The term “algae” has no formal taxonomic position. However, it is commonly applied when referring to polyphyletic (i.e., organisms with no common origin but evolving in multiple and independent lineages), random, and rather artificial associations of the lower taxa of photosynthetic eukaryotic organisms assimilating CO2 and releasing O2. Algae include both unicellular and multicellular forms; they may be aquatic or subaerial when exposed to the atmosphere rather than submerged in water. Moreover, they lack many types of cells and tissues that are characteristic of higher plants [1,2]. Algae ensure stability and the sustainable functioning of the freshwater and marine ecosystems. They are the principal agency by which the energy of sunlight is used to convert inorganic substances of low potential chemical energy to organic substances of high potential chemical energy, and other living organisms directly or indirectly depend on their metabolic activity. The ecological diversity of algae is very broad and reflected in a wide range of biochemical adaptations that determine the relationship of an organism with its environment [3].
Algae play a key role in the biogeochemical fate of many chemical elements and in the regulation of their cycling in the aquatic ecosystems [4]. The main part of algae biomass comprises six nutrients (C, O, H, N, S, and P) and minor elements (Ca, K, Na, Cl, Mg, Fe and Si). Other elements (Zn, Mg, Cu, Mn, Mo, Ni, Co, Se, et al.), which are necessary for a variety of catalytic processes, are found in trace amounts. All groups of algae are characterized by multi-element composition; in general, the latter reflects the chemical composition of the habitat. At the same time, some species are characterized by a unique (selective) accumulation of certain elements, whose content in the tissues of algae may be dozens, hundreds, and even thousands of times higher than that in the surrounding environment [5].
Elements included in the organic matter of algae are eventually recycled. The process of converting organics back into inorganic forms of chemical elements is called mineralization; it is the most important ecological aspect of the biogeochemical cycle. Mineralization takes place throughout the entire water column, as well as at/in the bottom of reservoirs, where most of the sedimentary matter of the overlying water masses accumulates over time [6]. Different chemical elements require different time scales, after which they again become metabolically available for algae, and thus, participate in the biogenic cycle (hours; days; months; years, etc.). During the process of primary production in photoautotrophs, differences in the assimilation efficiency of major and trace elements, in the ways of their trophic transfer, and in the mineralization rate of organic matter components, are the key processes of the global biogeochemical cycle performed by algae in freshwater and marine ecosystems [7].
The study of the chemical composition of various groups of algae has a long history, starting in the late XIX to the early XX century [8,9,10,11], and receiving much attention in the 1930–1940s [12]. Initially, these studies focused on determining the major component composition (proteins, fats, carbohydrates, and fiber) of macro- and microalgae, and on assessing of their energy capacity (feed value) for fish. By the middle of the XX century, a large amount of factual material was accumulated describing the features of the chemical composition of various marine organisms, including various taxonomic groups of algae and macrophytes [13]. Fundamental studies of the biochemical composition and biogeochemical role of algae have been widely applied, both in various scientific fields and in practice (Figure 1).
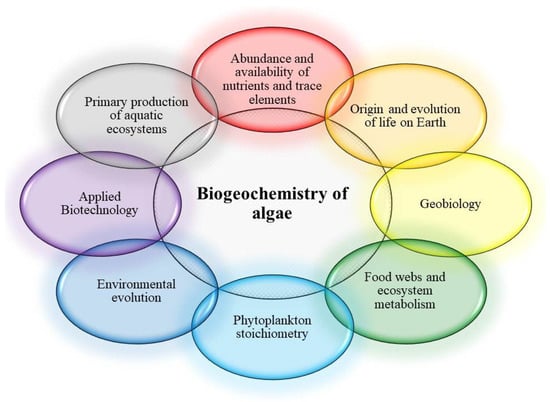
Figure 1.
Main applications of bioinorganic chemistry of algae.
In the 1940–1950s, studies of algae stoichiometry—primarily nutrients (carbon, nitrogen, and phosphorus), as well as trace elements—formed the basis of production hydrobiology [14,15]. These data then served for the development of modern ideas about the factors limiting the primary production in freshwater and marine ecosystems at various geological time scales. Subsequently, algae stoichiometry was widely applied in numerous mathematical models aiming to assess the global and regional cycles of chemical elements in the biosphere [16].
In the 1970–1980s, the development of general theoretical concepts of the geochemistry of sedimentary processes in the ocean became a new stage in the study of the biogeochemistry of algae (mainly plankton microalgae) [17,18,19]. According to these data, algae play a key role in the assimilation, transformation, and sedimentation of chemical elements in the course of their life activity. They change the forms of the occurrence of elements in the environment and increase the time of their residence in the photic layer of aquatic ecosystems [4].
Since the end of the 1980s, studying the role of algae in the regulation of nutrients has found wide application in climate studies. At present, algal climate regulation is called the CLAW hypothesis, which is of great importance for both understanding the cycle of certain nutrients in the biosphere and for assessing the effect of algae on the Earth’s radiation balance and climate in different geological epochs [20].
