Symbiont-Bearing Colonial Corals and Gastropods: An Odd Couple of the Shallow Seas
Abstract
1. Introduction
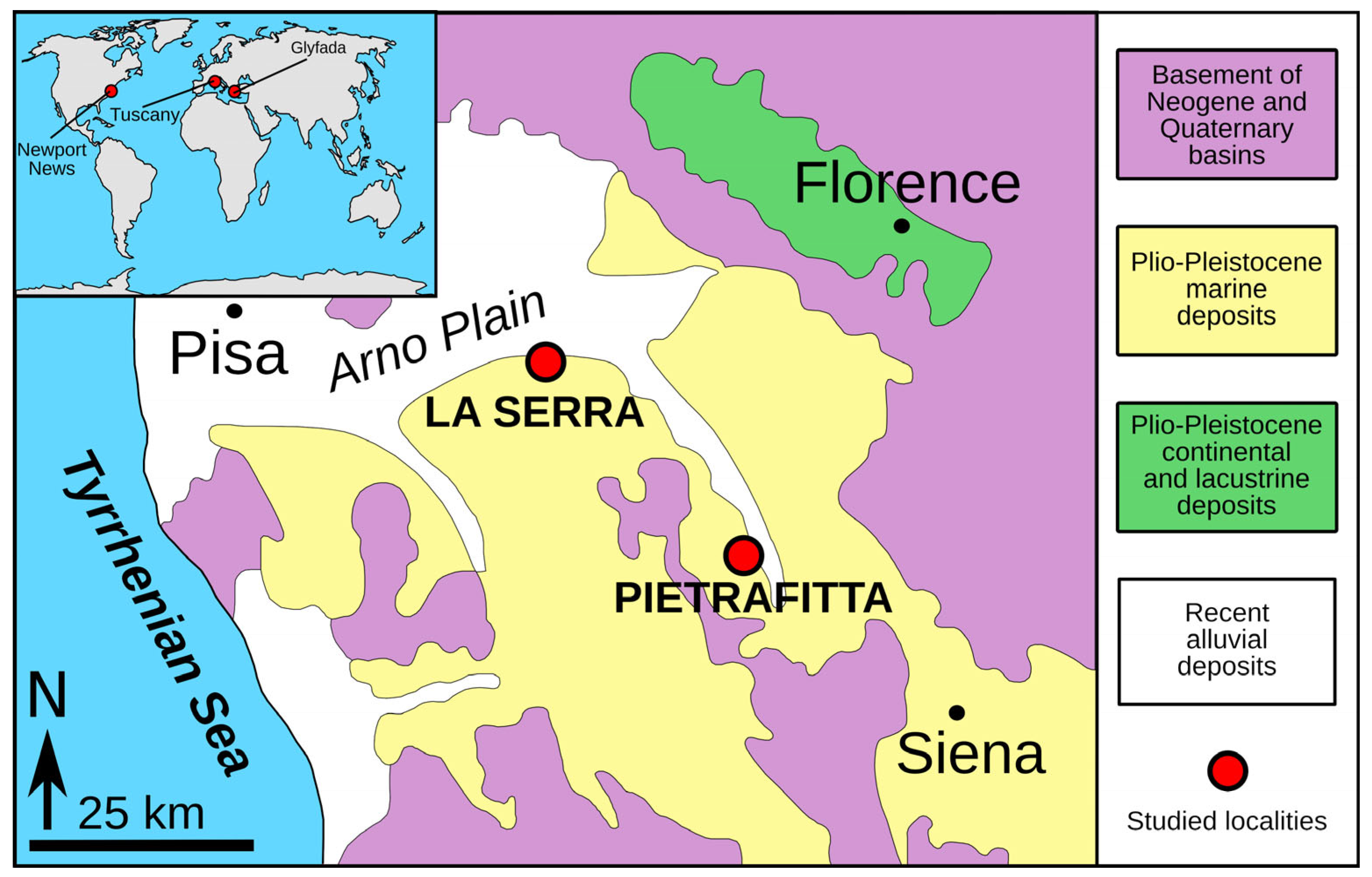
2. Materials and Methods
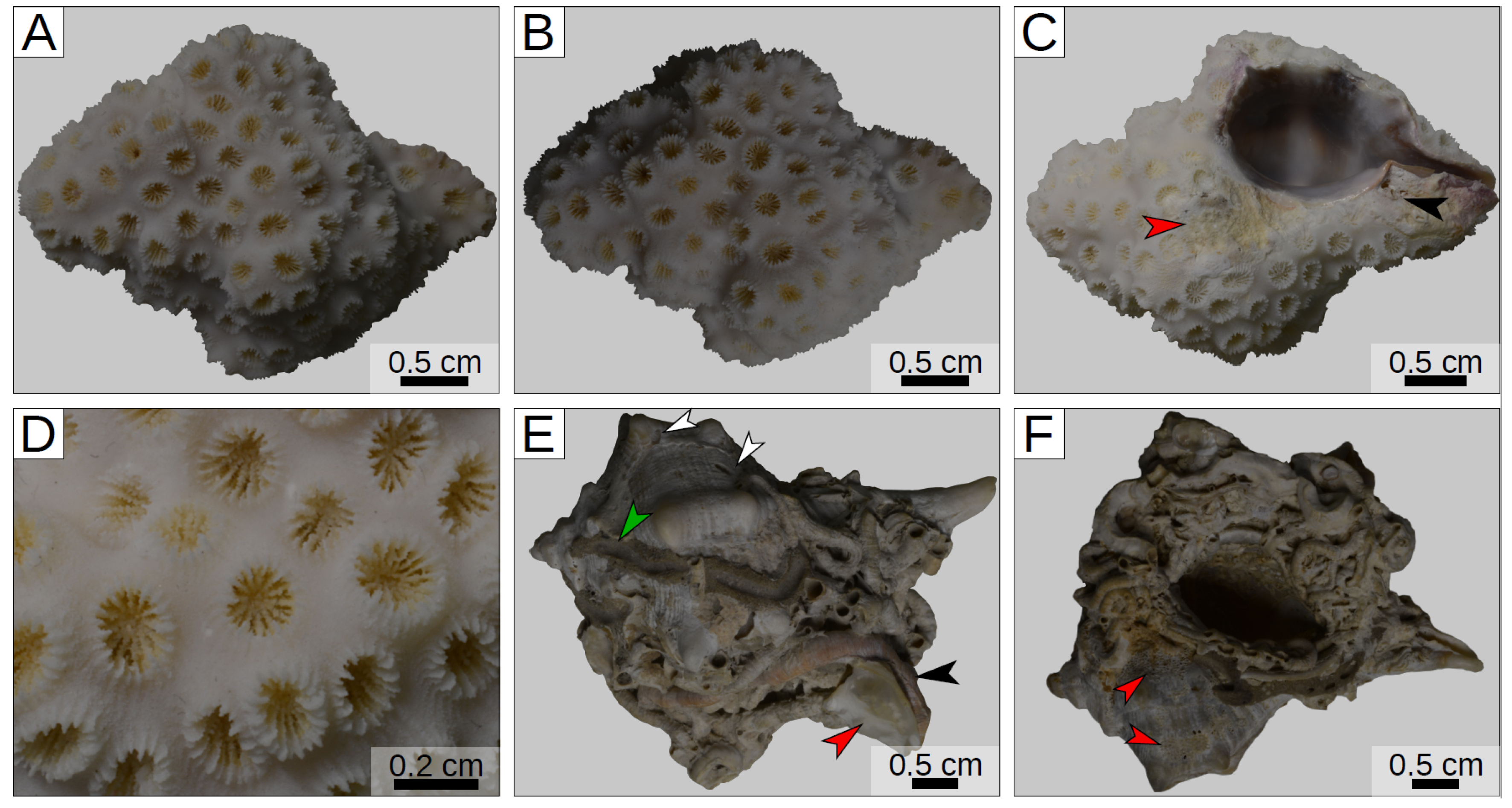
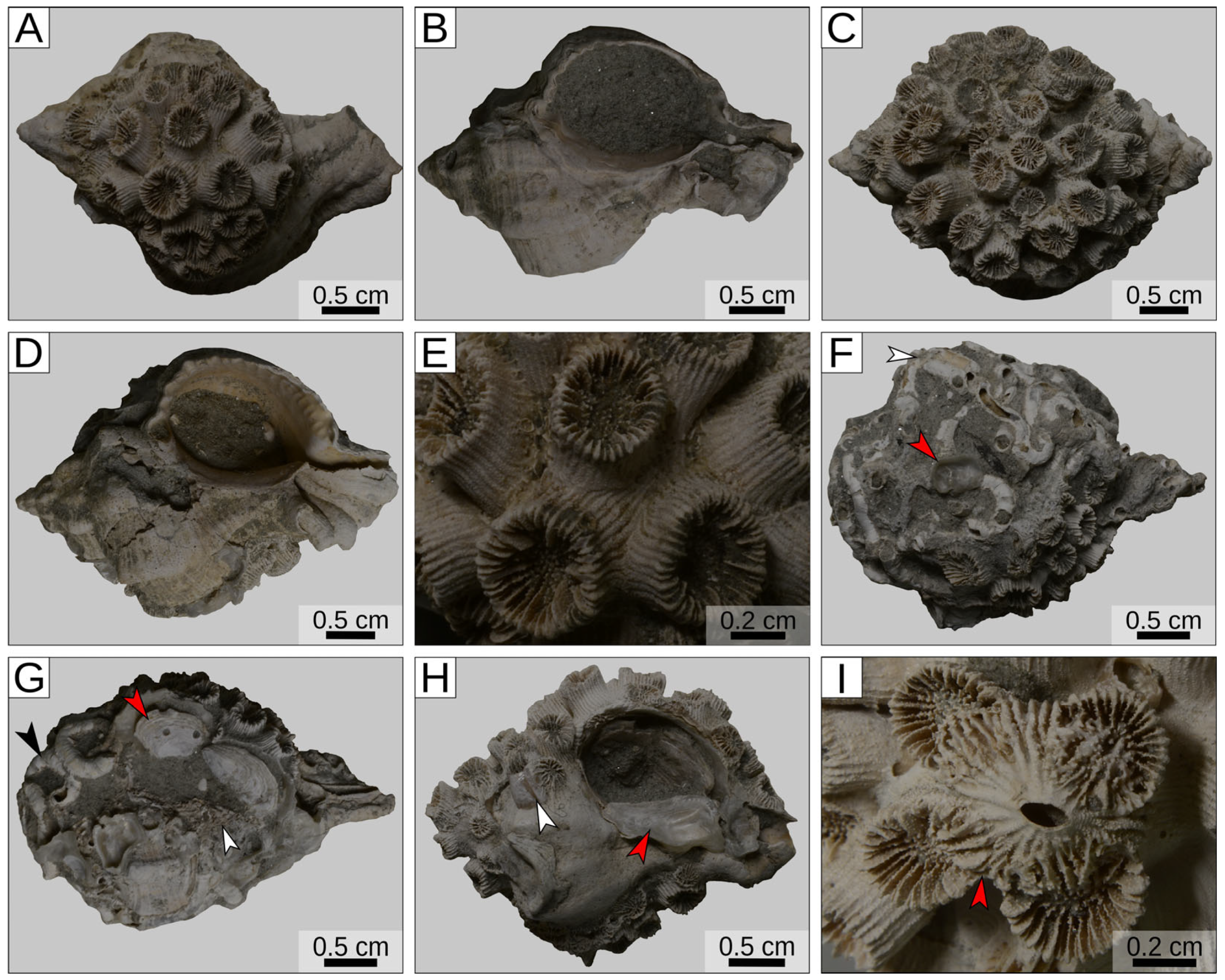
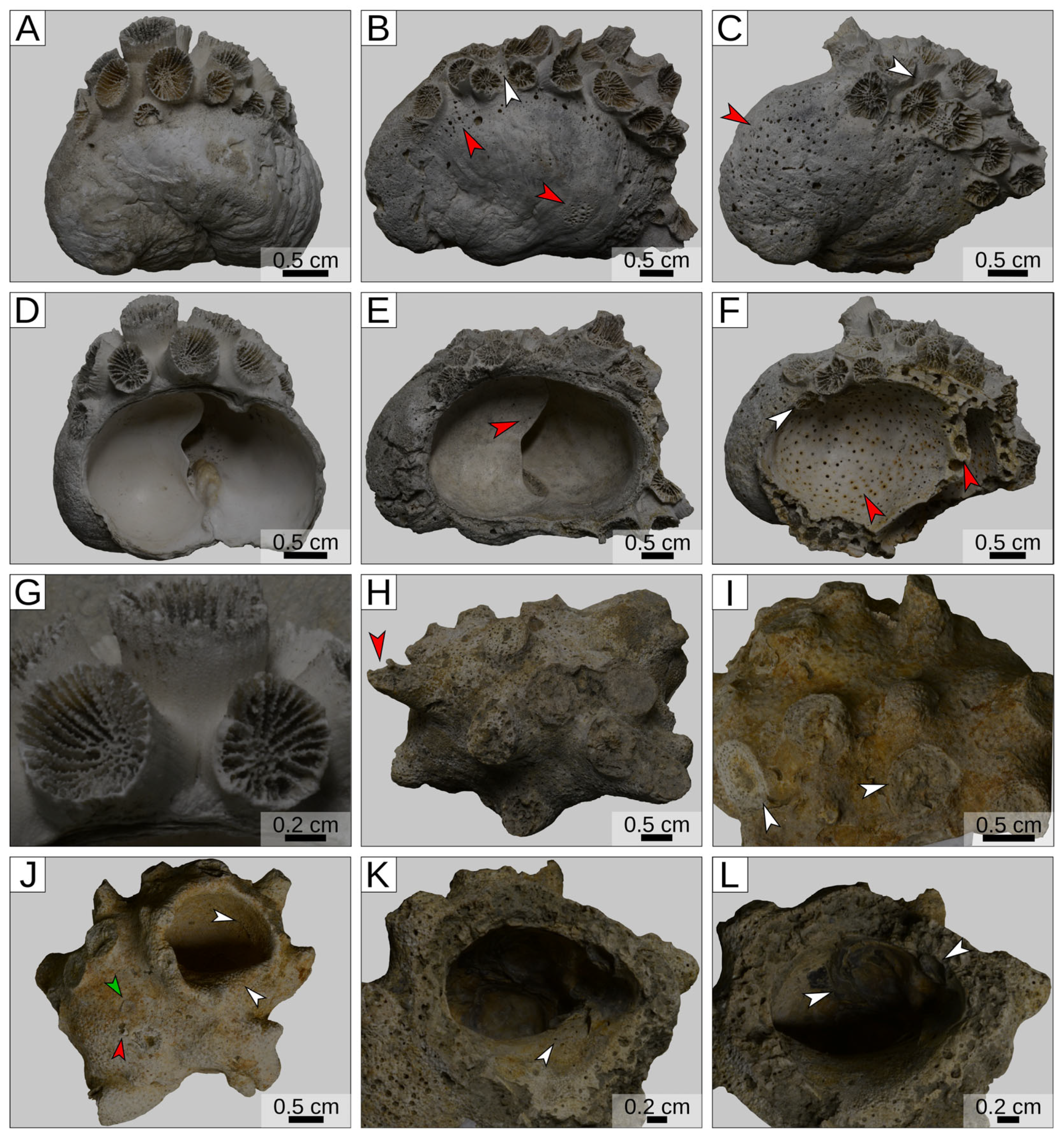
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, C.M.; Dennison, W.C.; Orth, R.J.; Carruthers, T.J. The charisma of coastal ecosystems: Addressing the imbalance. Estuaries Coasts 2008, 31, 233–238. [Google Scholar] [CrossRef]
- Schlager, W. The paradox of drowned reefs and carbonate platforms. Geol. Soc. Am. Bull. 1981, 92, 197–211. [Google Scholar] [CrossRef]
- Kružić, P.; Benković, L. Bioconstructional features of the coral Cladocora caespitosa (Anthozoa, Scleractinia) in the Adriatic Sea (Croatia). Mar. Ecol. 2008, 29, 125–139. [Google Scholar] [CrossRef]
- Kersting, D.K.; Linares, C. Cladocora caespitosa bioconstructions in the Columbretes Islands Marine Reserve (Spain, NW Mediterranean): Distribution, size structure and growth. Mar. Ecol. 2012, 33, 427–436. [Google Scholar] [CrossRef]
- Jiménez, C.; Hadjioannou, L.; Petrou, A.; Nikolaidis, A.; Evriviadou, M.; Lange, M.A. Mortality of the scleractinian coral Cladocora caespitosa during a warming event in the Levantine Sea (Cyprus). Reg. Environ. Changes 2016, 16, 1963–1973. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Casado-Amezúa, P.; Templado, J. Environmental drivers of distribution and reef development of the Mediterranean coral Cladocora caespitosa. Coral Reefs 2017, 36, 1195–1209. [Google Scholar] [CrossRef]
- Coletti, G.; Bracchi, V.; Corselli, C.; Marchese, F.; Basso, D.; Savini, A.; Vertino, A. Quaternary build-ups and rhodalgal carbonates along the Adriatic and Ionian coasts of the Italian Peninsula: A review. Riv. Ital. Paleontol. Stratigr. 2018, 124, 387–406. [Google Scholar]
- Bosio, G.; Di Cencio, A.; Coletti, G.; Casati, S.; Collareta, A. Exceptionally preserved coral bank and seagrass meadow from the lower Pleistocene of Fauglia (Tuscany, Italy). Alp. Mediterr. Quat. 2021, 34, 237–256. [Google Scholar]
- Kiessling, W.; Flügel, E.; Golonka, J. Paleo reef maps: Evaluation of a comprehensive database on phanerozoic reefs. Am. Assoc. Pet. Geol. Bull. 1999, 83, 1552–1587. [Google Scholar]
