Influence of Larval Diet and Adult Age on the Chemical Composition of Female Pheromone Glands of Copitarsia decolora (Lepidoptera: Noctuidae): Implications for Semiochemical-Based Crop Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Sampling and Rearing
2.2. Gland Extract Preparation and Treatments
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Diet Type on Reproductive Parameters of C. decolora
3.2. Female Pheromone Gland Components
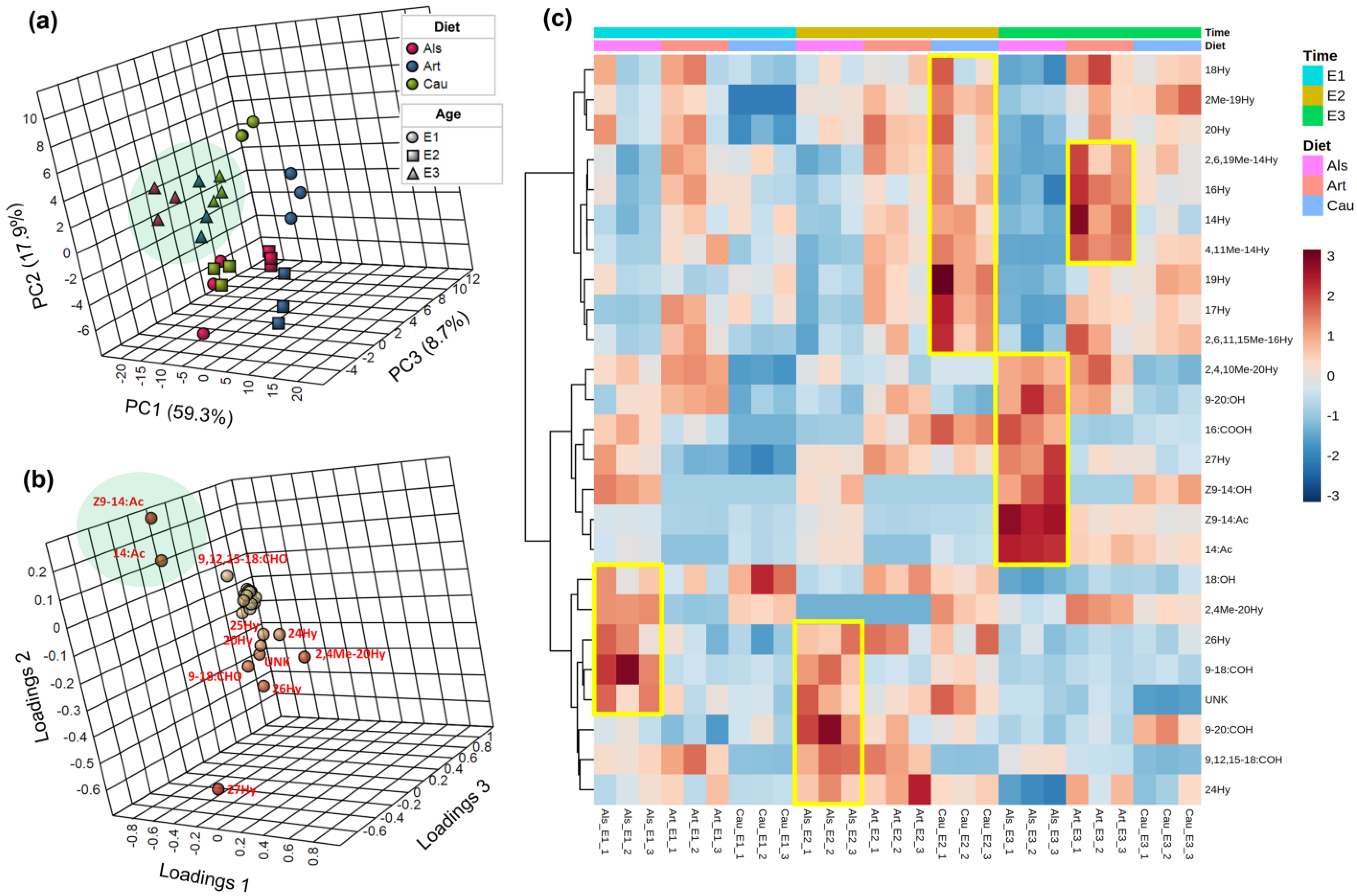
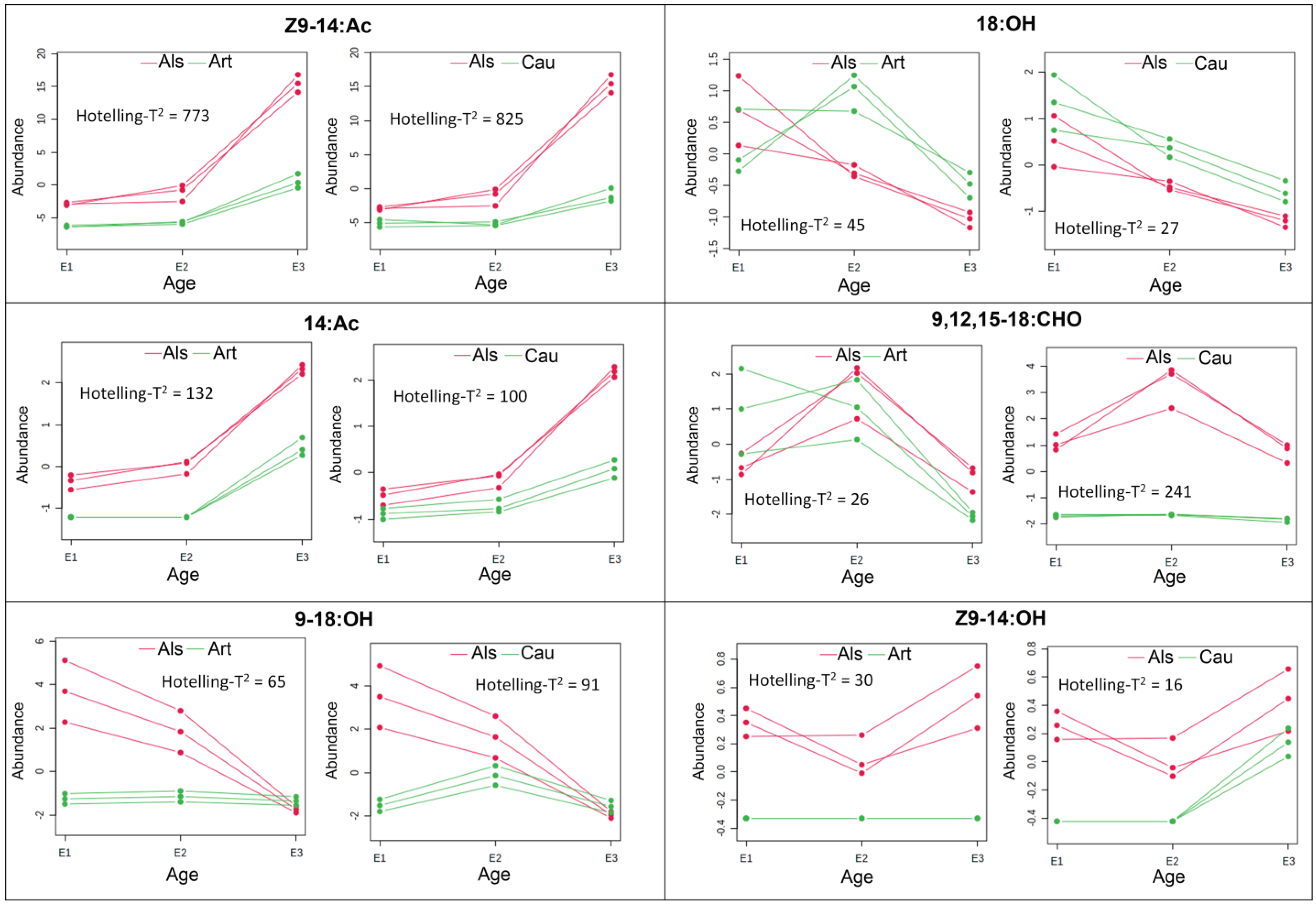
3.3. Multivariate Analysis of the Chemical Profiles of C. decolora Female Pheromone Glands
3.4. Implications for Rearing and Pest Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes-Prado, H.; Jiménez-Pérez, A.; Arzuffi, R.; Robledo, N. Copitarsia decolora Guenée (Lepidoptera: Noctuidae) Females Avoid Larvae Competition by Detecting Larvae Damaged Plants. Sci. Rep. 2020, 10, 5633. [Google Scholar] [CrossRef]
- Venette, R.C.; Gould, J.R. A Pest Risk Assessment for Copitarsia Spp., Insects Associated with Importation of Commodities into the United States. Euphytica 2006, 148, 165–183. [Google Scholar] [CrossRef]
