Abstract
The rhizosphere, a confined area of soil plant roots, is an intersection of microbial activity and root exudates. Known as the rhizosphere effect, it enhances crop yield and sustainability by improving nutrient availability, beneficial compounds, and pathogen control. This study combines a field-based rhizosphere–bulk soil comparison for peanut with a geostatistical approach to quantify the spatial variability of rhizosphere-driven changes in soil quality indicators in the Ghardaïa region (southern Algeria), which is known for its sandy–clay and sandy–loam soils. Samples of rhizosphere and bulk soils were prospected using a systematic plan. Subsequently, the pH, electrical conductivity, calcium carbonate, organic matter, total nitrogen, available phosphorus, total potassium, and soluble sodium were determined for each soil (rhizosphere and bulk soil). To assess the spatial variability of rhizosphere soil parameters, semi-variograms of the fitted models were generated using auto-kriging. The results showed that both types of soils were moderately alkaline, with a reduction of 5.52% in the pH of the rhizosphere compared to the bulk soils. Soils were relatively low in organic matter, with only 3.3% of soils having organic matter levels above 20 g kg−1. However, organic matter contents were consistently higher in the rhizosphere (8.51 ± 4.59 g kg−1) than in the bulk soil (6.78 ± 3.52 g kg−1). In the rhizosphere, an increase of 10% in labile phosphorus was noted. Total nitrogen was increased by 52.57%. T-tests suggested no significant difference in potassium and sodium levels, and they were moderately present in both soils. Significantly positive relationships were noted between available phosphorus and total nitrogen (R = 0.59, p < 0.001). However, negative correlations were revealed between pH and organic matter available phosphorus (R = −0.77, p < 0.001) and pH and total nitrogen (R = −0.56, p < 0.01). These results indicate the effects of rhizosphere interactions on soil property improvements and their implications for sustainable agricultural practices, including crop rotation, intercropping, and green manure applications.
1. Introduction
The rhizosphere is the narrow zone of soil directly influenced by plant roots and exudates, where nutrient cycling and microbial activity occur [1]. In leguminous crops, rhizosphere processes are especially important. They support symbiotic nitrogen fixation, beneficial microbial communities, and nutrient uptake under challenging soil conditions [2,3].
Peanut (Arachis hypogaea L.) is globally recognized as one of the major oilseed and protein crops, with significant agronomic, nutritional, and economic value, particularly in arid and semi-arid environments, by creating income at the farm level and also powering a whole chain of businesses (transport, processing, trade, and manufacturing) [4]. Its ability to fix atmospheric nitrogen via symbiosis and its adaptation to marginal soils make it an important crop for sustainable agriculture in dryland regions. The strains nodulating peanuts belong to Bradyrhizobium sp., several of which have been identified [5]. In these regions, however, soil physical and chemical constraints (e.g., low fertility, high pH or salinity, nutrient imbalance) frequently limit crop yield and quality. Recent research demonstrated that soil type (acidic, neutral, saline–alkaline) substantially influences both rhizosphere physicochemical properties and associated microbial communities in peanut cultivation [6,7,8].
Considering the sensitivity of rhizosphere processes to soil environmental conditions, understanding how soil properties (e.g., pH, electrical conductivity, organic matter, available nutrients, and enzyme activities) differ between the rhizosphere and bulk soil under field conditions is essential for optimizing peanut productivity and soil management. A field-based metagenomic and soil chemical study revealed that peanut rhizosphere samples were enriched in specific microbial taxa (Proteobacteria, Firmicutes, and Bacteroidota) and that rhizosphere pH and electrical conductivity significantly correlated with microbial community composition [9]. Other research has evolved from focusing on physiological responses to drought and nutrient uptake [10] to exploring microbial community dynamics and soil amendments that influence peanut productivity [11,12].
The spatial variability of soil properties is an important feature of contemporary agricultural research and environmental management. Comprehending the variability of soil qualities across landscapes is crucial for executing effective precision agriculture methods, optimizing resource utilization, and sustaining agricultural systems. Mapping spatial variability might enhance sustainable agroecosystem management by pinpointing regions necessitating particular interventions, such as targeted fertilization or soil amendments [13,14,15]. Local observations in the Sebseb region indicate that soils in places where peanuts are cultivated demonstrate enhanced fertility, typically facilitating improved crop development and yield. Zhang et al. [16] found that peanut enhances soil water availability, improves soil nutrient composition (organic matter, total carbon, total nitrogen, and accessible phosphorus), and promotes understory vegetation in arid ecosystems. Despite its significance, the interactions between peanut cultivation and soil characteristics, together with the geographical variability of these parameters, are still inadequately comprehended.
In southern Algeria and other arid regions, soil heterogeneity, low nutrient availability, and high evaporation further constrain peanut cultivation, yet field-based rhizosphere studies remain limited. Targeted research in these environments helps clarify the rhizosphere effect, identify key limiting soil factors, and guide site-specific soil management strategies to enhance peanut yield and soil health.
Therefore, this study aims to (i) quantify the physicochemical properties of peanut (Arachis hypogaea L.) rhizosphere soils under field conditions in southern Algeria, (ii) evaluate their spatial variability across arid-zone peanut fields, (iii) identify relationships between soil fertility indicators and cultivation conditions, and (iv) provide baseline data to inform site-specific soil management strategies in Saharan agroecosystems.
2. Materials and Methods
2.1. Study Area
The research was conducted in the Sebseb region, situated in Ghardaïa Province, southern Algeria (32°15′ N, 3°26′ E) (Figure 1). The region is characterized by a Lithosol class and exhibits a dry desert hot arid climate (BWh) [17], marked by substantial diurnal and seasonal temperature variations. The warm season spans from May to September, with July as the peak month, averaging 36.3 °C and reaching 47 °C. Conversely, January represents the coldest month, exhibiting an average temperature of 9.2 °C and an absolute minimum of −1 °C. Annual precipitation is limited and inconsistent, ranging from 13 to 68 mm and distributed over an average of 15 days. The region experiences an average annual rainfall of approximately 74.2 mm, with mean maximum and minimum temperatures recorded at 28.5 °C and 16.2 °C, respectively [18]. Soils develop on alluvial and aeolian deposits typical of Saharan environments. The parent geology comprises sedimentary rocks of the northern Sahara platform, including abundant limestone, dolomite, gypsum, and other evaporite minerals, which contribute to high dissolved mineral loads in soils and groundwater. Almost all the farms surveyed are equipped with drip or pivot irrigation systems, utilizing water from the groundwater system that interacts with the continental intercalary (from 250 to 1000 m) and phreatic groundwater systems (5–30 m) [19,20].
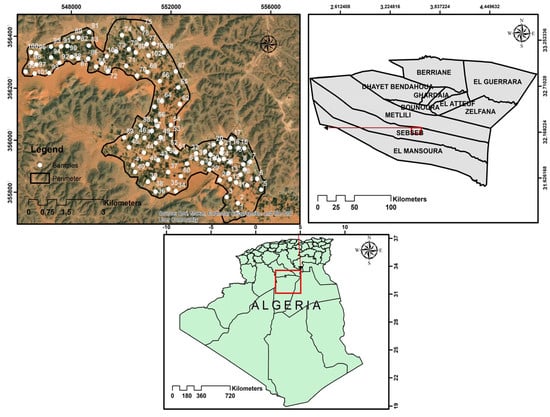
Figure 1.
Map of study area, rhizosphere, and bulk soil sampling.
