Abstract
Ultrasonic antifouling devices are installed globally on a variety of vessel types and are marketed as an environmentally friendly method for biofouling control. The aim of this study was to examine the effects of ultrasound on adults of three species of common solitary ascidians (Ciona intestinalis, Ascidiella aspersa and Styela plicata). After a brief (10 s) exposure to two ultrasound frequencies (30 and 35 kHz), alterations in the frequency of siphon closing events and the length of time the siphons remained closed/open were observed. The results revealed that ascidians are able to perceive ultrasound, showing frequency-dependent behavioural responses that vary depending on the species and size of individuals involving both tactile receptors and an acoustic system homologous to the vertebrate inner ear. Continuous (5 h) 30 kHz exposure caused other types of responses, the most interesting of which was the long-term opening of the oral siphon, indicating a lack of reactivity to mechanical stimuli. This effect suggests a stress condition that could lead to increased vulnerability to predators and filter-feeding impairment. Therefore, knowledge of the acoustic sensitivity of sessile marine species appears to be essential for better understanding the potential effects of noise pollution on marine ecosystems.
1. Introduction
The natural environment is characterised by a specific sound environment [1]. Animals use sound to communicate with conspecifics for reproduction, foraging, predation or escape from predators [1,2]. For many marine animals, sound is the most important sensory modality, and rising levels of noise pollution pose a risk to marine species that rely on sound [2,3,4]. Shipping, naval operations, offshore construction, seismic exploration, oceanographic research and fishing activities contribute to increasing anthropogenic noise pollution [5].
The first and most extensive studies on the effects of marine noise on animals have focused on the most iconic marine mammals [2,6,7]. Noise impacts on marine mammals range from physiological (e.g., shifts in hearing sensitivity and elevated stress hormone levels) to behavioural effects [8,9,10]. Southall and collaborators (2008) [7] reported that marine mammals might develop behavioural disorders when they are exposed to noise above 230 dB over a 24 h period. Regarding other marine vertebrates, the threshold audiogram measures for several species of fish [11,12] show that they cannot detect sound up to several tens of kHz. Thus, ultrasonic frequencies are well above the hearing ranges of almost all fish species. Although few studies have been carried out on diving seabirds and marine reptiles, many of these species appear susceptible to noise exposure [13,14,15,16].
On the other hand, research focused on the impact of sound on marine invertebrates is scant [17,18]. The great variety that is observed in the sensory organs of the various species suggests that, among these, there are differences in the auditory threshold and behavioural responses [19]. Invertebrates are more sensitive to the particle motion associated with sound rather than to sound pressure. They often use various types of mechanoreceptors and statocysts [18]. The component of sound related to particle motion is damped within a short distance from the source, whereas vibrations can propagate over great distances into the substratum and cause motion in overlying water particles that therefore can be perceived by benthic invertebrates [20]. Pacific oyster, Magallana gigas, exhibits transient valve closures in response to sound frequencies of 10 to <1000 Hz in a frequency-dependent manner, with maximum responses occurring between 10 to 200 Hz [21]. Lobster, Nephrops norvegicus, shows postural responses at frequencies between 20 and 120 Hz due to the perception of particle motion and not sound pressure [22]. Cephalopods are the most studied marine invertebrates, and their hearing relies on the presence of statocysts with sensitivity to frequencies beyond 400 Hz [23]; e.g., behavioural responses in squid, Doryteuthis pealeii, to stimuli can occur up to 1000 Hz [24].
Most studies have investigated the impact of anthropogenic sound on motile species of invertebrates such as squids [25] and decapod crustaceans [26,27]. Recently, it was discovered that the sounds produced by boats could have a negative impact on the settlement and metamorphosis of the larvae of various fouling organisms [2,16,28]. Every hard substratum submerged in the sea represents a new habitat that is rapidly colonised by a biofilm, followed by the settlement of a macroscopic community of marine organisms. This process is known as biofouling, which causes severe damage to ships’ hulls and submerged structures [29]. Since the 1980s, physical antifouling systems have been introduced to various types of vessels to protect hulls from biofouling during long mooring periods [30,31,32,33]. These systems focus on changing the physical properties of submerged surfaces or water hydrodynamics to remove settled organisms without using antifouling paints, which release chemical biocides into the environment [34]. Therefore, these devices are considered ‘eco-friendly’ antifouling systems [35], but there is no evidence to prove this. Guo and collaborators [36,37,38] observed the most effective inhibition of settlement in cyprid larvae of Amphibalanus amphitrite after a 23 kHz ultrasound treatment in comparison with other higher ultrasound frequencies (63 and 102 kHz). An ultrasound treatment operating at 20 kHz was able to kill barnacle larvae within 45 s [39]. This has also been confirmed by other studies, which have shown that frequencies of 20–22 kHz prevent fouling settlement [39,40].
The potential impact of ultrasound devices on coastal marine ecosystems has not been assessed. In particular, it is important to investigate the stress that sessile species might suffer. Motile organisms may respond to auditory disturbances with evasive behavioural responses [41,42], whereas sessile species cannot move or hide to minimise the effects of such disturbances. Among the benthic species of the coastal community, tunicates are the dominant taxon of the so-called ‘soft-macrofouling’ [43]. The first observations concerning the effects of anthropogenic noise on behaviour in adult tunicates have been reported by White and collaborators [44]. The authors exposed 48 specimens of the solitary ascidian Styela plicata for 8 s to three separate stimuli measured from the position (150 mm) of the ascidians in the tank, i.e., a recording of a boat motor (sound pressure level, the SPL peaked at 82–70 dB and a frequency of 100 Hz), a song recording (the SPL peaked at 81–57 dB and a frequency of 100 Hz) and a water current to simulate turbulence. Individuals showed an increase in the frequency of siphon closure when compared with the control.
The present study is the first to determine the behavioural effects of ascidians after exposure to the frequencies used by antifouling ultrasound systems commonly seen on the market. The responses to ultrasound have been investigated in adults of three species of solitary ascidians, i.e., Ciona intestinalis, Ascidiella aspersa and Styela plicata, considering two types of siphon responses (Figure 1). Various studies have shown that the siphon area is the most sensitive to tactile stimulation [45,46,47,48]. Primary sensory cells are located within a sensory field on the inside of the oral siphon between the velum and the rim [49,50,51]. They are hydrodynamic and vibration sensors sensitive to touch. Their stimulation evokes the squirt response (SqR), a strong, synchronous contraction of both siphons that causes a violent ejection of water from both the oral and the atrial siphon. Secondary sensory cells are mechanoreceptors located at the base of the oral siphon along the velum and the branched tentacles, where they are arranged in one row or in a few rows and described as the ‘coronal organ’ as a whole [52,53,54,55,56]. For their morphology and development, these sensory cells, flanked by supporting cells, are considered homologues of the hair cells of the vertebrate internal ear and lateral line system [57,58]. Like hair cells, they are mechanoreceptors that mediate vibrational and fluid-low sensing, which allow hearing and vibrational sensing [59]. Their stimulation, as a consequence of the mechanical stimulation of the tentacles, evokes the crossed response (CrR), a contraction of the atrial siphon while the oral siphon stays open [60,61].
