Abstract
Expansion of marine aquaculture into more remote areas will likely accelerate over the next decade. Integrating Marine Renewable Energy (MRE) generation technologies (e.g., wind turbines) into remote, off-grid aquaculture sites will reduce reliance on fossil fuels by allowing localised low-carbon power generation, but may also result in novel environmental pressures. In this study, we undertook a thought experiment to assess the potential for increased collision risks to local marine and coastal bird species of integrating small wind turbines (4 units; combined capacity of 200 MWh) into a generalised marine fish farm in western Scotland (UK). Potential risks to bird species were assessed using a bespoke Sensitivity Index (SI) based on 12 factors, including population size, adult survival rate, UK conservation status, flight manoeuvrability, nocturnal flight activity, habitat preference, sensitivity to wind farms, attraction to fish farms for feeding and/or resting, and attraction to other marine anthropogenic structures/activities. SI scores varied substantially between species, but large gulls (Larus sp.) and European shag (Phalacrocorax aristotelis) were expected to be at the greatest potential risk. The general lack of information on interactions between birds and fish farms represented a significant knowledge gap, and greater focus on these interactions is needed to improve future risk assessments.
1. Introduction
Significant growth of the global marine finfish aquaculture industry, as witnessed over the last 20 years in the face of continuing pressure on wild fish stocks, is expected to continue [1]. This expansion will likely be achieved at least partially by the sector moving into more remote, exposed coastal waters. Fish farms traditionally rely on diesel generators for electrical power, but there is an increasing interest in using different kinds of renewable energy (RE) systems, including wind turbines, solar photovoltaic (PV) modules, and/or wave-powered generators, to reduce the sector’s reliance on fossil fuels [2,3]. Co-locating RE technology and aquaculture in close proximity, including integrating them into a single ‘Multi-Purpose Platform’ (MPP) structure, is thus intuitively appealing [4,5]. The development of MPPs is at a very early stage; however, if implemented widely, they could also generate novel environmental impacts that need to be considered carefully.
Wind turbines are attractive RE candidates for integration into finfish aquaculture operations (fish farms hereafter) given their technological maturity, even in marine settings [6]. Wind turbines can, however, pose significant risks of injury and/or mortality to flying birds through collisions with rotating turbine blades [7,8,9]. There is a concern that such risks could be exacerbated by placing turbines near areas that are already inherently attractive to birds, such as fish farms. It is well documented that fish farms may attract particular bird species by providing structures on which to rest, as well as potential foraging opportunities on farmed stock and/or on wild fish species aggregated around farms [10,11]. In addition, fish farms, marker buoys, and similar floating structures may allow individual birds to feed for longer, in waters that would otherwise be less accessible to them. The consequences of this attraction in terms of individual fitness, translated through changes in foraging patterns, energy intake, and travel times to breeding colonies, are as yet poorly understood. The subsequent introduction of small wind turbines to fish farms may modify these existing relationships in unforeseen ways. Integrating wind turbines and fish farms may therefore increase overall risks to attracted birds, over and above those already posed by existing farm practices to reduce predation including entanglement in antipredator netting, shooting, and lethal poisoning [12,13,14].
In the current absence of operational MPPs containing both wind turbines and aquaculture, no empirical data are available to evaluate the relative significance of collision risk to attracted birds. The present study therefore undertook a theoretical thought experiment seeking to clarify which marine and coastal bird species might be at greatest relative risk of wind turbine integration The likelihood of bird species being at risk from co-locating wind turbines and fish farms was expressed by combining a series of factors into a single Sensitivity Index (SI), following the general method described by [15], focusing on fish farms in Scotland (UK) raising Atlantic salmon (Salmo salar; hereafter ‘salmon’) as an example.
2. Materials and Methods
2.1. Aquaculture
The Scottish salmon aquaculture sector currently produces >160,000 MT of salmon annually [16], with most farms situated in remote locations in western and northern Scotland (Figure 1). Farmed salmon are first raised in freshwater hatcheries for 12–18 months before being transferred to floating sea cages for a further 12–22 months prior to harvesting [17]. Farms typically consist of 4–12 circular net-pens (up to 120 m in circumference and 20 m deep), each capable of holding up to 300 mt of mature salmon, accompanied by a barge containing crew quarters, feed storage, generators etc. (Figure 1, inset). The salmon sector is forecast to expand substantially over the next decade, including into more offshore areas [18]. Given concurrent strong Scottish government support for marine RE technologies [19], this sector represents a potential testbed to consider effects of future integration of RE technologies into aquaculture operations.
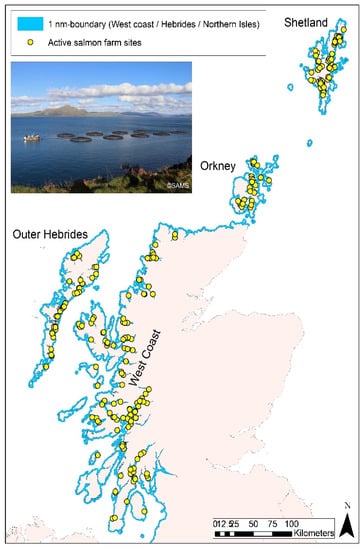
Figure 1.
Coastal waters of western and northern Scotland (incl. Outer Hebrides, Orkney and Shetland) out to a distance of 1 nm from land (in blue). Locations of active salmon growout farms are also indicated (March 2020 data; contains information from Scottish Government (Marine Scotland) licensed under the Open Government Licence v3.0. [25]. Inset: Example of a salmon growout farm in western Scotland, consisting of nine net pens and a barge (Photo copyright: SAMS 2016).
The present study only focused on the marine component of salmon production, assuming a hypothetical salmon farm (hereafter ‘fish farm’) of comparable capacity to current industry standard, but sited in a more exposed location to take better advantage of wind resources. The focus of this study, both in terms of aquaculture activity and bird distribution, was on western and northern Scottish waters out to 1 nm from shore (Figure 1). Waters off eastern Scotland were not considered in this study due to an absence of aquaculture activity in this area.
2.2. The Multi-Purpose Platform (MPP)
The MPP concept considered in the present study was developed as part of the INNO-MPP project [4,20,21]. It consisted of a generic reference feeding barge (30 m length; 19 m beam width; 3.6 m hull height) based on the AKVA Wavemaster AB650 design [22], selected for ease of modelling (Figure 2) [3]. The model barge was outfitted with four independently mounted horizontal axis wind turbines (2× Aeolos-H, 20 kW, 2× Polaris P10–20; [23,24]), with a total rated power of, 20 kW × 4 = 80 kW, and capable of collectively generating an annual energy yield of 200 MWh to contribute towards the ~260 MWh/year energy requirement of a 2500 MT salmon farm. Turbine tower heights were approximately 18–22 m above deck, with a 10-m rotor diameter in both cases [23,24].