2.2. Soil Sampling
A total of one hundred and three (103) soil samples for each type (rhizosphere and bulk soil) were collected during two years, 2023 and 2024. The rhizosphere soil samples (root-adhering soil) were collected after uprooting the peanut plant and shaking off the loose soil. Soil still stuck to the roots was gathered by brushing. The amount of soil recovered varies from 0.8 to 1.2 kg, depending on each plant’s root system. Bulk soil samples were obtained from outside the rooting area, near the same peanut plant, at a distance of 40–60 cm.
Furthermore, soil sampling was conducted in 03 replicates for each soil type and site. In all the sampled plots, farmers cultivate a local peanut genotype SEB 47026 belonging to the hypogaea subspecies.
2.3. Soil Analysis
Soil samples were subjected to sieving using a 2 mm mesh filter. The pH and electrical conductivity (EC) at 25 °C were measured in a 1:5 (w/v) aqueous extract. The total limestone (CaCO3) was evaluated using the Bernard calcimetry method described by Laoufi et al. [21]. To identify the organic matter (OM) content in soil, 5 g of sieved soil was placed into pre-weighed porcelain crucibles and then dried in an oven at 220 °C overnight. The crucibles containing oven-dried soil were subjected to a secondary ignition at 450 °C for a duration of 4 h, after which they were cooled and weighed [22]. Total nitrogen (TN) was determined after the digestion of soil samples with concentrated sulfuric acid and the distillation of the liberated ammonia using the Kjeldahl method [23]. Available phosphorus (AP) was quantified using UV-visible spectrometry at a wavelength of 882 nm, employing the sodium bicarbonate (NaHCO3) method [24]. Total potassium (TK) and soluble sodium (SNa) were quantified utilizing an aqueous extract derived from a saturated paste using a flame photometer [25]. The texture of some rhizosphere soils was determined using the hydrometer method.
2.4. Statistical Analysis
All statistical analyses were performed using the R software 4.5.1. Prior to analysis, data were checked for normality using the Shapiro–Wilk test and for homogeneity of variances using the Bartlett test for all variables. Significant differences between rhizosphere and bulk soil parameters were assessed using a paired two-sample t-test at p < 0.05 (Table 1). A principal component analysis (PCA) was performed using the FactoMineR and factoextra packages to explore relationships among soil parameters and identify the main factors contributing to the observed variability. The results were visualized using a PCA biplot, which showed both the projection of soil samples and the contributions of variables to each principal component. Additionally, correlation analyses among soil physicochemical parameters were performed using the corrplot package, which enabled visualization of both positive and negative associations. Soil texture classes were visualized using the ggsoiltexture package.

Table 1.
Statistical data of physicochemical properties and nutrients according to the soil type.
2.5. Geostatistical Analysis
Geostatistical methods were used to assess the spatial variability of soil parameters. Experimental semi-variograms were calculated for each variable to describe spatial dependence, and models (Gaussian, spherical, or exponential) were fitted based on the auto-kriging package of the rhizosphere parameter, utilizing R 4.5.1, along with interpolation performed in QGIS 3.34.9, a widely recognized approach for estimating the optimal weighting model.
3. Results
3.1. Soil Physicochemical Properties
The comparative analysis of soil parameters between the rhizosphere and bulk soil elucidated distinct disparities in chemical behavior. Among the parameters assessed, pH was the sole property demonstrating a highly significant difference (p < 0.0001), whereas electrical conductivity, organic matter, and total limestone exhibited no statistically significant variation across the soil compartments (Table 1). As illustrated in Figure 2, particle size analysis on specific soil samples revealed that the soil texture varies from sandy–clay to sandy–clay–loam. Data showed that both types of soils were moderately alkaline. The decreased pH of 5.52% observed in the rhizosphere, in contrast to bulk soil, suggests that root activities and concomitant microbial processes have modified the local chemical milieu.
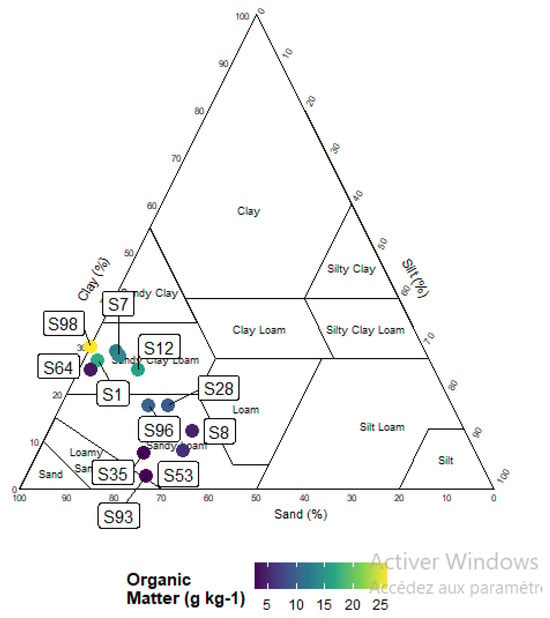
Figure 2.
Soil textural triangle with colored dots according to organic matter (g kg−1) content.
In contrast, the absence of significant differences in the contents of EC and OM indicates that rhizosphere processes did not significantly influence salinity or the enrichment of organic carbon under the examined field conditions. The comparatively low values of OM across both soil compartments are indicative of Saharan and semi-arid ecosystems, wherein rapid mineralization, minimal biomass inputs, and restricted moisture availability limit the accumulation of organic materials. In this study, only 3.3% of soils had organic matter levels above 20 g kg−1. Nevertheless, the concentrations of organic matter were persistently elevated within the rhizosphere (8.51 g kg−1) compared to those of the bulk soil (6.78 g kg−1) (Figure 3).
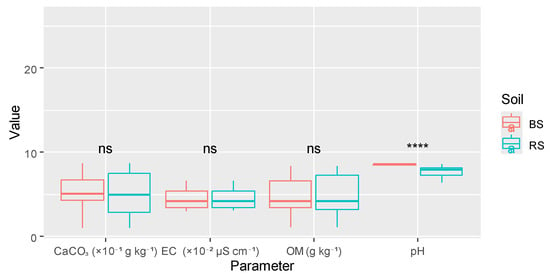
Figure 3.
Variation in physicochemical properties depending on soil type. Note: BS, bulk soil; RS, rhizosphere soil; **** represents the 0.0001 significant level; ns stands for not significant level.
3.2. Soil Nutrient Dynamics
As illustrated in Figure 4, t-tests suggested that among the analyzed parameters, only available phosphorus and total nitrogen showed significant differences (p < 0.001 and p < 0.0001), respectively, while soluble sodium and total potassium remained non-significant between the two types of soil.
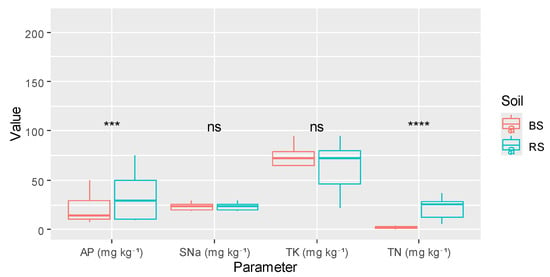
Figure 4.
Nutrient dynamics properties depending on soil type. Note: BS, bulk soil; RS, rhizosphere soil; **** represents the 0.0001 significant level; *** represents the 0.001 significant level; ns stands for not significant level.
The elevated concentration of available phosphorus (AP) in the rhizosphere signifies an augmented mobilization of phosphorus, facilitated by the roots of the peanut plant and their associated symbiotic microorganisms.
Similarly, the notable augmentation of total nitrogen within root systems can be ascribed to the mechanisms of biological nitrogen fixation (BNF) and the subsequent accumulation of nitrogenous root exudates. Fabacaea species, such as Arachis hypogaea, actively engage in the fixation of atmospheric N2 via rhizobial symbiosis, and the ensuing rhizodeposition significantly enriches the adjacent soil with amino compounds and microbial residues. This intricate process not only enhances local nitrogen fertility but also plays a crucial role in the long-term sustainability of agroecosystems predicated on legume cultivation.
