Abstract
Post-harvest bacterial infections pose a serious threat to modern agriculture, resulting in substantial financial losses and environmental issues. Every year, microbial spoiling causes a significant loss in fruit and vegetable production, and traditional control techniques are frequently insufficient. This review highlights novel delivery systems such as edible films and coatings while analyzing recent developments in bacteriophage-based post-harvest disease management techniques. Bacterial resistance, environmental stability, and regulatory issues restrict the use of bacteriophages, despite their high specificity, biodegradability, and low environmental impact. Phage viability in storage and the field has increased thanks to developments in formulation technologies, such as encapsulation and stabilization. The review highlights the practical implementation of phage applications in post-harvest disease management, with a particular focus on innovative delivery systems as well as integrating legal and regulatory viewpoints. By bridging scientific innovation with legal and commercial considerations, this work offers an interdisciplinary foundation for advancing sustainable, phage-based approach.
1. Introduction: The Need for Sustainable Post-Harvest Disease Management
Post-harvest losses remain a critical issue in modern agriculture, with significant economic, environmental, and social consequences. Despite improvements in harvesting, sorting, cooling, and storage technologies, bacterial diseases continue to cause substantial losses in perishable crops such as fruits and vegetables. These losses not only impact food availability and affordability but also contribute to resource waste and environmental degradation [1,2]. Fruits and vegetables are particularly prone to mechanical damage after harvest due to their freshness and fragility, which can serve as entry points for spoilage bacteria. As a result, postharvest losses can reach up to 12% in industrialized countries like the US, and as high as 30% in humid developing countries [3]. Conventional disease management strategies such as fungicides and biocontrol agents are facing increasing limitations, including environmental concerns, regulatory restrictions, and the emergence of resistant pathogen strains; in particular, the overuse of chemical fungicides in postharvest disease control has raised serious concerns due to their residual toxicity and ecological impact [4].
Animal immune responses are fundamentally different from the intricate host defensive systems that plant pathogenic microorganisms must overcome. Even though bacterial infection mechanisms in plants and animals were always thought to be different, new studies show that they are similar, particularly in terms of bacterial secretion systems and pathogenicity islands (PAIs). Plant resistance (R) and bacterial avirulence genes (avr) usually combine to give plants resistance to bacterial infection. When both are present, the plant triggers defence mechanisms such as the hypersensitive response (HR), which is characterized by localized cell death, reactive oxygen species generation, cell wall reinforcement, and antimicrobial chemicals. Numerous avr genes encode effector proteins, which are mostly transported into plant cells by the Type III secretion system [5]. Given the complexity of these interactions and the limitations of conventional management, there is growing interest in alternative, sustainable solutions for post-harvest disease control [6].
One promising biological approach is the use of bacteriophages, viruses that specifically infect and kill bacterial pathogens. Bacteriophages offer numerous advantages, including high specificity, adaptability, and minimal environmental impact, making them a promising tool for sustainable post-harvest disease management [7,8]. Due to their ability to replicate exclusively within living bacterial cells, bacteriophage-based biosensors can also distinguish between live and dead pathogens, reducing false-positive results [9]. Alongside these benefits, there is a need to select phages with a sufficiently broad host range to prevent false-negative results, since phages typically recognize specific receptors on bacterial cell surfaces [10]. This review highlights recent advancements in bacteriophage formulations, including encapsulation and stabilization techniques that enhance their viability under storage and field conditions. Practical aspects are examined of implementing bacteriophage-based strategies, focusing on delivery methods, real-world applications, formulation enhancements, and regulatory challenges (Table 1), which compares bacteriophage-based approaches with conventional post-harvest disease control methods, highlighting their practical advantages and limitations. Importantly, this work uniquely integrates legal and regulatory perspectives—identifying gaps in formal classification of phages as plant protection products—and discusses existing implementation pathways (e.g., through GAP) alongside several commercially available bacteriophage preparations. By also discussing commercially available phage products, this paper bridges theory with implementation, offering an interdisciplinary contribution to the development of sustainable agricultural solutions. By bridging theory with practice, this paper contributes to the growing knowledge on sustainable agricultural solutions.

Table 1.
Overview of Post-Harvest Disease Control Methods Including Bacteriophage-Based Approaches.
2. Methodology
This review was conducted using a systematic search of peer-reviewed articles from databases such as PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed on 7 May 2025), Scopus (https://www.scopus.com/pages/home?display=basic#basic, accessed on 7 May 2025), and Web of Science (https://clarivate.com/academia-government/scientific-and-academic-research/research-discovery-and-referencing/web-of-science/, accessed on 7 May 2025). Keywords included insect microbiome, plant growth promoting bacteria, PGPB, PGPR, biocontrol, and sustainable agriculture. The search was limited to articles published between 2000 and 2025, but most of the listed publications (48%) are from the years 2023–2024. Relevant studies were selected based on their focus on insect-associated bacteria with plant growth-promoting traits. Duplicate and non-English articles were excluded.
3. Delivery Methods for Bacteriophage Applications in Post-Harvest Systems
Delivery systems of bacteriophage application can be created using various techniques, enabling a wide range of applications in the food industry, such as food processing, packaging, and contact surfaces. Similar approaches have been used in biocontrol formulations, e.g., talc- or wood flour-based formulations based on Bacillus spp. and Pseudomonas fluorescens, which are used in plant protection to reduce the occurrence of diseases in greenhouse and field conditions [14,15]. The choice of technique depends on the intended purpose, such as sanitation, preservation, control, or modulation, and the active substance to be delivered, like phages, probiotics, or essential oils [16]. Food packaging plays a vital role in preserving food quality, ensuring safety, and extending shelf life by shielding against physical, chemical, and microbial damage. Traditional passive packaging, although effective at preventing contamination from environmental factors like light, oxygen, and moisture, cannot stop microbial growth if contamination occurs before or during packaging process. To address this, green and active food packaging has been developed, incorporating chemical, or biological agents that actively inhibit microbial growth, reduce oxidation, and manage moisture levels [17]. These innovations preserve freshness, reduce food waste, and also extend shelf life by advanced techniques like mould casting, electrospinning and coatings [18].