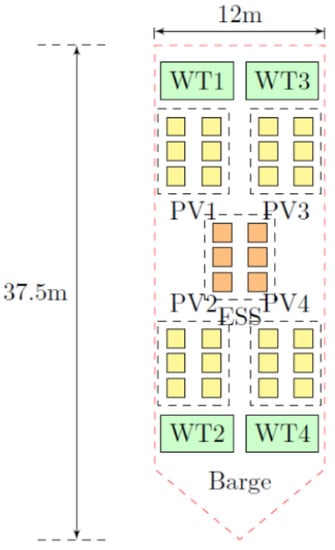
Figure 2.
Theoretical model of MPP barge developed as part of the INNO-MPP project [4,20,21]. WT = Wind Turbine; PV = Photovoltaic panel; ESS = Energy Storage System. © Dr L. Recalde Camarho, University of Strathclyde.
The choice of four smaller wind turbines rather than a single larger wind turbine was made in order to enhance the electricity generation reliability and exploit a wider range of wind speeds. The difference in tower heights was deliberately designed to allow downwind turbines to operate in upwind turbines’ wake, thereby increasing energy production.
2.3. Bird Species
Information on birds’ use of fish farms in Scotland was limited, although several species such as the large Larus gulls, great cormorant (Phalacrocorax carbo), European shag (P. aristotelis) and grey heron (Ardea cinerea) had been reported over the years [12,14,26,27]. The present study therefore relied on reviews of published and grey literature, as well as expert knowledge relating to birds’ distribution, flight capabilities and extent of their association with various marine industries for food and/or shelter, including aquaculture and offshore wind farms. Each bird species was selected based on whether it was regularly observed in or adjacent to Scottish coastal waters (including breeding species and wintering/passage migrants). This also included coastal populations of species that were more commonly associated with terrestrial or freshwater environments such as Grey heron, various ducks and geese, and several songbirds. A total of 82 species were selected, including representatives of aquatic groups such as divers (family Gaviidae), grebes (Podicipedidae), swans, geese and ducks (Anatidae), shearwaters, fulmars and petrels (“tubenoses”; Procellariidae and Hydrobatidae), gannets (Sulidae), cormorants (Phalacrocoracidae), raptors (Accipitridae, Pandionidae and Falconidae), herons (Ardeidae), waders (Scolopacidae, Charadriidae and Haematopodidae), gulls and terns (Laridae), skuas (Stercorariidae), and auks (Alcidae). Several terrestrial species, including representatives of pigeons (Columbidae), pipits (Motacillidae), finches (Fringillidae), starlings (Sturnidae), and corvids (Corvidae), were also included, following reports of species such as starlings (Sturnus vulgaris) and hooded crows (Corvus cornix) visiting salmon farms in western Scotland [14]. Nomenclature followed [28,29].
2.4. Sensitivity Index and Factors
The Sensitivity Index (SI) was calculated independently for each species following the general method described by [15]. For each species, a SI was calculated based on three groups of factors (twelve in total):
- (1)
- Conservation factors, which sought to capture population-level features of the species considered in the present study:
- A.
- Proportion of biogeographic population occurring in Scotland
- B.
- Life history
- C.
- UK threat status
- (2)
- Vulnerability factors, which aimed to incorporate important general aspects of bird species’ behaviour that might affect their sensitivity to collisions with wind turbines:
- D.
- Flight manoeuvrability
- E.
- Nocturnal flight activity
- F.
- Habitat preference
- (3)
- Interaction factors, which sought to capture the degree to which bird species might interact with fish farms and other anthropogenic structures/activities in the marine environment:
- G.
- Likelihood of foraging around fish farms
- H.
- Likelihood of resting on fish farms
- I.
- Likelihood of feeding on cultured shellfish
- J.
- Use of other floating structures for resting
- K.
- Propensity to feed on fishery discards
- L.
- Interactions with wind turbines in flight
The factors assessed are outlined in more detail below (Table 1). Each factor was scored on a five-point scale from 1 (equivalent to a negligible or low anticipated impact) to 5 (high anticipated impact). Except where specifically indicated, factors were based on those described by [15,30], but individual scores for such factors were checked to ensure that they remained up to date. Wherever possible, scores for each factor were based on available scientific literature. For some factors (e.g., factor H: Likelihood of resting on fish farms; factor J: Use of other floating structures for resting), this approach proved impractical and scores were allocated on the basis of authors’ expert knowledge.

Table 1.
Summary of factors used to compile overall Sensitivity Indices for marine and coastal bird species.
2.4.1. Conservation Factors
The following three factors sought to collectively describe population-level characteristics of bird species pertaining to their abundance, conservation status, and life history. The assumption was made that species scoring higher under some or all of these factors were considered to be at greater relative risk from accidental mortality due to wind turbines on fish farms.
A. Proportion of biogeographic population in Scotland
This factor sought to estimate the fraction of each species’ total Palearctic/European population (excluding Russia) found in Scotland, either as a breeding species or a migrant, on the premise that encounters between birds and MPPs were more important for those species for which Scotland was a particularly significant part of their range. For each species, numbers of breeding pairs and numbers of individual birds wintering/on passage were derived from [31], as reported by [32]. These numbers were divided by either (1) the estimated total Palearctic/European breeding population (excluding Russia) or (2) the estimated total number of individuals wintering/on passage in Europe, to estimate the proportion of the total population found in Scotland [33]. In cases where range estimates were available for either Scottish or European counts, both minimum and maximum estimated proportions were calculated. The larger of the two proportions (breeding or wintering/passage) was then assessed against threshold values as published by [30,34] (see also Table 1): 1 (proportion <1%), 2 (proportion= 1–4.9%), 3 (proportion = 5–9.9%), 4 (proportion = 10–19.9%), and 5 (proportion ≥20%). Historic uncertainty regarding the taxonomic status of hooded crow (C. cornix) meant that accurate Europe-wide population estimates were unavailable for this species; Scottish population estimates were amalgamated with those for carrion crow (C. corone) for the present analysis. For rock dove (Columba livia livia), the estimated total Palearctic breeding population figures used here also included feral birds (C. l. domestica) in the absence of reliable data pertaining explicitly to C. l. livia.
