Abstract
The Azov Sea estuaries play an important role in the reproduction of semi-anadromous fish species. Spawning efficiency is closely connected with overgrowing of those species spawning grounds; thus, the objective of the water vegetation research has vital fisheries importance. Thus, the main goal of the research was to develop a machine learning algorithm for the detection of water overgrowth with Phragmites australis based on Sentinel-2 data. The research was conducted based on field botanical and vegetation investigations in 2020–2021 in Soleniy and Chumyanniy firths. Collected field and remote sensing data were processed with the semi-automatic classification plugin for QGIS. For the classification of Azov Sea estuaries, a random forest algorithm was used. The obtained results showed that in 2020 the areas occupied by reeds reached 0.37 km2, while in 2021, they increased to 0.51 km2. There was a high level of Phragmites australis growth in the Soleniy and Chumyanniy firths. The rapid growth of Phragmites australis in the period of 2020–2021, where the area covered by the reed doubled, is primarily attributed to eutrophication. This is due to the nutrient enrichment from agricultural lands located in the northern part of the research area near Novonekrasovskiy village. Additionally, changes in water flows and hydrological conditions can also contribute to the favorable growth of the reed. This can result in a high growth rate of Phragmites australis, which can reach up to 2 m per year and can propagate both through vegetative and sexual means, leading to the formation of large and dense clusters.
1. Introduction
Most biological productivity indicators of the water bodies depend directly on the conditions and quality of the hydrobionts’ habitat areas. Thus, for sustainability and a high quantity of water organisms support, it is important to preserve their environment. Regarding this, littoral biotopes play a crucial role in protecting spawning grounds from wind and water waves. Littoral zones are natural spawning spots for several commercial fish species—zander, bream, pike, perch, and roach [].
The term «overgrowing» usually means the process of appearance and development of vegetation in aquatic areas of water bodies and streams. Moderate overgrowth (to 20% of the water area) has a positive impact on coastal fauna. However, significant speed (under 20% of the water area) leads to the accumulation of dead vegetation masses and a decrease in the dissolved oxygen level, both of which have a negative impact on aquatic animals. The process of overgrowth is usually caused by submerged vegetation (mainly Potamogeton) and air-water vegetation (mainly Phragmites). Among the latter group, the most widespread species is Phragmites australis (Cav.) Trin. ex Steud, whose communities can crowd out the other species [,].
The main indicators of reed conditions are structural characteristics—length, diameter, thicket density, and biomass. These characteristics greatly depend on climatic conditions and habitat features. The speed and peculiarities of water bodies overgrowing with macrophytes are mainly dependent on its shore configuration, types of sediments, absence, and the speed of the currents, waves, and chemical composition of the water. Water bodies overgrowing with macrophytes usually leads to physical, biological, and chemical changes in the water environment and sediments.
The Azov Sea estuaries are shallow water bodies with an average depth of 0.5 to 1 m, located on the eastern coast of the Sea of Azov; most of them have a connection between themselves and the Sea of Azov through a system of channels. In the modern period, there are 770 estuaries with a total area of about 77,700 hectares (from 0.9 to 6.7 thousand hectares). The Azov Sea estuaries can be characterized as a rapidly changing ecosystem, which mainly depends on the hydrological regime (the amount of fresh water and connection with the Sea of Azov) []. Other variables (depth, macrophyte overgrowth, hydrochemical regime, fish parasites, food resources, etc.) depend on the quantity and quality of fresh and seawater inflow. In this ecosystem, inter-annual and intra-annual fluctuations can be observed []. Estuaries are spawning water bodies for semi-anadromous and non-anadromous fish species. They reproduce 80% of zander and all rams, which form the main commercial stocks of the Sea of Azov. In the modern period, about 7.45 billion juvenile rams and pike perch migrate into the Sea of Azov from these estuaries; most of the juveniles (more than 75%) roll down from the estuaries of spawning and farms, in which the conditions for spawning and feeding of early juveniles are regulated []. Aquatic vegetation has a great influence on the reproduction of semi-anadromous fish [].
The first detailed research on the overgrowth of the Azov Sea estuaries with aquatic vegetation was conducted by A. Shekhov [,]. According to his research, the area of the Azov Sea estuaries in the 1970s equaled about 90,000 ha, and the flood zone that borders those estuaries occupied 86,000 ha. The most common «groups» of vegetation spread in this area were as follows:
- Phragmites australis—86,000 ha;
- Potamogeton—58,000 ha;
- Charophyta—5000 ha;
- Myriophyllum spicatum—4400 ha;
- Scirpus litoralis—3500 ha.
Based on the research of E. Tsunikova [], the area of heliophytes overgrowth (mostly by Phragmites australis) is regularly increasing, which leads to a decrease in the open area of the estuaries; in 1930–1988, it decreased almost by 25,000 ha.