Active packaging utilizing natural additives like organic acids, essential oils, enzymes, and bacteriocins has gained increasing attention over synthetic agents due to the latter’s potential health risks, environmental concerns, as well as contribution to antimicrobial resistance. Among natural bio additives, bacteriophages are especially promising due to their safety, cost-effectiveness and because they do not compromise the taste or texture of food [18,19]. Bacteriophages uniquely amplify in the presence of pathogens, providing a targeted antibacterial action unmatched by synthetic alternatives [18]. Techniques like spray drying, freeze drying, emulsification, extrusion, and electrospinning are commonly studied and utilized for developing delivery systems of bacteriophages. Spray drying involves spraying a liquid containing bacteriophages into hot air, leading to rapid water evaporation and the formation of dry particles, which ensures quick drying and stable powders [20]. Freeze drying (lyophilization is a low-pressure dehydration process that involves freezing the phage suspension and removing water by sublimation under vacuum, thereby maintaining the structural and biological integrity of bacteriophages. Initially designed for food preservation, lyophilization and related methods such as spray-drying, hot-air-drying, and drum-drying are now widely employed for the stabilization and long-term storage of bacteriophages [21,22,23]. Emulsification involves creating an emulsion, a mixture of two immiscible liquids, in which bacteriophages are encapsulated in microdroplets, stabilizing and protecting the bacteriophages in the emulsion [20,24,25]. Extrusion involves passing the material containing bacteriophages through a narrow opening under high pressure, forming fibres or granules, with the key being to control the shape and size of the particles [26]. Electrospinning involves producing nanofibers from a polymer solution containing bacteriophages using an electric field, resulting in very thin fibres with a large surface area [27,28,29]. These methods can produce structures of different scales, from nano to macro, allowing for effective retention and controlled release of phages at specific rates and locations. This enhances phage viability while reducing interactions with other elements in the same environment, such as the food matrix, making them less susceptible to environmental factors like pH and temperature, thus promoting stability [30]. Recent advancements in formulation and delivery technologies are summarized in Table 2, showcasing a variety of techniques and their specific advantages in improving bacteriophage viability and release profiles.

Table 2.
Recent Advancements in Bacteriophage Formulation and Delivery Methods.
When used for surface treatment or packaging, the type of delivery system and its application method (e.g., spraying, dipping, immersion, wrapping) should align with the manufacturer’s equipment and requirements. Bio-based structures, including films, multilayer films, emulsions, capsules, and hydrogels, can be tailored to create innovative phage delivery systems for various food applications [36,37,38]. The fabrication of bacteriophage-based packaging involves three primary components: the base material (such as hydrogels, films or coatings), phage incorporation as bioadditives, and specific processes like multilayer film or spray application. The choice of the base material is critical—it must not inhibit phage viability and should stabilize the phages in the innermost layer to ensure efficient antibacterial activity. These packaging systems are especially beneficial for ready-to-eat meats, fresh produce, and poultry, offering sustainable and natural solutions for food safety [18].
Edible dip coatings, as a type of biodegradable packaging, have been suggested as a method for applying antimicrobials to fresh produce. The process of preparing edible films or coatings using plasticizers is shown in Figure 1.
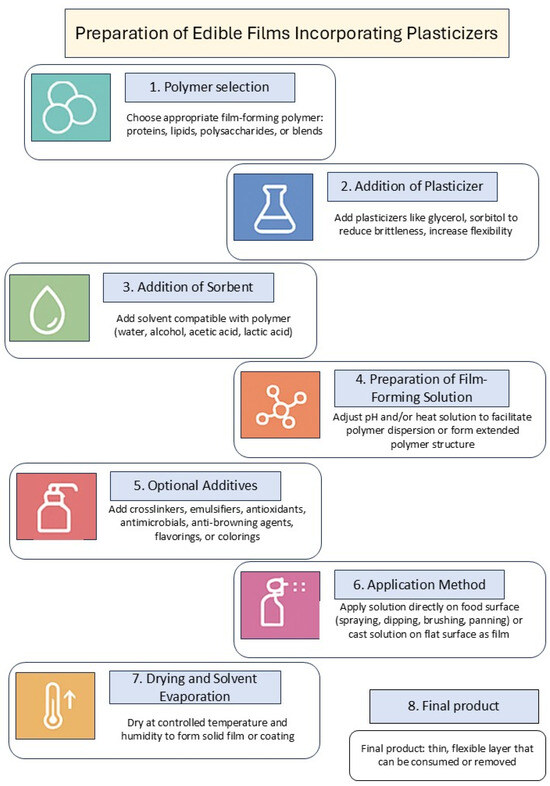
Figure 1.
Preparation process of edible films or coatings with plasticizers. Diagram created by the author and prepared based on data from [18].
These coatings act as barriers that prevent moisture loss, reduce gas exchange, and retain flavour volatiles, thereby maintaining the food’s quality and extending its shelf life. In addition to serving as physical barriers, dip coatings can also encapsulate antioxidants and antimicrobials [39].
Encapsulation or immobilization of phages using polymers is a common strategy for maintaining their antibacterial activity. The encapsulation process and material selection for bacteriophage stabilization are illustrated in Figure 2.
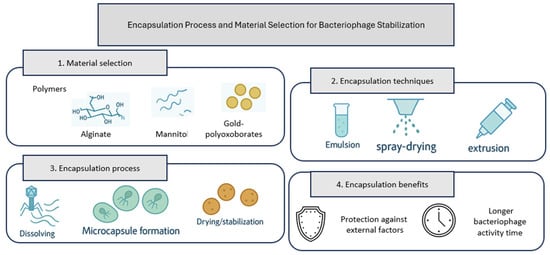
Figure 2.
Encapsulation process and material selection for bacteriophage stabilization. Siagram prepared by the author based on data from [21].
These methods enhance phage stability by creating a sustained release mechanism or forming protective barriers against pathogens on food surfaces. Both synthetic and natural polymers, such as agarose, alginate, chitosan, cellulose, whey proteins, PVA, PLGA, and PMMA, are widely employed to encapsulate or immobilize phages, improving their viability and activity. Advanced techniques like physisorption, microencapsulation, polymerization, electrospinning, electrospraying, and layer-by-layer assembly enable the development of edible films, microbeads, and spray or dip coatings for efficient antimicrobial performance in active food packaging [18]. Recent advances in biofilm control strategies and the use of microbial exopolysaccharides further expand the arsenal of biological tools for post-harvest disease management [40].
There are many benefits of using bacteriophage-based food packaging. As mentioned by, ref. [19] bacteriophages offer targeted antimicrobial action, specifically eliminating pathogenic bacteria while preserving beneficial microbial populations, making them a safer alternative to traditional antimicrobial agents. Their application in food packaging can significantly enhance food safety by reducing the microbial load throughout the food supply chain, thereby lowering the risk of foodborne illnesses. As natural viruses that do not harm humans or livestock, bacteriophages represent a green and sustainable solution for addressing antimicrobial resistance (AMR) issues.