- Angulo, A.O.; Olivares, T.S. The Moth Copitarsia Decolora: A Review of the Species Complex Based on Egg and Male Genital Morphology (Lepidoptera: Noctuidae). Rev. Biol. Trop. 2010, 58, 769–776. Available online: https://pubmed.ncbi.nlm.nih.gov/20527474/ (accessed on 24 October 2025). [PubMed]
- Angulo, A.O.; Olivares, T.S. Taxonomic Update of the Species of Copitarsia Hampson 1906, (Lepidoptera: Noctuidae: Cuculliinae). Gayana Concepc. 2003, 67, 33–38. [Google Scholar] [CrossRef]
- Gould, J.; Venette, R.; Winograd, D. Effect of Temperature on Development and Population Parameters of Copitarsia decolora (Lepidoptera: Noctuidae). Environ. Entomol. 2005, 34, 548–556. [Google Scholar] [CrossRef]
- Muñiz-Reyes, E.; Cibrián-Tovar, J.; Rojas-Leon, J.; Díaz-Gómez, O.; Valdés-Carrasco, J.; Bautista-Martínez, N. Captures of Copitarsia decolora (Lepidoptera: Noctuidae) with Traps Baited with Varying Ratios of Sex Pheromones. Agrociencia 2007, 41, 575–581. Available online: https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952007000500575&lng=es&tlng=en (accessed on 24 October 2025).