One of the important conditions for efficient fish breeding is a water body state, which is related to the quality and quantity of the macrophytes there. For example, for Stizostedion lucioperca (Linnaeus, 1758), the total productivity of the phytomass should not be higher than 10–15 t/ha, while for Rutilus heckeli (Nordmann, 1840), it is 30 t/ha. Overgrowing with aquatic vegetation reduces the productivity and stability of the spawning water bodies and leads to a decrease in accessible breeding areas for commercial fishes []. Present conditions of overgrowth with aquatic vegetation in the Azov Sea estuaries are characterized by an open field, mainly due to hard accessibility, low water levels, siltation, etc. Thus, one of the solutions can become the usage of remote sensing data [].
Remote sensing helps to evaluate an area of overgrowth by aquatic vegetation with high accuracy []. In this case, it is important to determine which area is occupied by heliophytes and which one is occupied by hydrophytes. In estuaries, the degree of overgrowth with helophytes is more than 10%, while the degree of overgrowth with hydrophytes is 30%; in order to remove excess vegetation, it is recommended to introduce a biological ameliorator of grass carp and mow vegetation, followed by removal of hay mowing from water bodies. The area overgrown with these vegetation types is an essential issue in case of recommendations development for the biological and mechanical reclamation of the Azov Sea estuaries.
The first experiments with remote sensing aiming at studying overgrowth by aquatic vegetation in the Azov Sea estuaries were held by Antonenko []. Using a series of Landsat data, with a spatial resolution of 30 m and Normalized Difference vegetation index (NDVI), the authors concluded that the area of most water bodies had decreased. In certain estuaries, the area overgrown by macrophytes varied from 2 to 100% (seasonal and inter-annual). However, the usage of NDVI did not allow the authors to separate helophytes and hydrophytes. Submerged hydrophytes are difficult to decipher on Landsat data, while heliophytes can be easily recognized even on low-resolution remote sensing data using different combinations of bands [,].
The problem of wetlands overgrowth is widespread throughout the world, and many research works address it in an attempt to find an optimal way to detect vegetation that causes overgrowing and leads to a decrease in the economic exploitation of water bodies.
One of the popular ways to detect overgrowing vegetation is using a Normalized Difference vegetation index (NDVI). In [], it was applied to assess the current state and dynamics of the lakes overgrowing in the «Narochansky» National Park (Belarus). For vegetation detection, different remote sensing data were used—Aster (Terra), Landsat-7 (ETM+), IRS(1C/1D), and WorldView-2, which were processed by means of Erdas Imagine, ENVI, eCognition, and ArcGIS software. As a result, they could detect several features of overgrowth:
- Shallow parts of the water bodies that are free from vegetation usually formed near the shoreline and deciphered in summer with maximum reflectance values about ≤450, 590–630 μm and 600–630 μm;
- Underwater vegetation was detected in summer using remote sensing data with maximum reflectance in the near-infrared (NIR) band and near 710 μm.
Another great case in reed detection was presented in [] for the north of the Bohai Sea. Analysis of the reed overgrowth was based on land-use land cover (LULC) methodology using Landsat 5, 7, and 8 images from 1986 to 2018. After downloading and preprocessing, they were atmospherically corrected, surface reflectance was generated, and cloud masking was performed. After reed detection, continuous change detection per pixel was applied based on a random forest classifier. Classification accuracy ranged from 0.89 to 0.94 for a reed field.
Another approach for reed detection is combining SAR and multispectral remote sensing data. As shown in [], this combination was used for mapping wetlands and deep waters of the Mid-Atlantic and Gulf Coast regions of the USA. In this study, Sentinel-1 C-band and Landsat-8 optical/IR imagery were used. The classification was made using a random forest classifier and reached >80% accuracy.
The same approach was also used in [] to find indicators of the expansion and retreat of Phragmites in the Danube Delta. Reed detection was based on NDVI calculation and the «ISO Cluster Unsupervised Classification» tool of ArcGIS software using Landsat 5, 7 multispectral images, and Sentinel-1 SAR data. As a result, radar data from Sentinel-1 images showed more accurate results against Landsat images while implementing reed detection tasks.
Thus, the main goal of the research was to develop a machine learning algorithm for the detection of water overgrowth with Phragmites australis based on Sentinel-2 data. Solving this problem will help by providing a reliable and efficient method for detecting and mapping areas of water overgrowth and aquatic vegetation. This information is useful for a variety of applications, including environmental monitoring, water management, and agriculture.
For example, in environmental monitoring, knowledge of the distribution and extent of aquatic vegetation can provide important information about the health of aquatic ecosystems. This information can be used to inform management decisions and track changes over time. In water management, detecting areas of water overgrowth and aquatic vegetation can help to identify areas where water resources may be at risk due to over-consumption or pollution. In agriculture, the information can be used to support decision-making for crop management and to reduce the risk of water-borne disease.
Overall, the development and validation of machine learning algorithms for the detection of water overgrowth and aquatic vegetation can provide valuable information for a wide range of applications, helping to improve our understanding of aquatic ecosystems and support the effective management of water resources and development of modern methodology for reed detection using multispectral remote sensing data and a machine learning approach based on the previous experience to improve the decision-making process in case of the biological melioration of the overgrown water bodies.