In contrast, no significant changes in soluble sodium or total potassium were detected between the rhizosphere and the bulk soil. This suggests that sodium and potassium dynamics are primarily controlled by soil parent material, irrigation quality, and mineral exchange equilibria rather than by short-term biological activity. Potassium in particular occurs in relatively immobile structural forms in arid soils, while sodium behavior depends more on evaporative concentration than on root influence. The uniformity of total limestone between rhizosphere and bulk soil reflects the mineral stability of carbonates, which remain largely unaffected by short-term biological activity or seasonal variations in such arid calcareous soils.
3.3. Spatial Variability and Multivariate Analysis
The spatial analysis of pH shows that the variability is rather low in rhizosphere soils (7.05%) (Table 2). Overall, pH values ranged narrowly between 6.55 and 8.33, indicating slightly to moderately alkaline soils throughout the region. Most of the map reflects a generally homogeneous alkaline environment, which is characteristic of arid soils rich in carbonates and low in organic matter, mainly in the central and southern sectors, which correspond to zones of relatively lower pH. These slightly less alkaline areas may be associated with higher organic matter content, greater biological activity, or localized irrigation effects leading to mild acidification (Figure 5). For the available phosphorus, a high variation (65.64%) was observed, which leads us to deduce that a large heterogeneity for the P-availability was observed in the study’s parcel, ranging from 9.78 to 175.71 mg kg−1. The overall pattern shows localized enrichment zones surrounded by larger expanses of low-to-moderate P levels. High concentrations are particularly evident in the central, western, and southeastern parts of the region, suggesting spatially heterogeneous phosphorus availability. The spatial interpolation map shows marked variability in soil electrical conductivity (EC) across the study area, with values ranging from 318.92 to 661.77 µS cm−1. Geographical distribution reveals moderate salinity levels with several localized zones of higher EC scattered throughout the landscape. These elevated EC areas are most evident in the central, western, and southeastern portions of the region, while the northern and northeastern sectors exhibit relatively lower conductivity. Data on organic matter indicate a predominantly low-to-moderate concentration throughout the research area, with values spanning from 1.17 to 26.01 g kg−1. A prevalence of green hues, signifying suboptimal organic soils characteristic of dry and semi-arid regions. However, localized hotspots are visible in the central, southwestern, and eastern parts of the region, suggesting spatially heterogeneous accumulation of organic residues (CV = 54.05%). Total nitrogen shows notable heterogeneity across the study area, with concentrations ranging from 5.61 to 56.04 mg kg−1. The soils have a low nitrogen concentration, a typical characteristic of dry and semi-arid environments where organic inputs are restricted, and mineralization rates are elevated. The map illustrates several localized enrichment zones mainly concentrated in the central, southwestern, and eastern sectors, contrasting with larger areas of low nitrogen content.

Table 2.
Fitted variogram models and variation properties.
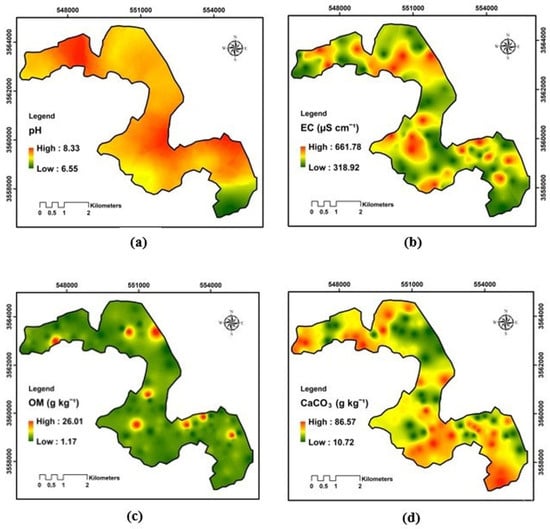
Figure 5.
Spatial variability of the rhizosphere physicochemical properties: (a) pH; (b) EC, electrical conductivity; (c) OM, organic matter; (d) CaCO3, total limestone.
The spatial distribution map illustrates a clear heterogeneity in total limestone content across the study area, with concentrations ranging from 10.72 to 86.57 g kg−1. Overall, the soils exhibit a predominantly calcareous character, indicating widespread high CaCO3 levels. The highest concentrations are observed in the southern, eastern, and northwestern sectors, while lower values occur sporadically in the central and northeastern zones. The distribution of soluble sodium in the research area appears in the spatial interpolation map, where values range from 19.02 to 29.16 mg kg−1. The general pattern reveals moderate to high sodium concentrations over most of the region, with localized enrichment zones scattered throughout the central, southeastern, and northwestern parts. In contrast, relatively lower concentrations occur mainly in the northeastern and southwestern sectors. As shown in Figure 6, total potassium concentrations vary across the study area, ranging from 30.16 to 90.67 mg kg−1. The general shape indicates a moderate to high level of K throughout most of the region, with localized enrichment zones evident particularly in the southern, southeastern, and northwestern sectors. Conversely, lower concentrations reside mainly in the central and northern parts of the area.
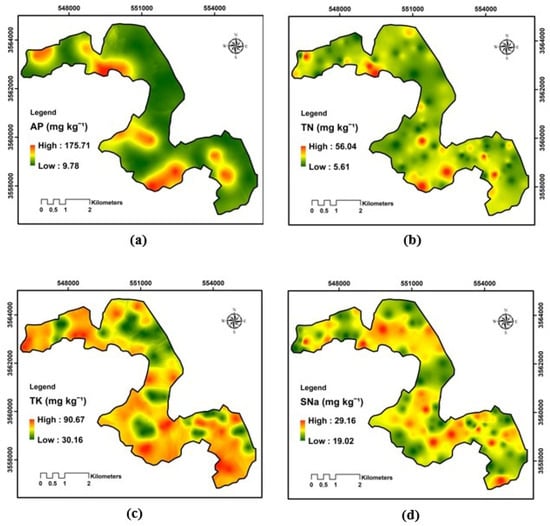
Figure 6.
Spatial variability of the rhizosphere nutrient dynamics: (a) AP, available phosphorus; (b) TN, total nitrogen; (c) TK, total potassium; (d) SNa, soluble sodium.
The principal component analysis (PCA) biplot shows that the first two axes explain a substantial portion of the variance in the soil dataset (Dim1 = 39.7%, Dim2 = 31.3%), indicating that these components effectively summarize the major gradients in soil physicochemical properties (Table 3). Along Dim1, electrical conductivity (EC) and available phosphorus (AP) load strongly and positively (R = 0.50, p < 0.001), while pH and total potassium (TK) load in the opposite direction (R = −0.49, p < 0.001), revealing a clear gradient from saline or mineral-rich soils toward more alkaline or potassium-enriched conditions. This pattern suggests that higher ion concentrations, potential salt accumulation, or fertilization inputs may influence soils with elevated EC and AP. In contrast, soils positioned on the negative side of Dim1 are characterized by greater base saturation and potassium availability. Dim2 is predominantly defined by total nitrogen (TN), which exhibits a significant positive loading, suggesting that this axis represents variations in soil nitrogen levels and potential fertility. Samples clustered in the upper section of the biplot correspond to nitrogen-rich soils that may facilitate elevated biological activity and organic matter turnover, whereas samples in the lower section are linked to nitrogen-poor conditions. The opposing directions of the variable vectors highlight important ecological trade-offs among nutrient pools and soil chemical processes, such as the antagonistic behavior between salinity and pH (R = −0.75, p < 0.001) or the decoupling of nitrogen from other macronutrients. Together, these patterns demonstrate that the soils in the study area vary along two principal ecological gradients—one related to salinity and nutrient ion accumulation, and the other linked to nitrogen-driven fertility—which likely reflect differences in land use, management intensity, or underlying parent material (Figure 7).