- Paz-Penagos, H.P.; Arenas, A.S.P.; Galarza, C.M. Effects of the Frequency Emissions of the Tadarita brasiliensis Simulated Using Electronic Device for Repelling Copitarsia decolora (Lepidoptera: Noctuidae) Adults. In Studies in Computational Intelligence; Springer: Berlin/Heidelberg, Germany, 2025; Volume 1215, pp. 327–352. [Google Scholar] [CrossRef]
- Areces-Berazain, F. Copitarsia decolora (Mexican Cabbage Heartworm). CABI Compend. 2022, 2022, 37945889. [Google Scholar] [CrossRef]
- Quimbayo, N.; Serna, F.; Olivares, T.S.; Angulo, A.O. Noctuids (Lepidoptera) in Colombian Flower Crops. Rev. Colomb. Entomol. 2010, 36, 38–46. [Google Scholar] [CrossRef]
- Castro-Márquez, A.M.C.; Caicedo, D.R. Life-Cycle Parameters of Copitarsia uncilata (Lepidoptera: Noctuidae) on Three Natural Diets. Rev. Fac. Nac. Agron. Medellín 2016, 69, 7763–7771. [Google Scholar] [CrossRef]
- Vázquez-Covarrubias, D.A.; Jiménez-Pérez, A.; Castrejón, F.; Figueroa-Brito, R.; Belmont, R.M. Effects of Five Species of Chenopodiaceae on the Development and Reproductive Potential of Copitarsia decolora (Lepidoptera: Noctuidae). Fla. Entomol. 2015, 98, 80–85. [Google Scholar] [CrossRef]
- Rojas, J.C.; Cruz-López, L.; Malo, E.A.; Díaz-Gómez, O.; Calyecac, G.; Cibrián-Tovar, J.C. Identification of the Sex Pheromone of Copitarsia decolora (Lepidoptera: Noctuidae). J. Econ. Entomol. 2006, 99, 797–802. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Díaz-Gómez, O.; Malo, E.A.; Patiño-Arrellano, S.A.; Rojas, J.C. Pheromone Trap for Monitoring Copitarsia decolora (Lepidoptera: Noctuidae) Activity in Cruciferous Crops in Mexico. Fla. Entomol. 2012, 95, 602–609. [Google Scholar] [CrossRef]
- Ahmad, S.N.; Kamarudin, N. Pheromone Trapping in Controlling Key Insect Pests: Progress and Prospects. Oil Palm Bull. 2011, 62, 12–24. Available online: https://palmoilis.mpob.gov.my/publications/OPB/opb62-Nurul.pdf/ (accessed on 28 October 2025).
- Blassioli-Moraes, M.C.; Laumann, R.A.; Michereff, M.F.F.; Borges, M. Semiochemicals for Integrated Pest Management. In Sustainable Agrochemistry: A Compendium of Technologies; Vaz, S., Jr., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 85–112. ISBN 978-3-030-17891-8. [Google Scholar]
- Callado-Galindo, M.M.; Villa-Ayala, P.; Castrejón, F.; Jiménez-Pérez, A. Effect of Age, Body Weight and Multiple Mating on Copitarsia decolora (Lepidoptera: Noctuidae) Reproductive Potential and Longevity. J. Insect Behav. 2013, 26, 860–872. [Google Scholar] [CrossRef]
- Suárez-Vargas, A.D.; Bautista-Martínez, N.; Valdéz-Carrasco, J.; Angulo-Ormeño, A.; Alatorre-Rosas, R.; Vera-Graziano, J.; Equilhua-Martínez, A.; Manuel-Pinto, V. Population Fluctuation of Copitarsia decolora (Guenée) and Its Association with Commercial Crucifers. Agrociencia 2006, 40, 501–509. Available online: https://www.redalyc.org/pdf/302/30240409.pdf (accessed on 24 October 2025).