2. Previous Work
At first, the estimation methodology of the area overgrown by heliophytes and hydrophytes using remote sensing data was based on field botanic studies in 2020–2021 in the Kushchevatiy firth (1505 ha), Gorkiy firth (2224 ha), Chumyaniy firth, and Soleniy-1 firth (water bodies of East-Akhtarskiy spawning grounds). For shoreline detection and water body area estimation, maps of “GosGISCenter” were used.
The area overgrown by aquatic vegetation was estimated by means of Sentinel-2 data. To detect the water surface, we used the modified normalized difference water index (mNDWI) [,]. The Normalized Difference Vegetation Index (NDVI) was also applied as a vegetation indicator.
The Normalized Difference Vegetation Index (NDVI) was also used as a vegetation index. For overgrown and transparent estuaries with a depth of 0.8 m or less, an uneven scale with four ranges of NDVI index values was developed to interpret satellite images in order to assess the overgrowth of aquatic vegetation:
- 0 class—from −1.0 to −0.1 (open surfaces, free from vegetation);
- 1 class—from −0.1 to 0.3 (hydrophytes located below the water surface);
- 2 class—from 0.3 to 0.5 (hydrophytes located on the water surface);
- 3 class—from 0.5 to 1.0 (emergent vegetation).
The use of a non-uniform scale with four NDVI classes made it possible to reduce the interclass dispersion of the projective cover of water areas with different overgrowth levels and simplified the classification of estuaries according to the overgrowth level with vegetation to determine the need for reclamation work.
River mouth areas are also subject to seasonal and interannual fluctuations, where it is difficult to determine the “true” water areas. In [], the authors calculate the area of Kulikovsky Bay using NDVI as the sum of the open water surface and the “islands” of vegetation (reed and cattail), but they do not take into account the thickets of macrophytes that grow along the coastline of the estuaries. In modern conditions, the coastal part of almost all estuaries is overgrown mainly with Phragmites australis, occasionally with Typha angustifolia L. and Schoenoplectus litoralis. Helophytes of Chumyaniy firth were represented by only one species, Phragmites litoralis, hydrophytes, Stuckenia pectinata L., Ceratophyllum demersum L., Charophyceae, and Ulothrix zonata (Web. et Mohr.) Kütz.
According to the botanical analysis in the Chumyaniy firth, in spring, the 1st class represents a part free from aquatic vegetation, the 2nd and 3rd classes represent a part occupied by both hydrophytes and reed, and the 4th class represents a part fully occupied by reed. In summer, the water level rose by 23 cm; thus, part of the hydrophytes sank to the bottom and became unavailable for remote sensing, so they were defined as the 1st, 2nd, and 3rd classes represented by hydrophytes and the 4th represented by heliophytes. In autumn, the estuary’s depth decreased by 30 cm compared to the depth in summer, and part of the hydrophytes came onto the water surface and was defined as the 4th class; moreover, most parts of the area overgrown by the reed were identified as the 3rd class due to their color change.
The maximum calculation accuracy of the overgrowth area by hydrophytes using the NDVI index in the Chumyaniy firth was made from images taken at the end of May, during the period of the smallest depth of estuaries and the phase of their active growth; however, in the same period, a part of the reed with dry leaves was identified as hydrophytes. In mid-July, the reed was identified as the third class of NDVI. However, in the same period, in the Chelbass group of estuaries, the water level dropped rapidly; as a result, submerged hydrophytes came to the surface of the water and, like reed, began to correspond to the NDVI 3rd class. Thus, studies in 2020 showed that in estuaries with an unstable hydrological regime, it is difficult to distinguish air-aquatic vegetation from real aquatic vegetation according to the NDVI index. In 2021, special studies were conducted to determine the area of overgrowth of Phragmites australis in the Azov estuaries.
3. Materials and Methods
3.1. Research Area
Estuaries of the Sea of Azov are characterized by significant fluctuations in water horizons both in annual and seasonal aspects []. In the winter–spring period, during the period of entry and spawning of semi-anadromous fish, in recent years, an insufficient amount of fresh water enters the reservoirs of the spawning and growing farms, especially in dry years. In summer, the volume of freshwater that inflows into the estuaries increases. The minimum depths in the estuaries are observed in the autumn–winter period. According to previous research [] data, in May 2020, the average depth in the Chumyaniy firth was 0.76 m, and Soleniy was 0.4 m in late June-early July due to the return of water from rice paddies; the depth increased by 0.23–0.28 m. In May 2021, the average water depth in the Chumyaniy firth at sampling locations increased by 48.7% or 1.13 m; in the summer period, the depth of “model” reservoirs was 38% higher compared to the low-water 2020 year, and seasonal fluctuations in spring–summer in 2021 were 0.17 m.
A high increase in the depth of estuaries in 2021 is associated with an increase in freshwater inflow into them due to an increase in the water content of the Kuban River basin, while in 2020, the water content was −53.5% of the normal values, then in 2021 it reached +0.7% (calculated from the long-term values of water resources for the period 1936–1980). In dry years, the area of overgrowing water bodies with macrophytes reaches 80–90%.
