Abstract
The tree is a fundamental living being. It contributes to nature and climate behaviour, as well to urban greening. It is also a source of wealth and employment. Most tree health inspection techniques are invasive or even destructive. Infrared thermography (IRT) is not invasive, and it has shown advantages when applied for inspection to trees and wood to detect deterioration or voids that could compromise its structure, stability, and durability. This study reviews the literature about IRT applied to a tree health inspection. It is framed in the context of the importance of trees for the balance of ecosystems, and the different techniques to detect tree deterioration. It highlights the difference when applied to wood or trees and the main factors that have been proven to cause disturbances in the thermal pattern of trees. The IRT, as other non-destructive methods, does not distinguish what type of damage it is, nor its causative agent. However, it enables identifying healthy and deteriorated tissues. The technology is very promising since it reveals that is efficient, fast, economical, and sustainable.
1. Introduction
The tree is a fundamental living being of ecosystems [1,2,3]. It contributes to regulating nature, climate, and urban greening [1]. It is also a source of wealth and employment [4]. Some trees are classified as natural and cultural heritage [5]. Trees provide enormous benefits, such as the ecosystems balance, prevention of desertification, and global warming, as well as the well-being of human populations and urban ecosystems [1]. However, trees are also prone to the risk of damage to people and assets associated with the falling of branches/trees, above all in urban spaces [6,7]. Risk increases when they have defects because it compromises their health and stability. Consequently, the safety of people and goods are compromised. To support decision making about trees, it is essential to monitor their health condition to understand their biological viability, cost-effectiveness, and associated risks.
The first discovery of the infrared spectrum was made by Herschel in 1800 during his study about optical filters to reduce the brightness of the sun in telescopes [8]. In his findings, Herschel also observed that these new rays had similar behaviour to the visible ones, i.e., they were absorbed, transmitted, refracted, and reflected [9]. The first thermal image (thermograph) was obtained by Herschel in 1840, by experiments with differential evaporation of a film of oil submitted to a heat pattern [8]. However, the crucial mark in the sensitivity of infrared detection was achieved by Langley in 1880 through the invention of the bolometer. The first infrared camera was developed by Tihanyi in 1929 and was applied for anti-aircraft defense by the British army [10]. The first uses of Infrared thermography (IRT) in trees was the assessment by infrared imaging of the crown condition, using monochrome and false colour films [11]. IRT is a non-contact technology that allows detection of damaged tissue in real-time. It is based on the detection of thermal differences between healthy and damaged zones.
This review paper concerns identifying the IRT effectiveness for inspection of the health of trees. For that purpose, this review was structured as follows. It starts with the research methods and then the importance of trees and their classification. The mechanism that trees use to protect themselves, as well as risks associated with them, and the way they should be managed, is referred to. Some methods and inspection techniques are also mentioned. The importance of sustainable techniques of inspection is highlighted. Principles of IRT, usefulness, and relevance are mentioned. A description of the IRT application for inspection of trees is done, and several experiments are described. The main differences between wood and tree properties are reviewed. Finally, the advantages and limitations associated with the use of IRT are described.
2. Materials and Methods
A database query research paper was carried out in B-on, DOAJ, Google Scholar, Microsoft Academic, ProQuest, SciELO, ScienceDirect, Scopus, and Web of Science. The review was done in Portuguese, English and Spanish. It was carried out between October 2018 and February 2019. The keywords were: Infrared thermography, inspection, inspection techniques, non-destructive, non-invasive, sustainable, monitoring, classification, deterioration detection, relevance, benefits, risks, management, tree, tree heritage, and timber. The keywords were combined with each other. Articles from the reference list of reviewed papers were also taken into account. Whenever combinations of keywords were found, the abstracts and keywords were read. When the abstract proved to be insufficient for classification, the full article was read. The selected articles were classified according to sections, subsections, and theoretical references. The gathered data was organized by type of item, title, authors, abstract, keywords, and classification. These procedures facilitated the identification and analysis of the main references used by the authors, as well as citation frequency. All possible contributions to the application of IRT for health tree monitoring were considered. Duplicate references were withdrawn. A total of 239 papers were collected, of which only 81 papers were selected.
3. Tree Relevance and Risks
In 1950, the world’s urban population was 30%; nowadays, the urban population reached 55%, and by 2050, 68% of the world population is to probjected to live in urban areas [12]. That is, the urbanization in the world population is increasing. Trees and green spaces play important roles in integrating the inhabitants into their urban communities. Trees provide well-being and quality of life; they establish the connection of inhabitants with the natural world and contribute to the mitigation of climate change. Urban trees provide numerous benefits for their inhabitants, but they are also prone to risk [6,13,14].
Trees interact with the surrounding environment, making it more pleasant. The basic function of trees is the improvement of air quality. Trees reduce the rainwater flow, allowing the infiltration into underground aquifer environments. Trees increase biodiversity by providing shelter for wildlife. Trees provide wind protection to people and animals since their leaves alter the direction and the speed of the wind. The tree roots hold the soil, and the tree canopy protects the soil from falling rain. Trees reduce urban noises and promote a sense of calm and rest. Trees create spaces for recreation and tourism. Consequently, trees favour the reduction of stress and even crime, since public open space with trees tends to be used much more than space without trees and this increases surveillance [15]. They influence on the decrease of energy consumption in the summer season, providing shade and a low incidence of solar radiation [16]. Trees have influence in the increasing the property value, if they are in or even outside the property. Trees produce aesthetic benefits because they decorate the urban landscape and enhance the season’ landscape [14,17,18,19,20].