- Kiessling, W.; Flügel, E.; Golonka, J.A.N. Patterns of Phanerozoic carbonate platform sedimentation. Lethaia 2003, 36, 195–225. [Google Scholar] [CrossRef]
- Coletti, G.; Commissario, L.; Mariani, L.; Bosio, G.; Desbiolles, F.; Soldi, M.; Bialik, O.M. Palaeocene to Miocene southern Tethyan carbonate factories: A meta-analysis of the successions of South-western and Western Central Asia. Depos. Rec. 2022, 8, 1031–1054. [Google Scholar] [CrossRef]
- Adey, W.H.; Macintyre, I.G. Crustose coralline algae: A re-evaluation in the geological sciences. Geol. Soc. Am. Bull. 1973, 84, 883–904. [Google Scholar] [CrossRef]
- Lokier, S.W.; Wilson, M.E.; Burton, L.M. Marine biota response to clastic sediment influx: A quantitative approach. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 281, 25–42. [Google Scholar] [CrossRef]
- Coletti, G.; Mariani, L.; Garzanti, E.; Consani, S.; Bosio, G.; Vezzoli, G.; Xiumian, H.; Basso, D. Skeletal assemblages and terrigenous input in the Eocene carbonate systems of the Nummulitic Limestone (NW Europe). Sediment. Geol. 2021, 425, 106005. [Google Scholar] [CrossRef]
- Seilacher, A. Whale barnacles: Exaptational access to a forbidden paradise. Paleobiology 2005, 31 (Suppl. S2), 27–35. [Google Scholar] [CrossRef]
- Collareta, A.; Newman, W.A.; Bosio, G.; Coletti, G. A new chelonibiid from the Miocene of Zanzibar (Eastern Africa) sheds light on the evolution of shell architecture in turtle and whale barnacles (Cirripedia: Coronuloidea). Integr. Zool. 2022, 17, 24–43. [Google Scholar] [CrossRef]
- Collareta, A.; Rasser, M.W.; Frey, E.; Harzhauser, M. Turtle barnacles have been turtle riders for more than 30 million years. PalZ 2022, 1–11. [Google Scholar] [CrossRef]
- Kase, T. Mode of life of the Silurian uncoiled gastropod Semitubina sakoi n. sp. from Japan. Lethaia 1986, 19, 327–337. [Google Scholar] [CrossRef]
- Marek, L.; Galle, A. The tabulate coral Hyostragulum, an epizoan with bearing on hyolithid ecology and systematics. Lethaia 1976, 9, 51–64. [Google Scholar] [CrossRef]
- Brett, C.E.; Cottrell, J.F. Substrate specificity in the Devonian tabulate coral Pleurodictyum. Lethaia 1982, 15, 247–262. [Google Scholar] [CrossRef]
- Petuch, E.J. The Pliocene reefs of Miami: Their geomorphological significance in the evolution of the Atlantic coastal ridge, southeastern Florida, USA. J. Coast. Res. 1986, 2, 391–408. [Google Scholar]
