Abstract
A divergent selection for resilience was carried out in rabbits over 12 generations. The selection criterion was increased (the HO line) and decreased litter size variability at birth (the HE line). The HO line (more resilient) shows higher litter size than the HE line (less resilient). The HO line sows higher litter size and embryo development than the HE line. The aim of this work is to investigate the plasma organic acid profile in both lines at mating and early gestation in order to analyze the effect of selection by resilience in the ovulation rate and early gestation. A total of 19 and 18 nonlactating multiparous females from the HE and HO lines were used. The ovulation rate, normal embryos, and percentage of compacted morulae at 72 h post-coitum (hpc) were studied, and blood samples were obtained at mating and 72 hpc. The organic profile was determined by HPLC. Bayesian methodology was used for statistical analysis. The HE line had 1.5% fewer normal embryos and 12.3% fewer compacted morulae than the HO line. The ovulation rate was similar in both lines. α-ketoglutaric acid and cis-aconitic acid were higher in the HE line than in the HO line. Citric acid, lactic acid, and pyruvic acid were higher at mating than at early gestation. In conclusion, the lower efficiency in the utilization of energy sources in the HE line could explain the reduced embryo production observed. The organic profile varies depending on the reproductive state in the female.
1. Introduction
The metabolism of the oocyte and early embryo depends on energetic substrates, such as pyruvic acid, lactic acid, NADH, and FADH2, which originate from glycolytic activity in cumulus cells and are transferred to the oocyte through gap junctions during antral follicle development [1,2,3]. These metabolic substrates are essential for the mitochondria of the oocyte or early embryo to produce ATP during oocyte maturation, fertilization, and embryo development up to the blastocyst stage [4,5,6]. Additionally, the beta-oxidation of fatty acids increases during ovulation and early gestation, providing substrates for the citric acid cycle [7,8,9,10,11]. Understanding the metabolic needs of preimplantation embryos is crucial for optimizing embryo growth. If the necessary energy substrates are not available in sufficient concentrations or at the appropriate time, the embryo will be unable to develop. Thus, the organic acid profile seems to be essential in this period to optimize the ovulation rate and the number of embryos and their quality.
A divergent selection program for resilience as an objective was developed in rabbits. But resilience is a trait that is difficult to measure directly. Thus, our team proposed measuring resilience as the environmental variance in litter size within females. The HO line (more resilient) was selected for decreasing litter size variability, and the HE line (less resilient) was selected for increasing litter size variability [12]. The genetic program was successful, where the variability in litter size is 2.5 kits2 for the HO line and 5.5 kits2 for the HE line after 12 generations of selection. Does of this line had lower inflammatory response to infectious processes and greater resistance to diseases than the heterogeneous line (HE). These findings align with the lower mortality rate of females at parturition, the reduced percentage of litter mortality at birth and weaning, and the greater uniformity in litter weight at weaning observed in the HO line [13]. In summary, the HO line demonstrated greater resilience compared to the HE line [14,15]. In addition, females from the HE line had a higher stress response and lower disease resistance and litter size than the HO line [13,14,15], so the HO line can be considered more resilient than the HE line. The studies carried out during early gestation indicate that the differences in litter size between the lines appear in the early stages of embryo development, showing that the HO line had a higher number of normal embryos and embryo development at 72 h post-coitum (hpc) than the HE line [16,17]. The rabbit is a suitable experimental model for the study of ovulation and early gestation because the timing of ovulation and embryo development is accurately known [18]. Therefore, these lines provide extraordinary genetic material for the study of energy requirements at ovulation and early gestation.
Apart from its use in the meat industry, the rabbit has been proposed as a model for other productive species and for humans [18]. So, identifying novel biomarkers that may play a significant role in monitoring the healthy progression of early gestation may be of particular interest (Ref. [19] in women, Ref. [20] in cows, Ref. [21] in sheep). Furthermore, these findings could contribute to the development of targeted supplements or therapeutic strategies aimed at ensuring optimal maternal and fetal outcomes. So, the objective of this study was to investigate the plasma organic acid profile in two lines selected divergently for resilience at mating and early gestation and to analyze the effect of selection in the ovulation rate, early gestation, and the plasma organic acid profile.