B. Life history score
The life history score was included to take account of the fact that anthropogenically-driven adult mortality represents a more serious threat to the survival of bird species with high natural adult survival rates and low annual reproductive rates; species with low adult survival rates tend to have comparatively high reproductive rates which can at least partially compensate for any additional mortality caused by human activity, thereby receiving a lower score [30,34]. Life history scores were calculated based on [33] by multiplying adult annual survival rates with the average number of broods per annum and the average clutch size to incorporate measures of both longevity and fecundity, with larger scores denoting reduced risk. Life history scores were classified on a scale from 1 to 5 (see also Table 1): 1 (score ≥ 6.0), 2 (score between 4.5 ≤ 6.0), 3 (score between 3.0 ≤ 4.5), 4 (score between 1.5 ≤ 3.0), 5 (score <1.5).
C. UK threat status
The conservation status of species in the UK was derived from the third and fourth ‘Birds of Conservation Concern’ reports (BOCC3 and BOCC4, respectively) [35,36]. Any changes in the status of a species during the time period between the publication of BOCC3 and BOCC4 were accounted for by allocating the following scores: 1 (green in BOCC3 and BOCC4), 2 (amber in BOCC3 and green in BOCC4), 3 (green in BOCC3 and amber in BOCC4, or red in BOCC3 and green in BOCC4), 4 (amber in BOCC3 and BOCC4, or red in BOCC3 and amber in BOCC4), and 5 (red in BOCC4), as outlined by [34] (see also Table 1).
2.4.2. Vulnerability Factors
The following three factors sought to capture elements in the behaviour of birds that might increase their vulnerability towards collision with aquaculture-associated wind turbines, independent of any interactions with infrastructure.
D. Flight manoeuvrability
Flight manoeuvrability was included to incorporate the variability in different species’ ability to avoid collisions with wind turbines. The underlying assumption, as derived from [15], was that birds with higher manoeuvrability scores would be more likely to successfully avoid collisions. Following [15], species were classified from 1 (very high flight manoeuvrability) to 5 (very low flight manoeuvrability; see also Table 1). Scores were either directly derived from [15] or inferred based on comparisons with related species, a review of the scientific literature and expert knowledge of the species’ anatomy and flight behaviour.
E. Nocturnal flight activity
This factor was based on species’ expected propensity for nocturnal flying, which was presumed to increase the risk of collision with wind turbines due to reduced detectability in low-light conditions. Following [15], species were classified subjectively from 1 (hardly any flight activity at night) to 5 (much flight activity at night; see also Table 1). Scores were derived from [15] or were based on a review of the scientific literature, comparisons with related species and expert knowledge.
F. Habitat preference
This factor was used to capture the varying likelihood of different species’ presence above expanses of salt water beyond the immediate vicinity of the shoreline. It was intended to allow differentiation between marine vs. coastal or even terrestrial bird species (e.g., herons, starlings), which have been observed on fish farms but have not traditionally been included in impact assessments of offshore marine wind farms [15,30,34]. Scores were derived from a review of the scientific literature, comparisons with related species, and expert knowledge. Species were classified as follows (see also Table 1):
- The species is coastal in its distribution, closely affiliated with shoreline or intertidal habitats and is seldom encountered more than a few 100 m offshore (except on migration; score = 1).
- The species spends all its life at sea including both coastal and offshore waters, and normally only returns to land to breed (score =3).
- The species is widespread in coastal waters but returns regularly to land to forage, rest, breed etc. (score = 5).
It is worth noting that, under this classification system, truly oceanic species such as storm petrels (Hydrobatidae) that only depend on access to shore for the breeding season would receive a lower ranking than more coastal species such as herring gulls (Larus argentatus).
2.4.3. Interaction Factors
The following six factors reflect the known or suspected willingness of bird species to interact with fish farms and other anthropogenic activities and structures at sea. Published references to birds’ behaviours among fish farms are scarce, resulting in a greater reliance on grey literature, expert knowledge, and anecdotal information. Because so little information was available referring explicitly to interactions in Scotland, scores for the following factors were based on a review of the scientific literature, comparisons with related species in other parts of the world, and expert knowledge. For interaction factors G-K, species were classified according to the following subjective scale (see also Table 1):
- The species is not known to ever [engage in behaviour X]
- The species has been recorded [engaging in behaviour X], but this is considered very unusual
- The species is occasionally observed [engaging in behaviour X]
- The species is regularly observed [engaging in behaviour X]
- Large numbers of individuals are frequently observed [engaging in behaviour X]
G. Foraging around fish farms
This factor was designed to capture published or anecdotal observations of birds foraging among fish farms for e.g., captive fish, spilled feed, and/or wild fish and invertebrates associated with the farm [12,14,26].
H. Resting on fish farms
Here, the emphasis was on capturing published information, anecdotal observations etc. about birds explicitly resting on fish farm infrastructure (whether brief rests in between foraging bouts or longer rests during the night) or seeking out the leeside of farms for shelter on water.
I. Feeding on shellfish in off-substrate aquaculture (e.g., mussel lines)
This factor was included to explicitly record the attraction of some molluscivorous species (e.g., seaducks) to shellfish mariculture sites, particularly vertical lines suspended beneath floats on which blue mussels (Mytilus sp.) are grown [37,38]. This behaviour indicates a degree of familiarisation with anthropogenic structures, and such species might therefore be more inclined to seek out similar structures with epifauna such as fish farms, thereby increasing their potential collision risk.
J. Use of other floating structures for resting
Although the resting behaviours of many bird species among fish farms are poorly documented, many of these species are nevertheless frequently observed resting on floating artificial structures such as anchored ships, pontoons, navigation buoys, and the like (e.g., gulls, terns, cormorants and herons). Species that commonly engage in such behaviour might therefore be expected to seek out similar structures among MPPs, thereby increasing their potential collision risk. Observations of birds’ perching on buoys are infrequently reported in the scientific literature, perhaps due to their familiarity. Google™ image searches on the Internet were therefore carried out using each species’ scientific name combined with the terms “buoy” and “float” as search terms.
K. Feeding on fishery discards
Some bird species are well known for seeking out fishing vessels to forage on discards [39,40,41], which is here considered indicative of a willingness to associate with human activities that provide a nutritional advantage of some kind (synanthropy; [42]). Such species might therefore be more willing to investigate other foraging opportunities associated with human activities, such as those found among fish farms.
L. Interactions with wind turbines in flight
This factor was included to explicitly account for observed avoidance of wind turbines, including offshore wind farms, by certain bird species such as divers (Gaviidae) and gannets (Morus bassanus) [43]. Scores were derived from a review of the scientific literature, comparisons with related species, and expert knowledge. Species were classified on a scale from 1 to 5 based on scores generated by [43] (see also Table 1):
- 1 = the species is known or thought to avoid wind turbines (equivalent to mean scores ≤2.5 as presented by [43]),
- 3 = the species does not display an obvious response to wind turbines, or the response is unknown (equivalent to mean scores between >2.5–3.5 as presented by [43]),
- 5 = the species is not afraid of turbines (equivalent to mean scores >3.5 as presented by [43]).