To study Phragmites australis, maps of the FSUE “Gosgiscenter” were used as a cartographic basis, which was used to determine the coastline and area of the studied water bodies. Since the reed grows in the coastal areas of the estuaries, entering their water area to a depth of 0.9 m, there is no visual coastline can be found. Therefore, when determining the area overgrown with helophytes, we used constant “masks” of the estuaries. With an average 1 m depth, even insignificant changes in the inflow amount can lead to sufficient bioecological transformations.
The first steps in vegetation research of the Azov Sea estuaries were made by Shekhov in the mid-twentieth century [,]. According to his research, they include about 103 macrophyte species and can be divided into six different types []. Based on the research of Tsunikova [], the biomass of soft vegetation, including the estuaries and fish spawning grounds, increased 1.5 times from 1960 to 2005 and reached 2550 thousand tons, or 39.6 t/ha, while the area of clear water surface decreased by 500 ha annually.
3.2. Field Data Collection
The research was conducted in June–July, when the estuaries’ depth decreased. In the low-water year of 2020, the field studies were performed in late May–June. Visual investigations were made by a detail-route method with a thorough description of water vegetation types (Figure 1 and Figure 2). Vegetation was collected using frame with 50 × 50 cm size and “Shekhov device”, specially developed for hydrophytes collection in estuaries. Except for vegetation, we also measured water depth, total vegetation biomass, water transparency, and biomass of dominant vegetation species.

Figure 1.
Research area.

Figure 2.
(A) Chumyaniy firth in July 2021, herbage of Phragmites australis. In front «Model» area covered with greed reed in vegetation phase and small areas of tall reeds with yellow stems and panicles. (B) Chumyaniy firth in July 2021, herbage of Phragmites australis. In front «Model» area covered with greed reed in vegetation phase and small areas of tall reeds with yellow stems and panicles. (C) Chumyaniy firth in July 2021, herbage of Phragmites australis. In front «Model» area covered with low-stemmed young reed, high-stemmed reed is visible in the center of the thickets.
In this research, we used water vegetation classification and botanical terms from V. Papchenko, A. Scherbakov, and A. Lapirov [,], and for species determination, A. Zernov [] and M. Gollerbach [] qualifiers.
3.3. Remote Sensing Data
Remote sensing data for the overgrowth assessment were obtained from Sentinel-2 images for the years 2020 and 2021. Images from the Sentinel-2 satellite were derived from the (Copernicus Open Access Hub) website. All Sentinel-2 data were acquired for late spring–summer seasons (May, June, and July). [,,,]. Reference data collection is described in 3.2. Properties of the Sentinel-2 bands are presented in Table 1.

Table 1.
Sentinel-2 bands description.
Collected samples of areas with Phragmites australis and free of it were merged into single geopackage file as a point layer. These points were used as an ROI for spectral signatures extraction from the stacked bands of the Sentinel-2 image.
3.4. Spectral Signatures Extraction
Spectral signatures can be defined as a response pattern that characterizes optical and electromagnetic properties of different materials on the Earth’s surface. In the GIS context, they can be extracted from different spectral bands of remote sensing data and calculated through the pixel values below each region of interest (ROI). ROI represents areas or points with known land cover class or object. As a result, extraction of pixel values in different bands allows for building a spectral signature plot to represent uniqueness or similarity of each object []. In this research, digitized reed areas collected from the field research were used.
3.5. Accuracy Assessment
As a measure of accuracy, «Intersection over Union» (IoU) was used. IoU is a measure that represents overlapping between true and predicted object areas [,]. Usually, for object detection tasks, it is necessary to calculate accuracy and response using IoU. If IoUpredicted > IoUthreshold, then prediction is classified as «true positive», and if IoUpredicted > IoUthreshold, prediction is classified as «false-positive».
Calculation of IoU can be represented as follows:
Area of overlap is an area between predicted ROI and ROI, collected from field research, while area of union is an area encompassed by them.
AoI helps to calculate other accuracy metrics to evaluate prediction models. To evaluate prediction model in this paper, we used precision measure. It is a measure of how many positive predictions are true (true positive) and is calculated as follows:
3.6. Reed Classification
In this study, a random forest classifier was used. Proposed by Breiman [], it showed good possibilities in image classification tasks [,,]. Random forest algorithm is generally a decision tree classifier with training parameters: number of trees in forest and number of random variables in each tree.
Breiman [] defines random forest as a classifier consisting of tree groups , where —independent equally distributed random vectors, where each tree contributes single vote in definition of class x.
Processing of random forest algorithm can be described as follows:
Let training dataset consist of N samples, with number of features M, then m is a partial number of features for training. The most widespread way for decision tree ensemble construction is bootstrap aggregation or bagging. Classification using random forest algorithm is based on classifiers voting. Accuracy in these cases mainly depends on classifiers’ diversity that composes ensemble or, in other words, on correlation between their decisions. This means that the more classifiers that are diverse, the higher total accuracy will be [].
