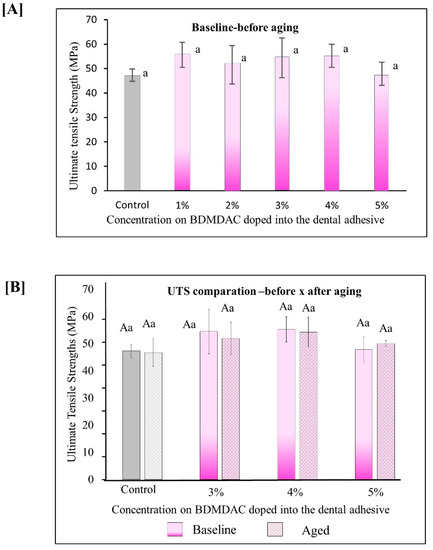
Figure 5.
Results for ultimate tensile strength (UTS). Data are presented as the mean ± SD. (A) shows the immediate results of ultimate tensile strength for all the initial concentrated evaluated from 0 to 5 wt.%. BDMDAC. (B) shows the results for the adhesive formulations containing BDMDAC greater than 3 wt.% before and after aging. Means marked with the same lowercase letters do not differ significantly among the BDMDAC concentrations, and means marked with the same uppercase letters do not differ significantly between before and after aging; (p < 0.05).
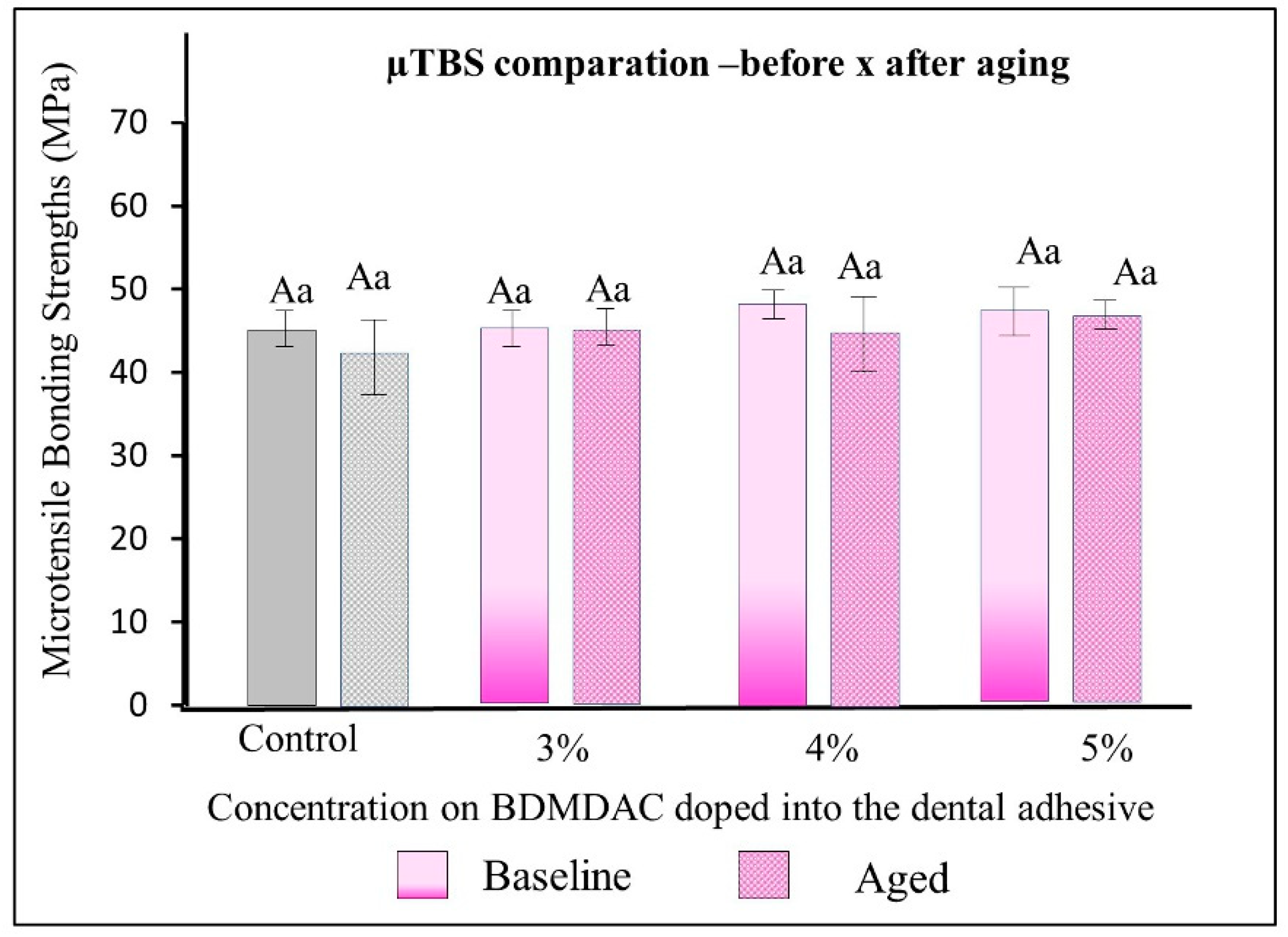
Figure 6 describes the µTBS results of the dental adhesives immediately and after aging. There were no statistically significant differences among the formulated adhesives with 1-to-5 wt.% BDMDAC concentrations (comparative expressed by lowercase letters) or between before or after aging (comparative expressed by uppercase letters) (p > 0.05).