2.5. Assessment Process
The twelve factors outlined above were amalgamated into a final Sensitivity Index (SI) following the general approach outlined by [15,30]. First, the scores for all factors within each subcategory (Conservation, Vulnerability, and Interaction) were averaged, as follows:
Total Conservation Score = (A + B + C)/3
Total Vulnerability Score = (D + E + F)/3
Total Interaction Score = ((G + H + ((I + J + K)/3) + L)/4
For the Interaction Score, the factors I, J, and K were amalgamated, assuming equal weighting, and averaged before being combined and averaged with the factors G, H, and L. This was done to reflect the greater significance of these factors (foraging and resting on fish farms, and wind turbine avoidance) for the present study, while still seeking to capture willingness of species to associate with human activities and infrastructure similar to those found among fish farms. The three resulting scores were then multiplied with each other to obtain the final Sensitivity Index, as follows:
Sensitivity Index = Total Conservation Score × Total Vulnerability Score × Total Interaction Score
On this basis, the Sensitivity Index for each species could range between 1 and 125. A higher Sensitivity Index thus indicated a higher potential negative impact on the species under consideration.
Due to the limited data available, an assessment of uncertainty associated with each interaction factor was calculated using a qualitative scaling process similar to that described by Wade et al. (2016). Each species’ scores for all factors A–L were assessed against the following three criteria, in each case receiving a numeric evaluation between 3 and 9 to express increasing uncertainty in the assessment:
- U1: Species specificity (Data explicitly refer to the species in question = 1; Data refer to a related species or a higher taxonomic classification = 2; No published data available = 3)
- U2: Reliability of observations (Observations were recorded through a focused study = 1; Observations were not the main focus of study = 2; No data, or only anecdotal observations = 3)
- U3: Local relevance (Observations from Scotland or adjacent northeast Atlantic countries = 1; Observations from comparable environments elsewhere such as North America or Asia = 2; No data, a general statement or observations from a different environment = 3)
Resulting uncertainty values were summed across all twelve interaction factors and could therefore range from 36 to 108, with higher values reflecting increasing uncertainty surrounding the assessment of each factor. The final uncertainty values were then used to identify data gaps within and across species, thereby seeking to identify potential new areas of research.
3. Results
The ranked SI values and associated uncertainty scores of all assessed species are presented in the Supplementary Material (Tables S1–S5). Species were organised by family as presented in Figure 3A–L. Estimated SI values varied substantially between species groups. Species with the highest overall SI values included the larger resident Larus gull species (specifically herring L. argentatus, lesser black-backed L. fuscus, and great black-backed gull L. marinus), as well as European shag (P. aristotelis; Table S5; Figure 3G–J). The high SI values obtained for these species were mainly driven by high conservation and interaction factors, capturing both the significance of these species’ Scottish populations at a European level and their confirmed propensity to seek out fish farms and other floating infrastructure for feeding and resting. Conversely, many species, particularly waders, swans, geese and ducks, and passerines received notably lower SI values (Table S5; Figure 3A,B,E,F,K,L). Species with low SI values tended to fall into one or more of the following categories:
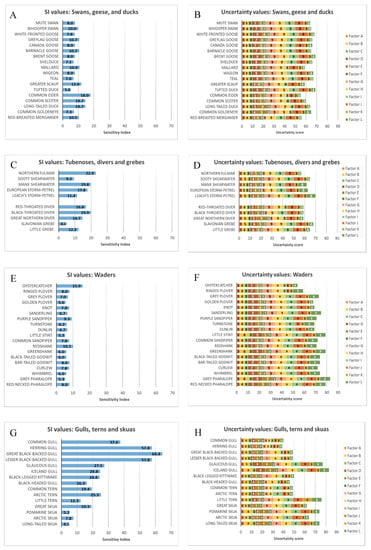
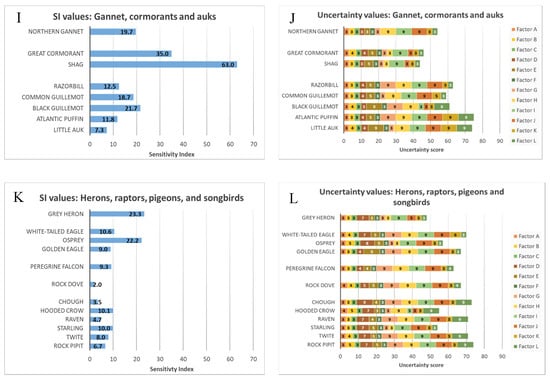
Figure 3.
Final Sensitivity Index values, and associated uncertainty values, for all 82 assessed bird species: (A,B) Swans, geese and ducks; (C,D) Shearwaters, petrels (“tubenoses”), divers and grebes; (E,F) waders; (G,H) Gulls, terns and skuas; (I,J) Gannets, cormorants and auks; (K,L) Herons, raptors, pigeons and various songbirds. Factor A = Proportion of biogeographic population in Scotland; Factor B = Life History score; Factor C = UK Threat status; Factor D = Flight manoeuvrability; Factor E = Nocturnal flight activity; Factor F = Habitat preference (not counting migration); Factor G = Foraging around fish farms; Factor H = Resting on fish farms; Factor I = Feeding on shellfish in off-substrate aquaculture; Factor J = Use of other floating structures for resting; Factor K = Feeding on fishery discards; Factor L = Avoidance of wind turbines. Groups in Figures C–D and I–L were aggregated solely for display purposes.
- Sensitive species that are known to often avoid marine infrastructure, such as red-throated divers (Gavia stellata).
- Pelagic species such as sooty shearwater (Puffinus griseus) and little auk (Alle alle) which, in Scotland, rarely approach nearshore waters in significant numbers.
- Species that spend a lot of time on land or in intertidal habitats, such as many waders, swans and geese, and coastal passerines such as rock pipit (Anthus petrosus).
While certain groups of species (gulls, cormorants) were considered more likely to be at risk than others, SI values also varied within species groups. These differences were often driven by genuine ecological differences between species, but also influenced by the amount of information available for review, which is reflected in the often-large associated uncertainty values. For example, the relatively high SI value for oystercatcher (Haematopus ostralegus) was partially influenced by information about this species’ ability to forage off epifauna attached to floating structures, as reported by [44]; similar behaviours have not been described for many related species of waders.