Final classifier can be calculated as follows:
where N is a number of trees in the random forest model, i is a tree numerator, b is a decision tree, and x is a generated dataset.
4. Results and Discussion
Extracted spectral curves that correspond to the reed areas for 2020 and 2021 are presented in Figure 3. Spectral signatures for reed in 2020 have the highest level of absorption or lowest reflection ranging from 0.443 to 0.56 μm or in a visible part of the spectrum, while the lowest level of absorption ranges from 0.705 to 0.842 μm or in the near-infrared part. This pattern is typical for vegetation that is usually characterized by a similar tendency: minimal reflection in the red part and maximum in the near-infrared, which can be problematic if there is a task to differentiate reed from the other vegetation.

Figure 3.
Spectral plot for reed signatures in 2020 and 2021.
Figure 3 Spectral plot for reed signatures in 2020 and 2021. Each color on the plot represents separate Phragmites australis spectral signature from sampling locations. In 2021, the pattern of spectral signatures changed. As in 2020, the highest level of absorption was in a visible part of the spectrum from 0.443 to 0.56 μm, but the lowest level of absorption ranged from 0.705 to 0.783 μm, and at 0.945 μm, the highest level of absorption can also be seen in wavelength 0.865 μm. It should also be noted that two signatures have higher values than the others, which could possibly be caused by a mix of reeds with different heights of stems or different levels of photosynthesis processes, which leads to an increase in the amount of chlorophyll (Figure 4).
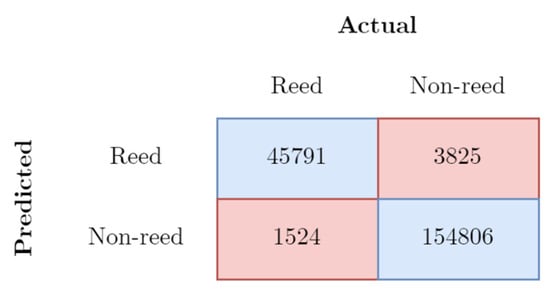
Figure 4.
Confusion matrix for accuracy calculation on testing data from Soleniy firth in pixels.
The collected dataset was split into train and test datasets with a proportion of 75% to 25%. The accuracy of the results was estimated using overlapping predicted reed locations with test data. As a result, the calculated accuracy reached 92%. The final resolution of the classified images was 10 m.
Despite this, the spectral reed signatures are quite similar and can be used for reed prediction from remote sensing data.
The investigated estuaries are usually used as semi-anadromous fish spawning grounds. Overgrowth processes here lead to a decrease in the state of spawning routes, spawning areas, and foraging grounds. Analysis of the overgrowth vegetation using remote sensing data and machine learning showed sufficient changes in the overgrowth speed, depending on the freshwater supply.
Data from Table 2 show the areas’ change matrices, where one class can change to another or stay the same in 2020–2021. From the table, a large increase in the overgrowth speed can be noted during this period.

Table 2.
Reed and water areas change matrix.
While in 2020, the areas with reed in the Chumyaniy and Soleniy firths reached 373.7 × 103 m2 or 0.37 km2, in 2021, these areas occupied 507.9 × 103 m2 or 0.51 km2. Thus, the area covered by reed increased by 1.36 times. The areas that turned from reed into water also changed; those areas, which were overgrown by reed in 2020, were occupied by water in 2021 and made up 0.162 km2. Thus, the total water area in 2021 was 8.136 km2 (Figure 5).

Figure 5.
Changes in water and reed area in 2020–2021.
Spatially, in 2020 (Figure 6), the reed areas mostly occupied western parts of the Chumyaniy firth, Chumyaniy spit, and the coast of the Soleniy firth. Northern and eastern areas of the Chumyaniy firth were free from reed. In 2021 (Figure 7) situation changed. The reed could be observed in the northern and western parts of the Chumyaniy firth; the reed areas in the west increased. In the Soleniy firth, the reed areas changed their spatial distribution, with a small reduction of areas free from reed in the northeastern part, but its total occupation remained the same.

Figure 6.
Area occupied by reed in Chumanskiy and Soleniy firths in 2020.
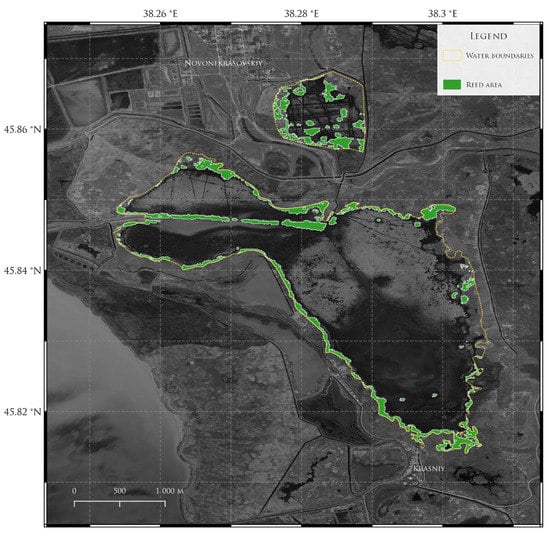
Figure 7.