Moreover, the incorporation of bacteriophages into food packaging does not adversely affect the physicochemical properties or sensory qualities of the food, ensuring consumer acceptance and maintaining food quality. For example, the study by [41] explored the application of phage vB_EcoMH2W incorporated into chitosan-based edible coatings for controlling E. coli O157:H7 on tomatoes. The results demonstrated that the phage remained viable within the coating throughout the storage period and significantly reduced the bacterial population on the tomato surface compared to the control. Researchers successfully isolated and characterized three phages with potential for E. coli O157:H7 biocontrol. Phage vB_EcoMH2W displayed the most promising results due to its broad host range and efficacy in reducing E. coli O157:H7 in chitosan coatings. The findings suggest the potential of phage-based edible coatings as a novel strategy for food safety applications. However, further research is needed to optimize phage incorporation techniques and ensure their long-term viability in food packaging [41].
Edible films and coatings with bacteriophages have shown potential for preserving vegetables, fruits, meat, and cheese by decreasing the growth of pathogenic bacteria. For instance, the study led by [42] demonstrated that phage-added edible coatings effectively reduced bacterial populations in various storage crops, such as cucumbers and potatoes, thereby extending shelf life and maintaining quality. However, the application of bacteriophages into edible films and coatings faces challenges, including the stability of bacteriophages within the film, their release from the film to the food, phage mobility, and the availability of bacteria to ensure effective physical contact [43].
Ref. [44] investigated the efficacy of composite phage nanofiber (fP-NF) films in active food packaging, particularly against E. coli O157:H7, by testing their application on fresh cucumbers and cherry tomatoes. The fruits and vegetables were first treated to remove surface microorganisms and then inoculated with E. coli O157:H7 before being wrapped with fP-NF films and stored at both 4 °C and 25 °C for four days. The results demonstrated significant antibacterial activity, with a rapid 60.61% reduction in free Escherichia coli O157:H7 within the first hour and a 99.74% reduction after 8 h. Remarkable anti-biofilm properties were also observed, achieving a 99.92% biofilm elimination rate on cucumbers at 4 °C and maintaining a 99.35% rate at 25 °C. Similar efficacy was noted on cherry tomatoes, indicating the films’ broad applicability across different produce types. Importantly, the phages in the films remained highly active for the first three days, with a release rate of 79.63% after 7 h at 25 °C, ensuring sustained antibacterial activity. Additionally, the incorporation of D-phenylalanine enhanced the films’ effectiveness against both free bacteria and biofilms. Crucially, the fP-NF films did not affect the colour, texture, or overall quality of the produce, underscoring their suitability for active food packaging. These findings suggest that fP-NF films could significantly reduce the risk of foodborne illnesses caused by E. coli O157:H7, providing a novel and effective solution for enhancing food safety in the fresh produce market. Another example is the study by [39] that evaluated the application of whey protein isolate WPI dip coatings on glass as a model surface to determine the distribution and efficacy of phages. Imaging measurements showed that fluorescently labelled phages were randomly distributed within the WPI coating without significant localisation, although some aggregation was observed. Antibacterial activity assays revealed that phages retained their antibacterial properties after being deposited on glass slides, with inhibition zones increasing in size with higher phage concentrations. The study also compared the phage loading efficiency of WPI coatings versus water coatings on sliced cucumbers, cut apple slices, and whole cherry tomatoes. The WPI coating consistently loaded more phages across all produce types compared to the water coating, achieving significantly higher phage counts (p < 0.05). Over a seven-day refrigerated storage period, the WPI coating provided enhanced phage stability on cut apples and whole cherry tomatoes but not on sliced cucumbers. Additionally, antimicrobial efficacy tests against E. coli BL21 demonstrated significant bacterial reduction on tomatoes with WPI coatings, while no significant reduction was observed on cut cucumbers, likely due to inhibitory effects of cucumber homogenate on phage activity. Phage survivability in a simulated gastric environment indicated that the WPI coating offered better protection on cucumbers compared to the control, but the differences were not significant for apples and tomatoes. Overall, the WPI dip coating proved to be a promising method for phage delivery on fresh produce, addressing key post-harvest pathogen control challenges by enhancing phage stability and antimicrobial efficacy, particularly under cold storage conditions [39].
Bacteriophages can be applied in the field using several methods, including immersion of seeds or seedlings in phage suspensions before planting, direct infiltration into plant tissues, foliar spraying on the phyllosphere, and soil drenching to target the rhizosphere [45]. These studies collectively highlight the versatility and effectiveness of bacteriophages in food preservation across various food types and storage conditions. The targeted nature of phage activity, combined with their natural origin and lack of impact on food quality, makes phages a promising alternative to traditional chemical preservatives.
Several commercial bacteriophage products have been developed and are currently commercially available for use, for combating bacterial diseases in crops. For instance, Intralytix, Inc. (Intralytix, Inc., Baltimore, MD, USA) offers Biolysate, a phage-based product aimed at controlling bacterial infections in various agricultural settings, including crops and livestock. These products include ListShield, which targets Listeria monocytogenes contamination in foods and food facilities, CampyShield for Campylobacter spp. and Campylobacter jejuni, Salmofresh for selected highly pathogenic Salmonella serotypes, and Shigashield for Shigella serotypes in foods and food processing facilities. These products have been approved by the FDA and other regulatory agencies (USA (FDA 21 CFR § 172.785, FSIS Directive 7120.1, GRAS GRN No. 528), USA (FDA FCN No. 1018, FSIS Directive 7120.1, Health Canada (iLONO), GRAS GRN No. 000672) [13,46]. Another notable product line is PhageGuard by Micreos, which targets specific pathogens such as Salmonella, E. coli, and Listeria, applicable in both plant and animal production systems. These products are USDA/FDA GRAS approved and are further accepted as processing aids in Australia, New Zealand, Israel, Switzerland, The Netherlands (EU), Canada, and others [13]. Other commercial bacteriophage products include Listex™ (Listex Global, Sunnyvale, CA, USA), which targets Listeria monocytogenes and is used in the food industry to control this pathogen in products like meat and cheese. Product has been approved by both the FDA and USDA. These types of products are used as biopreservatives in the processing of both animal products (e.g., raw milk cheeses, smoked salmon, raw meat, seafood) and plant products (e.g., ready-to-eat salad mixes, sprouts, fruit salads, strawberries) [12]. Additionally other commercial bacteriophage products include Secure Shield E1, developed by the German company FINK TEC GmbH, which specifically targets E. coli and has been approved by the FDA for use in food safety applications. Additionally, the Finalyse® (Brussels, Belgium) product, developed by Passport Food Safety Solutions and approved by the USDA, is designed to combat enterohemorrhagic Escherichia coli O157:H7 [47].