Table 3.
Eigenvalue of components, coordinates, and eigenvectors of variables.
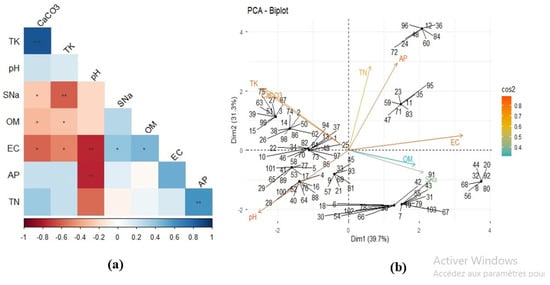
Figure 7.
(a) Corrplot and (b) PCA biplot illustrating the correlations between soil variables and soil samples from the rhizosphere. *** represents the 0.001 significant level; ** represents the 0.01 significant level; * represents the 0.05 significant level.
4. Discussion
4.1. Rhizosphere Soil Influence
In this study, a significant decrease in pH in the rhizosphere was obtained. The marked decrease in pH in the rhizosphere aligns with several previous findings in arid and semi-arid agroecosystems. For instance, Singh et al. [26] and El-sherbeny et al. [27] reported that peanut rhizospheres tend to exhibit slightly lower pH compared to bulk soils, primarily due to ion exchange processes and the release of acid exudates such as organic anions that mobilize phosphorus and calcium. Similarly, Baccari et al. [28] observed that root-induced acidification is a common feature in legume rhizospheres. In Fabacaea crops, such as peanut, the secretion of protons H+ during cation uptake, along with the exudation of organic anions that facilitate the mobilization of phosphorus and calcium, may contribute to the decrease in pH within the rhizosphere [29].
The absence of significant variation in EC and OM agrees with studies conducted in sandy and loamy soils of arid regions [30,31], where limited moisture and high temperature constrain microbial turnover and decomposition, thereby reducing OM enrichment around roots. In this context, it was noted that extreme climatic conditions and low carbon availability limit microbial turnover and organic matter dynamics in arid soils [32]. Moreover, limestone stability across soil compartments confirms that carbonate minerals are geochemically buffered and remain relatively unaffected by root activity at the temporal scale of a growing season [33,34]. In summary, while peanut root activity modifies soil pH, it exerts a limited influence on other physicochemical parameters, reflecting the low reactivity of arid calcareous soils. These findings highlight the selective yet ecologically meaningful impact of root processes on soil chemistry in dryland peanut systems [35,36,37].
The increase in available phosphorus (AP) in the rhizosphere compared with bulk soil highlights the ability of peanut roots and their associated microbiota to enhance P mobilization. Legume roots exude organic acids and phosphatase enzymes capable of solubilizing sparingly available Ca–P complexes, particularly in calcareous soils typical of arid regions. Moreover, symbiotic rhizobia and mycorrhizal fungi contribute to P acquisition by extending the effective root surface area and releasing chelating compounds. This mechanism leads to localized enrichment of plant-available phosphorus in the rhizosphere. These results are in agreement with previous findings. Similar rhizosphere enrichment of phosphorus and nitrogen was observed in peanut and cowpea soils of semi-arid regions, linking it to enhanced microbial mineralization and symbiotic fixation [38,39]. Gao et al. [40] also reported that organic acid exudation from legume roots increases P availability in calcareous soils.
Data pertaining to total nitrogen concentrations indicate that the rhizosphere exhibits elevated levels of nitrogen in comparison to the bulk soil. According to Grzyb et al. [41], it is uncommon for soil to possess more than 10% total inorganic nitrogen content. Mineral nitrogen is frequently found in the soil predominantly in the form of nitrate (NO3−) and ammonium (NH4+) [42]. Several studies reported that the extent of nitrogen fixation by peanuts ranges between 40 and 60% [43,44,45]. However, this fixation can diminish to 30% in soils abundant in mineral nitrogen, which corresponds to the prior crop yield of 60–120 kgN.ha−1, or it may exceed 80% in soils deficient in nitrate, such as inadequately fertilized sandy soils, provided that conditions conducive to nitrogen fixation are also present [46]. The availability of phosphorus enhances nitrogen accumulation within nodules and contributes to its density in the plant. Additional studies have elucidated a positive correlation between nitrogen and phosphorus concentrations across nine species of tropical food legumes [47]. Likewise, the presence of phosphorus facilitates nitrogenase activity and amplifies the quantity of nitrogen-fixing nodules [48].
Total potassium levels in the soil are moderate, while soluble sodium levels are low in both soil types. The concentration of potassium ions (K+) within plant tissues is intrinsically associated with the quantity and classification of salts present [49]. Analytical research indicates the existence of two homogenous groups that exhibit a statistically significant average impact concerning both the quantity and classification of salts; however, there is no notable impact of calcium amendments on potassium (K+) concentrations [50]. The findings regarding total potassium levels did not indicate any statistically significant variations among the concentrations observed during different sampling intervals. Nevertheless, a notable reduction in potassium levels within the soil was documented in the initial sampling [51]. In general, the levels of soluble potassium in the soil are exceedingly low, with more than 90% of this potassium residing within insoluble rock formations and silicate minerals [52,53].
The composition and organization of rhizosphere microbial communities, encompassing beneficial bacteria (e.g., Bradyrhizobium, Bacillus, Serratia) and fungi (e.g., arbuscular mycorrhizal fungi, Phomopsis liquidambari), are essential for nutrient cycling, disease suppression, nodulation, and nitrogen fixation. Manipulating these communities through inoculation, organic amendments, and biofertilizers improves peanut growth, nodulation, and resistance to soil-borne diseases. Microbial interactions and symbioses additionally affect plant physiological responses under stress conditions [54,55]. Several studies reinforce the critical role of rhizosphere soil physicochemical properties and microbial communities in modulating peanut yield and health, particularly under arid and semi-arid field conditions. The interplay between soil amendments, microbial diversity, and nutrient cycling emerges as a central mechanism influencing plant productivity and disease resistance [56,57].
4.2. Spatial Variability
Comparable distributions of phosphorus have been documented in arid and semi-arid regions within North Africa and the Middle East, where the presence of calcareous and alkaline soils significantly impacts the solubility of phosphorus. Research conducted in Saharan ecosystems has demonstrated that, despite sufficient total phosphorus content, the availability of phosphorus is frequently limited due to the immobilization of phosphate ions as calcium phosphates in alkaline environments [58,59,60]. A similar pattern of spatial variability has been observed in the oasis soils of Algeria, wherein factors such as irrigation water, organic amendments, and historical cropping practices establish localized zones of phosphorus enrichment [61,62,63]. These observations lend credence to the hypothesis that the availability of phosphorus in arid environments is governed more by biochemical processes and management practices than by the mere concentration of total P [64,65].
In the context of oasis and irrigated soils situated in southern Algeria, the EC is generally observed to range from 300 to 700 µS.cm−1, contingent upon the quality of irrigation water and the texture of the soil [66,67]. Similarly, the spatial heterogeneity that has been described in North African arid zones reflects salt redistribution driven by irrigation and evaporation processes. As articulated by Salem et al. [68] and Rufaut et al. [69], moderate EC values such as those recorded here are indicative of incipient salinization, a common feature in regions with limited rainfall and carbonate-rich soils. Thus, the generated EC map serves as a critical tool for the assessment of soil salinity dynamics and the formulation of site-specific management strategies aimed at mitigating the risk of prolonged salinization.