Area occupied by reed in Chumanskiy and Soleniy firths in 2021.
Seasonal fluctuations in the overgrowth areas of the estuaries were mainly caused by freshwater amount, vegetation phase, and water transparency []. Studies of the estuaries with the areas overgrown by reed, as well as hydrophytes phytomass, are important while determining the fishery value of water bodies. In scarcely overgrown water bodies with low transparency, the main identified fish species was Stizostedion lucioperca (Linnaeus, 1758); in overgrown with reed water bodies, the main identified fish species was Rutilus heckeli (Nordmann, 1840).
The high overgrowth speed of Phragmites australis in 2020–2021, when the reed area doubled in size, could be mainly caused by eutrophication due to the nutrient enrichment from agricultural lands located on the northern part of the research area near Novonekrasovskiy village. Another potential cause is alterations in water flows and hydrological regimes, which can create favorable conditions for reed growth. These can lead to the high growing speed of Phragmites australis, which can reach up to 2 m per year and can spread both vegetatively and sexually, leading to the formation of large, dense stands.
The rapid growth of Phragmites australis in Azov estuaries can have significant biological and ecological consequences, including:
- Phragmites can outcompete native vegetation and alter the composition and structure of wetland ecosystems, which can have a cascading effect on other species that depend on those habitats. The invasive species can form dense, monotypic stands that displace native vegetation, leading to the loss of biodiversity and changing the functional dynamics of the ecosystem.
- The invasion of Phragmites can lead to a decline in native plant and animal species, reducing biodiversity in affected areas. The displacement of native vegetation can reduce the habitat quality and availability of many species, including those that are threatened or endangered.
- Phragmites can change the hydrology of wetland ecosystems, leading to soil degradation and potentially altering water quality. The species has a deep root system that can affect soil stability and water flow, leading to changes in the ecosystem’s hydrological cycle and increasing the risk of erosion and sedimentation.
- The dense stands of Phragmites can increase the risk of fire, which can further alter wetland ecosystems. This can have a direct impact on the vegetation and wildlife of the area, altering the structure and composition of the ecosystem.
- Wetlands, including those dominated by Phragmites, can play an important role in carbon sequestration, but the rapid growth of Phragmites can alter the balance of carbon in wetland ecosystems. The species can significantly impact the carbon storage potential of wetland ecosystems, which can have wider implications for global climate change.
The fast growth of Phragmites australis can have far-reaching ecological consequences, making its control and management a crucial component of wetland conservation efforts. Effective management strategies are necessary to prevent the spread of the species, restore native vegetation, and maintain the health and functioning of wetland ecosystems.
Overgrowing reeds in water bodies used as fishing farms can also significantly impact fish species. The dense, monotypic stands of Phragmites can reduce the amount of open water and shade the underlying substrate, making it less suitable for many fish species. Additionally, the species can alter the water quality and hydrology of the ecosystem, leading to changes in the water temperature, dissolved oxygen levels, and nutrient availability that can negatively impact fish populations.
The loss of wetland habitats and the decline of fish species can have cascading effects on other species that depend on the ecosystem for their survival, including birds and mammals that rely on fish as a food source. Effective management strategies are needed to restore native vegetation and maintain the health and functioning of wetland ecosystems, including the preservation of fish populations and their habitats.
A positive role of the Phragmites australis is its participation in self-purification processes, where reed thickets can perform functions, such as mechanical cleaning, when they slow down suspended and slightly soluble particles; moreover, it possesses mineralization and oxidative properties as well as take part in detoxication of organic pollutants. Phragmites australis is usually characterized by high photosynthetic activity and active absorption of nitrogen and silicon.
A negative impact of the reed overgrowth is mainly related to its dying and decomposition of the phytomass, which leads to secondary pollution of the water body.
5. Conclusions
In recent decades, most of the Azov Sea estuaries have reduced spawning areas for commercial fishes, so it is important to conduct permanent monitoring of the overgrowth processes to optimize hydrophytes biomass. Overgrowth of the estuaries strongly depends on the annual water amount. Thus, it is essential to perform regular monitoring of the water bodies’ overgrowth using GIS and remote sensing. Under the remote research of vegetation, it is necessary to realize which part of the overgrown area is occupied by air–water vegetation or heliophytes and which one is occupied by true water vegetation or hydrophytes. Knowledge of the areas overgrown by these vegetation ecotypes is needed to develop recommendations for biological and mechanical melioration of the Azov Sea estuaries. As our previous research revealed, air–water vegetation can be detected with good accuracy using remote sensing data, but the accuracy of true water vegetation mainly depends on weather conditions, type, and the phase of growth. However, the separation of water vegetation into hydrophytes and heliophytes using NDVI is slightly difficult in shallow water estuaries with unstable conditions.
In this research, it has been demonstrated that the approach of using machine learning models to determine the areas of overgrowth with Phragmites australis, which makes up around 90% of heliophyte vegetation in the Azov Sea estuaries, showed 92% of accuracy.