- Darrell, J.G.; Taylor, P.D. Scleractinian symbionts of hermit crabs in the Pliocene of Florida. Mem. Assoc. Australas. Palaeontol. 1989, 8, 115–123. [Google Scholar]
- Walker, S.E. Criteria for recognizing marine hermit crabs in the fossil record using gastropod shells. J. Paleontol. 1992, 66, 535–558. [Google Scholar] [CrossRef]
- Allmon, W.D. Age, environment and mode of deposition of the densely fossiliferous Pinecrest Sand (Pliocene of Florida): Implications for the role of biological productivity in shell bed formation. Palaios 1993, 8, 183–201. [Google Scholar] [CrossRef]
- Pickerill, R.K.; Donovan, S.K. Ichnology and biotic interactions on a Pleistocene gastropod from south-east Jamaica. J. Geol. Soc. Jam. 1997, 32, 19–24. [Google Scholar]
- Bouvier, E.L. Un noveau cas de commensalisme: Association d’Aspidosiphon ace des polyplers madréporaires et unn mollusque bivalve. Comptes Rendus L’accademie Sci. 1894, 119, 96–98. [Google Scholar]
- Wilson, J.B. Attachment of the coral Caryophyllia smithii S. & B. to tubes of the polychaete Ditrupa arietina (Müller) and other substrates. J. Mar. Biol. Assoc. 1976, 56, 291–303. [Google Scholar]
- Hoeksema, B.; Best, M.B. New observations on scleractinian corals from Indonesia: 2. Sipunculan-associated species belonging to the genera Heterocyathus and Heteropsammia. Zool. Meded. 1991, 65, 221–245. [Google Scholar]
- Stolarski, J.; Zibrowius, H.; Loser, H. Antiquity of the scleractinian-sipunculan symbiosis. Acta Palaeontol. Pol. 2001, 46, 309–330. [Google Scholar]
- Fujii, T. A hermit crab living in association with a mobile scleractinian coral, Heteropsammia cochlea. Mar. Biodivers. 2017, 47, 779–780. [Google Scholar] [CrossRef]
- Igawa, M.; Kato, M. A new species of hermit crab, Diogenes heteropsammicola (Crustacea, Decapoda, Anomura, Diogenidae), replaces a mutualistic sipunculan in a walking coral symbiosis. PLoS ONE 2017, 12, e0184311. [Google Scholar] [CrossRef] [PubMed]
- Keshavmurthy, S.; Yang, S.Y.; Su, C.Y.; Chiu, Y.W.; Chen, C.A. Association between massive Porites and the hidden hermit crab, Calcinus latens (Decapoda, Diogenidae). Bull. Mar. Sci. 2019, 95, 115–116. [Google Scholar] [CrossRef]