In agriculture, OmniLytics company has developed the AgriPhage product line, designed to treat various bacterial plant pathogens. These products have shown effectiveness in managing diseases such as fire blight, citrus canker, and bacterial spot in tomatoes and peppers, without affecting beneficial microflora (Registered in USA (EPA Reg. No. 67986-1) [13]. Also, Integrated Bio Products LLC (Ault, CO, USA.) has developed the Eco product line, designed to treat plant pathogens. For example, EcoStonia targets Ralstonia solanacearum in bananas, GoldenEco targets bacterial speck and spot in tomato and pepper plants caused by Pseudomonas syringae pv. tomato and Xanthomonas vesicatoria, and EcoFire controls fire blight caused by Erwinia amylovora in pears and apples (EPA Reg. No. 102839-R) [48]. The U.S. Environmental Protection Agency (EPA) has approved the use of a phage product called AgriPhage™, which can be commercially used to prevent infections of young tomato and pepper plants by Pseudomonas syringae. In 2018, the EPA also approved Agriphage-Citrus canker™ as a biological control for use against Xanthomonas citri subsp. citri, which causes necrotic lesions on the leaves, stems, and fruits of citrus trees. Additionally, the EPA has approved other bacteriophage-containing products named Agriphage CMM™ and Agriphage-Fireblight™, used against Clavibacter michiganensis (causing tomato canker) and Erwinia amylovora (causing fire blight in apples and pears) [12]. Summarized in Table 3.

Table 3.
Methods of Bacteriophage Application—Commercial Products and Efficacy.
4. Case Studies and Recent Advances
Bacteriophages, employed to control phytopathogenic bacteria in post-harvest settings, have shown promising results [50]. Recent experimental and field studies have demonstrated the high potential of bacteriophages as a tool for managing phytopathogenic bacteria, particularly in post-harvest contexts [50]. Phytopathogenic bacteria are one of the most significant causes of crop yield losses. Until now, the direct treatment of bacteriosis was limited to the application of antibacterial compounds or resistance inducers. This is about to change due to the revolutionary discovery of phages.
In recent years, research on the use of bacteriophages for plant protection has gained significant momentum, as reflected by the steadily increasing number of scientific publications devoted to this topic. Bibliometric analyses from databases such as PubMed and Scopus reveal a clear, systematic upward trend in interest among both researchers and agricultural practitioners.
Between 2010 and 2014, the annual number of publications remained relatively low, corresponding to the early stage of development in this field. However, after 2015, there has been a dynamic increase in the number of scientific papers from just over a dozen per year to more than 250 in 2024. This trend highlights the growing importance and popularity of research on bacteriophage applications in plant protection, both from a scientific and practical perspective. The change in interest in this topic is shown in Figure 3.
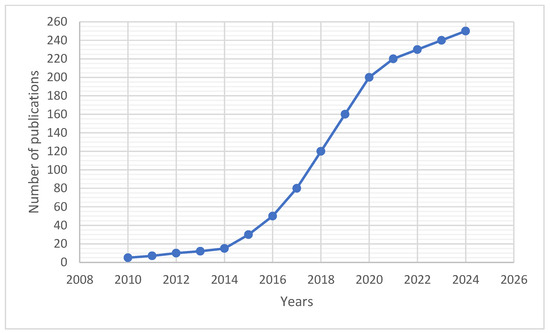
Figure 3.
Trend in the number of scientific publications on bacteriophages in plant protection (2010–2024), based on PubMed and Scopus data and literature review. (Estimated number of publications based on PubMed and Scopus search (keywords: ‘bacteriophage plant protection’), July 2025).
Indeed, bacteriophages look very promising as therapy agents: cheap, self-amplifying, self-eliminating, and safe for the host organism. However, phage therapy of plant diseases is still an emerging strategy with high potential, though only a few successful applications have been reported so far. Still as the demand for organic produce increases and restrictions on chemical treatments grow, phage therapy is emerging as an important alternative. With ongoing research, and with continued isolation and testing of new phages, their potential for use in both post-harvest and in-field applications is expanding, offering a promising solution for sustainable agricultural practices [50].
The phage treatment primarily serves as a preventive purpose, with phages applied using humidity maintenance system sprinklers. This method ensures that finely dispersed droplets penetrate deep into the bulk-stored potatoes. The concentration of phage PP16 remained stable at 104–105 PFU/tuber (ang. Plaque-Forming Units) for one week before gradually decreasing. The treatment resulted in a 10- to 12-fold reduction in the population of pectolytic bacteria on the tubers, with statistical analysis showing significant differences between treatment and control groups (p < 0.05) [49].
The study performed by [51] found that preliminary phage testing on inoculated fresh produce, such as cut green pepper and baby spinach leaves, revealed significant findings. The background microbiota on cut green pepper was eliminated using UV treatment, allowing inoculation with E. coli O157:H7 EDL 933. However, it was not feasible to eliminate background microbiota on spinach leaves without damaging them, so they were used without prior decontamination and inoculated with E. coli O157:H7 B6-914, which expresses green fluorescent protein (GFP) and antibiotic resistance. This allowed enumeration of the inoculated strain using selective agar medium and UV light. Phage treatments employed a crude lysate (108 PFU/mL) with a short application time of 2 min to minimize damage to spinach leaves. The preparation of fresh produce for testing took 4 h, covering the time from inoculation to storage placement. Initial differences in E. coli populations between phage treatment and control groups were due to phage-induced lysis and the rinsing action of the lysate solution. Results showed that the initial reduction in E. coli populations was 0.9 log cfu/g for cut green pepper and 1.7 log cfu/g for spinach leaves. After 72 h of storage at 4 °C, the average reduction attributed to phage action was 0.9 log for cut green pepper and 2.0 log for spinach leaves. E. coli populations remained relatively unchanged at 4 °C but increased at 25 °C, with a greater increase on spinach leaves [51]. Increasing the rinsing time of cut green pepper from 2 to 5 min enhanced the population reduction achieved by phage treatment. The optimized phage treatment involved using purified phage suspended in phosphate-buffered saline, with a 5 min rinse for green pepper and a 2 min rinse for spinach leaves. The optimized study showed significant differences in E. coli populations between treated and untreated produce, particularly after an initial 4 h storage at 25 °C followed by 68 h at 4 °C. This approach aimed to increase phage titter through replication in the host without significantly increasing the pathogen population [51].