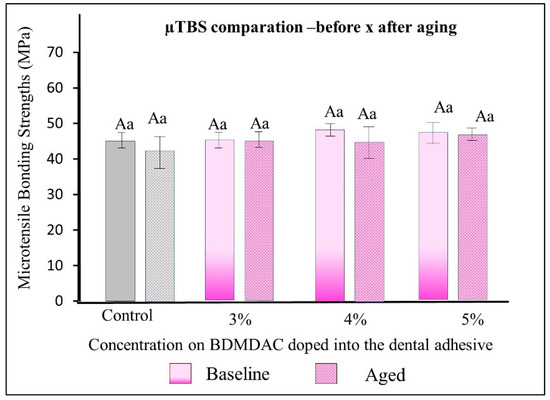
Figure 6.
µTBS results of the dental adhesives immediately and after aging. There were no statistically significant differences among the formulated adhesives with 1 to 5 wt.% BDMDAC concentrations (comparative expressed by lowercase letters) or between before or after aging (comparative expressed by uppercase letters) (p > 0.05).
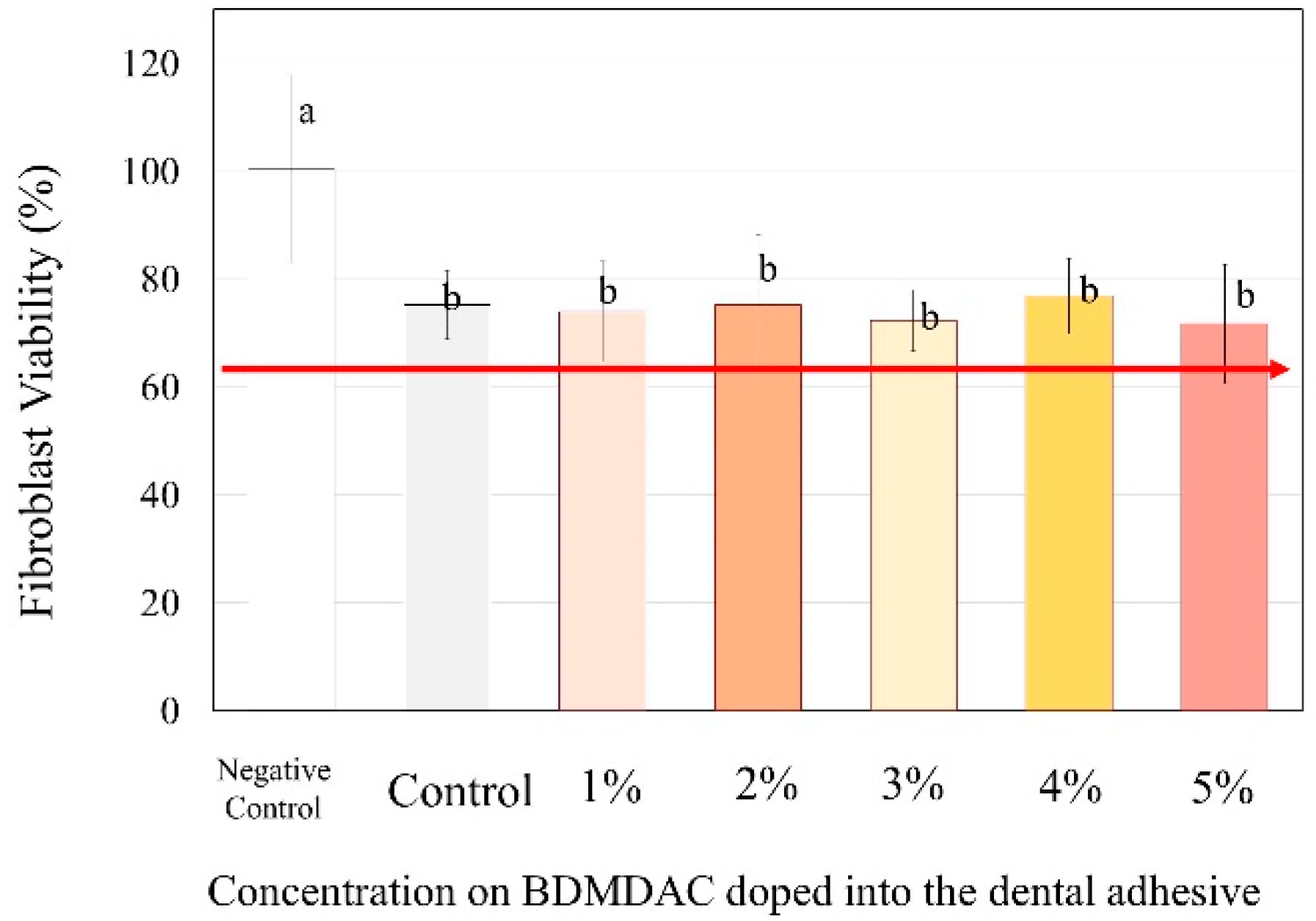
Figure 7 presents the cytotoxicity assays performed to evaluate the biosafety of the different concentrations of BDMDAC incorporated with dental adhesives. The percentage of viable fibroblast cells exposed to adhesive control (BDMDAC not added) was approximately 80%, and the cell number differed significantly from that in the negative control (no adhesive added) (p < 0.05). However, the increased concentration of BDMDAC did not promote any more significant reduction in viability. Furthermore, there was no difference in cell viability among the groups in contact with the adhesives, but the viability differed in relation to the negative control (p < 0.05).