- Herrán, N.; Narayan, G.R.; Doo, S.S.; Klicpera, A.; Freiwald, A.; Westphal, H. High-resolution imaging sheds new light on a multi-tier symbiotic partnership between a “walking” solitary coral, a sipunculan, and a bivalve from East Africa. Ecol. Evol. 2022, 12, e8633. [Google Scholar] [CrossRef]
- Khakhina, L. Review article Evolutionary Significance of Symbiosis; Development of the Symbiogenesis Concept. Symbiosis 1992, 14, 217–228. [Google Scholar]
- Collareta, A.; Varas-Malca, R.; Bosio, G.; Urbina, M.; Coletti, G. Ghosts of the Holobiont: Borings on a Miocene Turtle Carapace from the Pisco Formation (Peru) as Witnesses of Ancient Symbiosis. J. Mar. Sci. Eng. 2023, 11, 45. [Google Scholar] [CrossRef]
- Vinn, O. Symbiosis between Devonian corals and other invertebrates. Palaios 2017, 32, 382–387. [Google Scholar] [CrossRef]
- Benvenuti, M.; Del Conte, S.; Scarselli, N.; Dominici, S. Hinterland basin development and infilling through tectonic and eustatic processes: Latest Messinian–Gelasian Valdelsa Basin, Northern Apennines, Italy. Basin Res. 2014, 26, 387–402. [Google Scholar] [CrossRef]
- Benvenuti, M.; Bertini, A.; Conti, C.; Dominici, S.; Falcone, D. Analisi stratigrafica e paleoambientale integrata del Pliocene dei dintorni di San Miniato. Quad. Del Mus. Di Stor. Nat. Di Livorno 1995, 14, 29–49. [Google Scholar]
- Garassino, A.; Pasini, G.; De Angeli, A.; Charbonnier, S.; Famiani, F.; Baldanza, A.; Bizzarri, R. The decapod community from the Early Pliocene (Zanclean) of “La Serra” quarry (San Miniato, Pisa, Toscana, central Italy): Sedimentology, systematics, and palaeoenvironmental implications. Ann. De Paléontol. 2012, 98, 1–61. [Google Scholar] [CrossRef]
- Dominici, S.; Danise, S.; Benvenuti, M. Pliocene stratigraphic paleobiology in Tuscany and the fossil record of marine megafauna. Earth-Sci. Rev. 2018, 176, 277–310. [Google Scholar] [CrossRef]
- Collareta, A.; Casati, S.; Zuffi, M.A.L.; Di Cencio, A. First authentic record of the freshwater turtle Mauremys from the upper Pliocene of Italy, with a new occurrence of the rarely reported ichnotaxon Thatchtelithichnus holmani. Carnets Geol. 2020, 20, 301–313. [Google Scholar] [CrossRef]
- Collareta, A.; Merella, M.; Casati, S.; Di Cencio, A. Did titanic stingrays wander the Pliocene Mediterranean Sea? Some notes on a giant-sized myliobatoid stinger from the Piacenzian of Italy. Neues Jahrb. Geol. Paläontologie-Abh. 2020, 298, 155–164. [Google Scholar] [CrossRef]