Recent advancements in bacteriophage applications have showcased their potential as precise tools for modulating microbial communities, particularly in post-harvest settings and ecosystem restoration. Studies like those by [52] have demonstrated that phage treatments can significantly alter microbial diversity and composition. For instance, in degraded soils, the combination of phages and bacterial suspensions reduced the effective number of ASVs (ang. Amplicon Sequence Variants), revealing phage’s capacity to target and suppress dominant bacterial taxa. These findings align with broader experimental evidence supporting the efficacy of phages in reducing pathogen loads, controlling spoilage microbes, and selectively promoting beneficial microbial populations.
Field trials have further highlighted phage’s potential to improve agricultural and ecological outcomes. For example, phages have been successfully deployed to combat bacterial wilt in crops, reduce post-harvest spoilage in stored produce, and aid in the rehabilitation of degraded soils by selectively modulating microbial communities [52].
Emerging trends also indicate the potential of integrating phage therapy with established methods, such as crop rotation and the use of biostimulants, to maximize its impact. Together, these innovations highlight the promise of phages as a sustainable and environmentally friendly alternative to chemical pesticides, supporting global efforts to harmonize agricultural productivity with ecological conservation [53].
5. Phage Formulations and Enhancements
Advancements in molecular biology and genomic techniques are addressing the limitations in phage application in natural environment by enabling the creation of customized phage formulations. These innovations are pivotal for improving their therapeutic potential, ensuring stability, and facilitating effective delivery in diverse environments. Encapsulation strategies, such as those employing hydrogels, liposomes, and electroscopic fibres, create a protective barrier against environmental stressors, including heat, low pH, and enzymatic degradation. These methods are particularly valuable for maintaining phage viability in hostile environments, such as the gastrointestinal tract, where unprotected phages are prone to inactivation. Similarly, lyophilisation, a process that removes water through sublimation, transforms phages into stable powders. This approach retains their biological activity during long-term storage and offers versatility for delivery through capsules, tablets, or even inhalable powders designed for respiratory applications [31,33]. Lyophilisation, or freeze-drying, remains a commonly used method to produce dry, stable phage formulations that simplify storage and reduce refrigeration needs. However, the process involves technical complexities and added costs, which may limit its applicability on a larger scale. To address these challenges, stabilizers such as salts and sugars have been explored for their ability to maintain phage structural integrity and biological activity. For example, ions like sodium and magnesium enhance phage stability at room temperature and under elevated temperatures, offering practical solutions for post-harvest and food safety applications [34].
The use of excipients, such as sugars (e.g., trehalose, sucrose) and polymers (e.g., alginate, polyethylene glycol), further enhances phage stability during formulation and rehydration processes. Integrating phages with other antimicrobial agents, including antibiotics or natural antimicrobials, has demonstrated synergistic effects, offering a dual mechanism to combat multidrug-resistant pathogens. This combination is particularly effective in addressing biofilm-associated infections, where individual phages may face challenges in penetration and activity [31,33].
Encapsulation within nanoparticles, such as chitosan nanoparticles (CS-NPs), is another promising strategy for improving phage delivery and stability. For instance, encapsulation of phage HK6 in CS-NPs achieves a 97% entrapment efficiency, ensuring a high concentration of active phages while shielding them from environmental degradation. Encapsulated phages show enhanced thermal stability, maintaining efficacy across a temperature range of 25 °C to 80 °C. Additionally, encapsulated phage HK6 demonstrates rapid antibacterial activity within two hours, significantly outperforming free phages and CS-NPs alone. This synergistic combination of phages and nanoparticles improves bacterial targeting and infection control, highlighting the potential of nanoparticle-based encapsulation in advancing phage applications [23,32]. What is more, an integration of phages with antimicrobial agents, including bacteriocins and antibiotics, has shown notable synergistic effects, particularly in overcoming biofilm-associated infections and combating multidrug-resistant bacteria. Encapsulation strategies, lyophilisation, and synergistic combinations of phages with antimicrobial agents are essential for enhancing phage stability, delivery, and efficacy. These advancements hold particular promise for applications in food safety, healthcare, and environmental sanitation, ensuring bacteriophage success under diverse conditions [32]. Stability during post-harvest conditions such as fluctuating temperature, humidity, and sunlight remains a critical consideration for effective phage application. Innovative formulation methods, including encapsulation with biodegradable polymers (e.g., alginate, chitosan), lyophilisation with protective excipients (e.g., trehalose, gelatin), and the use of ion additives, have shown strong potential to preserve phage viability in such challenging environments [34,35].
Nowadays, encapsulation remains the dominant technique in the formulation of bacteriophages for plant disease control, as evidenced by numerous studies [23,31,32,33]. By encapsulation, the active agents are captured within a carrier material, creating monodisperse particles that maintain consistent size and physicochemical properties, preventing aggregation during production and application. Encapsulation is particularly effective in delivering bioactive molecules and living cells, with microcapsules, often spherical or irregular in shape, serving as protective carriers for the phages. This technique has proven beneficial in various fields, including agriculture, where it can protect phages from environmental factors and enhance their stability during application. Among the emerging advancements in bacteriophage formulation, hydrogels loaded with phages are gaining popularity. Hydrogels have shown promise in reducing biofilm formation caused by pathogenic bacteria, significantly lowering bacterial populations in vitro. As a result, research continues to focus on optimizing these formulations to improve phage stability, efficacy, and application methods, with promising implications for biocontrol in agriculture [31]. The latest research trends emphasize the need for development of more efficient, cost-effective, and environmentally friendly approaches, such as the use of hydrogels and other novel delivery systems, to further advance bacteriophage-based solutions for plant disease management [31]. Similar biocontrol concepts are used for mycorrhizal fungi such as Glomus spp., which may limit the development of verticillium wilt in cotton by competing with the pathogen for resources and space in the rhizosphere [54].
Formulation strategies such as encapsulation, lyophilisation, and the use of stabilizing excipients significantly improve bacteriophage stability by protecting them from environmental stressors including temperature fluctuations, UV radiation, desiccation, and enzymatic degradation [31,32,33]. Encapsulation in biopolymer matrices or nanoparticles creates a physical barrier that shields phages during storage and application, preserving their infectivity and enhancing delivery efficiency [23,32]. Lyophilisation transforms phages into stable powders, facilitating long-term storage without refrigeration [31]. Moreover, additives like salts and sugars maintain phage structural integrity and biological activity under challenging post-harvest conditions [55]. These advances in formulation enable practical and effective use of bacteriophages in diverse agricultural environments, addressing key limitations to their large-scale deployment [32].