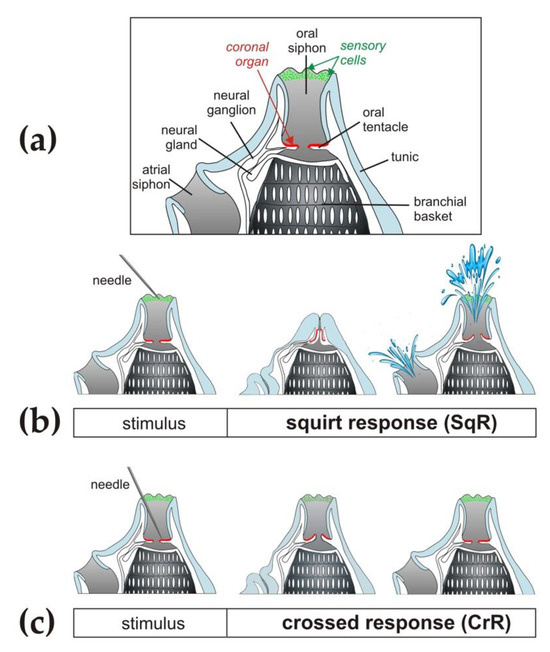
Figure 1.
Schematic drawing of the two main types of siphon responses to mechanical stimuli in ascidians according to the behavioural responses described in detail by Mackie and collaborators [60]. (a) General schematic morphology of siphons with the location of sensory cells. (b) Squirt response (SqR), i.e., fast closure of oral and atrial siphons and body contraction with ejection of water from both siphons due to stimulation of primary sensory cells in the inner epithelium of the siphon and along the rim. (c) Crossed response (CrR), i.e., closure of the atrial siphon due to the stimulation of secondary sensory cells along the coronal organ.
2. Materials and Methods
2.1. Animals
Three species of solitary ascidians (Figure 2) common in the intertidal and temperate zones were used for this study: Ciona intestinalis (Linnaeus, 1767), Ascidiella aspersa (Müller, 1776) and Styela plicata (Lesueur, 1823). The first two belong to the order Phlebobranchia, while S. plicata belongs to the order Stolidobranchia [62]. Individuals of A. aspersa and S. plicata were collected in the southern basin of the Lagoon of Venice (near Chioggia), while wild individuals of C. intestinalis were furnished by the Biological Station in Roscoff (France) and transported by air in an appropriate container. Once in the laboratory, ascidians were acclimated for one week (mortality less than 10%) and kept in aquaria inside thermostatic chambers under controlled conditions (temperature of 12 °C and salinity 35‰) in seawater filtered through 0.45 µm filters (FSW). The aquaria were kept well ventilated by oxygenators and replaced every 48 h, and the ascidians were fed with unicellular algae (1:1 ratio of Dunaliella sp. and Tetraselmis sp.). Before the experiment, the length of each individual was measured along the antero–posterior axis of the body, which is parallel to the endostyle and passes between the oral siphon and the digestive tract [63]. For S. plicata and A. aspersa, measurements were performed with a calliper. However, C. intestinalis individuals were placed in a glass Petri dish that was marked with a graphic scale of 1 cm on the bottom. When the animal was relaxed, a photo was taken, and then the size measure was obtained with the Infinity image analysis software Analyze Application v. 5.0.0 (Lumenera Co., Ottawa, Canada, 2002–2009). The body length can reach up to 20 cm in C. intestinalis, 13 cm in A. aspersa and 8 cm in S. plicata and is age-related [62]. However, it could also depend on other environmental and physiological conditions, such as nutrient availability, filtration rate and reproductive period.
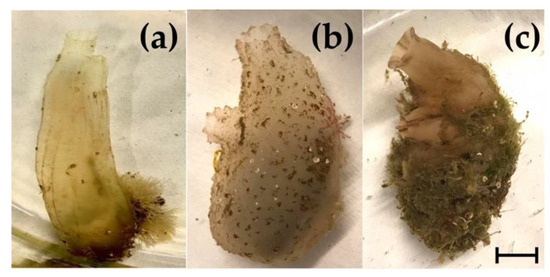
Figure 2.
Solitary ascidians employed in the present study. Vase tunicate, Ciona intestinalis (a); fluted sea squirt, Ascidiella aspersa (b); pleated sea squirt, Styela plicata (c). Relative dimensions of the individuals have been maintained. Bar length: 1 cm.
2.2. Ultrasound Devices
For this experiment, two types of continuous frequencies were used: (i) 30 kHz (90% amplitude), produced by a Sonoplus sonicator mini20 (Bandelin Electronic GmbH & Co. KG, Berlin, Germany); (ii) 35 kHz (90% amplitude), produced by a GN20Pro ultrasonic cleaning rod (Shenzhen Baryon Acoustic Technology Co, Ltd., Shenzhen, China).
The frequency of the ultrasound devices was validated with a hydrophone HydroMoth 1.0.0 (Open Acoustic Devices, https://www.openacousticdevices.info, accessed on 3 April 2023) with a sensitivity of 94 dB SPL@1 kHz. The recorded main frequencies of operation for the Sonoplus sonicator mini20 and the GN20Pro ultrasonic cleaning rod were 29.4 kHz and 35.1 kHz, respectively (Figure 3). Sonoplus sonicator mini20 and GN20Pro ultrasonic cleaning rod produced an environmental noise of 71.1 and 74.8 dB, respectively, which was measured with a Curconsa SL720 portable sound level phonometer (Shenzhen Putest Instrument Co., Ltd., Shenzhen, China).
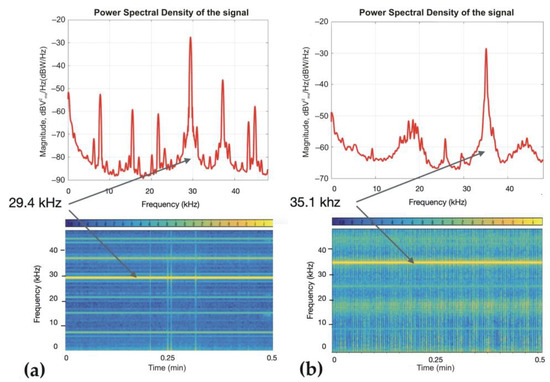
Figure 3.
Characteristics of ultrasound exposures produced by two devices. (a) Power spectral density and spectrogram of the Sonoplus sonicator mini20 recording over time. It is a continuous signal with mixed frequency content but with a main excited frequency at 29.4 kHz. (b) Power spectral density and spectrogram of the GN20Pro ultrasonic cleaning rod recording over time. It is a continuous signal with a main frequency content of 35.1 kHz.
2.3. Experimental Setup
The experiments were conducted in a soundproof thermostatic chamber with a low basal noise range (30–35 dB).