- Altamar-Varón, P.; Pérez-Maldonado, D.; Rodríguez-Caicedo, D.; Guerrero-Perilla, C.; Coy-Barrera, E. Chemical Composition of the Low-Polar Fraction of the Copitarsia uncilata Burgos & Leiva (Lepidoptera: Noctuidae) Eversible Pheromone Gland. Neotrop. Entomol. 2016, 45, 734–739. [Google Scholar] [CrossRef]
- Díaz, M.F.; Ramırez-Godoy, A.; Poveda, K. Efficiency of Different Egg Parasitoids and Increased Floral Diversity for the Biological Control of Noctuid Pests. Biol. Control 2012, 60, 182–191. [Google Scholar] [CrossRef]
- González-Chang, M.; Tiwari, S.; Sharma, S.; Wratten, S.D. Habitat Management for Pest Management: Limitations and Prospects. Ann. Entomol. Soc. Am. 2019, 112, 302–317. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Addison, M.F.; Terblanche, J.S. Mass-Rearing of Insects for Pest Management: Challenges, Synergies and Advances from Evolutionary Physiology. Crop Prot. 2012, 38, 87–94. [Google Scholar] [CrossRef]
- Schowalter, T.D. 3—Resource Acquisition. In Insect Ecology, 2nd ed.; Schowalter, T.D., Ed.; Academic Press: Burlington, VT, USA, 2006; pp. 53–93. ISBN 978-0-12-088772-9. [Google Scholar]
- Singh, P. Artificial Diets for Insects, Mites, and Spiders, 1st ed.; Springer: New York, NY, USA, 1977; Volume 1–594, ISBN 978-1-4684-8351-2. [Google Scholar]
- Cohen, A. Production of Insect Diets for Commercial Use. In California Conference on Biological Control IV, Berkeley, CA, USA, 13–15 July 2004; Hoddleeditor, M.S., Ed.; Center for Biological Control, College of Natural Resources, University of California: Berkeley, CA, USA, 2004; pp. 76–82. [Google Scholar]
- Reguilón, C.; Medina Pereyra, P.; Ordano, M.; Salvatore, A.; Barros, M.; Morsoletto Santos, E.; Vicente Cano, M. Assessment of artificial diet composition for laboratory rearing of Diatraea saccharalis (Lepidoptera: Crambidae) and Cotesia flavipes (Hymenoptera: Braconidae). Rev. Fac. Cienc. Agrar. Univ. Nac. Cuyo 2014, 46, 45–57. Available online: https://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1853-86652014000100004&lng=es&nrm=iso (accessed on 24 October 2025).
- Chaudhury, M.F. Insect Diet, Feeding and Nutrition. In Principles and Procedures for Rearing High Quality Insects; Schneider, J.C., Ed.; Mississippi State University: Starkville, MS, USA, 2009; pp. 121–143. ISBN 978-0-615-31190-6. [Google Scholar]
- Acatitla-Trejo, C.; Bautista-Martínez, N.; Vera-Graziano, J.; Romero-Nápoles, J.; Calyecac-Cortero, H.G. Biological Cycle and Rates of Survivorship and Reproduction of Copitarsia incommoda (Walker) (Lepidoptera: Noctuidae) in Five Artificial Diets. Agrociencia 2004, 38, 355–363. Available online: https://www.redalyc.org/pdf/302/30238309.pdf (accessed on 24 October 2025).
- Rojas, J.C.; Cibrian-Tovar, J.; Valde’z-Carrasco, J.; Nieto-Hernández, R. Analysis of Copitarsia consueta (Walker) Courtship Behavior and Isolation of its Sex Pheromone. Agrociencia 1993, 4, 23–39. [Google Scholar]
- Force, E.; Dacher, M.; Debernard, S. How the Diet Influences Lepidopteran Reproduction: Morpho-Functional, Behavioral, and Endocrine Aspects. J. Insect Physiol. 2025, 164, 104838. [Google Scholar] [CrossRef]
- Regmi, R.; Ali, R.; Akter, S.; Yousuf, F.; Mainali, B.; Park, S.J. The Role of Larval Nutrition in Shaping Pheromone Composition in Fall Armyworm. J. Chem. Ecol. 2025, 51, 79. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Kodama, S.; Ishikawa, Y.; Yamamoto, M.; Sakurai, T.; Fónagy, A. Lipid Droplets in the Pheromone Glands of Bombycids: Effects of Larval Diet on Their Size and Pheromone Titer. J. Insect Physiol. 2022, 142, 104440. [Google Scholar] [CrossRef] [PubMed]
- Colasurdo, N.; Gélinas, Y.; Despland, E. Larval Nutrition Affects Life History Traits in a Capital Breeding Moth. J. Exp. Biol. 2009, 212, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Cañas-Hoyos, N.; Lobo-Echeverri, T.; Saldamando-Benjumea, C.I. Chemical Composition of Female Sexual Glands of Spodoptera frugiperda Corn and Rice Strains from Tolima, Colombia. Southwest. Entomol. 2017, 42, 375–394. [Google Scholar] [CrossRef]
- Mujiono, K.; Witjaksono, W.; Putra, N.S. The Sex Pheromone Content of The Spodoptera exigua (Hubner) Under Artificial and Natural Diets. Int. J. Sci. Eng. 2015, 8, 146–150. Available online: https://ejournal.undip.ac.id/index.php/ijse/article/view/8681 (accessed on 28 October 2025).