This is particularly the case in agricultural settings, where the environment is not readily controlled and cold storage or controlled environment facilities do not exist. In typical post-harvest scenarios such as open storage, refrigerated shipping, or field-side packing phage preparations must be capable of withstanding temperature fluctuation, humidity, and sunlight. Encapsulation techniques using biodegradable polymers like alginate or chitosan, and lyophilized powders using excipients like trehalose or gelatin, have proven to promote phage viability under these conditions [35]. These formulation strategies bridge the gap between laboratory conditions and real-world agricultural applications, enabling phage use in uncontrolled environments such as field-side packing or open storage.
6. Regulatory and Safety Considerations
At the EU level, the Regulation (EC) No 1829/2003 on genetically modified food and feed, as well as the Commission Regulation (EC) No 2073/2005 (https://eur-lex.europa.eu/legal-content/PL/ALL/?uri=CELEX%3A32003R1829, accessed on 14 April 2025) on microbiological criteria for foodstuffs, provide additional regulatory guidelines that may apply to phage use in food systems. As a member of the European Union, Poland is bound by these regulations, ensuring harmonization of plant protection product usage across all EU member states.
The main legal act regulating the use of plant protection products in Poland is the Plant Protection Act of 8 March 2013 (Dz.U. z 2020 r. poz. 221) h (https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20200002021, accessed on 14 April 2025). This Act defines plant protection products and mandates that they be registered before being placed on the market or used. However, bacteriophages are not recognized as plant protection agents under this law, which means they are not required to be registered to be legally sold as ready plant protection products. Instead, bacteriophages can be used within the framework of good agricultural practice (GAP), a set of principles designed to ensure proper farm management, environmental protection, and food safety. The use of bacteriophages in GAP does not require formal registration or authorization.
Recent literature supports this approach, highlighting that phage-based biocontrol agents can be integrated into sustainable crop protection strategies, particularly when used as part of phage cocktails or encapsulated formulations that enhance stability and efficacy under field conditions [56].
These strategies are increasingly recognized as viable alternatives to chemical pesticides, especially in the context of EU Green Deal objectives and the Farm to Fork Strategy, which aim to reduce pesticide use and promote biological control methods. Current regulatory status of bacteriophages for plant protection are summarized in Table 4.

Table 4.
Current Regulatory Status of Bacteriophages for Plant Protection: EU and Poland.
7. Future Directions and Research Needs
Advancing bacteriophage therapy in agriculture involves addressing several key challenges. One primary concern is the development of scalable and cost-effective production methods. Research is needed to optimize large-scale phage propagation, formulation, and delivery systems for practical industrial application [57,58]. Additionally, it is crucial to evaluate and standardize application techniques, such as spraying, immersion, and packaging treatments, to identify the most effective methods for diverse agricultural environments. This standardization will ensure consistency and reliability in the use of phages across different crops and conditions [56,59].
As bacteriophage applications in agriculture expand, establishing regulatory frameworks to ensure their safe use become critical. These frameworks must address food safety, environmental impact, and the broader sustainability of phage therapy. This will help to pave the way for wider acceptance and adoption of bacteriophage-based products in the industry [60]. The ecological impact is the potential development of resistance in target bacterial pathogens. That is why, research focus on understanding molecular mechanisms behind phage resistance in phytopathogenic bacteria. Investigating strategies to combat resistance, such as developing phage cocktails, alternating phages, or combining phage therapy with other biocontrol agents, will be essential for enhancing the long-term effectiveness of phage-based treatments [8]. Additionally, the ecological impact of phage therapy on the plant microbiome is an important aspect for future research. Phage applications could alter microbial community dynamics on plant surfaces and in soil, potentially affecting beneficial microorganisms that contribute to plant health. It is vital to study the effects of phage treatments on non-target microorganisms and soil ecosystems to ensure that phage therapies do not disrupt these beneficial interactions [52].
By exploring those new research avenues, future studies will help unlock the full potential of bacteriophages as a sustainable and effective solution for post-harvest disease management and other agricultural applications.
8. Conclusions
Bacteriophage-based strategies hold great promise for improving post-harvest disease management, particularly through their integration into innovative delivery methods such as edible films and coatings [42]. These approaches provide natural, biodegradable, and highly effective solutions for reducing spoilage and enhancing food safety. However, several practical challenges must be addressed before widespread implementation.
One of the primary concerns is bacterial resistance to phages, which necessitates the development of phage cocktails to ensure broad-spectrum efficacy. Additionally, environmental factors including pH fluctuations, temperature variations, and UV exposure can significantly affect phage stability and performance, requiring optimized formulation strategies [46,61,62]. Furthermore, regulatory hurdles and commercial production complexities remain key barriers to large-scale adoption [19].
To fully realize the potential of bacteriophage applications, future efforts should focus on enhancing phage stability, optimizing their integration into post-harvest treatment systems, and addressing safety and regulatory concerns. By advancing interdisciplinary research and increasing public awareness, phage-based technologies can be effectively incorporated into sustainable agricultural practices, contributing to global food security. The integration of various microbial biocontrol agents, including PGPR and exopolysaccharide-producing bacteria, supports the development of resilient agroecosystems and complements the use of bacteriophages in sustainable plant protection [63].
Author Contributions
All authors contributed to the conception and design of the review. Literature search, analysis, and interpretation were performed A.H., K.S., W.Z. and K.K. All authors have read and agreed to the published version of the manuscript.