The experimental apparatus (Figure 4) consisted of a sound-absorbing polystyrene tank with a wall thickness of 40 mm to avoid sound wave diffraction from the walls and external vibrational interference. The tank was filled with 10 L FSW (temperature 12 °C, salinity 35‰ and density 1.0375 g cm−3) in the absence of both oxygenators and food particles. Each individual was placed inside a beaker to keep the body in a vertical position with both siphons clearly visible and facing upwards.

Figure 4.
A visual representation of the ultrasound tank set up. The walls of the tank were composed of polystyrene (40 mm in thickness). The tank was filled with 10 L seawater. Externally, mobile support allowed for the tip of the device to be held in place. The tank housed an ascidian individual placed in a glass beaker at 150 mm from the tip of the device.
Exposure to ultrasound occurred at a distance of 150 mm from the tip of the transducer, which was submerged at 20 mm and 12 mm for Sonoplus and GN20Pro, respectively.
The number of SqRs and CrRs after exposure to ultrasound was evaluated for 5 min after exposure to either 30 or 35 kHz for 10 s. For each frequency, 20 individuals per species were considered. At the beginning of the experiments, the ascidians were kept quiet until both siphons continuously stayed open. The oral siphon was then gently touched with a dissection needle, with the time of rapid closing and subsequent opening responses of both siphons (SqR) noted to confirm the animal reactivity. Only animals that promptly reacted to this stimulus were considered. The exposure to ultrasound began after waiting for the siphons to reopen, and the observations of responses were carried out for 5 min. During this time range, the number of SqR and CrR events was collected.
In two additional series of experimental assays, eight individuals per species were continuously exposed to 30 kHz for 5 h. Before the exposure trial, background siphon behaviour in the absence of stimuli was recorded for 5 h. The videos were recorded by attaching a GoPro Hero 5 Black digital camera, Full HD 1080p resolution, version 2.70 (GoPro, Inc., San Mateo, CA, USA) to a support in a vertical position over the animals. For each species, four specimens were analysed simultaneously, each one separately kept in an upright position in a 50 or 10 mL beaker. The GoPro was set to timelapse recording mode, taking one photo per second. These videos allowed us to measure the average frequency of SqRs in the total absence of external stimuli, including not only accidental impacts on the apparatus and the consequent movement of water but also the reflex responses due to suspended particulates that could touch the sensory cells.
During the first experimental exposure to ultrasound assays, the response behaviour (e.g., events of SqRs, events of CrRs, long-term opening of the oral siphon and long-term closing or opening of both siphons) was monitored by splitting the observations into 30 min sections and was matched with that of specimens in total absence of external stimuli (control).
In the second experimental exposure assays, a collection of immediate responses to a mechanical stimulus (e.g., long-term closing of both siphons, weak and slow SqR events and long-term opening of both siphons) was performed by touching the oral siphon with a dissection needle every 30 min during 5 h of continuous exposure at 30 kHz and was matched with that of unexposed specimens (control).
In all experiments, the temperature was constantly monitored and kept at 12 ± 1 °C for the entire duration of the trials.
2.4. Statistical Analysis
Regarding the first series of experiments, each experiment was replicated with twenty individuals per species (n = 20), and the results are expressed as the averages ± SD. The statistical analysis of the number and type of responses (SqR and CrR) collected for 5 min after exposure for 10 s was performed considering the individual size clustering (i.e., size classes) using the statistical program R Software Environment, version 3.5.3 [64]. The threshold for statistical significance was set at p < 0.05. Differences in the responses to the ultrasonic frequencies among size classes were investigated using nonmetric multidimensional scaling (NMDS) to graphically show differences or similarities among size classes. This allowed us to obtain, for all species tested, a distribution in three size classes with good scores of stress values (<0.2) [65], i.e., minor class (small individuals), intermediate class (medium-sized individuals) and major class (large individuals). Comparisons of SqRs and CrRs among size classes within the single exposure and within the same frequency were analysed with a glm with Poisson distribution (calculating p-values by using the likelihood ratio chi-square), followed by a post hoc test performed using the emmeans function (package emmeans [66]) to assess differences among the classes.
For the third experiment carried out with eight individuals per species (n = 8), to check for a statistical difference between control and treatments in the behavioural responses investigated, a permutational analysis of variance (PERMANOVA, with 9999 permutations, applying square root of dissimilarities, using the adonis2 function of the vegan package [67]) was performed on the entire dataset.
3. Results
3.1. Siphon Responses in the Absence of Stimuli
Ascidians in normal conditions keep their siphons open for a long time to favour filter-feeding activity. An SqR occasionally occurs, which consists of the rapid closure of both siphons, followed by equally rapid reopening and water ejection to promote the expulsion of faecal pellets from the atrial siphon and clean the branchial pharynx through the oral siphon. In the case of the observations carried out for 5 h to establish the background siphon behaviour in the absence of stimuli, the average frequency of a recorded SqR was 2.94 per h ± 0.09 for C. intestinalis, 2.7 per h ± 0.11 for A. aspersa and 5.7 per h ± 0.06 for S. plicata.
3.2. Siphon Responses after Exposure to a Brief Ultrasound Input
In these experiments, the number of SqR and CrR events was collected within a period of 5 min after a 10 s ultrasound input. Within the 30 kHz frequency assay, no differences were found among species in either the SqR (Chisq = 4.426, Df = 2, p-value = 0.1094) or the CrR (Chisq = 5.0582, Df = 2, p-value = 0.07973). Within the 35 kHz frequency assay, no differences were found among species in the SqR (Chisq = 3.398, Df = 2, p-value = 0.1829), whereas significant differences were highlighted in the CrR (Chisq = 7.4175, Df = 2, p-value = 0.02451).
3.2.1. Ciona intestinalis
On the basis of the distribution of responses to ultrasound with an NMDS analysis (Figure 5a,c), for both frequencies considered, a clear subdivision into three size classes of the treated individuals occurred, corresponding to minor (2–6 cm), intermediate (6–7.5 cm) and major (7.5–10 cm) classes.
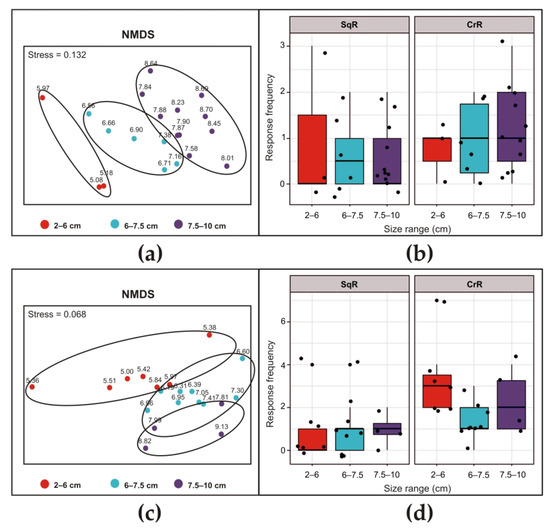
Figure 5.