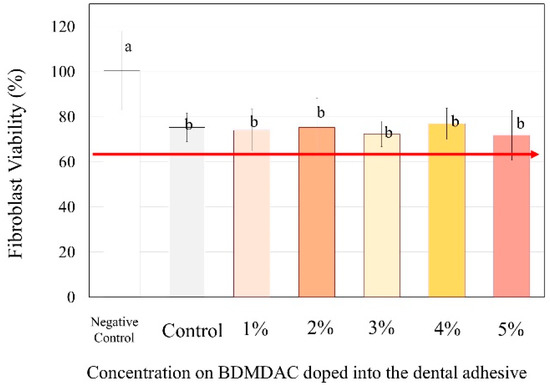
Figure 7.
The response of the human gingival fibroblasts to contact with the BDMDAC-doped adhesives. Data are presented as the mean ± SD. Means marked with different lowercase letters differ significantly (p < 0.05).
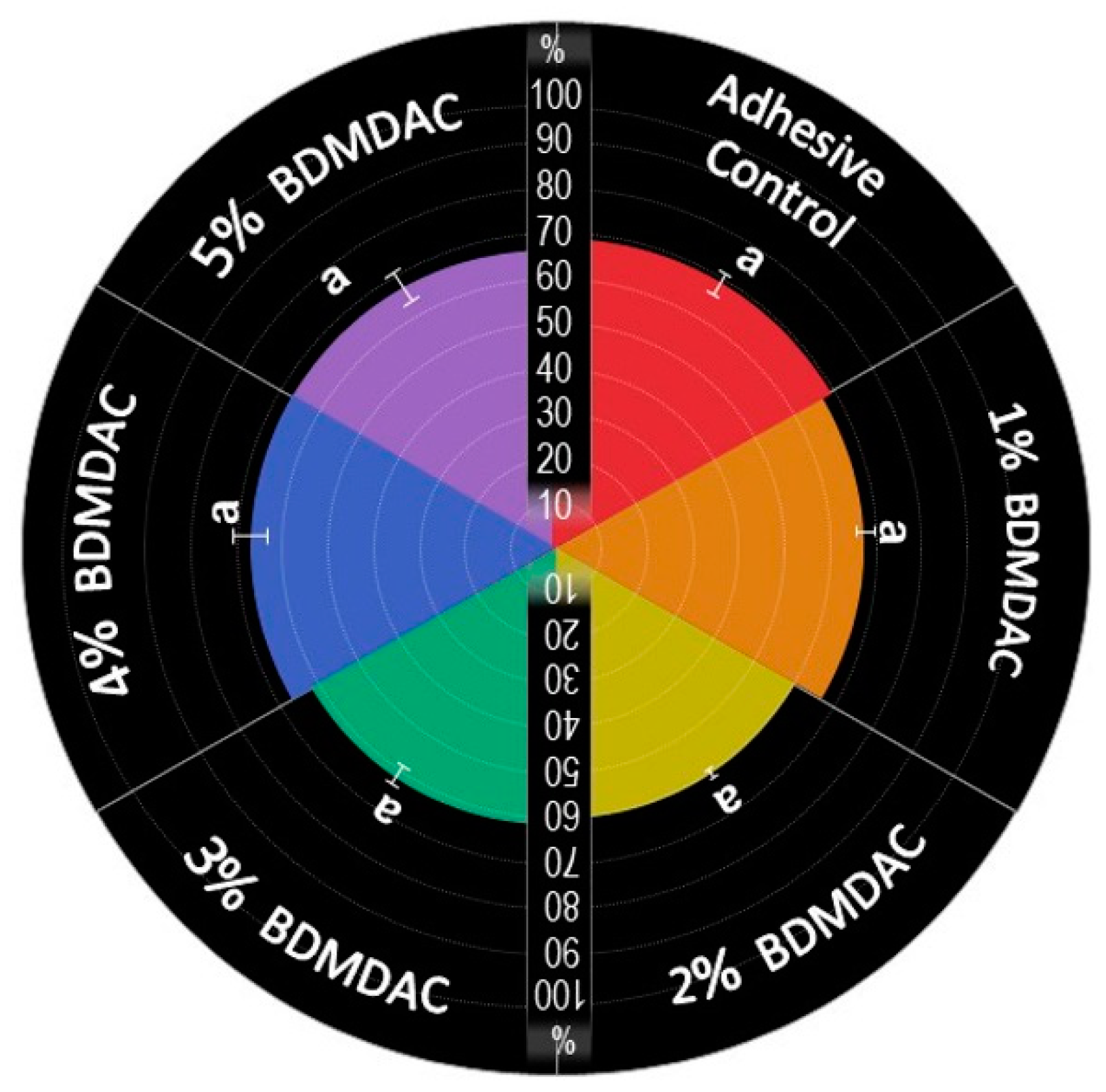
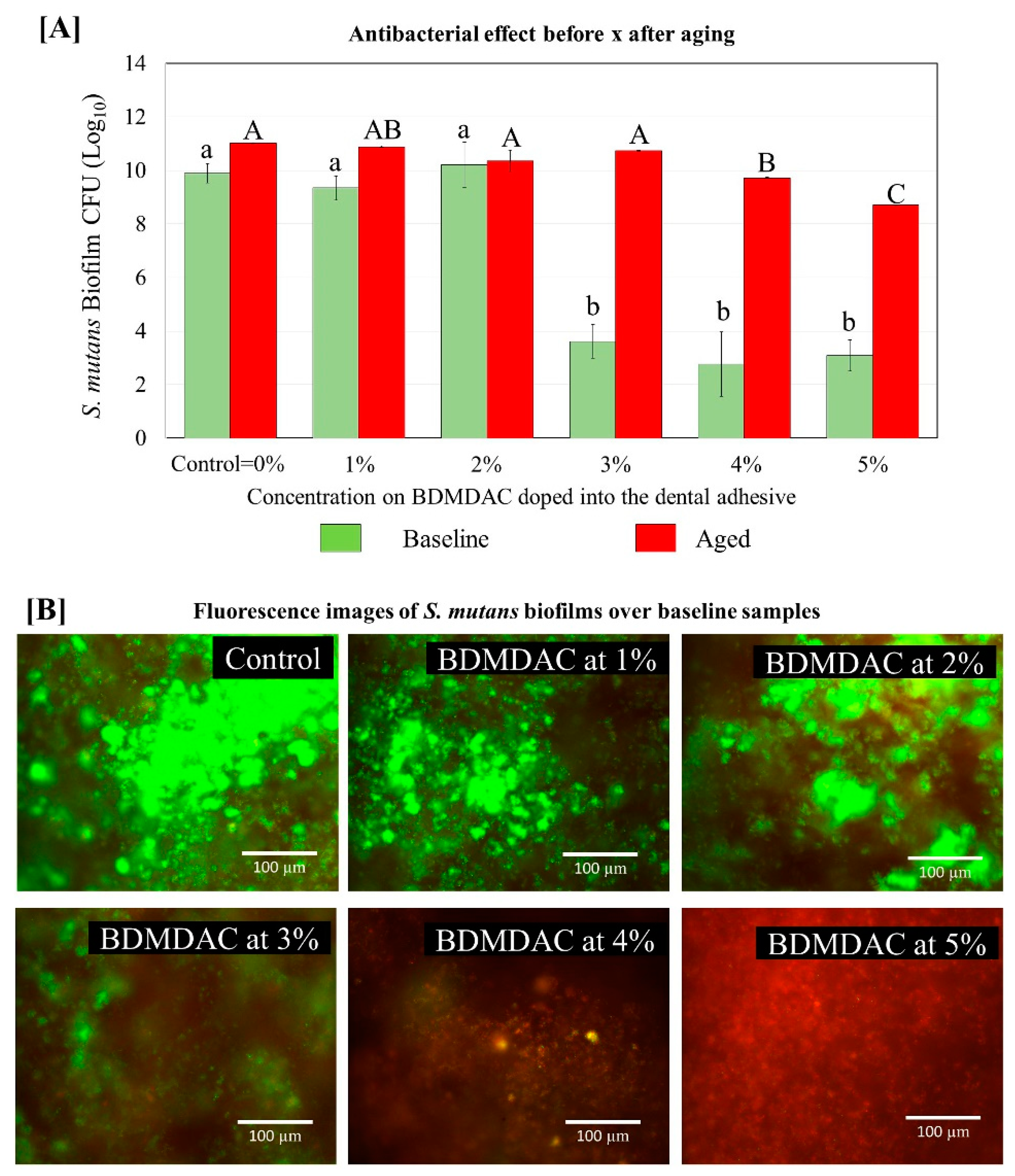
Figure 8 describes the antibacterial effect of the BDMDAC-doped adhesives against S. mutans biofilms before and after aging. Tests were performed at two different time points: immediately after the samples were prepared and after artificial aging simulating 1-year of service inside