2. Materials and Methods
2.1. Animals
The animals came from the twelfth generation of a divergent selection experiment for resilience, with the objective of selection with synthetic lines [12]. The selection criterion was increased (the HO line) and decreased litter size variability at birth (the HE line). Variability in the litter size was estimated as phenotypic variance in the litter size at birth within females, considering all parities, after correcting litter size for the effects of year–season and parity–lactation status (with 3 levels: primiparous females, multiparous nonlactating females, and multiparous lactating females). Generations are discrete, and each generation lasts approximately 12 months.
All animals were kept on the farm at the Miguel Hernández University (Spain). The rabbits were fed a standard commercial pelleted diet (16.5% CP, 15.8% fiber, 4% fat, 36% NDF, 18.5% ADF, 12% indigestible fiber, and 2.400 kcal digestible energy; Cunilactal, Nutreco, Spain). Food and water were provided ad libitum. The weight of the females was 3650 g, ranging between 3550 g. and 3735 g. Each female was housed in a cage with the following dimensions: 37.5 cm × 33 cm × 90 cm. The facilities were fully equipped with fans and cooling. The photoperiod was 16 h continuous illumination and 8 h continuous darkness.
2.2. Blood Sampling
A total of 19 and 18 nonlactating multiparous females from the HE and HO lines were mated at the end of the fourth lactation between September and December of 2019. Following the blood sampling procedure described in [15], two blood samples of 3 mL were drawn from the central artery of each doe’s ear at mating and 72 hpc. The blood sample was collected into a tube with tripotassium ethylenediaminetetraacetic acid (K3-EDTA). All samples were immediately centrifuged at 4000 rpm for 15 min, and the plasma was stored at −80 °C until required for organic acid analyses.
2.3. Traits
The females were euthanized at 72 hpc by intravenous administration of sodium thiopental at a dose of 50 mg/kg of body weight (Thiobarbital, B. Braun Medical S.A., Barcelona, Spain). The entire reproductive tract was immediately removed. The ovulation rate was estimated as the number of corpora lutea in both ovaries. The total number of embryos (TEs) was collected by perfusing the oviducts and uterine horns with 10 mL of Dulbecco’s phosphate-buffered saline containing 0.2% BSA. The embryos were classified as normal (NE) if they exhibited a homogeneous cellular mass and intact embryo coats, as observed under a binocular stereoscopic microscope (Leica Mz 9.5; ×600) [16]. The percentage of normal embryos was determined using the formula: ([NE/TE] × 100). At 72 hpc, the normal embryos were classified as early morulae or compacted morulae (Figure 1 and Figure 2). The compacted morulae were expressed as the percentage of normal embryos.
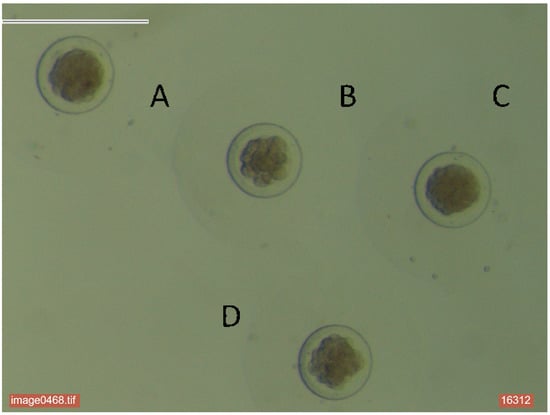
Figure 1.
Embryo classification. A: Abnormal compacted morulae. (B) Normal early morulae. (C) Normal compacted morulae. (D) Abnormal early morulae.
Figure 2.
Compact morulae classified as normal.
2.4. Organic Acid Analyses
All the samples were analyzed in duplicate. First, 100 µL of plasma was mixed with 150 µL ultrapure water and left for 30 min at a temperature of 37 °C. After extraction, the homogenized samples were centrifuged for 5 min at 16,000× g at 4 °C. The resulting extract was filtered through a 0.45 μm cellulose filter (Macherey–Nagel, Düren, Germany); thereby, the supernatant was obtained, which was transferred directly into vials of HPLC and stored at −20 °C.
Concentrations of acetic acid, α-ketoglutaric acid, cis-aconitic acid, citric acid, lactic acid, pyruvic acid, oxalic acid, and glucose were analyzed by High-Performance Liquid Chromatography (Agilent 1100 series HPLC System, Santa Clara, CA, USA) with UV detection at 50 °C, using an PLH-Plex-H Guard Column precolumn (50 × 7.7 mm) and Agilent Hi-Plex H column (300 × 7.7 mm particle size). The mobile phase was a 0.01 mM solution of H2SO4 at a flow rate of 0.4 mL/min with an injection volume of 20 µL.