Siphon responses of Ciona intestinalis after exposure to ultrasonic input at 30 kHz (a,b) and 35 kHz (c,d) for 10 s. NMDS of the average frequency of responses of 20 individuals showing differences among the three size classes (a,c). Boxplots showing the differences in sea squirt response frequency (SqR on the left and CrR on the right) among the three size classes of animals exposed to ultrasound frequencies. Above the boxplot, each point represents the value of a single specimen (b,d).
After exposure to the 30 kHz ultrasound (Figure 5b), both the SqR and CrR were not dependent on the size of the animals (Chisq = 0.68301, Df = 2, p-value = 0.7107 for SqR and Chisq = 0.67236, Df = 2, p-value = 0.7145 for CrR). After exposure to a 35 kHz ultrasound (Figure 5d), the SqR was not dependent on the size of the animals (Chisq = 0.2574, Df = 2; p-value = 0.8792). Conversely, in the case of the CrR, the size of the individuals significantly influenced the response (Chisq = 6.8377, Df = 2, p-value = 0.03275), with a significant difference between the smaller and intermediate group (emmeans post hoc, p-value = 0.0304).
3.2.2. Ascidiella aspersa
The NMDS analysis of the distribution of responses to stimuli with ultrasound led to three size classes. Because different animals were used and size uniformity could not be achieved, the three size classes were slightly different for the two exposure frequencies (Figure 6a,c). In particular, the dimensional ranges were determined to be 2–6 cm at 30 kHz and 2–5 cm at 35 kHz for the minor class, 6–8 cm at 30 kHz and 5–6.5 cm at 35 kHz for the intermediate class, and 8–10 cm at 30 kHz and 6.5–10 cm at 35 kHz for the major class. At 30 kHz (Figure 6b), the size of the individuals significantly influenced the SqR (Chisq = 6.2493, Df = 2, p-value = 0.04395). However, the post hoc analysis did not detect differences among the three groups. Similarly, in the case of CrR, a significant size-dependent effect was found (Chisq = 7.606, Df = 2, p-value = 0.0223), but the post hoc analysis following the glm did not reveal differences among the three groups. At 35 kHz (Figure 6d), the size of the individuals significantly influenced the SqR (Chisq = 7.373, Df = 2, p-value = 0.02506), although the post hoc analysis did not detect differences among the three groups. The CrR was not dependent on the size of the animals (Chisq = 1.2289, Df = 2; p-value = 0.5409), but it was significantly different compared to that of S. plicata (emmeans post hoc, p-value = 0.0222).
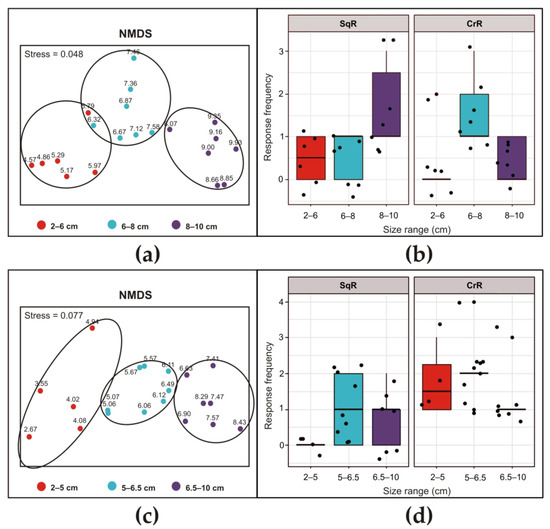
Figure 6.
Siphon responses of Ascidiella aspersa after exposure to ultrasonic input at 30 kHz (a,b) and 35 kHz (c,d) for 10 s. For details, see the legend in Figure 5.
3.2.3. Styela plicata
The size classes determined via the NMDS analysis (Figure 7a,c) were the same for the two exposure frequencies, i.e., minor (2–5 cm), intermediate (5–7 cm) and major (7–10 cm).
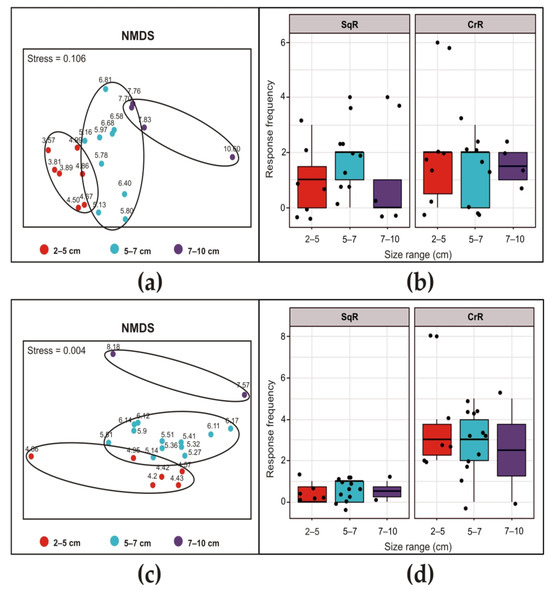
Figure 7.
Siphon responses of Styela plicata after exposure to an ultrasonic input at 30 kHz (a,b) and 35 kHz (c,d) for 10 s. For details, see the legend in Figure 5.
At 30 kHz (Figure 7b) and 35 kHz (Figure 7d), both the SqR and CrR were not dependent on the size of the animals (Chisq = 1.6818, Df = 2, p-value = 0.4313 for SqR and Chisq = 0.69316, Df = 2, p-value = 0.7071 for CrR at 30 kHz; Chisq = 0.53625, Df = 2, p-value = 0.7648 for SqR and Chisq = 1.2635, Df = 2, p-value = 0.5316 for CrR at 35 kHz).
3.3. Siphon Responses during Continuous Ultrasound Exposure
During the 5 h of exposure to the 30 kHz ultrasound, the responses were reported by splitting the observations into ranges of 30 min to highlight changes across time. Differences among species were observed throughout the exposure. The most remarkable behaviour was the long-term opening of both siphons, which occurred after 150 min of exposure in all species.
C. intestinalis was the species with the highest number of responses (n = 304) during the 5 h of exposure to ultrasound (Figure 8a). The main type of response was the long-term opening of the oral siphon. The responses of this type were almost double (n = 158) those observed in the other two species (n = 75 in A. aspersa and n = 81 in S. plicata). Moreover, the number of long-term closures of both siphons was a behavioural response that, in C. intestinalis, was approximately three times greater (n = 52) than in A. aspersa (n = 18) and S. plicata (n = 15). On the other hand, the episodes of the long-term opening of both siphons were similar to those observed in S. plicata (n = 19 and n = 21 in C. intestinalis and S. plicata, respectively) but were approximately half of those of A. aspersa (n = 48); A. aspersa proved to be the species with the greatest number of this type of response (Figure 8b). Unlike other species, the episodes of long-term closure of both siphons were observed in C. intestinalis throughout the exposure time and were very numerous (n = 52) (Figure 8a). S. plicata showed a greater number of episodes of CrR (n = 14) than C. intestinalis and A. aspersa (n = 5) during the whole period of ultrasound exposure (Figure 8c).