Trees interact in the ecosystem. They are exposed to aggression caused by birds, insects, animals, fire, atmospheric conditions, or even human beings during their activities, and these interactions could result in wounds, which act as entrance doors for pathogenic microorganisms. Trees have developed a system of compartmentalisation known as CODIT (Compartmentalization of Decay in Trees), described by Shigo and Marx [21]. In this system, chemical and physical barriers are created; cells undergo changes to form four types of walls around the wound; the walls are organized in axial, radial, and tangential directions. They act against the proliferation of pathogens by isolating the wounded tissues and thus impeding their dissemination to prevent/mitigate tree deterioration [22]. Wood deterioration and trees ageing are natural processes, but they carry on risks to the tree itself, as well as people and property [23].
On the other hand, some trees are of special interest because of age, size, physical characteristics, as well as cultural and historical connections [5]. Some examples of classification are:
- Heritage: trees that are linked to history and culture; trees that are rare and/or botanically relevant (see for instance Reference [24]).
- Notable: trees that have reached maturity stand out from their surroundings because they are larger than the trees around them (see for instance Reference [25]).
- Ancient: trees that have overpassed the maturity and despite the hollow trunk they continue to be healthy. Their value is intrinsically linked to their age (see for instance Reference [26]).
- Veterans: trees that survived wounds and deterioration (decay); young trees that developed old trees characteristics (see for instance Reference [27]).
Nevertheless, trees are also a risk factor for people and property. Large numbers of imposing trees characterize most urban gardens and avenues. These trees are subjected to undergoing stress hostile environment characteristic of urban centres, and very often under poor management. As a consequence, many are unhealthy, and a damaged tree poses a threat to people safety [11]. Three people die each year as a result of falling trees in public spaces in the United Kingdom. It is a low risk when considered that one in 10 million die as a result of it [6,7]. While there is a low rate of tree incidents, it ends up altering the risk perception. The risk perception of the falling tree has led tree managers (owners and managers of urban green spaces) to knock down trees at the slightest sign of danger. Tree felling is being aggravated, as who is responsible for tree management bear the costs and legal duties related to trees, meanwhile the public benefits of trees without knowing about their management issues [6]. In order to continue providing benefits, it is crucial to managing trees in such a way as to minimize risks and conflicts they may cause [20]. However, the safety of people and goods are not the only factor to take into account. Environmental and aesthetic factors are also considered when assessing costs and benefits [6].
As described above, trees represent enormous environmental and social benefits, but they are also a source of risk. The aim of tree health monitoring is, consequently, multifunctional. There are techniques to tree health inspection that support decision making related to trees management, in which the IRT presents several specific advantages [11].
4. Methods and Techniques of Inspection
VTA (Visual Tree Assessment) is an inspection method for tree diagnosis. It was developed by Mattheck and Breloer (1994), and according to VTA, interprets the body language of trees and provides the expert with failure criteria [28]. The method consists of three steps: a) External visual inspection in search of evidence of internal damage; b) the use of diagnostic tools for confirmation and measurement of damage; and c) assessment of damage extent and failure risk.
4.1. Instruments for Detecting Deterioration
Visual inspection alone does not provide enough evidence of internal damage extent. Then, additional diagnostic tools are required. According to several authors, those tools are classified as:
- Invasive and non-invasive: invasive instruments require drilling for deep penetration in the sapwood through one or more holes; the sapwood is living wood. Non-invasive instruments do not need contact, or they penetrate superficially in the sapwood. It is noted that any type of wound is an entry for pathogens into the tree [29].
- Destructive and non-destructive: Unlike the destructive instruments, non-destructive ones allow the identification of damage presence or not in the trees without causing harm [30]. Then, non-contact instruments are considered as non-destructive. Table 1 shows only non-contact instruments since they were considered non-destructive.
 Table 1. Classification of instruments for detecting deterioration in trees (adapted from Reference [31]).
Table 1. Classification of instruments for detecting deterioration in trees (adapted from Reference [31]). - Screening, diagnostic and evaluation: Screening tools allow a quick assessment to identify healthy and non-healthy trees. Diagnostic instruments allow a more accurate assessment, but require more time to identify the extent and type of damage in the tree. An intermediate method is an evaluation; it is a combination of screening speed and diagnostic accuracy methods [31].
Table 1 summarizes the main instruments for deterioration detection in trees, allowing to relate the purposes of the instruments to the invasive or destructive nature.
4.2. Sustainable Techniques Relevance
Besides IRT, the equipment performance evaluation and its advantages/disadvantages for detecting tree deterioration are briefly described in Table 2, and more details can be found in References [29,31]. It is noted that invasive techniques can increase tree damage [22], and radiation exposure is life detrimental for any living thing [11]. Inspection equipment must be safe for users and people around. It is therefore relevant to choose sustainable equipment. This type of equipment maintains a good relationship between portability, use speed, and they are not invasive or ionising. Besides, they have low running costs due to low energy consumption and low use of consumables [32].

Table 2.
A short summary of the techniques for detecting trees deterioration.
5. Infrared Thermography Applied to Trees
5.1. Principles of IRT
Infrared thermography (IRT) is a non-contact, non-destructive, and non-invasive technique that allows the detection of radiated heat energy from objects and bodies in the infrared range of the electromagnetic spectrum (wavelength range between 0.8–14 µm [43]). The conversion of infrared energy into visible imaging is possible through instruments that produce false-colour images, such as an infrared camera [44]. The principle underlying IRT is a method or equipment, which detects IR energy emitted from a surface, converts it to temperature, and displays the image of temperature field [43,45,46,47,48,49,50]. The fundamental concept behind it is that all bodies (alive and non-alive) have temperatures above absolute zero degrees (0 K) and emit infrared radiation that is captured by equipment capable of transforming that energy into pictures. The internal structure of the body shows different thermal behaviour depending on the health conditions of its parts. The differences in thermal behaviour result in differences in the colour pattern of the body surface image.
Figure 1 illustrates the heat transfer processes involved in a thermographic measurement. When the camera views the object, it receives radiation from the object, from the ambient between the camera and the object’s surface, and from the atmosphere itself [8].