2.5. Statistical Analyses
The ovulation rate, normal embryos, and compacted morulae were analyzed with a model, including the effects of the line (HE and HO lines).
The model for organic acids and glucose was the following:
where Yijk is the trait, Li is the line effect (i = 2; HE and HO lines), Rj is the reproductive state effect (j = 2; mating and early gestation), (LxR)ij is the interaction line by reproductive state (ij = 4; HE_mating, HE_gestation, HO_mating, HO_gestation), fijk is the female effect (37 levels, indicating repeated measures), and eijk is the error.
Yijk = µ + Li + Rj + (LxR)ij + fijk + eijk
Preliminary analyses were carried out, including weight in the model as a covariate. But this term was removed from the model because it did not present relevant results, maybe due to the low variation in female weight in the experiment.
The traits were analyzed using Bayesian methodology. Bounded flat priors were used for all unknowns, with the exception of the female effect, which was considered normally distributed with mean 0 and variance Iσf2, where I is a unity matrix and σf2 is the variance in the female effect. Residuals were normally distributed with mean 0 and variance Iσe2. The priors for the variances were also bounded uniform. Features of the marginal posterior distribution of differences between lines were estimated using Gibbs sampling. The Rabbit program developed by the Institute for Animal Science and Technology was used for all procedures [22]. Inferences were made from the estimated marginal posterior distributions of the differences between the HE and HO (DHE-HO) lines and between mating and early gestation. The probability of the difference between lines and moments being positive when the difference is positive or being negative when the difference is negative (P) and the highest posterior density (HPD95%) were calculated.
3. Results
3.1. Line Effect
Table 1 shows the features of the estimated marginal posterior distribution of the differences between the HE and HO lines (DHE-HO) for the ovulation rate, normal embryos, and percentage of compacted morulae. Both lines had a similar ovulation rate (10.7 and 10.3 corpora lutea for the HE and HO lines, P = 73%). The HE line had 1.5% fewer normal embryos (P = 90%) and 12.3% fewer compacted morulae than the HO line (P = 95%). Thus, the HE line showed lower embryo development at 72 hpc than the HO line. Hence, increasing litter size variability had a negative effect on early embryonic development.

Table 1.
Ovulation rate, normal embryos, and percentage of compacted morulae for the HE and HO lines.
Table 2 shows the features of the marginal posterior distributions of the difference between the HE and HO lines for the organic acid profile. The concentration of all organic acids was higher in the HE line than in the HO line, and this difference was different from zero for α-ketoglutaric acid and cis-aconitic acid (P > 90%). Both lines showed similar acetic acid, citric acid, lactic acid, pyruvic acid, oxalic acid, and glucose (4.59 mg/mL).

Table 2.
Organic profile for the HE and HO lines.
When the organic acid profile and glucose concentration were studied between the lines for each reproductive state, the results were similar (Figure 3). That is, α-ketoglutaric acid and cis-aconitic acid concentrations were higher in the HE line than in the HO line, both during mating and early gestation. Only during mating was the concentration of citric acid and lactic acid 18% and 15% higher, respectively, in the HE line than in the HO line.
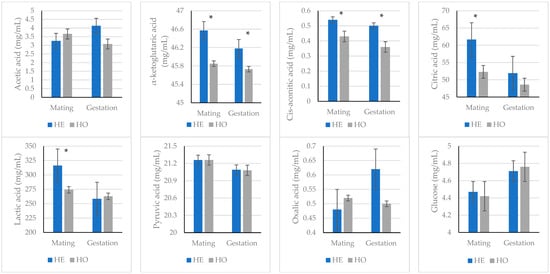
Figure 3.
Acetic acid, α-ketoglutaric acid, cis-aconitic acid, citric acid, lactic acid, pyruvic acid, oxalic acid, and glucose values (mean ± standard error of the posterior marginal distribution) for line and reproductive state. * indicates that the probability of the difference between lines in the same reproductive state being greater than 0 (if the difference is >0) or less than 0 (if the difference is <0) is higher than 90%.