The low organic matter contents recorded in this study are consistent with values reported for Saharan soils, which typically range between 1 and 30 g kg−1. Moreover, in southern oases, where intensive cultivation and limited organic amendments contribute to OM depletion, low OM levels in such environments stem from high oxidation rates and limited biomass return, leading to weak soil aggregation and reduced nutrient retention capacity [70,71]. In these areas, nitrogen rarely exceeds 1 g kg−1, and localized organic inputs or manure applications increase nitrogen in specific patches, while extensive areas remain poor in N. Furthermore, nitrogen deficiency in arid soils is primarily due to low organic matter turnover and weak microbial activity, which limit N retention and cycling [72]. Under arid climatic conditions, carbonate contents typically range between 20 and 90 g kg−1, where calcareous parent materials and low rainfall limit carbonate leaching and promote surface accumulation. Thus, high limestone levels are characteristic of young, weakly developed soils formed, resulting in carbonate accumulation standing as a dominant pedogenic feature in the region, controlling soil pH and nutrient dynamics [73].
Conversely, sodium buildup in arid soils results primarily from the evaporative concentration of groundwater salts and limited leaching due to low rainfall. The results, therefore, align with typical sodic soil development processes in arid environments, emphasizing the importance of periodic leaching, gypsum application, and improved irrigation management to mitigate sodicity risks [74]. The present results for total potassium, spanning 20 to 100 mg kg−1, reflect the geogenic nature of soil K in the region, suggesting that long-term fertility maintenance will depend on the balance between mineral weathering, crop uptake, and management interventions. As noted by AbdelRahman et al. [75], potassium in dryland soils is mainly derived from primary minerals and remains relatively stable unless leaching or intensive cropping occurs.
4.3. Effects of Management Practices
Management practices play a central role in shaping rhizosphere soil properties of peanut, especially under the fragile, low-organic-matter soils typical of Southern Algeria. In such an arid environment, tillage, residue management, cropping system, fertilization strategy, irrigation, and use of biofertilizers all interact to regulate the physical, chemical, and biological conditions around the roots, and therefore strongly influence nutrient availability and plant–microbe interactions. Studies in peanut-based systems show that reduced soil disturbance combined with the incorporation of crop residues, green manures, or other organic inputs generally improves rhizosphere conditions by increasing soil organic matter, enhancing total and available N, P, and K, and stimulating soil enzyme activities and microbial diversity [76,77]. Continuous monocropping was widely reported to degrade soil quality, reduce microbial diversity, and increase pathogen loads [78]. Integrated approaches combining organic amendments, microbial inoculants, and mineral fertilizers yielded synergistic benefits for soil and plant health [79]. Moreover, crop rotation, intercropping, and green manure applications were effective in mitigating continuous cropping obstacles, improving soil health, microbial diversity, and peanut productivity [80,81,82,83].
5. Conclusions
This study showed that, under arid conditions, peanut cultivation generates clear contrasts between rhizosphere and bulk soil, while overall fertility remains low. Soil pH was the main property that differed significantly between compartments, with a consistent acidification in the rhizosphere, likely driven by root exudation and microbial activity.
Nutrient patterns indicated that the rhizosphere functions as a local hotspot of fertility, with significantly higher available phosphorus and total nitrogen, reflecting enhanced P mobilization and biological N fixation by peanut–rhizobium symbiosis. In contrast, soluble sodium, total potassium, electrical conductivity, and total limestone were not significantly affected by root activity and appear mainly controlled by soil parent material and irrigation quality.
The strong spatial variability observed suggests that uniform fertilizer application is unlikely to be efficient. Site-specific management, based on spatial mapping of available P, N, OM, and EC, could optimize fertilizer inputs, reduce the risk of salt accumulation, and improve nutrient use efficiency. Practices that enhance rhizosphere activity—such as the use of effective rhizobial inoculants, improved root growth conditions, and the integration of organic amendments—could further exploit the natural capacity of peanuts to enrich the soil in N and mobilize P.
Future research should link soil gradients to yield and plant traits, monitor changes across seasons, and study microbial/enzyme mechanisms at larger scales.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by M.O.H., M.K., B.L., Z.S., I.L. and S.B. The first draft of the manuscript was written by M.O.H., and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported and funded by LVCEA—Valorization and Conservation of Arid Ecosystems Laboratory, University of Ghardaïa, Algeria.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hinsu, A.T.; Panchal, K.J.; Pandit, R.J.; Koringa, P.G.; Kothari, R.K. Characterizing Rhizosphere Microbiota of Peanut (Arachis hypogaea L.) from Pre-Sowing to Post-Harvest of Crop under Field Conditions. Sci. Rep. 2021, 11, 17457. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Vetukuri, R.R.; Kelbessa, B.G.; Gepts, P.; Heslop-Harrison, P.; Araujo, A.S.F.; Sharma, S.; Ortiz, R. Exploitation of Rhizosphere Microbiome Biodiversity in Plant Breeding. Trends Plant Sci. 2025, 30, 1033–1045. [Google Scholar] [CrossRef]
- Thepbandit, W.; Athinuwat, D. Rhizosphere Microorganisms Supply Availability of Soil Nutrients and Induce Plant Defense. Microorganisms 2024, 12, 558. [Google Scholar] [CrossRef]
- Oliva-Cruz, M.; Cabañas-López, J.R.; Altamirano-Tantalean, M.A.; Juarez-Contreras, L.; Vigo, C.N. Agronomic Behavior of Peanut (Arachis hypogaea L.) Cultivars Under Three Planting Densities in the Northeast of Peru. Agronomy 2024, 14, 1905. [Google Scholar] [CrossRef]
- Cesari, A.B.; Fernandez, M.; Paulucci, N.S.; Dardanelli, M.S. Long-Life Inoculant: Bradyrhizobium Stored in Biodegradable Beads for Four Years Shows Optimal Cell Vitality, Interacts with Peanut Roots, and Promotes Early Growth. Plants 2024, 13, 2983. [Google Scholar] [CrossRef]