the mouth. The CFU analysis showed that an expressive bacterial reduction occurred on the biofilm grown over samples of adhesives containing BDMDAC at concentrations greater than 3 wt.% for the baseline testing (p < 0.05). However, after aging, these results were lost, and the performance of adhesives containing BDMDAC at concentrations greater than 3 wt.% was similar to the control (p > 0.05). The formulations containing 4 and 5% BDMDAC promoted a significant biofilm reduction, respectively; however, the performance was not even similar to the baseline results.
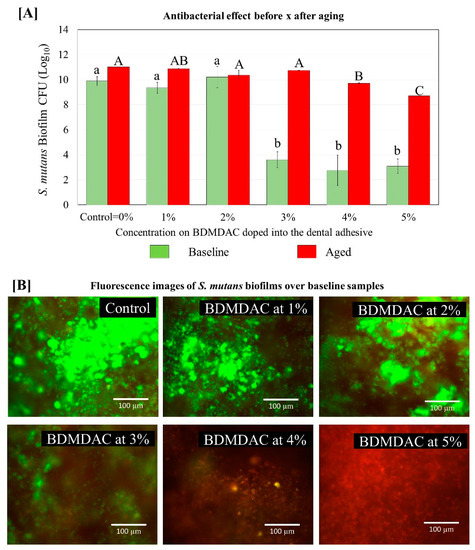
Figure 8.