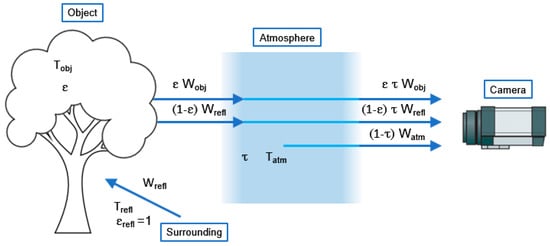
Figure 1.
Schematic representation of a generic thermographic measurement (adapted from [8]).
The total received radiation power that the camera receives is equal to the sum of the emission of the object (ε τ Wobj), the reflected emission of environmental sources ((1 − ε) τ Wrefl), and the emission from the atmosphere ((1 − τ) Watm). This is represented in the following equation (Equation (1)):
where:
Wtot = ε τ Wobj + (1 − ε) τ Wrefl + (1 − τ) Watm
| ε | Object emissivity |
| τ | Air transmittance |
| Wobj | Emission from the object |
| Wrefl | Reflected emission from ambient sources |
| Watm | Emission from the atmosphere |
The appropriate infrared camera selection depends on the characteristics of the object to be inspected, the atmospheric conditions, and the observation distance. Depending on the wavelength in the infrared range of the electromagnetic spectrum, much of the radiation is absorbed by the atmosphere. Lower absorption allows more radiation to reach the camera sensor. It happens at bands: Mid-wavelength infrared (MWIR) between 2 μm and 5 μm, and long-wavelength infrared (LWIR) between 8 μm and 14 μm. Limitations: Atmospheric humidity influences MWIR cameras, and LWIR cameras may capture optical and electronic noise [43].
The object emissivity depends on several factors such as wavelength. The emissivity is also highly sensitive to the nature and type of the material surface. As the emissivity increases, the influence of parasitic reflections diminishes. Metallic materials have low emissivity as they are highly reflective, whereas nonmetallic materials are high emissive and low reflection, leading to a better measurement [43,51]. Handling and interpretation of IRT information need cameras equipped with screens to visualise the energy emitted in the image format. The recording is an accessory that allows comparison between thermograms to follow the evolution of cases/pathologies [11]. It is also advantageous when the camera is equipped to capture thermograms and photographs simultaneously. It is preferably an integrated illumination accessory in the case of low illumination; this feature facilitates data interpretation [52].
There are two main thermographic procedures: Passive and active modes. In the active procedure, an external energy source is used to obtain the thermal contrast into the object of study. Different processes can trigger the heat flow, for example, thermal sources like lamps or heaters. The defects and damages on or near the object surface cause thermal discontinuities producing thermal contrast. It is detected during the thermographic inspection. In the passive procedure, the thermal contrast is generated by natural sources such as sunlight [44,50]. In most of the thermographic application scenarios, it is necessary to introduce an emissivity factor to calibrate the camera temperature measurement. The determination of the correct emissivity value of a specific wood remains a relevant issue [8].
IRT can be applied to both wood and tree plantlets to identify deterioration and voids that may compromise the structure, stability, and durability. Defects within the object disrupt the flow of energy and cause temperature differences on its surface. The thermal contrast results from differences in radiation emission that are registered by the thermal camera [43].
5.2. Wood and Trees
Wood and trees have significantly different thermal characteristics. That is, they differ in the variables that determine the temperature, density, humidity, thermal conductivity and thermal diffusivity, specific heat, convective coefficient, and emissivity [53,54,55].
After determining the density and the remaining properties of a species of wood, it is possible to predict its thermal behaviour. Therefore, when biodegradation changes the density and humidity of the wood, it also changes the thermal behaviour and temperature distribution along the surface [55,56,57,58]. Figure 2 shows the conventional photograph of a wood sample surface and its thermogram obtained in the active IRT procedure. By the active mode, this type of nodes and cracks show colour heterogeneity in relation to the general pattern denoting higher temperature. As shown in Figure 2, the cracks are barely visible in the photograph, but they are well seen in the thermogram [50].
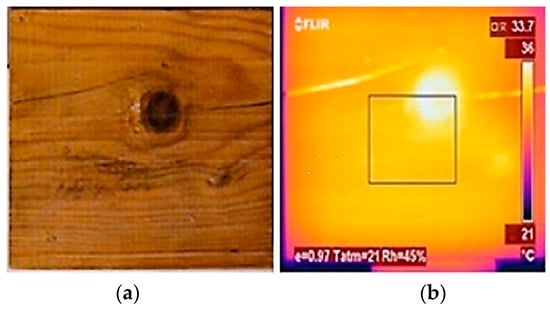
Figure 2.
Pine sample: (a) photograph and (b) IRT thermogram (active mode) [50].
The sap circulation causes the temperature gradient to vary along the trunk, and trees with more available water have better sap circulation [59]. This characteristic allows the differentiation between functional and dysfunctional tissue since the transport is done through the functional tissue. Therefore this feature allows that health and vitality can be verified, however, does not allow extrapolation of its thermal behaviour to the other trees, even of the same species, in order to identify damages. In fact, even for the same type of pathology, trees must be analyzed on a case-by-case basis [52,60]. Actually, two damaged trees of similar species, even when they have the same pathology, can generate different thermal patterns, because the availability of water to which the tree is subject is different and varies the temperature gradient along the trunk/tree. Thus, the temperature pattern, which allows the identification of functional or dysfunctional tissues, is unique for each tree.
Figure 3 shows the difference in surface temperature between a tree and a wooden stake [2]. The tree analysed was a specimen Prunus domestica L. (common name plum-tree) that was observed about 4 h after the sunset in the summer. The authors used an emissivity of 0.97 and a Rainbow colour pallet. During the observation, the atmospheric temperature was 22.5 °C, and the relative humidity was 55%. Most of the wooden stake presented a lower temperature than the tree, except its lower part. The lower part of the wood stake showed a slightly higher temperature compared to the lower trunk because the tree was irrigated before observation. The tree keeps a balanced relationship with the environment temperature, so tree temperatures are usually lower than the atmospheric temperature when the sun heating effect is over [61]. Therefore, the tree in Figure 3 showed higher temperatures in the healthier parts and lower temperatures in the deteriorated parts, as well as in parts where the tree was recently water wet [46].