Information on the behaviour of birds associating with fish farms in Scotland was limited, resulting in high uncertainty values for many of the SI values underpinning the final scores (Supplementary Material, Tables S1–S5). Factors A (% biogeographic population), C (UK threat status), and F (Habitat preference) were based on readily available species-specific data relevant to western and northern Scotland, generally resulting in very low uncertainty scores for each of these factors across all species. In contrast, uncertainty scores for the factors G–J (associated with foraging around fish farms and resting on fish farms/other floating infrastructure) were comparatively high for most, but not all, species. This was driven by genuine lack of information about how birds make use of fish farms and other floating infrastructure in Scottish waters and elsewhere. Factors B (life history score), D (flight manoeuvrability), E (nocturnal flight activity), and L (interactions with wind turbines in flight) mostly returned low to intermediate uncertainty values. Uncertainty values for factor K (feeding on fishery discards) were influenced by the likelihood that a given species would be able to feed on fishery discards based on its ecology and distribution; species considered unlikely to do so, such as Dunlin (Calidris alpina) or teal (Anas crecca), therefore received very low uncertainty values for this factor, despite the absence of published sources explicitly describing this relationship.
Summary uncertainty scores (Table S5) ranged from as low as 39 (herring gull) to as high as 83 (grey phalarope Phalaropus fulicarius, Iceland gull L.glaucoides) out of 108, reflecting large differences in the reliability of the associated final SI values. Species with high summary uncertainty scores tended to fall into the following, non-exclusive categories:
- Boreal/Arctic migrant species that only visit Scottish waters occasionally or in small numbers (including grey phalarope, glaucous gull L. hyperboreus, Iceland gull, pomarine and long-tailed skua Stercorarius pomarinus and S. longicaudus).
- Coastal species (waders, grebes, ducks, geese and swans, and assorted mostly-terrestrial species such as raptors and passerines), whose movements and in-flight behaviours in coastal waters at small spatiotemporal scales may be poorly documented.
- Species whose interactions with fish farms, if any, have not previously been reported in detail, including otherwise well-studied species such as Atlantic puffin Fratercula arctica and little tern Sternula albifrons.
Conversely, species with low summary uncertainty scores tended to live in coastal habitats, occur in Scotland in large numbers, and interact with fish farms, wind turbines or other marine infrastructure in ways that are comparatively well-understood. These included the large Larus gull species, cormorants and shags, grey heron (Ardea cinerea), and common and Arctic tern (Sterna hirundo, S. paradisaea).
4. Discussion
The results of the theoretical assessment confirm expectations that some seabird species (notably the large Larus gulls and European shag) might be at risk as a result of future integration of wind turbines and fish farms in western Scottish waters, and presumably elsewhere. The assessment method proved capable of selecting those species intuitively deemed to be at greatest risk due to their presence at existing fish farms, apparent lack of avoidance of wind turbines, widespread use of artificial structures for resting, and (in the case of the Larus gulls) attraction to fishery discards and other anthropogenic food sources. In the case of the European shag, the significance of the Scottish subpopulation at a European level was a further important factor. Western Scottish waters form a stronghold for these species at a UK level [45]; several species are facing population declines due to predation by invasive/introduced predators [46], human disturbance, reduction in food availability [47], and changes to the wider ecosystem driven through climate change [48].
This study did not attempt to spatiotemporally resolve relative variability of risk to bird species at small scales; essentially, risks were amalgamated across western and northern Scotland and across the year. More in-depth analysis was deemed to fall outside the scope of this study because it proved impossible to obtain sufficiently high-resolution data on abundance and distribution for all species across this area. One aspect that was not evaluated here relates to temporal variability in relative risk that might be experienced by social migratory species, as opposed to residents. The former might spend only a brief period in the vicinity of an MPP, but a propensity to travel in groups would mean that large numbers of migrants could still interact with, and be impacted by, wind turbines aboard fish farms. Similarly, widespread integration of wind turbines among salmon aquaculture sites could have at least a local effect on some bird populations, particularly if such MPPs were located near important breeding colonies; however, this was also not assessed in the present study. Since actual collision events are difficult to verify at sea due to the rapid disappearance of carcasses [49], any population-level effects of widespread development of MPPs in coastal waters off western Scotland would likely be difficult to assess. Further research at local scales is needed to evaluate whether MPPs might increase collision risk to particular colonies, and/or whether additional effects such as barriers to movement also need to be considered [50].
The present assessment methodology differs somewhat from previous studies on collision risk for seabirds with wind turbines in terms of factors selected [15,30,34]. In particular, flight altitude is widely considered to be highly significant in determining wind turbine collision risk to birds, as species that fly either below or above the area swept by the turbine blades will likely be at much lower risk of collision [15,51,52]. As a result, this parameter is often given prime importance when assessing collision risks for seabirds in offshore wind farms [30]. The wind turbines proposed in the present study were relatively small compared to current turbine models used in offshore wind farms, reducing the swept area but bringing it closer to the sea surface. Flight altitude information is available for a range of seabird species [52,53,54]. While this information could be used to assess risks for bird species that are not attracted to fish farms, it is likely that species that are attracted would display highly variable flight behaviours (by landing on/taking off from the fish farm infrastructure or surrounding water, circling around the fish farm, rapidly changing altitude, etc.). There are very few, if any, published data on such flight behaviours for the majority of species considered in this study. For this reason, the flight altitude factor, as used in [15,30,34], proved to be difficult to integrate into the assessment methodology in a consistent manner for all species.
The present study has attempted to integrate what is known about deterrent effects of operational wind turbines on birds within an operational salmon farm setting. Given the turbine sizes and designs used in this study, we have assumed that these impacts will be less pronounced when compared with commercial offshore wind farms. Divers and seaducks are examples of species known to be sensitive to disturbance [55,56], and an attempt was made to include this sensitivity through the interaction factor G: Foraging around fish farms. It is presently unclear whether wind turbine-derived deterrence effects might be different from those deterrent effects caused by salmon farms themselves, including through regular human presence, boat movements, artificial lighting, etc. Unfortunately, little is known about the nature and significance of these deterrent effects on birds, and an in-depth investigation was outside the scope of this study; however, given the more diverse spread of activities occurring at operational salmon farms, a more variable response among birds can be anticipated depending on specific local circumstances. The incorporation of wind turbines may result in an increased deterrence from the fish farm of some species due to e.g., sound or light flickering caused by the rotation of the turbines. Such an effect may be anticipated through the use of rotating gull deterrents among yacht owners (e.g., Scaregull™ seagull scarer; http://www.scaregull.com/). If wind turbines were to actively displace some bird species away from fish farms, this would represent a reduction in collision risk posed by MPPs to marine birds, although such displacement could have wider effects on the foraging success of the affected birds and their longer-term fitness.