3.2. Reproductive State
The concentration of citric acid was higher during mating than during early gestation (57.04 mg/mL and 50.26 mg/mL, respectively; P = 90%, Table 3). The lactic acid concentration was 13% higher during mating than at 72 hpc (P = 90%). Pyruvic acid was 21.21 mg/mL during mating and 21.09 during early gestation (P = 90%). The concentration of acetic acid, α-ketoglutaric acid, cis-aconitic acid, oxalic acid, and glucose was similar during both mating and early gestation (P < 87%).

Table 3.
Organic profile for mating and gestation stage.
4. Discussion
A divergent selection experiment on resilience was conducted in rabbits, where resilience was measured from litter size variability [12]. Line differences in immune response biomarkers (plasma cortisol, leukocytes, and acute-phase protein levels), in plasma cholesterol and triglycerides, management of energy reserves, and mortality were obtained [13,14,15,23]. Moreover, the most resilient line (HO) showed higher litter size at birth than the less resilient line (HE) [12].
We demonstrated that both lines exhibit similar ovulation rates; nevertheless, recent studies suggest that ovarian folliculogenesis is more efficient in the HO line than the HE line [24]. Furthermore, the HO line shows a higher embryo development and percentage of normal embryos at 72 hpc. These differences are maintained at 12 days of gestation and persist until parity [13,16,17]. Therefore, it is during the early stages of embryonic development that differences between the lines become evident.
Interestingly, the study of the organic acid profile indicates that the HE line shows a higher concentration of various substrates of the Krebs cycle, with particularly high levels of α-ketoglutaric acid, cis-aconitic acid, lactic acid, and citric acid. The oocyte stockpiles substrates of the Krebs cycle, which are used by mitochondria to produce ATP, essential for supporting processes such as oocyte maturation, fertilization, and embryo development up to the blastocyst stage [25,26,27]. It would appear that the energy requirements of the HE line are higher than in the HO line, but its productivity is lower in terms of viability and embryonic development. Our findings suggest a lower efficiency in the utilization of energy sources in the HE line, as its energetic demand, measured as the concentration of substrates of the Krebs cycle, is higher than that of the HO line for comparable oocyte production and lower embryo yield.
Thus, this study reveals that the HO line (more resilient) has better reproductive outcomes, which could be a direct consequence of more efficient energy use. This result could lay the groundwork for identifying new biomarkers in the organic acid profile that are essential for a healthy early gestation. Additionally, these findings may aid in developing targeted supplements or therapeutic approaches to support optimal health in gestating females.
Ovulation in rabbit females is induced by mating, making it possible to accurately determine the timing of ovulation and embryonic development [18]. This particularity suggests a lower efficiency in the utilization of energy sources in the HE line, as its energetic demand is higher than that of the HO line for similar oocyte production, and lower embryo production enables precise embryological studies to identify the main substrates required for energy production during ovulation and early gestation. Our results suggest that ovulation demands more energy than early gestation, as concentrations of citric acid, lactic acid, and pyruvic acid are higher at the time of mating. Before maturation, the oocyte accumulates metabolic substrates, such as pyruvate and lactate, through gap junctional transfer from the cumulus cells [26,27,28]. As it transitions from the germinal vesicle stage to metaphase II, mitochondrial activity increases to generate the ATP required for meiotic maturation [5,6,29,30]. During this process, energy production via oxidative phosphorylation is enhanced [31,32,33]. Additionally, Reiger and Loskutoff [34] reported that pyruvate metabolism through the Krebs cycle increases during the in vitro maturation of cattle oocytes, indicating that oxidative metabolism is the primary pathway for cellular energy production at this stage.
Our results indicate that energetic requirements are lower during early gestation than during ovulation. Rabbit zygotes can complete three cleavage divisions in the complete absence of nutrients [35], with similar findings reported for cattle zygotes [36]. Additionally, studies on mouse preimplantation embryos have demonstrated a low rate of energy metabolism, primarily due to the limited activity of specific Krebs cycle enzymes [37]. However, more advanced developmental stages, such as the blastocyst, exhibit significantly increased metabolic activity [38].
During preimplantation embryo development, glucose, pyruvate, and lactate serve as energy substrates [26,29]. The main source of energy varies depending on the species studied. For instance, developing murine embryos rely on pyruvate as their main energy source until they reach the blastocyst stage [39], while lactate is essential for the development of hamster embryos [40] and bovine embryos [41]. Conversely, in porcine embryos, pyruvate utilization remains minimal at all examined stages, both in vitro and in vivo, with significant increases observed only at the blastocyst stage [42,43]. The substrate with the highest concentration found in 72 hpc pregnant rabbits, when the embryos are at the morula stage, is lactic acid. Lactate serves not only as an energy source during preimplantation development but also as a potent cytosolic reductant via the activity of lactate dehydrogenase [44]. Additionally, lactate increases pyruvate metabolism in mouse embryos [45]. Nevertheless, lactate has been shown to inhibit the preimplantation development of pig embryos [46,47].