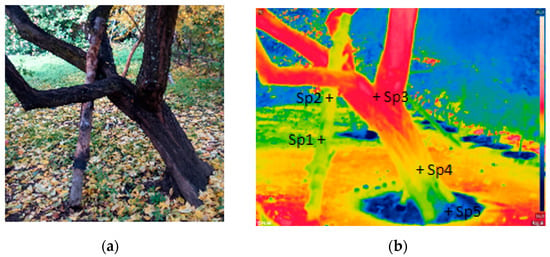
Figure 3.
A tree (Prunus domestica) and a wooden stake that supports it: (a) photograph; (b) IRT thermogram (passive mode). Temperature values (°C): Sp1 = 20.0; Sp2 = 20.5; Sp3 = 21.5; Sp4 = 20.5; and Sp5 = 19.5. Adapted from Reference [2].
IRT is commonly applied to wood in laboratory ambient. In fact, the lab is the first selected scenario because it is possible to control several environmental parameters (humidity, temperature, and luminosity), the thermal energy of the material, as well as the easy manipulation. Some studies in the lab have induced damage in the samples to verify the ability of IRT to detect wood degradation [55,56,62]. In the case of trees, most thermographic studies are performed on trees that are already programmed to be felled or when it is possible to observe the anomalies without causing damage [60,61].
The application of IRT to wood has been used for diverse purposes. Inspection of wood pieces quality in a production line is presented by Reference [63]. The results showed that TIV can be used to mark the debonded areas or to completely remove materials from the assembly line. IRT associated with ultrasonic equipment in the inspection of historic buildings is given by Reference [64]. Oratorio San Felipe Neri in Cadiz, Spain, was analyzed, and the authors conclude that the union of the ultrasound technique and the thermography represents a good tool for wooden structure inspection. IRT associated with ultrasonic equipment, supported by laboratory analyses of timber samples in the inspection of the Aslanhane Mosque in Ankara, Turkey, which can be found in Reference [65]. The combined analysis of these techniques was done to evaluate the condition of the structural elements of wood in terms of their state of preservation, moisture problems, and recent incompatible repairs that affect them. This combination of techniques was reported as useful in assessing the reliability of timber, increasing the accuracy and effectiveness of the survey, facilitating and distinguishing the work of urgent intervention from long-term conservation programs. The detection of termite pests in wood or wood-clad structures is analyzed by Reference [66]. The author points out that TIV enables finding two basic elements that an inspector looks to identify the presence of pest infestation: Areas exhibiting anomalous elements that could be associated with the presence of moisture or hot spots and the presence of subsurface defects. Additionally, in large wooden structures such as a railway bridge where, in addition to other techniques, the IRT was used to identify a rapidly significant amount of structural damage that was not identified by other techniques [67].
5.3. Analysis of Tree Health
The thermal contrast captured by the thermal camera only represents the temperature on the surface of the tree bark. However, the tree bark near deteriorated tissue and voids show a lower temperature than the area around it. As in wood, tree deteriorated tissue and voids undergo a change in their thermal properties. Whenever there are significant differences in the thermal properties of the wood and tree bark, they can be detected by IRT [60,68]. IRT applied to trees and offers a means to differentiate damaged and deteriorated tissue from healthy tissues. However, due to the specific characteristics of each species, whenever a species is evaluated for the first time, it is advisable to observe the representation of the thermal pattern of the tree bark in the thermal image for better results interpretation [11].
The first use of IRT in trees was for aerial surveillance of the canopies to detect the distribution and spread of forest damage [60]. Later, IRT was applied to tree bark (trunks and branches), and it is now possible to evaluate some types of damage in the lower trunk and to deduce the cause that affects roots (root system) [60,69].
Several factors affect tree health, and consequently, can change the thermal properties of the tree trunk and branches. Some of the conditions that IRT can identify are described below, namely, diseases, pest attack, water stress, and formation of new functional tissue.
Regarding diseases, the application of IRT in the detection of tree bark lesions such as hemorrhagic cancers caused by Phytophthora spp., even before its consequences can be detected with the naked eye [60,70]. Fungi such as Inonotus hispidus (Bull.) P. Karst., Phellinus punctatus (Fr.) Pilát, Coriolus pubescens (Schumach.) and Corticium sp. were found in samples taken from the woody material of platan previously analyzed by IRT [71].
IRT can also be used for detection of insect pests on trees. Insects attack both leaves for feeding as well as trunks for their habitat, feeding, and egg-laying. While trees have a certain tolerance to pest attack on their leaves, it repeated attacks and factors such as poor irrigation, flooding, and inadequate trimming generate greater stress on the tree and consequently compromise its ability to regenerate or resist. On the other hand, larvae and insects create wounds, and these wounds make the trees susceptible to other pathogens. Some larvae can dig deep into the trunk creating tunnels that ultimately affect the transportation of nutrients and water causing rapid tree deterioration. Some of the insects recently detected by IRT were: White Pine Cone Beetle, Australian ‘fire-beetle’, Citrus Long-horned Beetle, Woodworm, and Red Palm Weevil (RPW) [72]. However, the IRT was not able to detect larval stage insects in some young tree species, as in the case of goat moth larvae. A hypothesis was the thermal adaptation of the larvae to the environment, that even with the elevation of temperature due to the effect of the activity of the larvae, it was not sufficient to detect them [73].
IRT is also applied for trees water stress assessment. The temperature in the canopy is an indicator of the amount of water available in the soil because the leaves transpiration is a mechanism to dissipate excess energy (heat). The tree uses surplus energy to transform water into water vapour through the leaves to cool it. However, when the soil is in water shortage, the tree perspires less, and as a consequence, there is an increase in leaf temperature when compared to trees in soil adequately wet. Water stress is a relevant indicator because the monitoring provides information for the adequate water supply of each tree species [74,75,76,77].