Colony-forming unit (CFU) counts for S. mutans biofilms grown over the BDMDAC-doped adhesives. (A) shows the CFU values for the biofilms grown over the samples immediately after being prepared (not subject to aging) versus the biofilm grown over the samples that were subjected to artificial aging promoted by 10,000 thermal cycles to simulate one year of service inside the mouth. Data are presented as the mean ± SD. Means marked with different lowercase letters differ significantly among the BDMDAC concentrations and means marked with different uppercase letters differ significantly between before and after aging; (p < 0.05). (B) Representative live/dead staining images of biofilms grown over baseline samples using fluorescence microscopy live bacteria were stained green, and compromised bacteria were stained red.
4. Discussion
The addition of antibacterial agents can modify the physicochemical properties of dental adhesives, which may jeopardize their application. In this study, we observed that BDMDAC addition did not change the degree of conversion and ultimate tensile strength up to 5 wt.%, with suitable µTBS with dentin immediately and after aging. Furthermore, the antibacterial activity was dose-dependent with the maintenance of the adhesive’s reliability for application without cytotoxic effects on human cells.
During the photoactivation of dental adhesives, free radicals are created and responsible for converting carbon double bonds (C=C) into single bonds (C-C) of methacrylate groups. When the conversion is proper, the polymer achieves reliable physicochemical properties, such as high ultimate tensile strength and hardness and low sorption and solubility. Previous reports in the literature have addressed the incorporation of quaternary ammonium monomers into dental adhesives and investigated the degree of conversion [38,39]. Liang et al. [40] demonstrated that quaternary ammonium monomers negatively affect the photopolymerization of the relevant dental resin within the concentration range of 5–20 wt.%.
As a limiting factor, incorporating antibacterial agents into the organic matrix can decrease the conversion degree by influencing the polymer’s color or viscosity. BDMDAC is a quaternary ammonium-derivative molecule that does not present C=C on its structure and will not covalently bond with the monomers. Occasionally, the polymerization kinetics is modified by incorporating such molecules, decreasing the resin’s viscosity, increasing monomer chain mobility, and increasing the maximum polymerization rate. However, as previously observed with a quaternary ammonium-derivative molecule without methacrylate groups, the degree of conversion did not change, and all groups presented high values similar to commercial adhesives [38,39,40,41].
The degree of conversion provides critical data about the chemistry of the developed material [42]. However, this test does not show how the polymeric network is formed. Materials can achieve a high degree of conversion but achieve a more linear polymeric network with lower crosslink density [43]. The added agent can act as a plasticizer, pulling the chains apart. Therefore, the mechanical properties of the polymer must be tested, such as ultimate tensile strength. In the present research, besides no differences in the degree of conversion, the mechanical properties of the adhesives were also not influenced up to 5 wt.% addition of BDMDAC.
Despite good immediate results, the ability to maintain the adhesion must be analyzed over time, especially when dentin is involved in dental restoration. During aging, the cured adhesive is prone to swell and suffers hydrolysis and degradation [44]. Moreover, the collagen fibrils are also attacked by water and active MMP, cleaving the collagen structure [45]. Nevertheless, as observed in the degree of conversion and contact angle of the adhesives, the doping of BDMDAC has not impaired their performance since, overall, the BDMDAC-doped materials performed similarly to control regarding the physical–chemical properties.
The rationale for such results is based on the bacterial inhibition mechanism of quaternary ammonium compounds, in which the positively charged quaternary amine interacts with the negatively charged bacterial cell membrane, causing cytoplasmic leakage and bacterial death [46]. We chose to use BDMDAC due to its long alkyl chain with sixteen carbons, increasing the lipophilicity of the molecule and the chances of bacterial interaction. In addition, BDMDAC is a quaternary ammonium-derivative molecule without methacrylate groups. Therefore, the polymeric network’s part of the BDMDAC entrapped may be leached over time. Few studies analyze the antibacterial activity after aging [47,48]. However, long-term evaluation is critical as a preclinical assessment of the clinical performance during biomaterials development [49], especially when the antibacterial agent cannot chemically bond with the matrix. Therefore, we tested the adhesives samples immediately and after aging in artificial saliva. As expected, all groups with BDMDAC showed lower antibacterial activity after aging. This study’s limitation is that we did not quantify the antibacterial agent leached over time. However, it is possible to state that some part was lost over time.
Interestingly, the leaching did not affect the bonding to dentin, as they maintained a high µTBS over time, with no statistical differences for the immediate analysis. Similar results were observed in the literature. Pupo et al. [41] investigated the resin–dentin bond strength (μTBS), degree of conversion (DC), and antibacterial potential of commercial adhesive modified with quaternary ammonium methacrylate polymer (QAMP) at 5% and compared it with Clearfil™ Protect Bond. No statistically significant difference in μTBS was observed between Clearfil™ SE Bond containing 5% QAMP and control immediately and after 6 and 12 months of water storage. However, Clearfil™ Protect Bond showed a significant reduction of μTBS after 12 months of storage. In addition, QAMP provided no significant change in DC after incorporating it into Clearfil™ SE Bond.