- Force, E.; Suray, C.; Monsempes, C.; Danis, C.; Bonfils, G.; Debernard, S.; Dacher, M. Diet Acts on Sexual Behavior Development in a Male Moth. Insect Sci. 2025, 32, 1439–1452. [Google Scholar] [CrossRef]
- Zweerus, N.L.; van Wijk, M.; Schal, C.; Groot, A.T. Diet-Derived Male Sex Pheromone Compounds Affect Female Choice in a Noctuid Moth. Sci. Rep. 2023, 13, 19802. [Google Scholar] [CrossRef]
- Du, X.; Smirnov, A.; Pluskal, T.; Jia, W.; Sumner, S. Metabolomics Data Preprocessing Using ADAP and MZmine 2. In Computational Methods and Data Analysis for Metabolomics; Li, S., Ed.; Springer: New York, NY, USA, 2020; pp. 25–48. ISBN 978-1-0716-0239-3. [Google Scholar]
- Cárdenas-Laverde, D.; Rincón-Aceldas, S.; Coy-Barrera, E. Identification of Antifungal Compounds from Piper Plants Against Fusarium oxysporum: An Untargeted Metabolite Profiling-Based Approach. Nat. Prod. Commun. 2022, 17, 1934578X221089995. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Flores-Pérez, L.; Bautista-Martínez, N.; Vera-Graziano, J.; Valdez-Carrasco, J.; Angulo, A.O. Life Cycle and Survival and Reproduction Rates of Copitarsia incommoda Walker (Lepidoptera: Noctuidae) in Three Cultivars of Brassica oleracea L. Agrociencia 2004, 38, 517–523. Available online: https://www.redalyc.org/pdf/302/30238505.pdf (accessed on 24 October 2025).
- Cibrián-Tovar, J. Factors Influencing Insect Rearing under Controlled Conditions. In Insect Rearing Techniques; Bautista, M.N., Vejar, G., Carrillo, J.L., Eds.; Colegio de Posgraduados, Instituto de Fitosanidad: Montecillo, Mexico, 1994; pp. 11–23. [Google Scholar]
- Moreno Fajardo, O.L.; Serna Cardona, F.J. Biology of Copitarsia decolora (Lepidoptera: Noctuidae: Cuculliinae), on Cultivated Flowers of the Commercial Hybrid of Alstroemeria spp. Rev. Fac. Nac. Agron. Medellín 2006, 59, 3257–3270. Available online: https://www.redalyc.org/pdf/1799/179914074011.pdf (accessed on 24 October 2025).
- Awmack, C.S.; Leather, S.R. Host Plant Quality and Fecundity in Herbivorous Insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Wang, Z.; Andika, I.P.; Chung, H. Regulation of Insect Cuticular Hydrocarbon Biosynthesis. Curr. Opin. Insect Sci. 2025, 67, 101287. [Google Scholar] [CrossRef]
- Ngumbi, E.N.; Hanks, L.M.; Suarez, A.V.; Millar, J.G.; Berenbaum, M.R. Factors Associated with Variation in Cuticular Hydrocarbon Profiles in the Navel Orangeworm, Amyelois transitella (Lepidoptera: Pyralidae). J. Chem. Ecol. 2020, 46, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Subchev, M.; Jurenka, R.A. Sex Pheromone Levels in Pheromone Glands and Identification of the Pheromone and Hydrocarbons in the Hemolymph of the Moth Scoliopteryx libatrix L. (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 2001, 47, 35–43. [Google Scholar] [CrossRef]
- Naka, H.; Fujii, T. Chemical Divergences in the Sex Pheromone Communication Systems in Moths. In Insect Sex Pheromone Research and Beyond: From Molecules to Robots; Ishikawa, Y., Ed.; Springer: Singapore, 2020; pp. 3–17. ISBN 978-981-15-3082-1. [Google Scholar]