This study has attempted to assess the potential risk of future integration of wind turbines and fish farms to marine and coastal birds from a theoretical perspective. The study deliberately chose to assess a wide range of Scottish coastal bird species that might use fish farms, such as herons and crows, as well as more obligate marine species. If this study were to be replicated elsewhere in the world, it might be predicted that gulls, cormorants, and other opportunistic species (like herons) would be among the most likely candidate species to interact with fish farms, and thereby be at greatest risk of negative interactions with an MPP. As aquaculture moves further offshore, changes in the observed bird community will become apparent with a greater proportion of pelagic species, complemented by coastal species that are able to take advantage of the presence of the infrastructure to expand their range (e.g., cormorants; [43]). The results suggest that most coastal and marine bird species would only be experiencing low to moderate levels of relative risk, as expressed by overall SI values. This approach can, however, only indicate potential risk, given the near-total absence of current observational data on seabird behaviour around fish farms in Scotland and elsewhere. There were significant differences between species in terms of both quantity and quality of data available on which to base scores for the various factors.
The SI values and uncertainty values reported here have illustrated a number of data gaps related to marine and coastal bird ecology in relation to fish farms and wind turbines:
- Turbine-related risks: Most work on assessing risks posed by wind turbines to marine and coastal birds to date has focused on the increasingly large, widely spaced turbines arranged in ‘farms’ or ‘parks’, currently placed at increasing distances offshore. The present MPP concept instead proposes a cluster of small turbines placed close together, with rotors operating much closer to the sea surface. This may change the relative risks posed to bird species and needs to be carefully considered.
- Confirming interactions with fish farms: More information is needed to confirm whether marine and coastal bird species regularly use exisiting fish farms and other aquaculture-related infrastructure. More evidence, such as visual observations and telemetry records, is urgently needed to confirm which species are actively attracted to fish farms vs. those that actively avoid them, to reduce the uncertainty associated with many of the SI values reported above.
- Detailed observations of birds around fish farms: For those species that are attracted to existing fish farms, there are still numerous unanswered questions relating to their use of these structures. Detailed observational and telemetry studies are needed to understand the source(s) of many species’ attraction to fish farms (if any), to record details of foraging, flight and diving behaviours displayed around them and quantify spatiotemporal variability of occurrence, in relation to site-specific infrastructure and human activities.
- Ecological links: It is important to consider the wider food web developing around fish farms of which marine and coastal bird species are one visible component [10,11]. More information is required on the abundance and diversity of wild fish and macroinvertebrate species associated with fish farms at various stages of the production cycle, to clarify the prey field available to various bird species across different spatiotemporal scales.
- Effects on individual fitness: The extent to which fish farm infrastructure is being used only by particular individuals of a given species vs. the entire local population needs to be evaluated. Telemetry and dietary data could clarify the relative importance of foraging among fish farms to individuals, as well as clarify the energetic trade-offs made by individual birds to maximize their long-term fitness in response to these artificial structures, with potential population-level consequences.
- Population-level effects: At a regional scale, more long-term studies are needed to explore the consequences of changes to the number and distribution of aquaculture facilities over time to marine and coastal bird populations. This will allow us to understand connectivity between particular breeding colonies, to evaluate effects of farm-specific operational practices and to avoid potential habitat exclusion and barrier effects for vulnerable species. This information may be important for evaluating the overall population risks posed by widespread adoption of MPPs.
The resolution of the above data gaps would significantly enhance understanding of the interactions of fish farms (with or without wind turbines) and coastal and marine birds and the potential ecological role played by the fish farms in increasingly industrialised marine environments. As the global expansion of both aquaculture and renewable energy generation continues, it is important to understand their individual and synergistic effects on coastal and marine bird communities worldwide to develop and implement successful long-term sustainable use of the marine environment.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-1312/8/6/414/s1.
Author Contributions
Conceptualization, S.B. and M.C.; Formal analysis, S.B. and E.M.; Funding acquisition, S.B. and M.C.; Investigation, S.B. and E.M.; Methodology, S.B. and E.M.; Project administration, M.C.; Writing—original draft, S.B.; Writing—review & editing, E.M. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
S.B. and M.C. were supported by the Engineering and Physical Sciences Research Council UK (EPSRC) and the Natural Environment Research Council UK (NERC), through grant EP/R007497/1 and EP/R007497/2, and the Natural Science Foundation of China (NSFC) through grant 51761135013, under the INNO-MPP project.
Acknowledgments
R.W. Furness (University of Glasgow) provided key insights into parametrisation of the methodology described in this manuscript. M. Jessopp (University College Cork) provided insightful discussions about seabird-turbine interactions and gull deterrent tactics. L. Recalde Camacho (University of Strathclyde) kindly provided the MPP barge design diagram included as Figure 2.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Aquatera Ltd. Renewable Power Generation on Aquaculture Sites; A Report Commissioned by SARF and Prepared by Aquatera Ltd. P498—November 2013; Aquatera Ltd.: Stromness, UK, 2014. [Google Scholar]