Future studies could be addressed to synthesize drug delivery systems with BDMDAC able to control its release rate. In addition, Nanotubes, nanocapsules, microspheres, and core shells have increasingly emerged in dentistry and are encouraged to overcome the issue related to the fast leaching of antibacterial agents without methacrylate groups.
Finally, the adhesives were tested for possible cytotoxic effects on human cells. Incorporating antibacterial agents into dental materials should not affect their mechanical properties. Furthermore, the antibacterial agents should not generate toxicity in the surrounding tissues. Here we had no statistical differences among the experimental adhesives. However, in a previous study [50], myristyl trimethyl ammonium bromide was incorporated into a dental resin up to 2 wt.% and showed cytotoxic effects against human keratinocytes (HaCaT) compared to the control group and 0.5 wt.%, achieving percentages of cell viability below that recommended by the International Organization for Standardization (ISO), which states that biomaterials need to show at least 70% of cells viability not to be considered cytotoxic.
5. Conclusions
The present study combined a not-yet-explored quaternary ammonium-derivative molecule into a dental resin, which attained effective antibacterial activity against a Streptococcus mutans biofilm. The results showed that: (1) BDMDAC did not influence the physicochemical properties of the adhesives immediately or over time; (2) The lower the concentration of BDMDAC, the greater the antibacterial effect. However, this effect was lower after sample aging; (3) BDMDAC at a maximum concentration of 5 wt.% had no detrimental effect on the viability of human cells. In conclusion, BDMDAC is a promising antibacterial agent for dental applications, and future studies should be enrolled to keep this agent in the polymeric network or control its release over time.
Author Contributions
Conceptualization, M.A.S.M. and F.M.C.; methodology, A.A.B., L.S.M., and I.M.G.; validation, A.A.B., L.S.M., and I.M.G.; formal analysis, A.A.B., L.S.M., and I.M.G.; investigation, A.A.B.; L.S.M., and I.M.G.; resources, M.A.S.M.; data curation, F.M.C.; writing—original draft preparation, L.S.M.; writing—review and editing, A.A.B., L.S.M., I.M.G., F.M.C. and M.A.S.M.; visualization, L.S.M. and M.A.S.M.; supervision, M.A.S.M.; project administration, L.S.M.; funding acquisition, M.A.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
A.A.B acknowledges the scholarship during his Ph.D. studies from the Imam AbdulRahman bin Faisal University, Dammam, Saudi Arabia, and the Saudi Arabia Cultural Mission. I.M.G. acknowledges the scholarship during her Ph.D. studies from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001—scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Featherstone, J.D.B. Dental Caries: A Dynamic Disease Process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial Activity and Bonding Characteristics of an Adhesive Resin Containing Antibacterial Monomer MDPB. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Spałek, J.; Ociepa, P.; Deptuła, P.; Piktel, E.; Daniluk, T.; Król, G.; Góźdź, S.; Bucki, R.; Okła, S. Biocompatible Materials in Otorhinolaryngology and Their Antibacterial Properties. Int. J. Mol. Sci. 2022, 23, 2575. [Google Scholar] [CrossRef]
- de Melo, M.A.S. (Ed.) Designing Bioactive Polymeric Materials for Restorative Dentistry; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-11328-4. [Google Scholar]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717. [Google Scholar] [CrossRef]
- Garcia, I.M.; Souza, V.S.; Souza, J.D.; Visioli, F.; Leitune, V.C.B.; Scholten, J.D.; Collares, F.M. Zinc-Based Particle with Ionic Liquid as a Hybrid Filler for Dental Adhesive Resin. J. Dent. 2020, 102, 103477. [Google Scholar] [CrossRef]
- Montoya, C.; Jain, A.; Londoño, J.J.; Correa, S.; Lelkes, P.I.; Melo, M.A.; Orrego, S. Multifunctional Dental Composite with Piezoelectric Nanofillers for Combined Antibacterial and Mineralization Effects. ACS Appl. Mater. Interfaces 2021, 13, 43868–43879. [Google Scholar] [CrossRef]
- Collares, F.M.; Garcia, I.M.; Klein, M.; Parolo, C.F.; Sánchez, F.A.L.; Takimi, A.; Bergmann, C.P.; Samuel, S.M.W.; Melo, M.A.; Leitune, V.C. Exploring Needle-Like Zinc Oxide Nanostructures for Improving Dental Resin Sealers: Design and Evaluation of Antibacterial, Physical and Chemical Properties. Polymers 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yao, S.; Gu, L.; Huang, Z.; Mai, S. Antibacterial Effect and Bond Strength of a Modified Dental Adhesive Containing the Peptide Nisin. Peptides 2018, 99, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Balhaddad, A.A.; Kansara, A.A.; Hidan, D.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Toward Dental Caries: Exploring Nanoparticle-Based Platforms and Calcium Phosphate Compounds for Dental Restorative Materials. Bioact. Mater. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Garcia, I.M.; Balhaddad, A.A.; Ibrahim, M.S.; Weir, M.D.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Antibacterial Response of Oral Microcosm Biofilm to Nano-Zinc Oxide in Adhesive Resin. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2021, 37, e182–e193. [Google Scholar] [CrossRef] [PubMed]