A recurring pattern in embryo metabolism is the increased reliance on glycolysis and glucose utilization as preimplantation development progresses in pigs [48], sheep [49], and cattle [50]. However, species-specific metabolic preferences are evident. For example, glucose inhibits all stages of hamster embryo development [51]. Similarly, glucose can have an inhibitory effect on murine [52] and bovine [53] embryos when present before the maternal–zygotic transition. In hamster embryos, glucose is not the preferred energy substrate and inhibits preimplantation development in vitro [51]. Interestingly, our results indicate that glucose levels remain unchanged from ovulation to 72 hpc.
The results are clear despite the sample size of the experiment. However, a larger sample size would be desirable in future studies to strengthen the validity of the findings. We suggest future avenues for research based on the findings. For instance, investigating how the organic acid profile evolves throughout the entire gestation period and its correlation with the offspring’s health and development and examining the effect of supplementation or dietary changes on the organic acid profile and reproductive performance in the HE line.
5. Conclusions
In conclusion, the lower efficiency in the utilization of energy sources in the HE line compared to the HO line could explain the reduced embryo production observed in the HE line. The organic profile of plasma varies depending on the reproductive state in the rabbit female, adapting to the energetic demands of mating and early gestation.
Author Contributions
Conceptualization, M.-L.G. and M.-J.A.; methodology, M.-L.G.; formal analysis, M.-L.G.; resources, M.-L.G.; data curation, I.H. and I.A.; writing—original draft preparation, M.-L.G.; writing—review and editing, I.A., M.-L.G., M.-J.A., and I.H.; funding acquisition, M.-L.G. All authors have read and agreed to the published version of this manuscript.
Funding
This research was funded by the Valencia Regional Government, grant number AICO/2019/169.
Institutional Review Board Statement
All experimental procedures involving animals were approved by the Miguel Hernández University of Elche Research Ethics Committee (Reference number 2019/VSC/PEA/0017) on 25 February 2019, in accordance with the International Guiding Principles for Biomedical Research Involving Animals, as promulgated by the Society for the Study of Reproduction and EU Directive 2010/63/EU.
Data Availability Statement
The original contributions presented in this study are included in this article. Further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685. [Google Scholar] [CrossRef] [PubMed]
- Winterhager, E.; Kidder, G.M. Gap junction connexins in female reproductive organs: Implications for women’s reproductive health. Hum. Reprod. Update 2015, 21, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Richani, D.; Dunning, K.R.; Thompson, J.G.; Gilchrist, R.B. Metabolic co-dependence of the oocyte and cumulus cells: Essential role in determining oocyte developmental competence. Hum. Reprod. Update 2021, 27, 27–47. [Google Scholar] [CrossRef]
- Chappel, S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet. Gynecol. Int. 2013, 2013, 183024. [Google Scholar] [CrossRef] [PubMed]
- Dalton, C.M.; Szabadkai, G.; Carroll, J. Measurement of ATP in single oocytes: Impact of maturation and cumulus cells on levels and consumption. J. Cell. Physiol. 2014, 229, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Aguila, L.; Treulen, F.; Therrien, J.; Felmer, R.; Valdivia, M.; Smith, L.C. Oocyte selection for in vitro embryo production in bovine species: Noninvasive approaches for new challenges of oocyte competence. Animals 2020, 10, 2196. [Google Scholar] [CrossRef]