Author Contributions
Conceptualization, D.K. and S.C.; methodology, L.B.; software, E.M.; validation, D.K., A.Z. and E.Z.; formal analysis, S.C.; investigation, E.Z.; resources, L.B.; data curation, L.B.; project administration, D.K.; funding acquisition, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research is partially funded by the Ministry of Science and Higher Education of the Russian Federation as part of the World-class Research Center program: Advanced Digital Technologies (contract No. 075-15-2022-312 dated 20 April 2022).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mikhailova, K.B.; Mikhalap, S.G. Spawning Grounds Overgrowth Dynamics of the Pskov Lake on Anokhov Bay Example. Ecosyst. Transform. 2020, 1, 83–94. [Google Scholar]
- Tsunikova, E.P. Water Bodies of the Eastern Azov Region: Their Fishery Significance and Optimization of Their Practical Use; Mediapolis Publishing: Moscow, Russia, 2006. [Google Scholar]
- Matishov, G.G.; Matishov, D.G.; Namjatov, A.A.; Carroll, J.; Dahle, S. Artificial Radionuclides in Sediments of the Don River Estuary and Azov Sea. J. Environ. Radioact. 2002, 59, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Moses, W.J.; Gitelson, A.A.; Berdnikov, S.; Povazhnyy, V. Satellite Estimation of Chlorophyll-$a$ Concentration Using the Red and NIR Bands of MERIS—The Azov Sea Case Study. IEEE Geosci. Remote Sens. Lett. 2009, 6, 845–849. [Google Scholar] [CrossRef]
- Mosesyan, G.; Dudkin, S.; Strizhakova, T. Assessment of infection of Hamsa Engraulis encrasicolus nematode Hysterothylacium aduncum in the Sea of Azov in the summer and autumn periods 2015–2020. Fisheries 2021, 2021, 25–30. [Google Scholar] [CrossRef]
- Nabozhenko, M.V.; Kovalenko, E.P. Contemporary Distribution of Macrozoobenthic Communities of the Yeisk Estuary (Taganrog Bay of the Sea of Azov). Oceanology 2011, 51, 626. [Google Scholar] [CrossRef]
- Shekhov, A.G. Flora and Vegetation of Kuban Estuaries. Inland Water Biol. 1971, 10, 24–29. [Google Scholar]
- Shekhov, A.G. Influence of Watering Timing and Overgrowing of Fish Ponds in the Don Delta. Bot. Zhurnal 1970, 55, 1152–1157. [Google Scholar]
- Bondarenko, L.G.; Kulba, S.N.; Petrashov, V.I.; Smirnov, S.S.; Matveeva, E.I.; Rudakova, N.A. Assessment of Overgrowth of the Chelbas Group of the Azov Sea Limans with Aquatic Vegetation. Water Bioresour. Environ. 2021, 4, 14–26. [Google Scholar] [CrossRef]
- Borovskaya, R.; Krivoguz, D.; Chernyi, S.; Kozhurin, E.; Zinchenko, E. Surface Water Salinity Evaluation and Identification for Using Remote Sensing Data and Machine Learning Approach. J. Mar. Sci. Eng. 2022, 10, 257. [Google Scholar] [CrossRef]
- Antonenko, M.V.; Fedorova, S.I. Satellite Monitoring of the Kulikov-Kurchansk Group of Limans. Innov. Sci. 2016, 3, 59–61. [Google Scholar]
- Filonenko, I.V.; Komarova, A.S. Long-Term Dynamics of the Area Overgrown with Coastal Aquatic Vegetation of Lake Vozhe. Printsipy Ekol. 2015, 16, 63. [Google Scholar] [CrossRef]
- Kochetkova, A.I.; Bryzgalina, E.S.; Kalyuzhnaya, I.Y.; Sirotina, S.L.; Samoteyeva, V.V.; Rakshenko, E.P. Overgrowth Dynamics of the Tsimlyanskoe Reservoir. Princ. Ecol. 2018, 26, 60–72. [Google Scholar] [CrossRef]
- Vlasov, B.P.; Hryshchankava, N.D.; Sivenkou, A.Y.; Sukhovilo, N.Y.; Kolbun, D.A. Assessment of the Current State and Dynamics of the Overgrowing of Lakes in National Park “Narochansky” Using Remote Sensing Data. Acta Geogr. Sil. 2019, 13, 39–55. [Google Scholar]
- Peng, J.; Liu, S.; Lu, W.; Liu, M.; Feng, S.; Cong, P. Continuous Change Mapping to Understand Wetland Quantity and Quality Evolution and Driving Forces: A Case Study in the Liao River Estuary from 1986 to 2018. Remote Sens. 2021, 13, 4900. [Google Scholar] [CrossRef]
- Lamb, B.T.; Tzortziou, M.A.; McDonald, K.C. A Fused Radar–Optical Approach for Mapping Wetlands and Deepwaters of the Mid–Atlantic and Gulf Coast Regions of the United States. Remote Sens. 2021, 13, 2495. [Google Scholar] [CrossRef]