- Di Cencio, A.; Dulai, A.; Catanzariti, R.; Casati, S.; Collareta, A. First record of the brachiopod Lingula? from the Pliocene of Tuscany (Italy): The youngest occurrence of lingulides in the Mediterranean Basin. Neues Jahrb. Geol. Paläontologie-Abh. 2021, 299, 237–249. [Google Scholar] [CrossRef]
- Dowsett, H.J.; Wiggs, L.B. Planktonic foraminiferal assemblage of the Yorktown Formation, Virginia, USA. Micropaleontol. 1992, 38, 75–86. [Google Scholar] [CrossRef]
- Spivey, W.E.; Culver, S.; Mallinson, D.J.; Dowsett, H.J.; Buzas, M.A. Benthic Foraminiferal and Sedimentologic Changes in the Pliocene Yorktown Formation, Virginia, USA. J. Foram. Res. 2022, 52, 278–305. [Google Scholar] [CrossRef]
- Fulkerson, T. Systematics and Evolution of Western Atlantic Rocellaria Blainville, 1829 (Mollusca: Bivalvia: Gastrochaenidae). Ph.D. Thesis, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 2008. [Google Scholar]
- Pantanelli, D. Conchiglie plioceniche di Pietrafitta in provincia di Siena. Boll. Soc. Malac. Ital. 1880, 6, 265–276. [Google Scholar]
- Chirli, C.; Micali, P. Gibbula saeniensis n. sp. (Gastropoda: Trochidae) del Pliocene Toscano. Boll. Malacol. 2001, 37, 225–228. [Google Scholar]
- Chirli, C.; Micali, P. Su alcuni interessanti Pyramidellidae (Gastropoda) del Pliocene toscano e loro relazioni con specie attuali dell’Africa nord-occidentale. Boll. Malacol. 2008, 44, 39–44. [Google Scholar]
- Forli, M. Divagazioni intorno alla Mesalia cochleata (Brocchi, 1814), la mini turritella del Pliocene toscano. Notiz. SIM 2009, 27, 16–19. [Google Scholar]
- Forli, M.; Dell’Angelo, B.; Sosso, M.; Bonfitto, A. Note su alcune specie fossili di Williamia (Gastropoda: Siphonariidae) con descrizione di una nuova specie. Boll. Malacol. 2009, 45, 5–8. [Google Scholar]
- Ishida, S. An analysis of feeding aggregations in intertidal muricids: Species-specific modes of foraging—Initial predation and parasitism. Asian Mar. Biol. 2001, 18, 1–13. [Google Scholar]
- Mestre, N.C.; Brown, A.; Thatje, S. Temperature and pressure tolerance of larvae of Crepidula fornicata suggest thermal limitation of bathymetric range. Mar. Biol. 2013, 160, 743–750. [Google Scholar] [CrossRef]
- Trainito, E.; Baldacconi, R. Coralli del Mediterraneo, Il Castello; Cornaredo: Milano, Italy, 2016; p. 176. [Google Scholar]