- Lallaouna, R.; Ababsa, N.; Chenchouni, H. Soil Physicochemical Properties and Soil Fertility Indicators of Two Cropping Systems under Semiarid Climate Conditions. Environ. Adv. 2025, 21, 100663. [Google Scholar] [CrossRef]
- Lan, W.; Ding, H.; Zhang, Z.; Li, F.; Feng, H.; Guo, Q.; Qin, F.; Zhang, G.; Xu, M.; Xu, Y. Diversified Soil Types Differentially Regulated the Peanut (Arachis hypogaea L.) Growth and Rhizosphere Bacterial Community Structure. Plants 2025, 14, 1169. [Google Scholar] [CrossRef]
- Zou, X.; Jiang, X.; Jiang, H.; Li, C.; Cheng, J.; Ji, D.; Wang, J.; Ruan, J.; Zhou, T.; Kuang, C.; et al. Soil Biocrusts May Exert a Legacy Impact on the Rhizosphere Microbial Community of Plant Crops. Agronomy 2024, 14, 2548. [Google Scholar] [CrossRef]
- Wu, H.; Chen, S.; Huang, Z.; Huang, T.; Tang, X.; He, L.; Li, Z.; Xiong, J.; Zhong, R.; Jiang, J.; et al. Effects of Intercropping and Nitrogen Application on Soil Fertility and Microbial Communities in Peanut Rhizosphere Soil. Agronomy 2024, 14, 635. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Liu, C.; Shang, S.; Wang, C.; Tian, J.; Zhang, L.; Liu, X.; Bian, R.; He, Q.; Zhang, F.; Chen, L.; et al. Biochar Amendment Increases Peanut Production Through Improvement of the Extracellular Enzyme Activities and Microbial Community Composition in Replanted Field. Plants 2025, 14, 922. [Google Scholar] [CrossRef]
- Yu, T.; Hou, X.; Fang, X.; Razavi, B.; Zang, H.; Zeng, Z.; Yang, Y. Short-Term Continuous Monocropping Reduces Peanut Yield Mainly Via Altering Soil Enzyme Activity and Fungal Community. Environ. Res. 2024, 245, 117977. [Google Scholar] [CrossRef]
- Córdoba, M.A.; Hang, S.B.; Bozzer, C.; Alvarez, C.; Faule, L.; Kowaljow, E.; Vaieretti, M.V.; Bongiovanni, M.D.; Balzarini, M.G. Spatial Variability and Temporal Changes of Soil Properties Assessed by Machine Learning in Córdoba, Argentina. Soil. Syst. 2025, 9, 109. [Google Scholar] [CrossRef]
- Ellur, R.; Ankappa, A.M.; Dharumarajan, S.; Puttavenkategowda, T.; Nanjundegowda, T.M.; Sannegowda, P.S.; Pratap Mishra, A.; Đurin, B.; Dogančić, D. Soil Quality Assessment and Its Spatial Variability in an Intensively Cultivated Area in India. Land 2024, 13, 970. [Google Scholar] [CrossRef]
- Acir, N. Predicting Soil Fertility in Semi-Arid Agroecosystems Using Interpretable Machine Learning Models: A Sustainable Approach for Data-Sparse Regions. Sustainability 2025, 17, 7547. [Google Scholar] [CrossRef]
- Zhang, X.; Li, P.; Zhao, M.; Wang, S.; Sun, B.; Zhang, Y.; Wang, Y.; Chen, Z.; Xie, H.; Jiang, N.; et al. Organic Fertilizer with High Nutrient Levels Affected Peanut-Growing Soil Bacteria More Than Fungi at Low Doses. Agronomy 2024, 14, 765. [Google Scholar] [CrossRef]
- Andrade, C.; Fonseca, A.; Santos, J.A.; Bois, B.; Jones, G.V. Historic Changes and Future Projections in Köppen–Geiger Climate Classifications in Major Wine Regions Worldwide. Climate 2024, 12, 94. [Google Scholar] [CrossRef]
- Laouar, B.; Kraimat, M.; Benhammouda, H.; Oulad Heddar, M. A GIS-based Approach to Assessing the Spatial Variability and Rhizosphere Soil Properties of Retama raetam Forssk., Growing in Southern Algeria. J. Inf. Syst. Eng. Manag. 2025, 10, 988–1001. [Google Scholar] [CrossRef]
- Djili, B.; Hamdi-Aïssa, B. Characteristics and mineralogy of desert alluvial soils: Wadi Zegrir, Northern Sahara of Algeria. Arid. Land Res. Manag. 2018, 32, 1–19. [Google Scholar] [CrossRef]
- Dill Harald, G.; Buzatu, A. From the aeolian landform to the aeolian mineral deposit in the present and its use as an ore guide in the past. Constraints from mineralogy, chemistry and sediment petrography. Ore Geol. Rev. 2022, 141, 104490. [Google Scholar] [CrossRef]
- Laoufi, H.; Bachir, H.; Hadj-Miloud, S.; Clark, K. Comparative Assessment of Three Methods for Soil Organic Matter Determination in Calcareous Soils, Eastern Algeria. Land 2025, 14, 2030. [Google Scholar] [CrossRef]
- Krahl, I.; Tokarski, D.; Kučerík, J.; Schwitzky, E.; Siewert, C. New Approach to Experimental Soil Health Definition Using Thermogravimetric Fingerprinting. Agronomy 2025, 15, 487. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, J.E.; Back, I.; Lim, K.J.; Mo, C. Estimation of Total Nitrogen Content in Topsoil Based on Machine and Deep Learning Using Hyperspectral Imaging. Agriculture 2023, 13, 1975. [Google Scholar] [CrossRef]
- Mogashane, T.M.; Mapazi, O.; Motlatle, M.A.; Mokoena, L.; Tshilongo, J. A Review of Recent Developments in Analytical Methods for Determination of Phosphorus from Environmental Samples. Molecules 2025, 30, 1001. [Google Scholar] [CrossRef]
- Bekir, S.; Zoghlami, R.I.; Boudabbous, K.; Khelil, M.N.; Moussa, M.; Ghrib, R.; Nahdi, O.; Trabelsi, E.; Bousnina, H. Soil Physicochemical Changes as Modulated by Treated Wastewater after Medium-and Long-Term Irrigations: A Case Study from Tunisia. Agriculture 2022, 12, 2139. [Google Scholar] [CrossRef]
- Sharma, S.B.; Raverkar, K.P.; Wani, S.P.; Bagyaraj, D.J.; Kannepalli, A.; Kandula, D.R.W.; Mikaelyan, A.; Ansari, M.A.; Stock, S.P.; Davies, K.G.; et al. Role of the Plant–Microbiome Partnership in Environmentally Harmonious 21st Century Agriculture. Microorganisms 2025, 13, 2839. [Google Scholar] [CrossRef]
- El-sherbeny, T.M.S.; Mousa, A.M.; Zhran, M.A. Response of Peanut (Arachis hypogaea L.) Plant to Bio-Fertilizer and Plant Residues in Sandy Soil. Environ. Geochem. Health 2023, 45, 253–265. [Google Scholar] [CrossRef]
- Baccari, B.; Krouma, A. Rhizosphere Acidification Determines Phosphorus Availability in Calcareous Soil and Influences Faba Bean (Vicia faba) Tolerance to P Deficiency. Sustainability 2023, 15, 6203. [Google Scholar] [CrossRef]
- Turdaliev, A.; Askarov, K.; Abakumov, E.; Makhkamov, E.; Rahmatullayev, G.; Mamajonov, G.; Akhmadjonov, A.; Axunov, A. Biogeochemical State of Salinized Irrigated Soils of Central Fergana (Uzbekistan, Central Asia). Appl. Sci. 2023, 13, 6188. [Google Scholar] [CrossRef]
- Alnaimy, M.; Zelenakova, M.; Vranayova, Z.; Abu-Hashim, M. Effects of Temporal Variation in Long-Term Cultivation on Organic Carbon Sequestration in Calcareous Soils: Nile Delta, Egypt. Sustainability 2020, 12, 4514. [Google Scholar] [CrossRef]
- Xiao, Y.; Ye, M.; Zhang, J.; Chen, Y.; Sun, X.; Li, X.; Song, X. Significant Changes in Soil Properties in Arid Regions Due to Semicentennial Tillage—A Case Study of Tarim River Oasis, China. Sustainability 2025, 17, 4194. [Google Scholar] [CrossRef]