- Mitwalli, H.; Alsahafi, R.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. Emerging Contact-Killing Antibacterial Strategies for Developing Anti-Biofilm Dental Polymeric Restorative Materials. Bioengineering 2020, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Chwała, P. Synthesis and Anti-Microbial Activities of Choline-like Quaternary Ammonium Chlorides. Eur. J. Med. Chem. 2003, 38, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Syguda, A.; Mirska, I.; Pernak, A.; Nawrot, J.; Pradzyńska, A.; Griffin, S.T.; Rogers, R.D. Choline-Derivative-Based Ionic Liquids. Chemistry 2007, 13, 6817–6827. [Google Scholar] [CrossRef]
- Sousa-Silva, M.; Simões, M.; Melo, L.; Machado, I. Pseudomonas Fluorescens Tolerance to Benzyldimethyldodecyl Ammonium Chloride: Altered Phenotype and Cross-Resistance. J. Glob. Antimicrob. Resist. 2018, 15, 188–195. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Kist, T.L.; Takimi, A.; Samuel, S.M.W.; Collares, F.M. Quantum Dots as Nonagglomerated Nanofillers for Adhesive Resins. J. Dent. Res. 2016, 95, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Balhaddad, A.A.; Lan, Y.; Simionato, A.; Ibrahim, M.S.; Weir, M.D.; Masri, R.; Xu, H.H.K.; Collares, F.M.; Melo, M.A.S. Magnetic Motion of Superparamagnetic Iron Oxide Nanoparticles- Loaded Dental Adhesives: Physicochemical/Biological Properties, and Dentin Bonding Performance Studied through the Tooth Pulpal Pressure Model. Acta Biomater. 2021, 134, 337–347. [Google Scholar] [CrossRef]
- Collares, F.M.; Portella, F.F.; Leitune, V.C.B.; Samuel, S.M.W. Discrepancies in Degree of Conversion Measurements by FTIR. Braz. Oral Res. 2013, 27, 453–454. [Google Scholar] [CrossRef]
- Stürmer, M.; Garcia, I.M.; Souza, V.S.; Visioli, F.; Scholten, J.D.; Samuel, S.M.W.; Leitune, V.C.B.; Collares, F.M. Titanium Dioxide Nanotubes with Triazine-Methacrylate Monomer to Improve Physicochemical and Biological Properties of Adhesives. Dent. Mater. 2021, 37, 223–235. [Google Scholar] [CrossRef]
- Tian, F.-C.; Wang, X.-Y.; Huang, Q.; Niu, L.-N.; Mitchell, J.; Zhang, Z.-Y.; Prananik, C.; Zhang, L.; Chen, J.-H.; Breschi, L.; et al. Effect of Nanolayering of Calcium Salts of Phosphoric Acid Ester Monomers on the Durability of Resin-Dentin Bonds. Acta Biomater. 2016, 38, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymer. J. Appl. Polym. Sci. 1969, 13, 1741–1747. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/app.1969.070130815 (accessed on 10 March 2022). [CrossRef]
- Tani, C.; Manabe, A.; Itoh, K.; Hisamitsu, H.; Wakumoto, S. Contact Angle of Dentin Bonding Agents on the Dentin Surface. Dent. Mater. J. 1996, 15, 39–44. [Google Scholar] [CrossRef]
- Katyal, D.; Subramanian, A.K.; Venugopal, A.; Marya, A. Assessment of Wettability and Contact Angle of Bonding Agent with Enamel Surface Etched by Five Commercially Available Etchants: An In Vitro Study. Int. J. Dent. 2021, 2021, 9457553. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Ibrahim, A.S.; Balhaddad, A.A.; Weir, M.D.; Lin, N.J.; Tay, F.R.; Oates, T.W.; Xu, H.H.K.; Melo, M.A.S. A Novel Dental Sealant Containing Dimethylaminohexadecyl Methacrylate Suppresses the Cariogenic Pathogenicity of Streptococcus Mutans Biofilms. Int. J. Mol. Sci. 2019, 20, 3491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.-X.; Xu, H.H.K. Effects of Quaternary Ammonium Chain Length on the Antibacterial and Remineralizing Effects of a Calcium Phosphate Nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef]
- Zhang, N.; Melo, M.A.S.; Chen, C.; Liu, J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Development of a Multifunctional Adhesive System for Prevention of Root Caries and Secondary Caries. Dent. Mater. 2015, 31, 1119–1131. [Google Scholar] [CrossRef]