IRT detects the formations of new functional tissue. It was confirmed after dissection that the thermogram showed a band of higher wood formation in the area of the thermogram. This happens as a consequence of the adaptive growth of the change, because as Shigo (1984) has defined, the vascular cambium is the cell generator, and the new cells have different thermal properties from the other tissues [22]. This formation of new cells has different thermal properties than the other tissues. This tree had emptiness along the trunk that could also be observed in the thermogram. Catena and Catena [60] suggest that an adequate prognosis of the actual state of the tree requires the identification of the cause for tissue forming, that is, as result of damage and/or mechanical stress.
5.4. Advantages and Limitations of IRT Applicated to Trees
It is an advantage that IRT allows the tree to be observed as a whole, then the damage is early identified, even in trees that do not yet have external visual signs [52]. IRT requires little time to perform the inspection and is relatively easy to interpret the results. It does not require contact with the tree, and observations can be made at a distance of up to 25 m (depending on the camera and lens) safely. It is a safe technology for both the trees and the examiner as it does not emit harmful radiation. It is not necessary to use ladders or lifting platforms to observe the high parts of the tree; it reduces time and costs [60,68,78]. IRT also allows real-time assessment of root damage by observation of the lower trunk. It is a tool for stability and safety evaluation [69,79]. It eases to monitor the health status of trees over time, following up the evolution of pathologies previously identified as well as the identification of new patterns that indicate the development of new pathologies [69]. However, the major advantage of IRT compared to other inspection methods is the ability to differentiate functional tissue from dysfunctional tissue. In fact, IRT provides information for analysing the vitality and health status of a tree in a non-destructive, rapid, and cost-effective manner [60]. In essence, agriculture has been mechanized by the green revolution and now, like any other industry, agriculture is being digitalized [80]. According to this study, most farmers are ready to accept technology if it is profitable, less complex, and makes their life easier. IRT may be one of the relevant components of smart farming and agriculture, as can better manage and help to carry out real-time events.
As in other non-invasive methods, the main limitation is that IRT does not identify whether the damage detected is a void or a deteriorated tissue, nor its causative agent. Nor can it give precise indications of the magnitude of the damage. However, the fact that IRT identifies damaged areas early, it reduces the tests time and thus the damage progression. The acquired data leads to a more precise investigation of the pathologies, and identify the locations where invasive techniques are required [11,81]. Interferences are observed in thermograms when the surface of the tree is obscured by mosses or other vegetation, and when the tree is wet, or when IRT has been carried out directly to sunlight. Some of these limitations can be overpassed by avoiding inspections after rainy days or carry them out before the trees are watered. In the case of sunlight, the tests should preferably be performed at night. Another commonly noted limitation is the cost of the equipment when compared to simpler equipment. This is not the case when compared to more sophisticated equipment, or when multiple applications beyond the inspection of tree health are taken into account [60,68,78].
A brief search result summary of IRT applied to tree health analysis is presented in Table 3.

Table 3.
A short summary of infrared thermography applied to tree health analysis.
6. Conclusions
The IRT was presented as a tool to inspect tree health, and it has proved to be an efficient tool in the early detection of damages even though it is not possible to identify the type of damage. As well as other non-invasive and non-destructive techniques, the causative agent can be identified when resorting to invasive methods. However, the comparison of inspection methods has shown that IRT has great advantages in terms of the capacity to differentiate functional tissue from dysfunctional tissue, and thus to inspect the vitality and health status of trees. It allows monitoring the evolution of pathologies in a fast, economical, and non-destructive way. Result improvement can be achieved with the evolution and increased application of IRT.
7. Recommendations
Despite its merits, IRT is still a relatively new technique in assessing tree health and remains residually implemented in agriculture, but it needs more detailed studies to establish a solid application basis, which can guide practitioners. This would allow developing the IRT potential for applicability on a larger scale. Some challenges such as the complexity of the technique, the new and atypical aspects of the problem, and several knowledge gaps related to technical issues and applicability specificities the affect of the technique performance. The latest high-definition thermal cameras record thermal images of high resolution and sensitivity will also contribute to overcoming these challenges, which will help to turn the IRT technique as a decision-making tool to assess the health status of trees.
Author Contributions
D.V. and R.P. developed the methodology, carried out the review, made the analysis and wrote the manuscript.
Funding
This work is framed in the activities of the “TreeM—Trees Advanced Monitoring & Maintenance” project No. 023831, 02/SAICT/2016, co-funded by CENTRO 2020 and FCT/Portugal 2020, and EU-ERDF structural funds.
Acknowledgments
The authors would thank João Crisóstomo for technical support and some study materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pitarma, R.; Crisóstomo, J.; Ferreira, M.E. Learning About Trees in Primary Education: Potentiality of IRT Technology in Science Teaching. In Proceedings of the EDULEARN18 Conference, Palma, Spain, 2–4 July 2018; pp. 208–213. [Google Scholar]
- Ferreira, M.; Crisóstomo, J.; Pitarma, R. Infrared thermography technology to support science teaching-meaningful learning about trees with university students. In Proceedings of the 13th International Technology, Education and Development Conference (INTED2019), Valencia, Spain, 11–13 March 2019. [Google Scholar]
- Ferreira, M.E.; André, A.C.; Pitarma, R. Potentialities of Thermography in Ecocentric Education of Children: An Experience on Training of Future Primary Teachers. Sustainability 2019, 11, 2668. [Google Scholar] [CrossRef]
- Lier, M.; Parviainen, J. Integration of Nature Protection in Forest Policy in Finland; INTEGRATE Country Report; EFICENT-OEF: Freiburg, Germany, 2013. [Google Scholar]
- Ancient Tree Forum & The Woodland Trust. Ancient Tree Guide No. 4: What Are Ancient, Veteran and Other Trees of Special Interest; The Woodland Trust: Grantham, UK, 2008. [Google Scholar]
- National Tree Safety Group. Common Sense Risk Management of Trees: Guidance on Trees and Public Safety in the UK for Owners, Managers and Advisers; Forestry Commission: Edinburgh, Scotland, 2011; ISBN 978-0-85538-840-9. [Google Scholar]
- Health and Safety Executive. Management of the Risk from Falling Trees; Health & Safety Executive/Local Authorities Enforcement Liaison Committee (HELA): Bootle, UK, 2007.