- Khan, M.T.; Supronienė, S.; Žvirdauskienė, R.; Aleinikovienė, J. Climate, Soil, and Microbes: Interactions Shaping Organic Matter Decomposition in Croplands. Agronomy 2025, 15, 1928. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, J.; Liao, F.; Xu, X.; Li, A.; Chen, L.; Zhao, T.; Long, T.; Li, S.; Li, H. Mineralogical and Geochemical Evolution During Limestone Weathering and Pedogenesis in Shimen, Hunan Province, South China. Minerals 2025, 15, 1109. [Google Scholar] [CrossRef]
- Du, Y.; Ge, X.; Du, Y.; Ding, H.; Lu, A. Mineral–Soil–Plant–Nutrient Synergism: Carbonate Rock Leachate Irrigation Enhances Soil Nutrient Availability, Improving Crop Yield and Quality. Minerals 2025, 15, 825. [Google Scholar] [CrossRef]
- Tlili, A.; Dridi, I.; Mlaiki, F.; Schillaci, C.; Saia, S. Description of Representative “In-Situ” Soil Profiles in Northwestern Tunisia. Discov. Soil 2025, 2, 31. [Google Scholar] [CrossRef]
- González-Valoys, A.C.; Chong, T.; Arrocha, J.; Lloyd, J.; Olmos, J.; Vergara, F.; Denvers, M.; Jaén, J.; Jiménez-Oyola, S.; García-Navarro, F.J. Geochemical Characterization of Soil and Water in an Agricultural Area for the Sustainable Use of Natural Resources. Agriculture 2025, 15, 702. [Google Scholar] [CrossRef]
- Zhao, C.X.; Jia, L.H.; Wang, Y.F.; Wang, M.L.; McGiffen, M.E. Effects of Different Soil Texture on Peanut Growth and Development. Commun. Soil Sci. Plant Anal. 2015, 46, 2249–2257. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, L.; Liang, H.; Liu, M.; Chen, Y.; Chen, D.; Shen, P. Impacts of Soil Compaction and Phosphorus Levels on the Dynamics of Phosphate-Solubilizing and Nitrogen-Fixing Bacteria in the Peanut Rhizosphere. Agronomy 2024, 14, 1971. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Z.; Sun, D.; Lei, Y.; Li, Z.; Zheng, Y. Advances in Water and Nitrogen Management for Intercropping Systems: Crop Growth and Soil Environment. Agronomy 2025, 15, 2000. [Google Scholar] [CrossRef]
- Gao, C.; Kong, W.; Zhao, F.; Ju, F.; Liu, P.; Li, Z.; Liu, K.; Zhao, H. Enhancing Soil Phosphorus Availability in Intercropping Systems: Roles of Plant Growth Regulators. Agronomy 2025, 15, 1748. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The Significance of Microbial Transformation of Nitrogen Compounds in the Light of Integrated Crop Management. Agronomy 2021, 11, 1415. [Google Scholar] [CrossRef]
- Han, B.; Chen, W.Q.; Jiao, Y.Q.; Yang, R.; Niu, L.L.; Chen, X.R.; Ji, C.Y.; Yin, D.X. Effects of Nitrogen Fertilizer Application on Soil Properties and Arsenic Mobilization in Paddy Soil. Sustainability 2024, 16, 5565. [Google Scholar] [CrossRef]
- Ding, B.; Feng, M.; Wang, R.; Chang, L.; Jiang, Y.; Xie, J.; Tian, D. A Study of Growth and Yield of Four Peanut Varieties with Rhizobia Inoculation under Field Conditions. Agronomy 2024, 14, 1410. [Google Scholar] [CrossRef]
- Hu, J.; Yang, Y.; Zhang, H.; Li, Y.; Zhang, S.; He, X.; Huang, Y.; Ye, Y.; Zhao, Y.; Yan, J. Reduction in Nitrogen Rate and Improvement of Nitrogen Use Efficiency Without Loss of Peanut Yield by Regional Mean Optimal Rate of Chemical Fertilizer Based on a Multi-Site Field Experiment in the North China Plain. Plants 2023, 12, 1326. [Google Scholar] [CrossRef]
- Hou, L.; Lin, R.X.; Wang, X.J.; Li, H.; Zhao, C.Z.; Zhu, X.J.; Li, C.S.; Li, G.H. The Mechanisms of Pod Zone Nitrogen Application on Peanut Pod Yield. Russ. J. Plant Physiol. 2022, 69, 51. [Google Scholar] [CrossRef]
- Cugnon, T.; De Toffoli, M.; Mahillon, J.; Lambert, R. Improving Nitrogen Fertilization Recommendations in Temperate Agricultural Systems: A Study on Walloon Soils Using Anaerobic Incubation and POxC. Nitrogen 2025, 6, 91. [Google Scholar] [CrossRef]
- Zhong, Y.; Tian, J.; Li, X.; Liao, H. Cooperative Interactions Between Nitrogen Fixation and Phosphorus Nutrition in Legumes. New Phytol. 2023, 237, 734–745. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Yang, Z.; Xing, X.; Fu, Z.; Li, X.; Kong, Y.; Li, W.; Du, H.; Zhang, C. Overexpression of GmPAP4 Enhances Symbiotic Nitrogen Fixation and Seed Yield in Soybean under Phosphorus-Deficient Condition. Int. J. Mol. Sci. 2024, 25, 3649. [Google Scholar] [CrossRef]
- Shani, M.Y.; Ashraf, M.Y.; Butt, A.K.; Abbas, S.; Nasif, M.; Khan, Z.; Mauro, R.P.; Cannata, C.; Gul, N.; Ghaffar, M.; et al. Potassium Nutrition Induced Salinity Mitigation in Mungbean [Vigna radiata (L.) Wilczek] by Altering Biomass and Physio-Biochemical Processes. Horticulturae 2024, 10, 549. [Google Scholar] [CrossRef]
- Kaymak Bayram, G.; Can, M.; Tunalı, U.; Acar, Z.; Ayan, İ. Growth and Physiological Responses and Selection of Tedera (Bituminaria bituminosa L.) Genotypes Under Salt Stress Conditions. Plants 2025, 14, 3618. [Google Scholar] [CrossRef]
- Sharmin, S.; Arfin, M.N.H.; Tareque, A.M.M.M.U.; Kafi, A.A.; Miah, M.S.; Hossen, M.Z.; Talukder, M.A.S.; Robin, A.H.K. Reduction of Potassium Supply Alters the Production and Quality Traits of Ipomoea batatas cv. BAU Sweetpotato-5 Tubers. Stresses 2024, 4, 883–895. [Google Scholar] [CrossRef]
- El-Egami, H.M.; Hegab, R.H.; Montaser, H.; El-Hawary, M.M.; Hasanuzzaman, M. Impact of Potassium-Solubilizing Microorganisms with Potassium Sources on the Growth, Physiology, and Productivity of Wheat Crop under Salt-Affected Soil Conditions. Agronomy 2024, 14, 423. [Google Scholar] [CrossRef]
- Kholdarov, D.; Sobitov, U.; Zakirova, S.; Mirzaev, U.; Kholdarova, M.; Sotiboldieva, G.; Azimov, Z.; Abdukhakimova, K.; Jabbarov, Z.; Kenjaev, Y.; et al. Current State of Saline Soils in the Fergana Valley. E3S Web Conf. 2024, 563, 03053. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, B.; Deng, J.; Li, L.; Yi, T.; Hong, Y. The resistance of peanut to soilborne pathogens improved by rhizosphere probiotics under calcium treatment. BMC Microbiol. 2021, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, H.; Zhang, G.C.; Li, Z.; Guo, Q.; Feng, H.; Qin, F.; Dai, L.X.; Zhang, Z. Green manure increases peanut production by shaping the rhizosphere bacterial community and regulating soil metabolites under continuous peanut production systems. BMC Plant Biol. 2023, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, C.B.; He, X.; Wu, Q.; Sun, X.W.; Liu, M.; Shen, P. Multi-year crop rotation and quicklime application promote stable peanut yield and high nutrient-use efficiency by regulating soil nutrient availability and bacterial/fungal community. Front. Microbiol. 2024, 15, 1367184. [Google Scholar] [CrossRef]