- Karlson, R.H.; Cariolou, M.A. Hermit crab shell colonization by Crepidula convexa Say. J. Exp. Mar. Biol. Ecol. 1982, 65, 1–10. [Google Scholar] [CrossRef]
- Van Ofwegen, L.P.; Häussermann, V.; Försterra, G. A new genus of soft coral (Octocorallia: Alcyonacea: Clavulariidae) from Chile. Zootaxa 2006, 1219, 47–57. [Google Scholar] [CrossRef]
- Sarti, G.; Testa, G.; Zanchetta, G. A new stratigraphic insight of the Upper Pliocene-Lower Pleistocene succession of Lower Valdarno (Tuscany, Italy). Geoacta 2008, 7, 27–41. [Google Scholar]
- Zanchetta, G.; Bini, M.; Montagna, P.; Montagna, P.; Regattieri, E.; Ragaini, L.; Da Prato, S.; Ciampalini, A.; Dallai, L.; Leone, G. Stable isotope composition of fossil ahermatypic coral Cladocora caespitosa (L.) from Pleistocene raised marine terraces of the Livorno area (Central Italy). Alp. Mediterr. Quat. 2019, 32, 73–86. [Google Scholar] [CrossRef]
- Baldanza, A.; Bizzarri, R.; Famiani, F.; Faralli, L. First report of Cladocora arbuscula in Early Pleistocene deposits (Western Umbria, Italy) and its relationships with the distribution of Cladocora caespitosa. Atti Soc. Tosc. Sci. Nat. Mem. Ser. A 2021, 128, 37–53. [Google Scholar]
- Mariani, L.; Coletti, G.; Mateu-Vicens, M.; Bosio, G.; Collareta, A.; Khokhlova, A.; Di Cencio, A.; Casati, S.; Malinverno, E. Testing an indirect palaeo-seagrass indicator: Benthic foraminifera from the Lower Pleistocene Posidonia meadow of Fauglia (Tuscany, Italy). Mar. Micropaleontol. 2022, 173, 102126. [Google Scholar] [CrossRef]
- Williams, J.D.; McDermott, J.J. Hermit crab biocoenoses: A worldwide review of the diversity and natural history of hermit crab associates. J. Exp. Mar. Biol. Ecol. 2004, 305, 1–128. [Google Scholar]
- Kauffman, E.G. Benthic environments and paleoecology of the Posidonienschiefer (Toarcian). Neues Jahrb. Geol. Paläontologie-Mon. 1978, 157, 18–36. [Google Scholar]
- Seilacher, A. Ammonite shells as habitats in the Posidonia Shales of Holzmaden-floats or benthic islands? Neues Jahrb. Geol. Paläontologie-Mon. 1982, 159, 98–114. [Google Scholar] [CrossRef]
- Taylor, P.D.; Wilson, M.A. Palaeoecology and evolution of marine hard substrate communities. Earth-Sci. Rev. 2003, 62, 1–103. [Google Scholar]