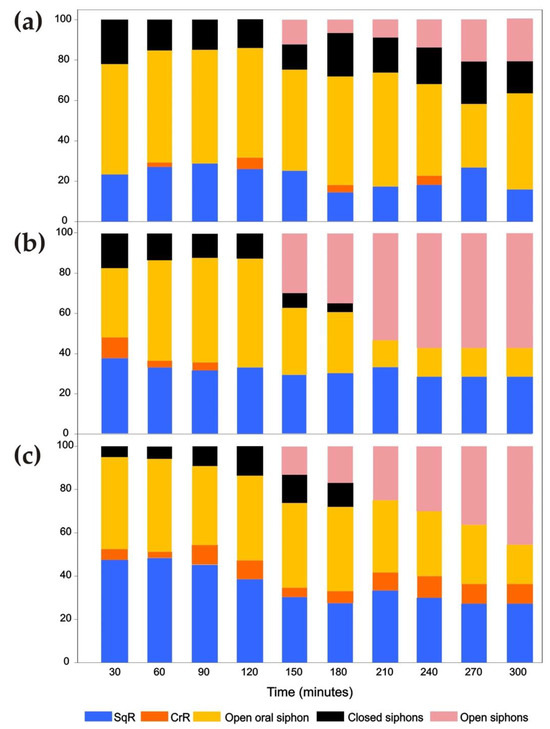
Figure 8.
Percent of responses observed every 30 min during 5 h of exposure to 30 kHz ultrasound in Ciona intestinalis (a), Ascidiella aspersa (b) and Styela plicata (c). The average results for eight specimens of each species are shown.
3.4. Responses of the Oral Siphon to a Mechanical Stimulus during Continuous Ultrasound Exposure
C. intestinalis, A. aspersa and S. plicata responded in a similar way to the mechanical stimuli carried out by touching the rim of the oral siphon with a needle every 30 min within 5 h of ultrasound exposure (Figure 9). The behavioural modes were only directly annotated and not recorded on the camera to time the response speed. For all species, the PERMANOVA revealed a statistically significant effect of the condition of exposure (C. intestinalis: F1,18 = 49.028, R2 = 0.73146, p-value < 0.001; A. aspersa: F1,18 = 64.395, R2 = 0.78154, p-value < 0.001; S. plicata: F1,18 = 64.395, R2 = 0.69653, p-value < 0.001).
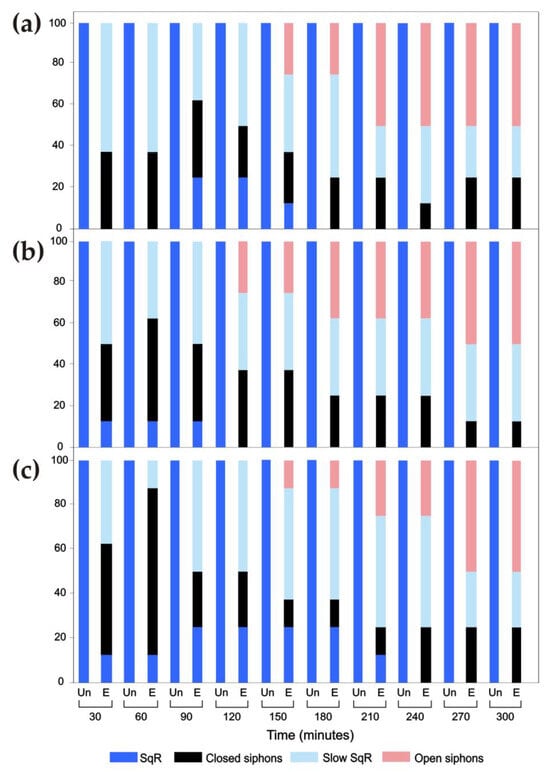
Figure 9.
Percent value of responses to a mechanical stimulus of the oral siphon observed every 30 min for 5 h in normal conditions (Un, unexposed specimens) and during continuous exposure to 30 kHz ultrasound (E, exposed specimens) in Ciona intestinalis (a), Ascidiella aspersa (b) and Styela plicata (c). The average results of eight specimens for each species are shown. Note that responses in normal conditions are SqRs only (100%).
In the first 30 min for all species, the episodes of SqR occurred more slowly than in the controls not exposed to ultrasound. Approximately the same number (~20) of the prolonged closing responses of both siphons was detected. Beginning from 150 min, siphons were observed to remain open despite the mechanical stimulus, and this response prevailed over the other types of response. This lack of response to the stimulus was mostly presented by A. aspersa beginning at 120 min.
4. Discussion
This study represents the first to investigate behavioural effects in adult ascidians after exposure to the frequencies created by new-generation ultrasonic antifouling systems. The siphon responses that were observed in the short-term ultrasonic exposure experiment at frequencies of 30 and 35 kHz were both SqRs and CrRs. SqRs are behavioural responses that occur even at rest, but in the case of sound stimuli, they are significantly more numerous and intense and are explained by the stimulation of the epidermal sensory cells of the rim of the oral siphon, probably due to acoustic pressure, as observed in the case of noise [44].
CrRs were the most interesting responses from an acoustic point of view since they are caused by the direct stimulation of the coronal organ by sound vibrations. All species tested showed high sensitivity, especially at the highest frequency. Regarding the distribution based on the size class, the highest number of CrRs was observed at 35 kHz in the minor class of C. intestinalis. It is possible that juvenile forms have a greater number of sensory cells in the coronal organ, similarly to what occurs in the inner ear of mammals, which tends to lose sensory cells and consequently hearing ability with age [68].
In the experiments in which individuals were continuously exposed to 30 kHz for 5 h, a variety of behavioural responses occurred. In addition to SqRs and CrRs, events of the long-term opening of the oral siphon and the long-term closing or opening of both siphons were observed. Particularly in A. aspersa and S. plicata, a greater number of episodes of siphon closure were more concentrated in the first part of the exposure. Then, a new behaviour appeared from two and a half hours onwards, represented by the long-term opening of both siphons. Unlike the other two species, in S. plicata, more episodes of CrR during 5 h of exposure were observed, confirming the previous behavioural responses observed after short exposure. This feature of prevailing CrRs may reflect the difference in the extent of the coronal organ. S. plicata has the largest number of tentacles, up to 60, compared to 25–45 in C. intestinalis [69] and 16–31 in A. aspersa [70].
The long-term opening of the siphons observed during the continuous exposure to the 30 kHz ultrasound could be interpreted as stimulus-specific habituation [71]. However, by changing the nature of the stimulus, such as a physical contact (i.e., the mechanical touch of the oral siphon), a new response did not occur. Both the siphon and body stopped reacting, and the SqRs disappeared. This alteration in behavioural response could be due to muscle fatigue, which functions similarly to chronic treatment, as a generation of stress caused, in this case, by continuous siphon responses to ultrasound stimuli. Somatic, smooth muscles are particularly abundant around the siphons, especially the oral siphon, where they form a siphonal sphincter [50] innervated by cholinergic nerves [72]. Keeping the siphons open or the occurrence of a limited closing capacity could make the animals more vulnerable to predators or parasites and to branchial damage caused by large food particles that enter the pharynx with the water flow and cannot be expelled by the ejection reaction [60,73]. Thus, the constant opening of siphons has potentially negative consequences on filter-feeding activity and survival. Recently, in other filter-feeding organisms such as bivalves, valve closure and reduced gill function have been considered protective responses when they are stressed by exposure to pollutants [74] and anthropogenic noise [21,75]. This behaviour had the positive result of less pollutant accumulation but the negative outcome of reductions in feeding and growth [76].