- Qin, W.; Li, G.; Chen, X.; Liu, J. Organic amendments enhance peanut nodulation by influencing interactions between rhizobia and arbuscular mycorrhizal fungi in the peanut rhizosphere. Agronomy 2024, 14, 3004. [Google Scholar] [CrossRef]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus Transformation in Soils Following Co-Application of Charcoal and Wood Ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Elbasiouny, H.; Elbehiry, F.; El-Ramady, H.; Brevik, E.C. Phosphorus Availability and Potential Environmental Risk Assessment in Alkaline Soils. Agriculture 2020, 10, 172. [Google Scholar] [CrossRef]
- Silva, L.I.d.; Pereira, M.C.; Carvalho, A.M.X.d.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Tomaz, A.; Martins, I.; Catarino, A.; Mourinha, C.; Dôres, J.; Fabião, M.; Boteta, L.; Coutinho, J.; Patanita, M.; Palma, P. Insights into the Spatial and Temporal Variability of Soil Attributes in Irrigated Farm Fields and Correlations with Management Practices: A Multivariate Statistical Approach. Water 2022, 14, 3216. [Google Scholar] [CrossRef]
- Benslama, A.; Khanchoul, K.; Benbrahim, F.; Boubehziz, S.; Chikhi, F.; Navarro-Pedreño, J. Monitoring the Variations of Soil Salinity in a Palm Grove in Southern Algeria. Sustainability 2020, 12, 6117. [Google Scholar] [CrossRef]
- Retta, A.N.; Kebede, F.; Hailen, M.; Gebresamuel, G.; Zenebe, A.; Girmay, G. Assessing the Spatial Variability of Soil Properties in the Semiarid Areas of Hintalo Wejerat District, Tigray region, Ethiopia. Discov. Sustain. 2025, 6, 502. [Google Scholar] [CrossRef]
- Ahmad, M.; Ishaq, M.; Shah, W.A.; Adnan, M.; Fahad, S.; Saleem, M.H.; Khan, F.U.; Mussarat, M.; Khan, S.; Ali, B.; et al. Managing Phosphorus Availability from Organic and Inorganic Sources for Optimum Wheat Production in Calcareous Soils. Sustainability 2022, 14, 7669. [Google Scholar] [CrossRef]
- Mushtaq, R.; Sharma, M.K.; Mir, J.I.; Mansoor, S.; Mushtaq, K.; Popescu, S.M.; Malik, A.R.; El-Serehy, H.A.; Hefft, D.I.; Bhat, S.A.; et al. Physiological Activity, Nutritional Composition, and Gene Expression in Apple (Malus domestica Borkh) Influenced by Different ETc Levels of Irrigation at Distinct Development Stages. Water 2021, 13, 3208. [Google Scholar] [CrossRef]
- Kim, H.N.; Park, J.H. Monitoring of Soil EC for The Prediction of Soil Nutrient Regime Under Different Soil Water and Organic Matter Contents. Appl. Biol. Chem. 2024, 67, 1. [Google Scholar] [CrossRef]
- Kargas, G.; Londra, P.; Sotirakoglou, K. The Effect of Soil Texture on the Conversion Factor of 1:5 Soil/Water Extract Electrical Conductivity (EC1:5) to Soil Saturated Paste Extract Electrical Conductivity (ECe). Water 2022, 14, 642. [Google Scholar] [CrossRef]
- Salem, O.H.; Jia, Z. Evaluation of Different Soil Salinity Indices Using Remote Sensing Techniques in Siwa Oasis, Egypt. Agronomy 2024, 14, 723. [Google Scholar] [CrossRef]
- Rufaut, C.; Pillai, D.; Craw, D. Enhancement of alkaline saline soil-free bare substrates and specialist ecosystems, southern New Zealand. Environ. Earth Sci. 2023, 82, 440. [Google Scholar] [CrossRef]
- Voltr, V.; Menšík, L.; Hlisnikovský, L.; Hruška, M.; Pokorný, E.; Pospíšilová, L. The Soil Organic Matter in Connection with Soil Properties and Soil Inputs. Agronomy 2021, 11, 779. [Google Scholar] [CrossRef]
- Githongo, M.; Kiboi, M.; Muriuki, A.; Fliessbach, A.; Musafiri, C.; Ngetich, F.K. Organic Carbon Content in Fractions of Soils Managed for Soil Fertility Improvement in Sub-Humid Agroecosystems of Kenya. Sustainability 2023, 15, 683. [Google Scholar] [CrossRef]
- Farhan, M.; Sathish, M.; Kiran, R.; Mushtaq, A.; Baazeem, A.; Hasnain, A.; Hakim, F.; Atif, S.; Naqvi, H.; Mubeen, M.; et al. Plant Nitrogen Metabolism: Balancing Resilience to Nutritional Stress and Abiotic Challenges. Phyton-Int. J. Exp. Bot. 2024, 93, 581–609. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, C.; Yang, S.; Liu, Y.; Zhao, S.; Zhang, C. Characteristics of Soil Calcium Content Distribution in Karst Dry-Hot Valley and Its Influencing Factors. Water 2023, 15, 1119. [Google Scholar] [CrossRef]
- Mohanavelu, A.; Naganna, S.R.; Al-Ansari, N. Irrigation Induced Salinity and Sodicity Hazards on Soil and Groundwater: An Overview of Its Causes, Impacts and Mitigation Strategies. Agriculture 2021, 11, 983. [Google Scholar] [CrossRef]
- AbdelRahman, M.A.E.; Metwaly, M.M.; Afifi, A.A.; D’Antonio, P.; Scopa, A. Assessment of Soil Fertility Status Under Soil Degradation Rate Using Geomatics in West Nile Delta. Land 2022, 11, 1256. [Google Scholar] [CrossRef]
- Țopa, D.-C.; Căpșună, S.; Calistru, A.-E.; Ailincăi, C. Sustainable Practices for Enhancing Soil Health and Crop Quality in Modern Agriculture: A Review. Agriculture 2025, 15, 998. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; Madejón, P.; Madejón, E.; de Sosa, L.L. Compost and Vegetation Cover Drive Soil Fertility, Microbial Activity, and Community in Organic Farming Soils. Plant Soil. 2025, 516, 299–321. [Google Scholar] [CrossRef]
- Niu, Y.; Luo, Z.; Cai, L.; Coulter, J.A.; Zhang, Y.; Berti, M. Continuous Monoculture of Alfalfa and Annual Crops Influence Soil Organic Matter and Microbial Communities in the Rainfed Loess Plateau of China. Agronomy 2020, 10, 1054. [Google Scholar] [CrossRef]
- Cai, Z.; Shi, J.; Fu, S.; Li, F.; Lv, L.; Liu, Q.; Zhang, H.; Bao, S. Effects of Microbial Fertilizer Combined with Organic Fertilizer on Forage Productivity and Soil Ecological Functions in Grasslands of the Muli Mining Area. Plants 2025, 14, 3156. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Gu, X.; Wei, Q.; Liu, L.; Gou, J. Green Manure Rotation Combined with Biochar Application Improves Yield and Economic Stability of Continuous Cropping of Peppers in Southwest China. Plants 2024, 13, 3387. [Google Scholar] [CrossRef]
- Wang, Z.; Xuan, H.; Liu, B.; Zhang, H.; Zheng, T.; Liu, Y.; Dai, L.; Xie, Y.; Shang, X.; Zhang, L.; et al. Diversified Cropping Modulates Microbial Communities and Greenhouse Gas Emissions by Enhancing Soil Nutrients. Agronomy 2025, 15, 1472. [Google Scholar] [CrossRef]
- Al-Musawi, Z.K.; Vona, V.; Kulmány, I.M. Utilizing Different Crop Rotation Systems for Agricultural and Environmental Sustainability: A Review. Agronomy 2025, 15, 1966. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, J.; Qiao, H.; Du, M.; Hu, Q.; Wan, S.; Dong, H.; Zhang, J.; Dong, Z.; Li, T.; et al. Diversified Crop Rotation Improves Soil Quality by Increasing Soil Organic Carbon in Long-Term Continuous Cotton Fields. Agronomy 2025, 15, 2698. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.