- Terblanche, J.S.; Lehmann, P. Metabolic Flexibility in Insects: Patterns, Mechanisms, and Implications. Annu. Rev. Entomol. 2025, 71. [Google Scholar] [CrossRef]
- Wedell, N. Female Receptivity in Butterflies and Moths. J. Exp. Biol. 2005, 208, 3433–3440. [Google Scholar] [CrossRef]
- Matsumoto, S. Molecular Mechanisms Underlying Sex Pheromone Production in Moths. Biosci. Biotechnol. Biochem. 2010, 74, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpa, F.A.; Jacquin-Joly, E. Sex in the Night: Fatty Acid-Derived Sex Pheromones and Corresponding Membrane Pheromone Receptors in Insects. Membr. Pathol. 2014, 107, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Blankers, T.; Lievers, R.; Plata, C.; van Wijk, M.; van Veldhuizen, D.; Groot, A.T. Sex Pheromone Signal and Stability Covary with Fitness. R. Soc. Open Sci. 2021, 8, 210180. [Google Scholar] [CrossRef] [PubMed]
- Schal, C.; Charlton, R.E.; Cardé, R.T. Temporal Patterns of Sex Pheromone Titers and Release Rates in Holomelina lamae (Lepidoptera: Arctiidae). J. Chem. Ecol. 1987, 13, 1115–1129. [Google Scholar] [CrossRef]
- Jurenka, R. Regulation of Pheromone Biosynthesis in Moths. Neurosci. Pheromones 2017, 24, 29–35. [Google Scholar] [CrossRef]
- Rafaeli, A. Mechanisms Involved in the Control of Pheromone Production in Female Moths: Recent Developments. Entomol. Exp. Appl. 2005, 115, 7–15. [Google Scholar] [CrossRef]
- Foster, S.P.; Anderson, K.G.; Casas, J. The Dynamics of Pheromone Gland Synthesis and Release: A Paradigm Shift for Understanding Sex Pheromone Quantity in Female Moths. J. Chem. Ecol. 2018, 44, 525–533. [Google Scholar] [CrossRef]
- Ding, B.-J.; Lager, I.; Bansal, S.; Durrett, T.P.; Stymne, S.; Löfstedt, C. The Yeast ATF1 Acetyltransferase Efficiently Acetylates Insect Pheromone Alcohols: Implications for the Biological Production of Moth Pheromones. Lipids 2016, 51, 469–475. [Google Scholar] [CrossRef]
- Umbers, K.D.L.; Symonds, M.R.E.; Kokko, H. The Mothematics of Female Pheromone Signaling: Strategies for Aging Virgins. Am. Nat. 2015, 185, 417–432. [Google Scholar] [CrossRef]
- Grayson, K.L.; Parry, D.; Faske, T.M.; Hamilton, A.; Tobin, P.C.; Agosta, S.J.; Johnson, D.M. Performance of Wild and Laboratory-Reared Gypsy Moth (Lepidoptera: Erebidae): A Comparison between Foliage and Artificial Diet. Environ. Entomol. 2015, 44, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.P.; Rani, S.; Birah, A.; Raghuraman, M. Improved Artificial Diet for Mass Rearing of the Tobacco Caterpillar, Spodoptera litura (Lepidoptera: Noctuidae). Int. J. Trop. Insect Sci. 2005, 25, 55–58. [Google Scholar] [CrossRef]
- Rizvi, S.A.; George, J.; Reddy, G.V.P.; Zeng, X.; Guerrero, A. Latest Developments in Insect Sex Pheromone Research and Its Application in Agricultural Pest Management. Insects 2021, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Reddy, G.V.P. Chemical Communication in Insects: New Advances in Integrated Pest Management Strategies. Insects 2023, 14, 799. [Google Scholar] [CrossRef] [PubMed]

| Compound a | Rt b | RI c | RI d | Alstroemeria | Artificial | Cauliflower | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative Abundance (%) e | |||||||||||||