5. Conclusions
Increasing attention has recently been given to marine noise pollution with regard to both its sources and its damage to marine mammals [77]. Nevertheless, knowledge of the acoustic sensitivity of various marine species, fish and invertebrates to the anthropogenic sound frequencies that are released into the environment is essential to better understand the effects that noise pollution might have on marine organisms. Although the responses of marine organisms to sound are complex, appearing to be both species- and sound-specific, the continuous emission of ultrasound from antifouling devices over time such as that occurring in harbours and marinas—where ships and boats are moored for a long time—could cause stress in local benthic animals with unpredictable negative consequences on populations and ecosystems. From the results obtained in this preliminary study based on frequencies used by acoustic antifouling systems, it has been possible to observe for the first time how ascidians are able to perceive ultrasound. Various behavioural responses of their siphons, varying according to species and the size of the individuals, were observed. The most important features appear to be the involvement of the coronal organ, which is based on axonless hair cells resembling those of the vertebrate acusticolateralis system, and the establishment of stress conditions able to cause muscle fatigue, both of which warrant further in-depth studies.
Author Contributions
Conceptualisation, resources and supervision, F.C.; investigation and data curation, R.V.; formal analysis, D.A.; methodology and writing—original draft preparation and editing, D.A., J.B., F.C. and R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Italian MIUR (DOR 2021) to F.C. and by a funding agreement between P. Zarantonello’s “Resimix s.r.l.” (Brendola, Vicenza, Italy, http://www.resimix.com/it/ (accessed on 20 May 2023)) and the University of Padova, Italy for a Ph.D. fellowship in Biosciences to R.V. (Rep. #1488, Prot. #186788, 6 May 2019).
Institutional Review Board Statement
The authors followed all applicable international, national and/or institutional guidelines for the care and use of animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Acknowledgments
The authors wish to thank Alberto Citton for research assistance and skilful technical help in this study and Andrea Sambo and Cristina Breggion, technicians at the ‘Umberto D’Ancona’ Hydrobiological Station of Chioggia (Venice, Italy), for assistance in ascidian collection and boat driving.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pavan, G. Bioacustica e ecologia acustica. In Acustica. Fondamenti e Applicazioni; Spagnolo, R., Ed.; UTET Università: Turin, Italy, 2015; pp. 803–828. [Google Scholar]
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Erbe, C.; Gordon, T.A.C.; Halpern, B.S.; Harding, H.R.; et al. The soundscape of the Anthropocene Ocean. Science 2021, 371, eaba4658. [Google Scholar] [CrossRef] [PubMed]
- William, R.; Wright, A.J.; Ashe, E.; Blight, L.K.; Bruintjes, R.; Canessa, R.; Clark, C.W.; Cullis-Suzuki, S.; Dakin, D.T.; Erbe, C.; et al. Impacts of anthropogenic noise on marine life: Publication patterns, new discoveries, and future directions in research and management. Ocean Coast. Manag. 2015, 115, 17–24. [Google Scholar] [CrossRef]
- Chu, D. Technology evolution and advances in fisheries acoustic. J. Mar. Sci. Technol. 2011, 19, 2. [Google Scholar] [CrossRef]
- Hildebrand, J.A. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 2009, 395, 5–20. [Google Scholar] [CrossRef]
- Erbe, C. Effects of underwater noise on marine mammals. Adv. Exp. Med. Biol. 2012, 730, 17–22. [Google Scholar]
- Southall, B.L.; Bowles, A.E.; Ellison, W.T.; Finneran, J.J.; Gentry, R.L.; Greene, C.R., Jr.; Kastak, D.; Ketten, D.R.; Miller, J.H.; Nachtigall, P.E.; et al. Marine mammal noise-exposure criteria: Initial scientific recommendations. Bioacoustics 2008, 17, 273–275. [Google Scholar] [CrossRef]
- Nowacek, D.P.; Thorne, L.H.; Johnston, D.W.; Tyack, P.L. Responses of cetaceans to anthropogenic noise. Mamm. Rev. 2007, 37, 81–115. [Google Scholar] [CrossRef]
- Weilgart, L.S. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 2007, 85, 1091–1116. [Google Scholar] [CrossRef]
- Erbe, C.; Dunlop, R.; Dolman, S. Effects of noise on marine mammals. In Effects of Anthropogenic Noise on Animals; Slabbekoorn, H., Dooling, R., Popper, A., Fay, R., Eds.; Springer: New York, NY, USA, 2018; Volume 66, pp. 277–309. [Google Scholar]
- Ketten, D.R. Marine mammal auditory systems: A summary of audiometric and anatomical data and implications for underwater acoustic impacts. Polarforschung 2004, 72, 79–92. [Google Scholar]
- Popper, A.N. Effects of Mid- and High-Frequency Sonars on Fish; Naval Undersea Warfare Center Division (U.S.A.): Providence, RI, USA, 2008; Contract No. N66604-07M-6056. [Google Scholar]
- Kunc, H.P.; McLaughlin, K.E.; Schmidt, R. Aquatic noise pollution: Implications for individuals, populations, and ecosystems. Proc. R. Soc. B-Biol. Sci. 2016, 283, 20160839. [Google Scholar] [CrossRef]
- Pichegru, L.; Nyengera, R.; McInnes, A.M.; Pistorius, P. Avoidance of seismic survey activities by penguins. Sci. Rep. 2017, 7, 16305. [Google Scholar] [CrossRef] [PubMed]
- Popper, A.N.; Hawkins, A.D. An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J. Fish Biol. 2019, 94, 692–713. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, E.; Pierson, P.; Di Iorio, L.; Calò, A.; Cottalorda, J.M.; Derijard, B.; Guidetti, P. Effects of marine noise pollution on Mediterranean fishes and invertebrates: A review. Mar. Poll. Bull. 2020, 159, 111450. [Google Scholar] [CrossRef] [PubMed]
- Morely, E.L.; Jones, G.; Radford, A.N. The importance of invertebrates when considering the impacts of anthropogenic noise. Proc. R. Soc. B-Biol. Sci. 2014, 281, 20132683. [Google Scholar] [CrossRef] [PubMed]