- Daood, D.; Yiu, C.K.Y.; Burrow, M.F.; Niu, L.-N.; Tay, F.R. Effect of a Novel Quaternary Ammonium Silane Cavity Disinfectant on Durability of Resin-Dentine Bond. J. Dent. 2017, 60, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Zhou, C.-C.; Weir, M.D.; Zhou, X.-D.; Xu, H.H.K. One-Year Water-Ageing of Calcium Phosphate Composite Containing Nano-Silver and Quaternary Ammonium to Inhibit Biofilms. Int. J. Oral Sci. 2016, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, Y.-H.; Xing, X.-D.; Li, F.; Ma, S.; Qi, L.-L.; Chen, J.-H. Antibacterial Activity and Cytotoxicity of Two Novel Cross-Linking Antibacterial Monomers on Oral Pathogens. Arch. Oral Biol. 2011, 56, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H.K. Comparison of Quaternary Ammonium-Containing with Nano-Silver-Containing Adhesive in Antibacterial Properties and Cytotoxicity. Dent. Mater. 2013, 29, 450–461. [Google Scholar] [CrossRef]
- Association for the Advancement of Medical Instrumentation. Biological Evaluation of Medical Devices, Part 12: Sample Preparation and Reference Materials; ANSI/AAMI/ISO 10993-12: 2007; AAMI: Arlington, VA, USA, 2007. [Google Scholar]
- Sigusch, B.W.; Pflaum, T.; Völpel, A.; Gretsch, K.; Hoy, S.; Watts, D.C.; Jandt, K.D. Resin-Composite Cytotoxicity Varies with Shade and Irradiance. Dent. Mater. 2012, 28, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials Guidance on In Vitro Testing of Dental Composite Bonding Effectiveness to Dentin/Enamel Using Micro-Tensile Bond Strength (ΜTBS) Approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Baracco, B.; Fuentes, M.; Garrido, M.A.; Gonzalez-Lopez, S.; Ceballos, L. ISO/TS 11405: Dental materials-testing of adhesion to tooth structure. ISO/TS 11405: Dental materials-testing of adhesion to tooth structure, 2003. Odontology 2013, 101, 177–185. [Google Scholar] [CrossRef]
- Gale, M.S.; Darvell, B.W. Thermal Cycling Procedures for Laboratory Testing of Dental Restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Daood, U.; Sauro, S.; Pichika, M.R.; Omar, H.; Liang Lin, S.; Fawzy, A.S. Novel Riboflavin/VE-TPGS Modified Universal Dentine Adhesive with Superior Dentine Bond Strength and Self-Crosslinking Potential. Dent. Mater. 2020, 36, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Omar, H.; Qasim, S.; Nogueira, L.P.; Pichika, M.R.; Mak, K.-K.; Steier, L.; Cky, Y.; Lin, S.L.; Fawzy, A.S. New Antimicrobial and Collagen Crosslinking Formulated Dentin Adhesive with Improved Bond Durability. J. Mech. Behav. Biomed. Mater. 2020, 110, 103927. [Google Scholar] [CrossRef]
- Liang, X.; Söderling, E.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Optimizing the Concentration of Quaternary Ammonium Dimethacrylate Monomer in Bis-GMA/TEGDMA Dental Resin System for Antibacterial Activity and Mechanical Properties. J. Mater. Sci. Mater. Med. 2014, 25, 1387–1393. [Google Scholar] [CrossRef]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Simão, L.C.; Esmerino, L.A.; Gomes, O.M.M.; Gomes, J.C. Effect of a Novel Quaternary Ammonium Methacrylate Polymer (QAMP) on Adhesion and Antibacterial Properties of Dental Adhesives. Int. J. Mol. Sci. 2014, 15, 8998–9015. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Antoniolli, F.; Sauro, S.; Tay, F.R.; Di Lenarda, R.; Prati, C.; Biasotto, M.; Contardo, L.; Breschi, L. Degree of Conversion and Permeability of Dental Adhesives. Eur. J. Oral Sci. 2005, 113, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in Polymeric Materials: Resolving the Intersection of Thermoplastics and Thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef] [PubMed]
- Koin, P.J.; Kilislioglu, A.; Zhou, M.; Drummond, J.L.; Hanley, L. Analysis of the Degradation of a Model Dental Composite. J. Dent. Res. 2008, 87, 661–665. [Google Scholar] [CrossRef]
- Sabatini, C.; Pashley, D.H. Mechanisms Regulating the Degradation of Dentin Matrices by Endogenous Dentin Proteases and Their Role in Dental Adhesion. A Review. Am. J. Dent. 2014, 27, 203–214. [Google Scholar] [PubMed]
- Kwaśniewska, D.; Chen, Y.-L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Balhaddad, A.A.; Mokeem, L.S.; Weir, M.D.; Xu, H.; Melo, M.A.S. Sustained Antibacterial Effect and Wear Behavior of Quaternary Ammonium Contact-Killing Dental Polymers after One-Year of Hydrolytic Degradation. Appl. Sci. 2021, 11, 3718. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Zhu, C.G.; Zhou, X.; Wang, H.; Han, Q.; Ren, B.; Cheng, L. Anti-Bacterial and Anti-Microbial Aging Effects of Resin-Based Sealant Modified by Quaternary Ammonium Monomers. J. Dent. 2021, 112, 103767. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.C. Correlation of Clinical Performance with ‘In Vitro Tests’ of Restorative Dental Materials That Use Polymer-Based Matrices. Dent. Mater. 2012, 28, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Mena Silva, P.A.; Garcia, I.M.; Nunes, J.; Visioli, F.; Castelo Branco Leitune, V.; Melo, M.A.; Collares, F.M. Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus Mutans Grown over Dental Resins: An In Vitro Study. J. Funct. Biomater. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).