Currently, the development of so-called “green” and “blue” technologies is one of the key areas of technological development in the world, which enables ensuring the necessary level of economic growth without introducing additional environmental risks [21,22]. The ability of algae to assimilate inorganic forms of chemical elements and convert them into organometallic forms, forming a wide range of biologically active substances, is intensively utilized by human beings. Since ancient times, various groups of algae have served as a source of food, feed, medicines, fertilizers, biosorbents, etc. Modern biotechnology makes it possible to use algae as a source of biologically active forms of trace elements and as producers of inexpensive carotenoids, pigments, proteins, vitamins, and fatty acids for the production of nutraceuticals, pharmaceuticals, food additives, cosmetics, etc. (Table 1) [2,23].

Table 1.
Main applications of algae in biotechnology.
Algae are also being considered as a potential third-generation biofuel source due to their numerous advantages over other crops. The idea of using algae for energy production is quite old. Currently, the cost of producing biofuels from algae is high due to limited cultivation systems. However, over time, new technologies will be developed that allow algae cultivation on a large scale in various climatic zones throughout the year [24].
Algae are able to efficiently assimilate CO2; thus, they are considered promising catchers of the carbon footprint emitted from various anthropogenic sources. One kilogram of dry algae biomass is capable of retaining ~1.8 kg of CO2 [25,26]. Such algae may be grown in wastewater containing high concentrations of nitrogen and phosphorus. The absorption of dissolved biogenic elements necessary for the growth and development of algae ensures high purification of water masses, preventing N and P from entering natural waters, and their subsequent eutrophication [27].
Being able to concentrate various chemical elements from the environment, algae may serve as biological sorbents, promoting the development of biotechnologies for the removal of inorganic contaminants from industrial waters [2,22,23]. Due to their small size and high surface area to volume ratio, they have a large contact area interacting with the metal ions present in solution. Due to the efficiency of heavy metal absorption by algae, the environmental safety of raw materials, relative simplicity and mode of cultivation, using live and/or inactivated algae is currently considered a promising biotechnological approach in water treatment aimed at preventing environmental pollution [22,23].
In recent decades, there has been great concern about the change in regional and global cycles of the major and trace elements, caused primarily by climate change and anthropogenic impact [23,28]. Negative consequences of such changes include eutrophication, hydrogen sulfide contamination, heavy metal pollution, microbial decomposition of frozen carbon pool, etc., which are potential feedback processes affected by global climate dynamics accelerating global warming. In this regard, it is of great interest to study the role of algae in biogeochemical processes—in particular, their response to the changing environmental conditions and the development of environmental monitoring programs [29,30].
Thus, modern studies of the bio(in)organic chemistry of primary producers of aquatic ecosystems serve as a theoretical basis for the development of green biotechnologies in such practical fields as controlled photobiosynthesis, production hydrobiology, the environmental monitoring of aquatic ecosystems, the production of biologically active substances, biofuels, the bioremediation of water bodies, waste and industrial water treatment, the sequestration of CO2 and key nutrients (N and P), as well as the search for alternative, biological methods for the concentration of rare and noble metals and metalloids.
This Special Issue will uniquely focus on the bio(geo)chemical interactions between algae and the environment. We welcome original research papers presenting experimental work, field studies, new methods and equipment, theoretical approaches, and mathematical modeling, in addition to review papers. We especially encourage contributions that use multidisciplinary approaches to explore biogeochemical processes in the natural environment and applied biotechnology.