- Luci, L.; Lazo, D.G. Living on an island: Characterization of the encrusting fauna of large pectinid bivalves from the Lower Cretaceous of the Neuquén Basin, west-central Argentina. Lethaia 2014, 48, 205–226. [Google Scholar] [CrossRef]
- Smyth, M.J. Bioerosion of gastropod shells: With emphasis on effects of coralline algal cover and shell microstructure. Coral Reefs 1989, 8, 119–125. [Google Scholar] [CrossRef]
- Abrams, P. Shell selection and utilization in a terrestrial hermit crab, Coenobita compressus (H. Milne Edwards). Oecologia 1978, 34, 239–253. [Google Scholar] [CrossRef]
- Brett, C.E.; Parsons-Hubbard, K.M.; Walker, S.E.; Ferguson, C.; Powell, E.N.; Staff, G.; Ashton-Alcox, K.A.; Raymond, A. Gradients and patterns of sclerobionts on experimentally deployed bivalve shells: Synopsis of bathymetric and temporal trends on a decadal time scale. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 312, 278–304. [Google Scholar] [CrossRef]
- Brett, C.E.; Smrecak, T.; Hubbard, K.P.; Walker, S. Marine sclerobiofacies: Encrusting and endolithic communities on shells through time and space. In Earth and Life; Springer: Dordrecht, The Netherlands, 2012; pp. 129–157. [Google Scholar]
- James, N.P.; Kendall, A.C. Introduction to carbonate and evaporate facies models. In Facies Models, Response to Sea Level Change; Walker, R.G., James, N.P., Eds.; Geological Association of Canada: Stittsville, ON, Canada, 1992; pp. 265–275. [Google Scholar]
| Occurrence | Time | Coral | Substrate | References |
|---|---|---|---|---|
| Yokokurama Formation (Japan) | Silurian | Favosites (tabulate coral) | Shells of the gastropod Semitubina sakoi | [18] |
| Hamilton Group (New York, USA) | Devonian | Pleurodictyum americanum (tabulate coral) | Mainly shells of gastropod Palaeozygopleura hamiltoniae occupied by a secondary inhabitant, but also other molluscs and brachiopods | [20] |
| Bohemia (Czech Republic) | Devonian | Hyostragulum (tabulate coral) | Mainly hyolithids but also nautiloids and gastropods | [19] |
| Tropical to sub-tropical shallow water | Early Cretaceous—Recent | Heterocyanthus, Heteropsammia (symbiont-bearing solitary scleractinian corals) | Gastropod shells inhabited by sipunculid worms and, more rarely, by hermit crabs | [26,28,29,30,31,32] |
| Buckingham, Duplin, Tamiami and Yorktown formations (East Coast, USA) | Pliocene | Septastrea marylandica (colonial scleractinian coral) | Various gastropod shells occupied by secondary inhabitants | [21,22,23,24,25] |