- Syse, H.L. Investigating Off-Grid Energy Solutions for the Salmon Farming Industry; University of Strathclyde: Glasgow, UK, 2016. [Google Scholar]
- Abhinav, K.A.; Collu, M.; Ke, S.; Zhou, B. Frequency domain analysis of a hybrid aquaculture-wind turbine offshore floating system. In Proceedings of the American Society of Mechanical Engineers (ASME) 37th International Conference on Ocean, Offshore, and Arctic Engineering (OMAE), Glasgow, UK, 8–14 June 2019; p. 8. [Google Scholar]
- Lagasco, F.; Collu, M.; Mariotti, A.; Safier, E.; Arena, F.; Atack, T.; Brizzi, G.; Tett, P.; Santoro, A.; Bourdier, S.; et al. New engineering approach for the development and demonstration of a Multi-Purpose Platform for the Blue Growth economy. In Proceedings of the American Society of Mechanical Engineers (ASME) 37th International Conference on Ocean, Offshore, and Arctic Engineering (OMAE), Glasgow, UK, 8–14 June 2019. [Google Scholar]
- Leung, D.Y.C.; Yang, Y. Wind energy development and its environmental impact: A review. Renew. Sustain. Energy Rev. 2012, 16, 1031–1039. [Google Scholar] [CrossRef]
- Desholm, M.; Kahlert, J. Avian collision risk at an offshore wind farm. Biol. Lett. 2005, 1, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Drewitt, A.L.; Langston, R.H.W. Assessing the impacts of wind farms on birds. Ibis (Lond. 1859) 2006, 148, 29–42. [Google Scholar] [CrossRef]
- Marques, A.T.; Batalha, H.; Rodrigues, S.; Costa, H.; Pereira, M.J.R.; Fonseca, C.; Mascarenhas, M.; Bernardino, J. Understanding bird collisions at wind farms: An updated review on the causes and possible mitigation strategies. Biol. Conserv. 2014, 179, 40–52. [Google Scholar] [CrossRef]
- Callier, M.D.; Byron, C.J.; Bengtson, D.A.; Cranford, P.J.; Cross, S.F.; Focken, U.; Jansen, H.M.; Kamermans, P.; Kiessling, A.; Landry, T.; et al. Attraction and repulsion of mobile wild organisms to finfish and shellfish aquaculture: A review. Rev. Aquac. 2018, 10, 924–949. [Google Scholar] [CrossRef]
- Barrett, L.T.; Swearer, S.E.; Dempster, T. Impacts of marine and freshwater aquaculture on wildlife: A global meta-analysis. Rev. Aquac. 2019, 11, 1022–1044. [Google Scholar] [CrossRef]
- Carss, D.N. Killing of piscivorous birds at Scottish fin fish farms, 1984–1987. Biol. Conserv. 1994, 68, 181–188. [Google Scholar] [CrossRef]
- Price, I.M.; Nickum, J.G. Aquaculture and birds: The context for controversy. Colon. Waterbirds 1995, 18, 33–45. [Google Scholar] [CrossRef]
- Quick, N.J.; Middlemas, S.J.; Armstrong, J.D. A survey of antipredator controls at marine salmon farms in Scotland. Aquaculture 2004, 230, 169–180. [Google Scholar] [CrossRef]
- Garthe, S.; Hüppop, O. Scaling possible adverse effects of marine wind farms on seabirds: Developing and applying a vulnerability index. J. Appl. Ecol. 2004, 41, 724–734. [Google Scholar] [CrossRef]
- Scottish Government. Marine Scotland Science—Scottish Fish Farm Production Survey 2016; Scottish Government: Edinburgh, UK, 2017.
- Ellis, T.; Turnbull, J.F.; Knowles, T.G.; Lines, J.A.; Auchterlonie, N.A. Trends during development of Scottish salmon farming: An example of sustainable intensification? Aquaculture 2016, 458, 82–99. [Google Scholar] [CrossRef]
- Scotland Food and Drink. Aquaculture Growth to 2030. A Strategic Plan for Farming Scotland’s Seas; Scotland Food and Drink: Edinburgh, UK, 2016. [Google Scholar]
- Scottish Government Policy: Renewable and Low Carbon Energy. Available online: https://www.gov.scot/policies/renewable-and-low-carbon-energy/ (accessed on 26 April 2020).
- Recalde-Camacho, L.; Yue, H.; Leithead, W.; Anaya-Lara, O.; Liu, H.; You, J. Hybrid renewable energy systems sizing for offshore Multi-Purpose Platforms. In Proceedings of the American Society of Mechanical Engineers (ASME) 37th International Conference on Ocean, Offshore, and Arctic Engineering (OMAE), Glasgow, UK, 8–14 June 2019. [Google Scholar]
- UK and China Centre for Offshore Renewable Energy (CORE) Investigation of the Novel Challenges of an Integrated Offshore Multi-Purpose Platform—EP/R007497/1. Available online: https://www.ukchn-core.com/project/inno-mpp/ (accessed on 25 April 2020).
- AKVA Group. Cage Farming Aquaculture Prospectus; AKVA Group: Klepp, Norway, 2018. [Google Scholar]
- Aeolos Wind Energy. Datasheet Aeolos-H 20kW; Aeolos Wind Energy: London, UK, 2018. [Google Scholar]
- Polaris America LLC. P10-20 Specification Sheet; Polaris America LLC: Allenwood, NJ, USA, 2015. [Google Scholar]
- Scottish Government Aquaculture—Finfish and Shellfish Farms (Including Fishery Sites): Marine_Scotland_FishDAC_1249. Available online: https://spatialdata.gov.scot/geonetwork/srv/eng/catalog.search#/metadata/Marine_Scotland_FishDAC_1249 (accessed on 26 April 2020).
- Carss, D.N. Grey heron, Ardea cinerea L., predation at cage fish farms in Argyll, western Scotland. Aquac. Res. 1993, 24, 29–45. [Google Scholar] [CrossRef]
- Furness, R.W. Interactions between seabirds and aquaculture in sea lochs. In Aquaculture and Sea Lochs. Proceedings of a Joint Meeting of the Scottish Association for Marine Science and the Challenger Society at Dunstaffnage, 1995; Black, K.D., Ed.; Scottish Association for Marine Science: Oban, UK, 1996; pp. 50–55. ISBN 0952908905. [Google Scholar]
- Svensson, L. Collins Bird Guide, 2nd ed.; HarperCollins Publishers: London, UK, 2009; ISBN 9780007268146. [Google Scholar]
- Handbook of the Birds of the World; Del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., Eds.; Lynx Edicions: Barcelona, Catalunya, Spain, 2010. [Google Scholar]
- Furness, R.W.; Wade, H.M.; Masden, E.A. Assessing vulnerability of marine bird populations to offshore wind farms. J. Environ. Manag. 2013, 119, 56–66. [Google Scholar] [CrossRef] [PubMed]
- The Birds of Scotland; Forrester, R., Andrews, I., Eds.; Scottish Ornithologists’ Club: Aberlady, UK, 2007; ISBN 978-0951213902. [Google Scholar]
- Holden, P.; Housden, S. RSPB Handbook of Scottish Birds, 1st ed.; Bloomsbury Publishing Plc: London, UK, 2009; ISBN 9781408112328. [Google Scholar]
- Birdlife International Birds in Europe: Population Estimates, Trends and Conservation Status. Available online: https://www.bto.org/understanding-birds/birdfacts (accessed on 26 April 2020).