- FLIR Tools+ User´s Guide; Flir Systems, Inc.: Wilsonville, OR, USA, 2016.
- Barr, E.S. Historical Survey of the Early Development of the Infrared Spectral Region. Am. J. Phys. 1960, 28, 42–54. [Google Scholar] [CrossRef]
- Kylili, A.; Fokaides, P.A.; Christou, P.; Kalogirou, S.A. Infrared thermography (IRT) applications for building diagnostics: A review. Appl. Energy 2014, 134, 531–549. [Google Scholar] [CrossRef]
- Catena, A. Thermography Reveals Hidden Tree Decay. Arboric. J. 2003, 27, 27–42. [Google Scholar] [CrossRef]
- United Nations. World Urbanization Prospects: The 2018 Revision; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2018. [Google Scholar]
- Pokorny, J.; O’Brien, J.; Hauer, R.; Johnson, G.; Albers, J.; Bedker, P.; Mielke, M. Urban Tree Risk Management: A Community Guide to Program Design and Implementation; USDA Forest Service Northeastern Area State and Private Forestr: St. Paul, MN, USA, 2003.
- Rotherham, I.D. Editorial: Trees In A Changing World. Arboric J. 2010, 33, 1–2. [Google Scholar] [CrossRef]
- Kuo, F.E.; Sullivan, W.C. Environment and Crime in the Inner City: Does Vegetation Reduce Crime? Environ. Behav. 2001, 33, 343–367. [Google Scholar] [CrossRef]
- Coder, K.D. Identified Benefits of Community Trees and Forests; University of Georgia School of Forest Resources: Athens, GA, USA, 1996. [Google Scholar]
- International Society of Arboriculture Benefits of Trees; International Society of Arboriculture: Champaign, IL, USA, 2011.
- Roy, S.; Byrne, J.; Pickering, C. A systematic quantitative review of urban tree benefits, costs, and assessment methods across cities in different climatic zones. Urban For. Urban Green. 2012, 11, 351–363. [Google Scholar] [CrossRef]
- Song, X.P.; Tan, P.Y.; Edwards, P.; Richards, D. The economic benefits and costs of trees in urban forest stewardship: A systematic review. Urban For. Urban Green. 2018, 29, 162–170. [Google Scholar] [CrossRef]
- Johnston, M.; Hirons, A. Urban Trees. In Horticulture: Plants for People and Places; Dixon, G.R., Aldous, D.E., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, ISBN 978-94-017-8580-8. [Google Scholar]
- Shigo, A.L.; Marx, H.G. Compartmentalization of Decay in Trees; U. S. Government Printing Office: Washington, DC, USA, 1977; pp. 4–15.
- Shigo, A.L. Compartmentalization: A Conceptual Framework for Understanding How Trees Grow and Defend Themselves. Annu. Rev. Phytopathol. 1984, 22, 189–214. [Google Scholar] [CrossRef]
- Shortle, W.C.; Dudzik, K.R. Wood Decay in Living and Dead Trees: A Pictorial Overview; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2012. [Google Scholar]
- Sherwood Forest. Available online: https://www.visitsherwood.co.uk/things-to-do/the-major-oak/ (accessed on 18 June 2019).
- Undiscovered Scotland. Available online: https://www.undiscoveredscotland.co.uk/blairgowrie/meikleourhedge/index.html (accessed on 18 June 2019).
- Woodland Trust. Available online: https://www.woodlandtrust.org.uk/visiting-woods/trees-woods-and-wildlife/woodland-habitats/ancient-trees/ (accessed on 18 June 2019).
- National Trust. Available online: https://www.nationaltrust.org.uk/ashridge-estate/features/looking-after-our-veteran-trees-at-ashridge-estate (accessed on 18 June 2019).
- Mattheck, C.; Breloer, H. Field guide for visual tree assessment (Vta). Arboric. J. 1994, 18, 1–23. [Google Scholar] [CrossRef]
- Goh, C.L.; Abdul Rahim, R.; Fazalul Rahiman, M.H.; Mohamad Talib, M.T.; Tee, Z.C. Sensing wood decay in standing trees: A review. Sens. Actuators A Phys. 2018, 269, 276–282. [Google Scholar] [CrossRef]
- Hellier, C. Introduction to Nondestructive Testisng. In Handbook of Nondestructive Evaluation; Hellier, C., Ed.; McGraw-Hill: New York, NY, USA, 2003; ISBN 978-0-07-139947-0. [Google Scholar]
- Leong, E.-C.; Burcham, D.C.; Fong, Y.-K. A purposeful classification of tree decay detection tools. Arboric. J. 2012, 34, 91–115. [Google Scholar] [CrossRef]
- Crisóstomo, J.; Pereira, C.; Roque, E.; Jorge, L.; Pitarma, R. Considerações na Observação do Estado de Salubridade de Árvores Através da Termografia por Infravermelhos. In Proceedings of the 1st Iberic Conference on Theoretical and Experimental Mechanics and Materials/11th National Congress on Experimental Mechanics, Porto, Portugal, 4–7 November 2018; pp. 745–748. [Google Scholar]
- Mattheck, C.; Bethge, K.; Albrecht, W. How To Read The Results Of Resistograth M. Arboric. J. 1997, 21, 331–346. [Google Scholar] [CrossRef]
- Shigo, A.L.; Shortle, W.C. Spruce Budworms Handbook: Shigometry–A Reference Guide; Agric. Handb.; U.S. Department of Agriculture, Forest Service, Cooperative State Research Service: Washington, DC, USA, 1985.