- Hyttel, P.; Callesen, H.; Greve, T. Ultrastructural features of preovulatory oocyte maturation in superovulated cattle. J. Reprod. Fertil. 1986, 76, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Dom. Anim. 2009, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, M.; Silva, E.; Schoolcraft, W.B.; Krisher, R.L. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol. Reprod. 2013, 88, 111. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and β-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- Dalbies-Tran, R.; Cadoret, V.; Desmarchais, A.; Elis, S.; Maillard, V.; Monget, P.; Monniaux, D.; Reynaud, K.; Saint-Dizier, M.; Uzbekova, S. A comparative analysis of oocyte development in mammals. Cells 2020, 9, 1002. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.; Martínez-Álvaro, M.; García, M.L.; Ibáñez-Escriche, N.; Argente, M.J. Selection for environmental variance of litter size in rabbit. Genet. Sel. Evol. 2017, 49, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Argente, M.J.; Calle, E.W.; García, M.L.; Blasco, A. Correlated response in litter size components in rabbits selected for litter size variability. J. Anim. Breed. Genet. 2017, 134, 505–511. [Google Scholar] [CrossRef]
- Argente, M.J.; García, M.L.; Zbyňovká, K.; Petruška, P.; Capcarová, M.; Blasco, A. Correlated response to selection for litter size environmental variability in rabbit’s resilience. Animal 2019, 13, 2348–2355. [Google Scholar] [CrossRef] [PubMed]
- Beloumi, D.; Blasco, A.; Muelas, R.; Santacreu, M.A.; García, M.L.; Argente, M.J. Inflammatory correlated response in two populations of rabbit selected divergently for litter size environmental variability. Animals 2020, 10, 1540. [Google Scholar] [CrossRef] [PubMed]
- García, M.L.; Blasco, A.; Argente, M. Embryologic changes in rabbit lines selected for litter size variability. Theriogenology 2016, 86, 1247–1250. [Google Scholar] [CrossRef]
- Calle, E.W.; García, M.L.; Blasco, A.; Argente, M.J. Correlated response in early embryonic development in rabbits selected for litter size variability. World Rabbit Sci. 2017, 25, 323–327. [Google Scholar] [CrossRef][Green Version]
- García, M.L. Embryo manipulation techniques in the rabbit. In New Insights into Theriogenology, 1st ed.; Payan-Carreira, R., Ed.; IntechOpen: London, UK, 2018; pp. 113–133. Available online: https://www.intechopen.com/chapters/63632 (accessed on 1 February 2025).
- Carlin, A.; Alfirevic, Z. Physiological changes of pregnancy and monitoring. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 801–823. [Google Scholar] [CrossRef] [PubMed]
- Szenci, O. Recent Possibilities for the Diagnosis of Early Pregnancy and Embryonic Mortality in Dairy Cows. Animals 2021, 11, 1666. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Fan, Y.; Nie, H.; Wang, R.; Wang, F. Metabolic profiling of stages of healthy pregnancy in Hu sheep using nuclear magnetic resonance (NMR). Theriogenology 2017, 92, 121–128. [Google Scholar] [CrossRef]
- Blasco, A. The use of Bayesian statistics in meat quality analyses: A review. Meat Sci. 2005, 69, 115–122. [Google Scholar] [CrossRef] [PubMed]
- García, M.L.; Blasco, A.; García, M.E.; Argente, M.J. Correlated response in body condition and energy mobilisation in rabbits selected for litter size variability. Animal 2019, 13, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, I.; Fabová, Z.; García, M.L.; Loncová, B.; Morovic, M.; Makovicky, P.; Sirotkin, A.V.; Argente, M.J. Effects of selection for litter size variability on ovarian folliculogenesis, ovarian cell proliferation, apoptosis, and production of regulatory peptides in rabbits. Ital. J. Anim. Sci. 2024, 23, 1290–1304. [Google Scholar] [CrossRef]
- Johnson, M.T.; Freeman, E.A.; Gardner, D.K.; Hunt, P.A. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol. Reprod. 2007, 77, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Pool, T.B. ART failure: Oocyte contributions to unsuccessful fertilization. Hum. Reprod. Update 2008, 14, 431–446. [Google Scholar] [CrossRef]
- Zhang, K.; Smith, G.W. Maternal control of early embryogenesis in mammals. Reprod. Fertil. Dev. 2015, 27, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Franciosi, F. Acquisition of oocyte competence to develop as an embryo: Integrated nuclear and cytoplasmic events. Hum. Reprod. Update 2018, 24, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Fair, T.; Hulshof, S.; Hyttel, P.; Greve, T.; Boland, M. Oocyte ultrastructure in bovine primordial to early tertiary follicles. Anat. Embryol. 1997, 195, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.J.; Cho, S.J.; Lee, H.S.; Deb, G.K.; Lee, Y.S.; Kwon, T.H.; Kong, I.K. Effect of cytoplasmic lipid content on in vitro developmental efficiency of bovine IVP embryos. Theriogenology 2009, 72, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Biggers, J.D.; Whittingham, D.G.; Donahue, R.P. The pattern of energy metabolism in the mouse oocyte and zygote. Proc. Natl. Acad. Sci. USA 1967, 58, 560–567. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.M.; Dunning, K.R.; Sutton-McDowall, M.; Gilchrist, R.B.; Thompson, J.G.; Russell, D.L. Failure to launch: Aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction 2017, 153, R109–R120. [Google Scholar] [CrossRef] [PubMed]