Funding
Preparation of the manuscript was funded from the statutory project WB-KK, inanced by the Polish Ministry of Science.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anusha, M.; Sri, K.R.; Lokesh, M.; Mrudula Deepthi, N.; Durga Malleswari, M. Postharvest Management Techniques for Improved Shelf Life of Horticultural Crops: A Review. J. Exp. Agric. Int. 2024, 46, 362–380. [Google Scholar] [CrossRef]
- Satheesan, A. Environmental Impacts of Post-Harvest Loss of Onions. Int. Res. J. Mod. Eng. Technol. Sci. 2024, 6, 89–95. [Google Scholar] [CrossRef]
- Ling, L.; Wang, Y.; Cheng, W.; Jiang, K.; Luo, H.; Pang, M.; Yue, R. Research Progress of Volatile Organic Compounds Produced by Plant Endophytic Bacteria in Control of Postharvest Diseases of Fruits and Vegetables. World J. Microbiol. Biotechnol. 2023, 39, 149. [Google Scholar] [CrossRef] [PubMed]
- Raynaldo, F.A.; Xu, Y.; Yolandani, Y.; Wang, Q.; Wu, B.; Li, D. Biological Control and Other Alternatives to Chemical Fungicides in Controlling Postharvest Disease of Fruits Caused by Alternaria alternata and Botrytis cinerea. Food Innov. Adv. 2024, 3, 135–143. [Google Scholar] [CrossRef]
- Kjemtrup, S.; Nimchuk, Z.; Dangl, J.L. Delivery of Virulence Genes: Type III Secretion. Curr. Opin. Microbiol. 2000, 3, 73–78. [Google Scholar] [CrossRef]
- Jamil, A. Antifungal and Plant Growth Promoting Activity of Trichoderma Spp. Against Fusarium oxysporum f. Sp. Lycopersici Colonizing Tomato. J. Plant Prot. Res. 2021, 61, 243–253. [Google Scholar] [CrossRef]
- Obradovic, A.; Jones, J.B.; Momol, M.T.; Olson, S.M.; Jackson, L.E.; Balogh, B.; Guven, K.; Iriarte, F.B. Integration of Biological Control Agents and Systemic Acquired Resistance Inducers against Bacterial Spot on Tomato. Plant Dis. 2005, 89, 712–716. [Google Scholar] [CrossRef]
- Halawa, E.M. Challenges of Bacteriophages Application in Controlling Bacterial Plant Diseases and How to Overcome Them. J. Genet. Eng. Biotechnol. 2023, 21, 98. [Google Scholar] [CrossRef]
- Altintas, Z.; Pocock, J.; Thompson, K.A.; Tothill, I.E. Comparative Investigations for Adenovirus Recognition and Quantification: Plastic or Natural Antibodies? Biosens. Bioelectron. 2015, 74, 996–1004. [Google Scholar] [CrossRef]
- Al-Hindi, R.R.; Teklemariam, A.D.; Alharbi, M.G.; Alotibi, I.; Azhari, S.A.; Qadri, I.; Alamri, T.; Harakeh, S.; Applegate, B.M.; Bhunia, A.K. Bacteriophage-Based Biosensors: A Platform for Detection of Foodborne Bacterial Pathogens from Food and Environment. Biosensors 2022, 12, 905. [Google Scholar] [CrossRef]
- Merabishvili, M.; Vervaet, C.; Pirnay, J.P.; de Vos, D.; Verbeken, G.; Mast, J.; Chanishvili, N.; Vaneechoutte, M. Stability of Staphylococcus aureus Phage ISP after Freeze-Drying (Lyophilization). PLoS ONE 2013, 8, e68797. [Google Scholar] [CrossRef] [PubMed]
- Stobnicka-Kupiec, A. Bakteriofagi Jako Czynniki Biokontroli Populacji Niepożądanych Bakterii. Bezp. Pr. Nauka Prakt. 2024, 637, 22–27. [Google Scholar] [CrossRef]
- Połaska, M.; Sokołowska, B. Review Bacteriophages—A New Hope or a Huge Problem in the Food Industry. AIMS Microbiol. 2019, 5, 324–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Jung, H.; Koo, B.K.; Han, J.A.; Lee, H.S. Exploiting Bacterial Genera as Biocontrol Agents: Mechanisms, Interactions and Applications in Sustainable Agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Sallam, N.A.; Nagy Riad, S.; Mohamed, M.S.; Seef El-Eslam, A. Formulations of Bacillus spp. and Pseudomonas fluorescens for biocontrol of cantaloupe root rot caused by Fusarium solani. J. Plant Prot. Res. 2013, 53, 296–300. [Google Scholar] [CrossRef]
- Costa, M.J.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Bacteriophage Delivery Systems for Food Applications: Opportunities and Perspectives. Viruses 2023, 15, 1271. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Zhang, Y.; Zeng, H.; Li, W.; Wang, Y. Design and Application of Antibacterial Bioactive Films for Food Preservation: A Review. J. Agric. Food Chem. 2025, 73, 19244–19261. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Bhaskar, R.; Han, S.S. Bacteriophages: Natural Antimicrobial Bioadditives for Food Preservation in Active Packaging. Int. J. Biol. Macromol. 2024, 276, 133945. [Google Scholar] [CrossRef]
- Wagh, R.V.; Priyadarshi, R.; Rhim, J.W. Novel Bacteriophage-Based Food Packaging: An Innovative Food Safety Approach. Coatings 2023, 13, 609. [Google Scholar] [CrossRef]
- Malik, D.J. Bacteriophage Encapsulation Using Spray Drying for Phage Therapy. Curr. Issues Mol. Biol. 2021, 40, 303–316. [Google Scholar] [CrossRef]
- Wdowiak, M.; Paczesny, J.; Raza, S. Enhancing the Stability of Bacteriophages Using Physical, Chemical, and Nano-Based Approaches: A Review. Pharmaceutics 2022, 14, 1936. [Google Scholar] [CrossRef] [PubMed]
- Komárková, M.; Benešík, M.; Černá, E.; Sedláčková, L.; Moša, M.; Vojtová, L.; Franc, A.; Pantůček, R. The Pharmaceutical Quality of Freeze-Dried Tablets Containing Therapeutic Bacteriophages against Pseudomonas aeruginosa and Staphylococcus aureus. Int. J. Pharm. 2025, 671, 125199. [Google Scholar] [CrossRef] [PubMed]
- Śliwka, P.; Skaradziński, G.; Dusza, I.; Grzywacz, A.; Skaradzińska, A. Freeze-Drying of Encapsulated Bacteriophage T4 to Obtain Shelf-Stable Dry Preparations for Oral Application. Pharmaceutics 2023, 15, 2792. [Google Scholar] [CrossRef] [PubMed]
- Vinner, G.K.; Richards, K.; Leppanen, M.; Sagona, A.P.; Malik, D.J. Microencapsulation of Enteric Bacteriophages in a pH-Responsive Solid Oral Dosage Formulation Using a Scalable Membrane Emulsification Process. Pharmaceutics 2019, 11, 475. [Google Scholar] [CrossRef]