- Furness, R.W.; Wade, H.M.; Robbins, A.M.C.; Masden, E.A. Assessing the sensitivity of seabird populations to adverse effects from tidal stream turbines and wave energy devices. ICES J. Mar. Sci. 2012, 69, 1466–1479. [Google Scholar] [CrossRef]
- Eaton, M.A.; Brown, A.F.; Noble, D.G.; Musgrove, A.J.; Hearn, R.D.; Aebischer, N.J.; Gibbons, D.W.; Evans, A.; Gregory, R.D. Birds of Conservation Concern 3: The population status of birds in the United Kingdom, Channel Islands and the Isle of Man. Br. Birds 2009, 102, 296–341. [Google Scholar]
- Eaton, M.; Aebischer, N.; Brown, A.; Hearn, R.; Lock, L.; Musgrove, A.; Noble, D.; Stroud, D.; Gregory, R. Birds of Conservation Concern 4: The population status of birds in the UK, Channel Islands and Isle of Man. Br. Birds 2015, 108, 708–746. [Google Scholar]
- Dionne, M.; Lauzon-Guay, J.-S.; Hamilton, D.J.; Barbeau, M.A. Protective socking material for cultivated mussels: A potential non-disruptive deterrent to reduce losses to diving ducks. Aquac. Int. 2006, 14, 595–613. [Google Scholar] [CrossRef]
- Varennes, É.; Hanssen, S.A.; Bonardelli, J.; Guillemette, M. Sea duck predation in mussel farms: The best nets for excluding common eiders safely and efficiently. Aquac. Environ. Interact. 2013, 4, 31–39. [Google Scholar] [CrossRef]
- Garthe, S.; Huppop, O. Distribution of ship-following seabirds and their utilization of discards in the North Sea in Summer. Mar. Ecol. Prog. Ser. 1994, 106, 1–10. [Google Scholar] [CrossRef]
- Camphuysen, C.J.; Calvo, B.; Durinck, J.; Ensor, K.; Follestad, A.; Furness, R.W.; Garthe, S.; Leaper, G.; Skov, H.; Tasker, M.L.; et al. Consumption of Discards by Seabirds in the North Sea; Final Report to the European Commission DG XIV Research Contract BIOECO/93/10, NIOZ-Report 1995-5; Den Burg: Texel, The Netherlands, 1995. [Google Scholar]
- Garthe, S.; Walter, U.; Tasker, M.L.; Becker, P.H.; Chapdelaine, G.; Furness, R.W. Evaluation of the role of discards in supporting bird populations and theireffects on the species composition of seabirds in the North Sea. In ICES Cooperative Research Report No. 232: Diets of Seabirds and Consequences of Changes in Food Supply; Furness, R.W., Tasker, M.L., Eds.; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1999; pp. 29–41. [Google Scholar] [CrossRef]
- Johnston, R.F. Synanthropic birds of North America. In Avian Ecology and Conservation in an Urbanizing World; Marzluff, J.M., Bowman, R., Donelly, R., Eds.; Springer: New York, NY, USA, 2001; pp. 49–67. ISBN 978-0792374589. [Google Scholar]
- Dierschke, V.; Furness, R.W.; Garthe, S. Seabirds and offshore wind farms in European waters: Avoidance and attraction. Biol. Conserv. 2016, 202, 59–68. [Google Scholar] [CrossRef]
- Roycroft, D.; Kelly, T.C.; Lewis, L.J. Birds, seals and the suspension culture of mussels in Bantry Bay, a non-seaduck area in Southwest Ireland. Estuar. Coast. Shelf Sci. 2004, 61, 703–712. [Google Scholar] [CrossRef]
- Mitchell, P.I.; Newton, S.F.; Ratcliffe, N.; Dunn, T.E. Seabird Populations of Britain and Ireland: Results of the Seabird 2000 Census (1998–2002); T. and A.D. Poyser Ltd.: London, UK, 2004. [Google Scholar]
- Jones, H.P.; Holmes, N.D.; Butchart, S.H.M.; Tershy, B.R.; Kappes, P.J.; Corkery, I.; Aguirre-Muñoz, A.; Armstrong, D.P.; Bonnaud, E.; Burbidge, A.A.; et al. Invasive mammal eradication on islands results in substantial conservation gains. Proc. Natl. Acad. Sci. USA 2016, 113, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Miles, W.T.S.; Mavor, R.; Riddiford, N.J.; Harvey, P.V.; Riddington, R.; Shaw, D.N.; Parnaby, D.; Reid, J.M. Decline in an Atlantic Puffin population: Evaluation of magnitude and mechanisms. PLoS ONE 2015, 10, e0131527. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Ratcliffe, N.; Suddaby, D.; Ribbands, B.; Smith, C.; Ellis, P.; Meek, E.; Bolton, M. Combined bottom-up and top-down pressures drive catastrophic population declines of Arctic skuas in Scotland. J. Anim. Ecol. 2018, 87, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Desholm, M.; Fox, A.D.; Beasley, P.D.L.; Kahlert, J. Remote techniques for counting and estimating the number of bird-wind turbine collisions at sea: A review. Ibis (Lond. 1859) 2006, 148, 76–89. [Google Scholar] [CrossRef]
- Masden, E.A.; Haydon, D.T.; Fox, A.D.; Furness, R.W. Barriers to movement: Modelling energetic costs of avoiding marine wind farms amongst breeding seabirds. Mar. Pollut. Bull. 2010, 60, 1085–1091. [Google Scholar] [CrossRef]
- Band, W. Strategic Ornithological Support Services programme (SOSS), Project SOSS-02. Using a Collision Risk Model to Assess Bird Collision Risks for Offshore Windfarms; SOSS: London, UK, 2012. [Google Scholar]
- Cook, A.S.C.P.; Johnston, A.; Wright, L.J.; Burton, N.H.K. BTO Research Report Number 618. Strategic Ornithological Support Services Project SOSS-02: A Review of Flight Heights and Avoidance Rates of Birds in Relation to Offshore Wind Farms; SOSS: London, UK, 2012. [Google Scholar]
- Tasker, M.L.; Jones, P.H.; Dixon, T.I.M.; Blake, B.F. Counting seabirds at sea from ships: A review of methods employed and a suggestion for a standardized approach. Auk 1984, 101, 567–577. [Google Scholar] [CrossRef]
- Camphuysen, C.J.; Fox, A.D.; Leopold, M.F.; Petersen, I.K. Towards Standardised Seabirds at Sea Census Techniques in Connection with Environmental Impact Assessments for Offshore Wind Farms in the UK: A Comparison of Ship and Aerial Sampling Methods for Marine Birds and Their Applicability to Offshore Wind Farm a; Den Burg: Texel, The Netherlands, 2004. [Google Scholar]
- Mendel, B.; Schwemmer, P.; Peschko, V.; Müller, S.; Schwemmer, H.; Mercker, M.; Garthe, S. Operational offshore wind farms and associated ship traffic cause profound changes in distribution patterns of Loons (Gavia spp.). J. Environ. Manag. 2019, 231, 429–438. [Google Scholar] [CrossRef]
- Fliessbach, K.L.; Borkenhagen, K.; Guse, N.; Markones, N.; Schwemmer, P.; Garthe, S. A ship traffic disturbance vulnerability index for Northwest European seabirds as a tool for marine spatial planning. Front. Mar. Sci. 2019, 6, 1–15. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).