- Monk, B. Evaluation of Decay Detection Equipment in Standing Trees. Available online: https://www.fs.fed.us/t-d/programs/im/tree_decay/tree_decay_detect_equip.shtml (accessed on 17 June 2019).
- Ross, R.J.; Pellerin, R.F. Inspection of Timber Structures Using Stress Wave Timing Nondestructive Evaluation Tools. In Wood and Timber Condition Assessment Manual: Second Edition; White, R.H., Ross, R.J., Eds.; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2014. [Google Scholar]
- Oliva, J.; Romeralo, C.; Stenlid, J. Accuracy of the Rotfinder instrument in detecting decay on Norway spruce (Picea abies) trees. For. Ecol. Manag. 2011, 262, 1378–1386. [Google Scholar] [CrossRef]
- Nicolotti, G.; Socco, L.V.; Martinis, R.; Godio, A.; Sambuelli, L. Application And Comparison Of Three Tomographic Techniques For Detection Of Decay In Trees. J. Arboric. 2003, 29, 66–78. [Google Scholar]
- Bogosanovic, M.; Al Anbuky, A.; Emms, G.W. Overview and comparison of microwave noncontact wood measurement techniques. J. Wood Sci. 2010, 56, 357–365. [Google Scholar] [CrossRef]
- Wang, P.C.; Chang, S.J. Nuclear Magnetic Resonance Imaging of Wood. Wood Fiber Sci. 1986, 18, 308–314. [Google Scholar]
- Baietto, M.; Wilson, A.; Bassi, D.; Ferrini, F. Evaluation of Three Electronic Noses for Detecting Incipient Wood Decay. Sensors 2010, 10, 1062–1092. [Google Scholar] [CrossRef]
- Habermehl, A.; Ridder, H.-W. Computerised Tomographic Investigationa Of Street And Park Trees. Arboric. J. 1995, 19, 419–437. [Google Scholar] [CrossRef]
- Usamentiaga, R.; Venegas, P.; Guerediaga, J.; Vega, L.; Molleda, J.; Bulnes, F. Infrared Thermography for Temperature Measurement and Non-Destructive Testing. Sensors 2014, 14, 12305–12348. [Google Scholar] [CrossRef]
- Maldague, X.P.V.; Streckert, H.H.; Trimm, M.W. Introduction to Infrared and Thermal Testing. In Infrared and Thermal Testing; Maldague, X.P.V., Moore, P.O., Eds.; Nondestructive Testing Handbook; American Society for Nondestructive Testing: Columbus, OH, USA, 2001; ISBN 978-1-57117-044-6. [Google Scholar]
- Snell, J.R., Jr. Thermal Infrared Testing. In Handbook of Nondestructive Evaluation; Hellier, C., Ed.; McGraw-Hill: New York, NY, USA, 2003; ISBN 978-0-07-139947-0. [Google Scholar]
- Meola, C. (Ed.) Carosena Origin and Theory of Infrared Thermography. In Infrared Thermography Recent Advances and Future Trends; Bentham Science Publishers: New York, NY, USA, 2012; pp. 3–28. ISBN 978-1-60805-143-4. [Google Scholar]
- Ibarra-Castanedo, C.; Maldague, X.P.V. Infrared Thermography. In Handbook of Technical Diagnostics; Czichos, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 175–220. ISBN 978-3-642-25849-7. [Google Scholar]
- Holst, G.C. Common Sense Approach to Thermal Imaging; JCD Pub.; Co-Published by SPIE Optical Engineering Press: Winter Park, FL, USA; Bellingham, WA, USA, 2000; ISBN 978-0-9640000-7-0. [Google Scholar]
- Pitarma, R.; Crisóstomo, J.; Jorge, L. Analysis of Materials Emissivity Based on Image Software. In New Advances in Information Systems and Technologies; Rocha, Á., Correia, A.M., Adeli, H., Reis, L.P., Mendonça Teixeira, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 444, pp. 749–757. ISBN 978-3-319-31231-6. [Google Scholar]
- Crisóstomo, J.; Pitarma, R. The Importance of Emissivity on Monitoring and Conservation of Wooden Structures Using Infrared Thermography. In Advances in Structural Health Monitoring; Hassan, M., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Maldague, X.P.V. Nondestructive Evaluation of Materials by Infrared Thermography; Springer: London, UK, 1993; ISBN 978-1-4471-1997-5. [Google Scholar]
- Crisóstomo, J.; Pereira, C.; Roque, E.; Jorge, L.; Pitarma, R. Análise da Salubridade de Árvores Através da Termografia por Infravermelhos; Gomes, J.F.S., Ed.; INEGI/FEUP: Porto, Portugal, 2018; pp. 749–758. [Google Scholar]
- Meola, C.; Carlomagno, G.M. Recent advances in the use of infrared thermography. Meas. Sci. Technol. 2004, 15, R27–R58. [Google Scholar] [CrossRef]
- Wyckhuyse, A.; Maldague, X. A Study of Wood Inspection by Infrared Thermography, Part I: Wood Pole Inspection by Infrared Thermography. Res. Nondestruct. Eval. 2001, 13, 1–12. [Google Scholar] [CrossRef]
- Conde, M.J.M.; Liñán, C.R.; de Hita, P.R.; Gálvez, F.P. Infrared Thermography Applied to Wood. Res. Nondestruct. Eval. 2012, 23, 32–45. [Google Scholar] [CrossRef]
- Rodríguez-Liñán, C.; Morales-Conde, M.J.; Rubio-de Hita, P.; Pérez-Gálvez, F. Análisis sobre la influencia de la densidad en la termografía de infrarrojos y el alcance de esta técnica en la detección de defectos internos en la madera. Mater. de Construcción 2012, 62, 99–113. [Google Scholar] [CrossRef][Green Version]