- Rieger, D.; Loskutoff, N.M. Changes in the metabolism of glucose, pyruvate, glutamine and glycine during maturation of cattle oocytes in vitro. J. Reprod. Fertil. 1994, 100, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.T. Minimal nutrient requirements for culture of one-cell rabbit embryos. Biol. Reprod. 1987, 37, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.M.; Leese, H.J. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2006, 73, 1195–1201. [Google Scholar] [CrossRef]
- Barbehenn, E.K.; Wales, R.G.; Lowry, O.H. Measurement of metabolites in single preimplantation embryos; a new means to study metabolic control in early embryos. J. Embryol. Exp. Morphol. 1978, 43, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.M., Jr.; Brinster, R.L. Oxygen consumption of preimplantation mouse embryos. Expl. Cell Res. 1967, 47, 337–344. [Google Scholar] [CrossRef]
- Leese, H.J.; Barton, A.M. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J. Reprod. Fertil. 1984, 72, 9–13. [Google Scholar] [CrossRef]
- McKiernan, S.H.; Bavister, B.D.; Tasca, R.J. Energy substrate requirements for in-vitro development of hamster 1- and 2-cell embryos to the blastocyst stage. Hum. Reprod. 1991, 6, 64–75. [Google Scholar] [CrossRef]
- Rosenkrans, C.F., Jr.; Zeng, G.Q.; MCNamara, G.T.; Schoff, P.K.; First, N.L. Development of bovine embryos in vitro as affected by energy substrates. Biol. Reprod. 1993, 49, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.; Bormann, C.; Clark, S.; Walters, E.; Wheeler, M.; Krisher, R. Use of energy substrates by various stage preimplantation pig embryos produced in vivo and in vitro. Reproduction 2002, 123, 253–260. [Google Scholar] [CrossRef]
- Petters, R.M.; Johnson, B.H.; Reed, M.L.; Archibong, A.E. Glucose, glutamine and inorganic phosphate in early development of the pig embryo in vitro. Reproduction 1990, 89, 269–275. [Google Scholar] [CrossRef]
- Reader, K.L.; Stanton, J.L.; Juengel, J.L. The Role of oocyte organelles in determining developmental competence. Biology 2017, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.; Gardner, D.K. Lactate regulates pyruvate uptake and metabolism in the preimplantation mouse embryo. Biol. Reprod. 2000, 62, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.L.; Day, B.N. Cleavage and blastocyst formation by pig eggs in vitro. J. Anim. Sci. 1978, 46, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.L. Culture and storage of pig embryos. J. Reprod. Fert. 1985, 33, 115–124. [Google Scholar] [CrossRef]
- Flood, M.R.; Weibold, J.L. Glucose metabolism by preimplantation pig embryos. J. Reprod. Fertil. 1988, 84, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.G.; Simpson, A.C.; Pugh, P.A.; Wright Jr, R.W.; Tervit, H.R. Glucose utilization by sheep embryos derived in vivo and in vitro. Reprod. Fertil. Devel. 1991, 3, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Rieger, D.; Loskutoff, N.M.; Betteridge, K.J. Developmentally related changes in the metabolism of glucose and glutamine by cattle embryos produced and co-cultured in vitro. J. Reprod. Fertil. 1992, 95, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Seshagiri, P.B.; Bavister, B.D. Glucose inhibits development of hamster 8-Cell embryos in vitro. Biol. Reprod. 1989, 40, 599–606. [Google Scholar] [CrossRef]
- Chatot, C.L.; Tasca, R.J.; Ziomek, C.A. Glutamine uptake and utilization by preimplantation mouse embryos in CZB medium. J. Reprod. Fertil. 1990, 89, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Funahashi, H.; Niwa, K.; Okuda, K. Glucose requirement at different developmental stages of in vitro fertilized bovine embryos cultured in semi-defined medium. Theriogenology 1993, 39, 875–886. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).