- Solé, M.; Kaifu, K.; Mooney, T.A.; Nedelec, S.L.; Olivier, F.; Radford, A.N.; Vazzana, M.; Wale, M.A.; Semmens, J.M.; Simpson, S.D.; et al. Marine invertebrates and noise. Front. Mar. Sci. 2023, 10, 1129057. [Google Scholar] [CrossRef]
- Lucke, K.; Popper, A.N.; Hawkins, A.D.; Akamatsu, T.; André, M.; Branstetter, B.K.; Lammers, M.; Radford, C.A.; Stansbury, A.L.; Aran Mooney, T. Auditory sensitivity in aquatic animals. J. Acoust. Soc. Am. 2016, 139, 3097–3101. [Google Scholar] [CrossRef] [PubMed]
- Wale, M.A.; Briers, R.A.; Diele, K. Marine invertebrate anthropogenic noise research: Trends in methods and future directions. Mar. Poll. Bull. 2021, 173, 112958. [Google Scholar] [CrossRef]
- Charifi, M.; Sow, M.; Ciret, P.; Benomar, S.; Massabuau, J.C. The sense of hearing in the pacific oyster, Magallana gigas. PLoS ONE 2017, 12, e0185353. [Google Scholar] [CrossRef]
- Popper, A.N.; Salmon, M.; Horch, K.W. Acoustic detection and communication by decapod crustaceans. J. Comp. Physiol. 2001, 187A, 83–89. [Google Scholar] [CrossRef]
- de Soto, N.A. Peer-reviewed studies on the effects of anthropogenic noise on marine invertebrates: From scallop larvae to giant squid. In The Effects of Noise on Aquatic Life II; Popper, A., Hawkins, A., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 875, pp. 17–26. [Google Scholar]
- Mooney, T.A.; Samson, J.E.; Schlunk, A.D.; Zacarias, S. Loudness-dependent behavioural responses and habituation to sound by the longfin squid (Doryteuthis pealeii). J. Comp. Physiol. 2016, 202A, 489–501. [Google Scholar] [CrossRef]
- Fewtrell, J.L.; McCauley, R.D. Impact of air gun noise on the behaviour of marine fish and squid. Mar. Poll. Bull. 2012, 64, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.R.; Mann, D.A.; Kimbro, D.L. Predatory fish sounds can alter crab foraging behaviour and influence bivalve abundance. Proc. R. Soc. B-Biol. Sci. 2014, 281, 20140715. [Google Scholar] [CrossRef] [PubMed]
- Solan, M.; Hauton, C.; Godbold, J.A.; Wood, C.L.; Leighton, T.G.; White, P. Anthropogenic sources of underwater sound can modify how sediment-dwelling invertebrates mediate ecosystem properties. Sci. Rep. 2016, 6, 20540. [Google Scholar] [CrossRef]
- Lillis, A.; Eggleston, D.B.; Bohnenstiehl, D.R. Oyster larvae settle in response to habitat-associated underwater sounds. PLoS ONE 2013, 8, e79337. [Google Scholar] [CrossRef]
- Gittenberger, A.; van Stelt, R.C. Artificial structures in harbours and their associated ascidian fauna. Aquat. Invasions 2011, 6, 413–420. [Google Scholar] [CrossRef]
- Townsin, R.L. The ship hull fouling penalty. Biofouling 2003, 19, 9–15. [Google Scholar] [CrossRef]
- Mazue, G.; Viennet, R.; Hihn, J.Y.; Carpentier, L.; Devidal, P.; Albaina, I. Large-scale ultrasonic cleaning system: Design of a multi-transducer device for boat cleaning (20 kHz). Ultrason. Sonochem. 2011, 18, 895–900. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Choi, C.H.; Scardino, A.J.; Dylejko, P.G.; Fletcher, L.E.; Juniper, R. The effect of vibration frequency and amplitude on biofouling deterrence. Biofouling 2013, 29, 195–202. [Google Scholar] [CrossRef]
- Taylor, A.; Rigby, G.; Gollasch, S.; Voight, M.; Hallegraeff, G.M.; McCollin, T.; Jelmert, A. Preventive treatment and control techniques for ballast water. In Invasive Aquatic Species of Europe. Distribution, Impacts and Management; Leppakoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 484–507. [Google Scholar]
- Legg, M.; Yücel, M.K.; Garcia del Carrelan, I.; Kappatos, V.; Selcuk, C.; Gan, T.H. Acoustic methods for biofouling control: A review. Ocean Eng. 2015, 103, 237–247. [Google Scholar] [CrossRef]
- Guo, S.F.; Lee, H.P.; Chaw, K.C.; Miklas, J.; Teo, S.L.M.; Dickinson, G.H.; Birch, W.R.; Khoo, B.C. Effect of ultrasound on cyprids and juvenile barnacles. Biofouling 2011, 27, 185–192. [Google Scholar] [CrossRef]
- Guo, S.; Lee, H.P.; Khoo, B.C. Inhibitory effect of ultrasound on barnacle (Amphibalanus amphitrite) cyprid settlement. J. Exp. Mar. Biol. Ecol. 2011, 409, 253–258. [Google Scholar] [CrossRef]
- Guo, S.; Lee, H.P.; Teo, S.L.M.; Khoo, B.C. Inhibition of barnacle cyprid settlement using low frequency and intensity ultrasound. Biofouling 2012, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Seth, N.; Chakravarty, P.; Khandeparker, L.; Anil, A.C.; Pandit, A.B. Quantification of the energy required for the destruction of Balanus amphitrite larva by ultrasonic treatment. J. Mar. Biol. Assoc. UK 2010, 90, 1475–1482. [Google Scholar] [CrossRef]
- Kitamura, H.; Takahashi, K.; Kanamaru, D. Inhibitory effect of ultrasonic waves on the larval settlement of the barnacle, Balanus amphitrite in the laboratory. Mar. Fouling 1995, 12, 9–13. [Google Scholar] [CrossRef]
- Simpson, S.D.; Radford, A.N.; Tickle, E.J.; Meekan, M.G.; Jeffs, A.G. Adaptive avoidance of reef noise. PLoS ONE 2011, 6, e16625. [Google Scholar] [CrossRef]
- Weilgart, L.S. The Impact of Ocean Noise Pollution on Fish and Invertebrates; Report by Ocean Care & Dalhousie University; Ocean Care: Wädenswil, Switzerland, 2018; pp. 1–34. [Google Scholar]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 210–244. [Google Scholar] [CrossRef]
- White, K.N.; Ambrosio, L.J.; Edwards, C. Anthropogenic sound in the sea: Are ascidians affected? Gulf Caribb. Res. 2021, 32, 1–7. [Google Scholar] [CrossRef]
- Hecht, S. The physiology of the Ascidia atra Leseuer. I. General physiology. J. Exp. Zool. 1918, 25, 229–259. [Google Scholar] [CrossRef]
- Hecht, S. The physiology of the Ascidia atra Leseuer. II. Sensory physiology. J. Exp. Zool. 1918, 25, 261–299. [Google Scholar] [CrossRef]
- Day, E.C. The physiology of the nervous system of the tunicates. I: The relation of the nerve ganglion to sensory response. J. Exp. Zool. 1919, 28, 307–335. [Google Scholar] [CrossRef]