Funding
This work was performed in the framework of the State assignment of the Ministry of Science and Higher Education of the Russian Federation (theme no. 122042700045-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Evolution of Oxygenic Photosynthesis. Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Pisa, Italy, 2014; 362p. [Google Scholar]
- Yoon, H.S.; Hackett, J.D.; Ciniglia, C.; Pinto, G.; Bhattacharya, D. A Molecular Timeline for the Origin of Photosynthetic Eukaryotes. Mol. Biol. Evol. 2004, 21, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef]
- Lobus, N.V.; Kulikovskiy, M.S.; Maltsev, Y.I. Multi-element composition of diatom Chaetoceros spp. from natural phytoplankton assemblages of the Russian Arctic Seas. Biology 2021, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Romankevich, E.A. Geochemistry of Organic Matter in the Ocean; Springer: Berlin, Germany, 1984; 336p. [Google Scholar]
- Anbar, A.D. Elements and Evolution. Science 2008, 322, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Hensen, V. Uber die Bestimmung des Planktons Oder des im Meere Treibenden Materials an Pflanzen und Thieren. In Fünfter Bericht der Kommission zur Wissenschaftlichen Untersuchung der Deutschen Meere in Kiel für die Jahre 1882 bis 1886; Paul Parey: Berlin, Germany, 1887; pp. 1–109. [Google Scholar]
- Brandt, K. Beiträge zur Kenntniss der chemischen Zusammensetzung des Planktons. In Wissenschaftliche Meeresuntersuchungen Herausgegeben von der Kommission zur Wissenschaftliche Untersuchung der Deutschen Meere in Kiel und der Biologischen Anstalt auf Helgoland; Verlag von Lipsius and Tischen: Kiel/Leipzig, Germany, 1898; pp. 43–90. [Google Scholar]
- Delff, C. Beiträge zur Kenntnis der chemischen zusammensetzung wirbelloser Meerestiere. In Wissenschaftliche Meeresuntersuchungen Herausgegeben von der Kommission zur Wissenschaftliche Untersuchung der Deutschen Meere in Kiel und der Biologischen Anstalt auf Helgoland; Verlag von Lipsius and Tischen: Kiel/Leipzig, Germany, 1912; pp. 51–100. [Google Scholar]
- Meyer, J.A. Beiträge zur Kenntnis der chemischen Zusammensetzung wirbelloser Tiere. In Wissenschaftliche Meeresuntersuchungen Herausgegeben von der Kommission zur Wissenschaftliche Untersuchung der Deutschen Meere in Kiel und der Biologischen Anstalt auf Helgoland; Verlag von Lipsius and Tischen: Kiel/Leipzig, Germany, 1914; pp. 231–286. [Google Scholar]
- Vinogradov, A.P. The chemical composition of marine plankton. In Papers on the Biology and Chemistry of Marine Organisms: Transactions of the Institute of Marine Fisheries and Oceanography of the USSR; VNIRO: Moscow, Russia, 1938; Volume VII, pp. 97–112. [Google Scholar]
- Vinogradov, A.P. The Elementary Chemical Composition of Marine Organisms; Sears Foundation for Marine Research, Yale University: New Haven, CT, USA, 1953; 647p. [Google Scholar]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Flemming, R.H. Composition of Plankton and Units for Reporting Populations and Production. In Proceedings of the Sixth Pacific Scientific Congress, Berkley, CA, USA, 24 July–12 August 1940; pp. 535–540. [Google Scholar]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; 464p. [Google Scholar]
- Lisitzin, A.P.; Vinogradov, M.E. Global Regularities in Distribution of Life in the Ocean and Their Reflexion in the Composition of Bottom Sediments. Formation and Distribution of Biogenic Sediment. Izv. Akad. Nauk SSSR. Ser. Geol. 1982, 1, 5–24. (In Russian) [Google Scholar]
- Martin, J.H.; Knauer, G.A. The elemental composition of plankton. Geochim. Cosmochim. Acta 1973, 37, 1639–1653. [Google Scholar] [CrossRef]
- Boström, K.; Joensuu, O.; Brohm, I. Plankton: Its chemical composition and its significance as a source of pelagic sediments. Chem. Geol. 1974, 14, 255–271. [Google Scholar] [CrossRef]
- Charlson, R.J.; Lovelock, J.E.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Peter, A.P.; Yew, G.Y.; Tang, D.Y.Y.; Koyande, A.K.; Chew, K.W.; Show, P.L. Microalgae’s prospects in attaining sustainable economic and environmental development. J. Biotechnol. 2022, 357, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of Microalgae for Biomaterial Production and Evironmental Applications at Biorefineries. Sustainability 2021, 13, 3063. [Google Scholar] [CrossRef]
- Griffiths, M.; Harrison, S.T.L.; Smit, M.; Maharajh, D. Major Commercial Products from Micro- and Macroalgae. In Algae Biotechnology. Green Energy and Technology; Springer International Publishing AG: Cham, Switzerland, 2016; pp. 269–300. [Google Scholar]
- Bux, F.; Chisti, Y. (Eds.) Algae Biotechnology: Products and Processes; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; 344p. [Google Scholar]
- Passow, U.; Carlson, C.A. The biological pump in a high CO2 world. Mar. Ecol. Prog. Ser. 2012, 470, 249–271. [Google Scholar] [CrossRef]
- Basu, S.; Mackey, K.R.M. Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate. Sustainability 2018, 10, 869. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef] [PubMed]
- Wollast, R.; Mackenzie, F.T. Global Biogeochemical Cycles and Climate. In Climate and Geo-Sciences; Springer: Dordrecht, The Netherlands, 1989; pp. 453–473. [Google Scholar]
- Robinson, C. Phytoplankton Biogeochemical Cycles; Castellani, C., Edward, M., Eds.; Oxford University Press: Oxford, UK, 2017; Volume 1, pp. 42–51. [Google Scholar]
- Litchman, E.; Tezanos Pinto, P.; Edwards, K.F.; Klausmeier, C.A.; Kremer, C.T.; Thomas, M.K. Global biogeochemical impacts of phytoplankton: A trait-based perspective. J. Ecol. 2015, 103, 1384–1396. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).