- Oteman, B.; Scrieciu, A.; Bouma, T.J.; Stanica, A.; van der Wal, D. Indicators of Expansion and Retreat of Phragmites Based on Optical and Radar Satellite Remote Sensing: A Case Study on the Danube Delta. Wetlands 2021, 41, 72. [Google Scholar] [CrossRef]
- Krivoguz, D.; Mal’ko, S.; Borovskaya, R.; Semenova, A. Automatic Processing of Sentinel-2 Image for Kerch Peninsula Lake Areas Extraction Using QGIS and Python. E3S Web Conf. 2020, 203, 3011. [Google Scholar] [CrossRef]
- Krivoguz, D.; Bespalova, L.; Zhilenkov, A.; Chernyi, S. A Deep Neural Network Method for Water Areas Extraction Using Remote Sensing Data. J. Mar. Sci. Eng. 2022, 10, 1392. [Google Scholar] [CrossRef]
- Bondarenko, L.G.; Kulba, S.N.; Petrashov, V.I.; Smirnov, S.S.; Matveeva, E.I. The influence of changes in the hydrological parameters of limans of the Eastern-Akhtarsky growing farm with the overgrowth of hydrophytes. Bull. KSMTU 2022, 3, 24–39. [Google Scholar]
- Papchnkov, V.G.; Scherbakov, A.V.; Lapirov, A.G. Main Hydrobotanical Definitions and Terms. In Proceedings of the Hydrobotany: Methodology, Methods, Moscow, Russia, 19 May 2003; pp. 27–38. [Google Scholar]
- Lapirov, A.G. Ecological Groups of Aquatic Vegetation. In Proceedings of the Hydrobotany: Methodology, Methods, Moscow, Russia, 19 May 2003; pp. 5–22. [Google Scholar]
- Zernov, A.S. Illustrated Flora of the Russian Black Sea South Region; KMK: Rostov, Russia, 2013. [Google Scholar]
- Gollerbach, M.M.; Kosinskaya, E.K.; Polyanskiy, V.I. Qualifier of Freshwater Algae in USSR; Soviet Science: Moscow, Russia, 1953. [Google Scholar]
- Joshi, S.; Rai, N.; Sharma, R.; Baral, N. Land Use/Land Cover (LULC) Change in Suburb of Central Himalayas: A Study from Chandragiri, Kathmandu. J. For. Environ. Sci. 2021, 37, 44–51. [Google Scholar] [CrossRef]
- Krivoguz, D.O.; Burtnik, D.N. Neural Network Modeling of Changes in the Land Cover of the Kerch Peninsula in the Context of Landslides Occurence. Nauchno-Tekhnicheskiy Vestn. Bryanskogo Gos. Univ. 2018, 1, 113–121. [Google Scholar] [CrossRef]
- Souza, J.M.d.; Morgado, P.; Costa, E.M.d.; Vianna, L.F.d.N. Modeling of Land Use and Land Cover (LULC) Change Based on Artificial Neural Networks for the Chapecó River Ecological Corridor, Santa Catarina/Brazil. Sustainability 2022, 14, 4038. [Google Scholar] [CrossRef]
- Thiam, S.; Salas, E.A.L.; Hounguè, N.R.; Almoradie, A.D.S.; Verleysdonk, S.; Adounkpe, J.G.; Komi, K. Modelling Land Use and Land Cover in the Transboundary Mono River Catchment of Togo and Benin Using Markov Chain and Stakeholder’s Perspectives. Sustainability 2022, 14, 4160. [Google Scholar] [CrossRef]
- Congedo, L. Semi-Automatic Classification Plugin: A Python Tool for the Download and Processing of Remote Sensing Images in QGIS. J. Open Source Softw. 2021, 6, 3172. [Google Scholar] [CrossRef]
- Chernyi, S.; Emelianov, V.; Zinchenko, E.; Zinchenko, A.; Tsvetkova, O.; Mishin, A. Application of Artificial Intelligence Technologies for Diagnostics of Production Structures. J. Mar. Sci. Eng. 2022, 10, 259. [Google Scholar] [CrossRef]
- Zhilenkov, A.; Chernyi, S.; Emelianov, V. Application of Artificial Intelligence Technologies to Assess the Quality of Structures. Energies 2021, 14, 8040. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Corcoran, J.M.; Knight, J.F.; Gallant, A.L. Influence of Multi-Source and Multi-Temporal Remotely Sensed and Ancillary Data on the Accuracy of Random Forest Classification of Wetlands in Northern Minnesota. Remote Sens. 2013, 5, 3212–3238. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C., Jr.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests for Classification in Ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Feng, Q.; Gong, J.; Liu, J.; Li, Y. Monitoring Cropland Dynamics of the Yellow River Delta Based on Multi-Temporal Landsat Imagery over 1986 to 2015. Sustainability 2015, 7, 14834–14858. [Google Scholar] [CrossRef]
- Chistiakov, S. Random Forests: An Overview. Trans. KarRC RAS 2013, 12, 117–136. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).