| Yorktown Formation (Virgina, USA) | Pliocene | Astrangia lineata (colonial scleractinian coral) | Shells of the gastropod Crepidula | This work |
| La Serra, Valdelsa Basin (Tuscany, Italy) | Piacenzian | Cladocora caespitosa (symbiont-bearing colonial scleractinian coral) | Shells of muricid gastropods, both living and occupied by secondary inhabitants | This work |
| Port Morrant Formation (Southeastern Jamaica) | Upper Pleistocene | Siderastrea radians (symbiont-bearing colonial scleractinian coral) | A large shell of the gastropod Aliger gigas | [25] |
| Intertidal shelfal sand patches (Great Britain) | Recent | Caryophyllia smithii (non-symbiont-bearing solitary scleractinian coral) | Mainly tubes of the polychaete Ditrupa arietina, but also gastropod shells inhabited by sipunculid worms | [27] |
| Lagoon of the Dongsha Atoll (South China Sea) | Recent | Porites (symbiont-bearing colonial scleractinian coral) | Shells of gastropods inhabited by hermit crabs | [32] |
| Shallow sub-tidal of Glyfada (Athens, Greece) | Recent | Oculina patagonica (symbiont-bearing colonial scleractinian coral) | Shells of a living muricid gastropod of the genus Hexaplex | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coletti, G.; Collareta, A.; Di Cencio, A.; Bosio, G.; Casati, S. Symbiont-Bearing Colonial Corals and Gastropods: An Odd Couple of the Shallow Seas. J. Mar. Sci. Eng. 2023, 11, 260. https://doi.org/10.3390/jmse11020260
Coletti G, Collareta A, Di Cencio A, Bosio G, Casati S. Symbiont-Bearing Colonial Corals and Gastropods: An Odd Couple of the Shallow Seas. Journal of Marine Science and Engineering. 2023; 11(2):260. https://doi.org/10.3390/jmse11020260
Chicago/Turabian StyleColetti, Giovanni, Alberto Collareta, Andrea Di Cencio, Giulia Bosio, and Simone Casati. 2023. "Symbiont-Bearing Colonial Corals and Gastropods: An Odd Couple of the Shallow Seas" Journal of Marine Science and Engineering 11, no. 2: 260. https://doi.org/10.3390/jmse11020260
APA StyleColetti, G., Collareta, A., Di Cencio, A., Bosio, G., & Casati, S. (2023). Symbiont-Bearing Colonial Corals and Gastropods: An Odd Couple of the Shallow Seas. Journal of Marine Science and Engineering, 11(2), 260. https://doi.org/10.3390/jmse11020260