| E1 f | E2 f | E3 f | E1 f | E2 f | E3 f | E1 f | E2 f | E3 f | |||||
| 1 | tetradecane | 14.2 | 1400 | 1400 | 0.86 ± 0.19 | 0.97 ± 0.21 | 0.68 ± 0.10 | 1.52 ± 0.30 | 1.66 ± 0.22 | 2.56 ± 0.57 | 1.47 ± 0.28 | 1.92 ± 0.31 | 1.21 ± 0.22 |
| 2 | 4,11-dimethyltetradecane | 16.3 | 1491 | 1483 | 1.17 ± 0.19 | 0.87 ± 0.15 | 0.59 ± 0.02 | 2.04 ± 0.44 | 2.13 ± 0.27 | 2.91 ± 0.20 | 1.32 ± 0.31 | 2.48 ± 0.36 | 1.92 ± 0.24 |
| 3 | 2,6,19-trimehyltetradecane | 17.6 | 1547 | 1548 | 0.68 ± 0.19 | 0.61 ± 0.12 | 0.52 ± 0.02 | 1.11 ± 0.18 | 1.14 ± 0.13 | 1.27 ± 0.22 | 0.89 ± 0.14 | 1.07 ± 0.18 | 1.00 ± 0.11 |
| 4 | hexadecane | 18.9 | 1600 | 1600 | 1.91 ± 0.51 | 1.96 ± 0.28 | 1.27 ± 0.33 | 2.94 ± 0.37 | 2.42 ± 0.22 | 3.63 ± 0.38 | 2.17 ± 0.35 | 3.02 ± 0.43 | 2.45 ± 0.16 |
| 5 | tetradec-9-en-1-ol | 20.4 | 1667 | 1664 | 0.76 ± 0.12 | 0.51 ± 0.14 | 0.94 ± 0.22 | nd | nd | nd | nd | nd | 0.64 ± 0.11 |
| 6 | heptadecane | 21.2 | 1700 | 1700 | 1.01 ± 0.05 | 0.94 ± 0.21 | 0.65 ± 0.11 | 2.13 ± 0.53 | 1.99 ± 0.34 | 2.05 ± 0.16 | 1.71 ± 0.33 | 2.74 ± 0.55 | 1.87 ± 0.22 |
| 7 | 2,6,11,15-tetramethylhexadecane | 22.2 | 1746 | 1753 | 1.41 ± 0.18 | 1.23 ± 0.31 | 1.13 ± 0.31 | 2.11 ± 0.34 | 1.82 ± 0.41 | 2.29 ± 0.55 | 1.24 ± 0.08 | 2.52 ± 0.52 | 2.04 ± 0.17 |
| 8 | tetradec-9-en-1-yl acetate | 23.2 | 1795 | 1793 | 4.97 ± 0.22 | 6.74 ± 1.25 | 23.32 ± 1.33 | 1.55 ± 0.14 | 2.11 ± 0.19 | 8.41 ± 1.08 | 2.79 ± 0.55 | 2.67 ± 0.29 | 6.86 ± 0.98 |
| 9 | octadecane | 23.4 | 1800 | 1800 | 3.15 ± 0.80 | 3.13 ± 0.33 | 2.02 ± 0.23 | 3.76 ± 0.92 | 3.42 ± 0.35 | 4.26 ± 0.66 | 2.61 ± 0.25 | 3.68 ± 0.97 | 3.54 ± 0.27 |
| 10 | tetradec-1-yl acetate | 23.5 | 1805 | 1793 | 1.01 ± 0.18 | 1.38 ± 0.16 | 3.71 ± 0.11 | nd | nd | 1.83 ± 0.22 | 0.64 ± 0.12 | 0.79 ± 0.14 | 1.61 ± 0.20 |
| 11 | nonadecane | 25.7 | 1900 | 1900 | 1.26 ± 0.28 | 0.97 ± 0.26 | 0.72 ± 0.09 | 1.11 ± 0.18 | 1.69 ± 0.34 | 1.45 ± 0.27 | 1.49 ± 0.22 | 2.57 ± 0.66 | 1.85 ± 0.29 |
| 12 | 2-methyl nonadecane | 26.5 | 1950 | 1557 | 1.02 ± 0.21 | 1.17 ± 0.04 | 0.73 ± 0.11 | 1.23 ± 0.23 | 1.28 ± 0.22 | 1.33 ± 0.26 | nd | 1.61 ± 0.18 | 1.66 ± 0.33 |
| 13 | n-hexadecanoic acid | 26.6 | 1956 | 1942 | 1.32 ± 0.24 | 0.34 ± 0.01 | 1.85 ± 0.34 | 0.70 ± 0.17 | 1.26 ± 0.34 | 0.37 ± 0.03 | nd | 1.87 ± 0.23 | 0.63 ± 0.04 |
| 14 | eicosane | 27.4 | 2000 | 2000 | 4.11 ± 1.14 | 4.01 ± 0.43 | 2.37 ± 0.23 | 4.55 ± 1.08 | 5.04 ± 0.63 | 4.35 ± 0.94 | 2.04 ± 0.35 | 4.85 ± 1.13 | 4.12 ± 0.29 |
| 15 | octadeca-1-ol | 29.0 | 2086 | 2070 | 2.56 ± 0.55 | 1.61 ± 0.09 | 0.84 ± 0.12 | 1.99 ± 0.52 | 2.87 ± 0.29 | 1.39 ± 0.22 | 3.41 ± 0.62 | 2.42 ± 0.20 | 1.47 ± 0.23 |
| 16 | octadec-9,12,15-trienal | 30.4 | 2023 | 2010 | 3.26 ± 0.31 | 5.5 ± 0.82 | 2.91 ± 0.36 | 4.82 ± 1.22 | 4.86 ± 0.85 | 1.81 ± 0.11 | 0.49 ± 0.05 | 0.53 ± 0.02 | 0.34 ± 0.08 |
| 17 | 2,4-dimethyl eicosane | 31.1 | 2095 | 2085 | 6.35 ± 0.14 | nd | 2.62 ± 0.44 | 0.97 ± 0.15 | nd | 6.26 ± 0.53 | 4.56 ± 0.32 | 5.18 ± 1.12 | 3.84 ± 0.39 |
| 18 | octadec-9-enal | 32.5 | 2137 | 2129 | 6.83 ± 1.42 | 4.97 ± 0.96 | 1.39 ± 0.15 | 1.88 ± 0.24 | 1.99 ± 0.25 | 1.78 ± 0.21 | 1.82 ± 0.28 | 3.20 ± 0.45 | 1.77 ± 0.28 |