- Manni, L.; Pennati, R. Tunicata. In Structure and Evolution of Invertebrate Nervous Systems; Schmidt-Rhaesa, A., Harzsch, S., Purschke, G., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 699–718. [Google Scholar]
- Fedele, M. Sulla organizzazione e le caratteristiche funzionali dell’attività nervosa dei Tunicati. I. Ricerche sul sistema nervoso periferico degli Ascidiacea. In Atti della Reale Accademia dei Lincei Rendiconti; Classe di Scienze Fisiche Matematiche e Naturali; Accademia Nazionale dei Lincei: Rome, Italy, 1923; Volume 32, pp. 98–102. [Google Scholar]
- Millar, R.H. Ciona. In L.M.B.C. Memoirs on Typical British Marine Plants and Animals; University Press of Liverpool: Liverpool, UK, 1953; Volume 35, pp. 1–122. [Google Scholar]
- Mackie, G.O.; Wyeth, R.C. Conduction and coordination in deganglionated ascidians. Can. J. Zool. 2000, 78, 1626–1639. [Google Scholar] [CrossRef]
- Burighel, P.; Lane, N.J.; Gasparini, F.; Tiozzo, S.; Zaniolo, G.; Carnevali, M.D.; Manni, L. Novel, secondary sensory cell organ in ascidians: In search of the ancestor of the vertebrate lateral line. J. Comp. Neurol. 2003, 461, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Burighel, P.; Caicci, F.; Manni, L. Hair cells in non-vertebrate models: Lower chordates and molluscs. Hear. Res. 2011, 273, 14–24. [Google Scholar] [CrossRef]
- Manni, L.; Caicci, F.; Gasparini, F.; Zaniolo, G.; Burighel, P. Hair cells in ascidians and the evolution of lateral line placodes. Evol. Dev. 2004, 6, 379–381. [Google Scholar] [CrossRef]
- Manni, L.; Lane, N.J.; Joly, J.S.; Gasparini, F.; Tiozzo, S.; Caicci, F.; Zaniolo, G.; Burighel, P. Neurogenic and non-neurogenic placodes in ascidians. J. Exp. Zool. B Mol. Dev. Evol. 2004, 302, 483–504. [Google Scholar] [CrossRef]
- Manni, L.; Mackie, G.O.; Caicci, F.; Zaniolo, G.; Burighel, P. Coronal organ of ascidians and the evolutionary significance of secondary sensory cells in chordates. J. Com. Neurol. 2006, 495, 363–373. [Google Scholar] [CrossRef]
- Mackie, G.O.; Burighel, P. The nervous system in adult tunicates: Current research directions. Can. J. Zool. 2005, 83, 151–183. [Google Scholar] [CrossRef]
- Rigon, F.; Stach, T.; Caicci, F.; Gasparini, F.; Burighel, P.; Manni, L. Evolutionary diversification of secondary mechanoreceptor cells in tunicata. BMC Evol. Biol. 2013, 13, 112. [Google Scholar] [CrossRef]
- Manni, L.; Anselmi, C.; Burighel, P.; Martini, M.; Gasparini, F. Differentiation and induced sensorial alteration of the coronal organ in the asexual life of a tunicate. Integr. Comp. Biol. 2018, 58, 317–328. [Google Scholar] [CrossRef]
- Mackie, G.O.; Burighel, P.; Caicci, F.; Manni, L. Innervation of ascidian siphons and their responses to stimulation. Can. J. Zool. 2006, 84, 1146–1162. [Google Scholar] [CrossRef]
- Caicci, F.; Degasperi, V.; Gasparini, F.; Zaniolo, G.; Del Favero, M.; Burighel, P.; Manni, L. Variability of hair cells in the coronal organ of ascidians (Chordata, Tunicata). Can. J. Zool. 2010, 88, 567–578. [Google Scholar] [CrossRef]
- Brunetti, R.; Mastrototaro, F. Ascidiacea of the European Waters; Edagricole: Milan, Italy, 2017; pp. 1–430. [Google Scholar]
- Hotta, K.; Dauga, D.; Manni, L. The ontology of the anatomy and development of the solitary ascidian Ciona: The swimming larva and its metamorphosis. Sci. Rep. 2020, 10, 17916. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 October 2019).
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.8.5. 2023. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 6 March 2023).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 2 June 2022).
- Paplou, V.; Schubert, N.; Pyott, S.J. Age-related changes in the cochlea and vestibule: Shared patterns and processes. Front. Neurosci. 2021, 15, 680856. [Google Scholar] [CrossRef]
- Boon, J.R. The ascidians (Tunicata) from Chindo Islands, Korea. Anim. Syst. Evol. Divers. 1995, 11, 125–145. [Google Scholar]
- Tatián, M.; Schwindt, E.; Lagger, C.; Varela, M.M. Colonization of Patagonian harbours (SW Atlantic) by an invasive sea squirt (Chordata, Ascidiacea). Spixiana 2010, 33, 111–117. [Google Scholar]
- Clark, R.B. Habituation of the polychaete Nereis to sudden stimuli. 1. General properties of the habituation process. Anim. Behav. 1960, 8, 82–91. [Google Scholar] [CrossRef]
- Burighel, P.; Sorrentino, M.; Zaniolo, G.; Thorndyke, M.C.; Manni, L. The peripheral nervous system of an ascidian, Botryllus schlosseri, as revealed by cholinesterase activity. Invertebr. Biol. 2001, 120, 185–198. [Google Scholar] [CrossRef]
- Hoyle, G. The response mechanism in ascidians. J. Mar. Biol. Ass. UK 1952, 31, 287–305. [Google Scholar] [CrossRef]
- Charifi, M.; Miserazzi, A.; Sow, M.; Perrigault, M.; Gonzalez, P.; Ciret, P.; Benomar, S.; Massabuau, J.C. Noise pollution limits metal bioaccumulation and growth rate in a filter feeder, the pacific oyster Magallana gigas. PLoS ONE 2018, 13, e0194174. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.; Cheesman, S.; Breithaupt, T.; Elliott, M. Sensitivity of the mussel Mytilus edulis to substrate-borne vibration in relation to anthropogenically generated noise. Mar. Ecol. Prog. Ser. 2015, 538, 185–195. [Google Scholar] [CrossRef]
- Ledoux, T.; Clements, J.C.; Comeau, L.A.; Cervello, G.; Tremblay, R.; Olivier, F.; Chauvaud, L.; Bernier, R.Y.; Lamarre, S.G. Effects of anthropogenic sounds on the behavior and physiology of the Eastern oyster (Crassostrea virginica). Front. Mar. Sci. 2023, 10, 1104526. [Google Scholar] [CrossRef]
- Trickey, J.S.; Cárdenas-Hinojosa, G.; Rojas-Bracho, L.; Schorr, G.S.; Rone, B.K.; Hidalgo-Pla, E.; Rice, A.; Baumann-Pickering, S. Ultrasonic antifouling devices negatively impact Cuvier’s beaked whales near Guadalupe Island, México. Commun. Biol. 2022, 5, 1005. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).