- Tanaka, T.; Divós, F. Wood Inspection by Thermography. In Proceedings of the 12th International Symposium on Nondestructive Testing, Sopron, Hungary, 13–15 September 2000. [Google Scholar]
- Pereira, J.C.A. Contribuição para a Análise de Manifestações Patológicas em Madeira na Construção com Recurso à Termografia; Instituto Politécnico de Castelo Branco: Castelo Branco, Portugal, 2014. [Google Scholar]
- Burcham, D.C.; Leong, E.-C.; Fong, Y.-K. Passive infrared camera measurements demonstrate modest effect of mechanically induced internal voids on Dracaena fragrans stem temperature. Urban For. Urban Green. 2012, 11, 169–178. [Google Scholar] [CrossRef]
- Catena, A.; Catena, G. Overview of Thermal Imaging For Tree Assessment. Arboric. J. 2008, 30, 259–270. [Google Scholar] [CrossRef]
- Bellett-Travers, M.; Morris, S. The Relationship Between Surface Temperature And Radial Wood Thickness Of Twelve Trees Harvested In Nottinghamshire. Arboric. J. 2010, 33, 15–26. [Google Scholar] [CrossRef]
- López, G.; Basterra, L.-A.; Ramón-Cueto, G.; Diego, A. de Detection of Singularities and Subsurface Defects in Wood by Infrared Thermography. Int. J. Archit. Herit. 2014, 8, 517–536. [Google Scholar] [CrossRef]
- Meinlschmidt, P. Thermographic Detection of Defects in Wood and Wood-based Materials. In Proceedings of the 14th International Symposium of Nondestructive Testing of Wood, Hannover, Germany, 2–4 May 2005. [Google Scholar]
- Rodríguez Liñán, C.; Morales Conde, M.J.; Rubio de Hita, P.; Pérez Gálvez, F. Inspección mediante técnicas no destructivas de un edificio histórico: Oratorio San Felipe Neri (Cádiz). Inf. de la Construcción 2011, 63, 13–22. [Google Scholar] [CrossRef]
- Kandemir-Yucel, A.; Tavukcuoglu, A.; Caner-Saltik, E.N. In situ assessment of structural timber elements of a historic building by infrared thermography and ultrasonic velocity. Infrared Phys. Technol. 2007, 49, 243–248. [Google Scholar] [CrossRef]
- Grossman, J.L. Advanced techniques in IR thermography as a tool for the pest management professional. In Proceedings of the SPIE, Orlando, FL, USA, 18 April 2006; Volume 6205. [Google Scholar]
- Grossman, J.L. Trestles anyone? A Thermographic Nightmare. In Proceedings of the SPIE, Orlando, FL, USA, 9 April 2007; Volume 6541. [Google Scholar]
- Catena, G. A new application of thermography. Atti Della Fond. Giorgio Ronchi 1990, 6, 947–952. [Google Scholar]
- Catena, A.; Catena, G.; Lugaresi, D.; Gasperoni, R. La Termografia rivela la presenza di danni anche nell’apparato radicale degli alberi. Agric. Ric. 2002, 81–100. [Google Scholar]
- Burcham, D.C.; Leong, E.-C.; Fong, Y.-K.; Tan, P.Y. An Evaluation of Internal Defects and Their Effect on Trunk Surface Temperature in Casuarina equisetifolia L. (Casuarinaceae). Arboric. Urban For. 2012, 38, 277–286. [Google Scholar]
- Catena, G. Une Appication De La Thermographie En Phytopathologie. Phytoma-La Défense Des Végétaux 1992, 439, 46–48. [Google Scholar]
- Al-doski, J.; Mansor, S.B.; Shafri, H.Z.B.M. Thermal Imaging For Pests Detecting-A Review. Int. J. Agric. For. Plant. 2016, 2, 10–30. [Google Scholar]
- Hoffmann, N.; Schröder, T.; Schlüter, F.; Meinlschmidt, P. Potenzial von Infrarotthermographie zur Detektion von Insektenstadien und -schäden in Jungbäumen. J. Für Kult. 2013, 65, 2013. [Google Scholar]
- Ballester, C.; Jiménez-Bello, M.A.; Castel, J.R.; Intrigliolo, D.S. Usefulness of thermography for plant water stress detection in citrus and persimmon trees. Agric. For. Meteorol. 2013, 168, 120–129. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978. [Google Scholar] [CrossRef]
- García-Tejero, I.; Durán-Zuazo, V.H.; Arriaga, J.; Hernández, A.; Vélez, L.M.; Muriel-Fernández, J.L. Approach to assess infrared thermal imaging of almond trees under water-stress conditions. Fruits 2012, 67, 463–474. [Google Scholar] [CrossRef]
- Giuliani, R.; Flore, J.A. Potential Use Of Infra-Red Thermometry For The Detection Of Water Stress In Apple Trees. Acta Hortic. 2000, 537, 383–392. [Google Scholar] [CrossRef]
- Ibarra-Castanedo, C.; Tarpani, J.R.; Maldague, X.P.V. Nondestructive testing with thermography. Eur. J. Phys. 2013, 34, S91–S109. [Google Scholar] [CrossRef]
- Catena, A. Thermography Shows Damaged Tissue and Cavities Present in Trees. In Nondestructive Characterization of Materials XI.; Green, R.E., Djordjevic, B.B., Hentschel, M.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 515–522. ISBN 978-3-540-40154-4. [Google Scholar]
- Sharma, S.; Kaushik, A. Views of Irish Farmers on Smart Farming Technologies: An Observational Study. AgriEngineering 2019, 1, 164–187. [Google Scholar]
- Bellett-Travers, M. A Risk Assessment Methodology For Trees In Parkland Based On Comparative Population Analysis. Arboric. J. 2010, 33, 3–14. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).