| 19 | eicos-9-enal | 32.7 | 2184 | 2190 | 1.70 ± 0.15 | 2.85 ± 0.43 | 1.41 ± 0.14 | 1.21 ± 0.24 | 1.95 ± 0.34 | 1.55 ± 0.13 | 1.63 ± 0.24 | 1.77 ± 0.17 | 2.25 ± 0.26 |
| 20 | 2,4,10-trimethyl eicosane | 32.9 | 2189 | 2185 | 2.55 ± 0.31 | 2.16 ± 0.34 | 3.15 ± 0.16 | 3.33 ± 0.11 | 1.89 ± 0.33 | 3.33 ± 0.52 | 0.83 ± 0.08 | 2.01 ± 0.01 | 1.25 ± 0.10 |
| 21 | eicos-9-en-1-ol | 33.9 | 2213 | 2220 | 1.21 ± 0.34 | 0.87 ± 0.19 | 2.12 ± 0.36 | 1.79 ± 0.09 | 1.56 ± 0.25 | 1.65 ± 0.30 | 0.68 ± 0.17 | 0.74 ± 0.21 | 0.87 ± 0.12 |
| 22 | tetracosane | 35.2 | 2400 | 2400 | 5.13 ± 0.63 | 6.76 ± 0.72 | 2.62 ± 0.41 | 5.79 ± 1.07 | 7.55 ± 1.51 | 6.26 ± 1.11 | 4.65 ± 0.11 | 6.04 ± 0.31 | 4.65 ± 1.19 |
| 23 | unknown | 37.5 | 2458 | 2442 | 4.67 ± 1.01 | 4.51 ± 1.01 | 2.29 ± 0.28 | 3.04 ± 0.72 | 3.36 ± 0.44 | 2.16 ± 0.42 | 2.33 ± 0.54 | 4.55 ± 1.03 | 0.89 ± 0.06 |
| 24 | pentacosane | 39.4 | 2500 | 2500 | 5.61 ± 1.11 | 5.38 ± 1.32 | 4.44 ± 1.18 | 6.24 ± 1.08 | 7.20 ± 0.94 | 6.67 ± 0.48 | 4.50 ± 0.19 | 6.49 ± 1.15 | 4.41 ± 1.13 |
| 25 | hexacosane | 41.2 | 2600 | 2600 | 8.08 ± 1.39 | 7.84 ± 0.91 | 5.17 ± 0.73 | 5.51 ± 1.17 | 7.77 ± 1.64 | 5.20 ± 0.23 | 4.61 ± 0.77 | 7.52 ± 1.47 | 5.51 ± 0.52 |
| 26 | heptacosane | 43.2 | 2700 | 2700 | 14.02 ± 2.52 | 12.54 ± 0.31 | 18.72 ± 2.16 | 6.66 ± 1.11 | 15.35 ± 1.85 | 11.57 ± 1.78 | 4.22 ± 0.91 | 12.58 ± 1.59 | 10.48 ± 1.51 |
| Diet | Alstroemeria | Artificial | Cauliflower | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | E1 b | E2 b | E3 b | E1 b | E2 b | E3 b | E1 b | E2 b | E3 b |
| Z9-14:OH a | 0.012 ± 0.002 | 0.004 ± 0.0005 | 0.038 ± 0.005 | nd | nd | nd | nd | nd | 0.013 ± 0.001 |
| Z9-14:Ac a | 1.05 ± 0.21 | 2.45 ± 0.19 | 9.52 ± 1.23 | 0.095 ± 0.012 | 0.37 ± 0.02 | 0.95 ± 0.03 | 0.85 ± 0.03 | 2.88 ± 0.16 | 1.96 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, L.; Rodríguez, D.; Coy-Barrera, E. Influence of Larval Diet and Adult Age on the Chemical Composition of Female Pheromone Glands of Copitarsia decolora (Lepidoptera: Noctuidae): Implications for Semiochemical-Based Crop Protection. Agriculture 2025, 15, 2262. https://doi.org/10.3390/agriculture15212262
Díaz L, Rodríguez D, Coy-Barrera E. Influence of Larval Diet and Adult Age on the Chemical Composition of Female Pheromone Glands of Copitarsia decolora (Lepidoptera: Noctuidae): Implications for Semiochemical-Based Crop Protection. Agriculture. 2025; 15(21):2262. https://doi.org/10.3390/agriculture15212262
Chicago/Turabian StyleDíaz, Luis, Daniel Rodríguez, and Ericsson Coy-Barrera. 2025. "Influence of Larval Diet and Adult Age on the Chemical Composition of Female Pheromone Glands of Copitarsia decolora (Lepidoptera: Noctuidae): Implications for Semiochemical-Based Crop Protection" Agriculture 15, no. 21: 2262. https://doi.org/10.3390/agriculture15212262
APA StyleDíaz, L., Rodríguez, D., & Coy-Barrera, E. (2025). Influence of Larval Diet and Adult Age on the Chemical Composition of Female Pheromone Glands of Copitarsia decolora (Lepidoptera: Noctuidae): Implications for Semiochemical-Based Crop Protection. Agriculture, 15(21), 2262. https://doi.org/10.3390/agriculture15212262