- Aziz, N.S.; Ibrahim, S.; Zaharinie, T.; Tang, S.S. Bacteriophage Encapsulation—Trends and Potential Applications in Aquaculture. Aquaculture 2025, 594, 741398. [Google Scholar] [CrossRef]
- Castro-Mejía, J.L.; Muhammed, M.K.; Kot, W.; Neve, H.; Franz, C.M.A.P.; Hansen, L.H.; Vogensen, F.K.; Nielsen, D.S. Optimizing Protocols for Extraction of Bacteriophages Prior to Metagenomic Analyses of Phage Communities in the Human Gut. Microbiome 2015, 3, 64. [Google Scholar] [CrossRef]
- Sugimoto, R.; Lee, J.H.; Lee, J.H.; Jin, H.E.; Yoo, S.Y.; Lee, S.W. Bacteriophage Nanofiber Fabrication Using near Field Electrospinning. RSC Adv. 2019, 9, 39111–39118. [Google Scholar] [CrossRef]
- Martinez-Soto, C.E.; Zaitoon, A.; Wang, C.; Barbut, S.; Balamurugan, S.; Lim, L.-T.; Khursigara, C.M.; Anany, H. Phage-Loaded Electrospun Nonwovens with Antimicrobial Properties Against Salmonella enteritidis. PHAGE 2025, 6, 212–221. [Google Scholar] [CrossRef]
- Matinfar, G.; Ye, H.; Bashiry, M.; Hashami, Z.; Yang, T. Electrospinning-Based Sensing Technologies: Opportunities for Food Applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13415. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Kim, M.; Park, Y.; Ryu, S. Development of New Strategy Combining Heat Treatment and Phage Cocktail for Post-Contamination Prevention. Food Res. Int. 2021, 145, 110415. [Google Scholar] [CrossRef]
- Bolsan, A.C.; Sampaio, G.V.; Rodrigues, H.C.; Silva De Souza, S.; Edwiges, T.; Celant De Prá, M.; Gabiatti, N.C. Phage Formulations and Delivery Strategies: Unleashing the Potential against Antibiotic-Resistant Bacteria. Microbiol. Res. 2024, 282, 127662. [Google Scholar] [CrossRef] [PubMed]
- Temsaah, H.R.; Abdelkader, K.; Ahmed, A.E.; Elgiddawy, N.; Eldin, Z.E.; Elshebrawy, H.A. Chitosan Nano-Formulation Enhances Stability and Bactericidal Activity of the Lytic Phage. BMC Biotechnol. 2025, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Meshram, S. The Evolution of Phage Therapy: A Comprehensive Review of Current Applications and Future Innovations. Cureus 2024, 16, e70414. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lim, M.C.; Woo, M.A.; Kim, B.S.; Lim, J.A. Development of Stabilizing Solution for Long-Term Storage of Bacteriophages at Room Temperature and Application to Control Foodborne Pathogens. Viruses 2024, 16, 1155. [Google Scholar] [CrossRef]
- Manohar, P.; Ramesh, N. Improved Lyophilization Conditions for Long-Term Storage of Bacteriophages. Sci. Rep. 2019, 9, 15242. [Google Scholar] [CrossRef]
- Korehei, R.; Kadla, J. Incorporation of T4 Bacteriophage in Electrospun Fibres. J. Appl. Microbiol. 2013, 114, 1425–1434. [Google Scholar] [CrossRef]
- Balcão, V.M.; Glasser, C.A.; Chaud, M.V.; del Fiol, F.S.; Tubino, M.; Vila, M.M.D.C. Biomimetic Aqueous-Core Lipid Nanoballoons Integrating a Multiple Emulsion Formulation: A Suitable Housing System for Viable Lytic Bacteriophages. Colloids Surf. B Biointerfaces 2014, 123, 478–485. [Google Scholar] [CrossRef]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a Phage Cocktail and Cinnamaldehyde on Sodium Alginate Emulsion-Based Films to Fight Food Contamination by Escherichia coli and Salmonella enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef]
- Vonasek, E.L.; Choi, A.H.; Sanchez, J.; Nitin, N. Incorporating Phage Therapy into WPI Dip Coatings for Applications on Fresh Whole and Cut Fruit and Vegetable Surfaces. J. Food Sci. 2018, 83, 1871–1879. [Google Scholar] [CrossRef]
- Kilic, T.; Bali, E.B. Biofilm Control Strategies in the Light of Biofilm-Forming Microorganisms. World J. Microbiol. Biotechnol. 2023, 39, 131. [Google Scholar] [CrossRef]
- Boggione, D.M.G.; Batalha, L.S.; Gontijo, M.T.P.; Lopez, M.E.S.; Teixeira, A.V.N.C.; Santos, I.J.B.; Mendonça, R.C.S. Evaluation of Microencapsulation of the UFV-AREG1 Bacteriophage in Alginate-Ca Microcapsules Using Microfluidic Devices. Colloids Surf. B Biointerfaces 2017, 158, 182–189. [Google Scholar] [CrossRef]
- García-Anaya, M.C.; Sepulveda, D.R.; Zamudio-Flores, P.B.; Acosta-Muñiz, C.H. Bacteriophages as Additives in Edible Films and Coatings. Trends Food Sci. Technol. 2023, 132, 150–161. [Google Scholar] [CrossRef]
- Vila, M.M.D.C.; Cinto, E.C.; Pereira, A.O.; Baldo, D.Â.; Oliveira, J.M., Jr.; Balcão, V.M. An Edible Antibacterial Coating Integrating Lytic Bacteriophage Particles for the Potential Biocontrol of Salmonella enterica in Ripened Cheese. Polymers 2024, 16, 680. [Google Scholar] [CrossRef]
- Cui, H.; Yang, X.; Li, C.; Ye, Y.; Chen, X.; Lin, L. Enhancing Anti-E. coli O157:H7 Activity of Composite Phage Nanofiber Film by D-Phenylalanine for Food Packaging. Int. J. Food Microbiol. 2022, 376, 109762. [Google Scholar] [CrossRef]
- Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.A.; Momol, M.T.; Jones, J.B.; Vallad, G.E. Soil-Based Systemic Delivery and Phyllosphere in Vivo Propagation of Bacteriophages. Bacteriophage 2012, 2, e23530. [Google Scholar] [CrossRef]
- Liu, S.; Quek, S.; Huang, K. Advanced Strategies to Overcome the Challenges of Bacteriophage-Based Antimicrobial Treatments in Food and Agricultural Systems. Crit. Rev. Food Sci. Nutr. 2024, 64, 12574–12598. [Google Scholar] [CrossRef]
- Gillies, K.; Gaynor, P.; Gillies, K.O.; Gaynor, P. OFAS/GNP for Their Use in Making a Suitability Determination for the Described Use as Provided for Under Appendix A, Section C of the MOU. Please Promptly Contact Me Should You Have Any Questions Regarding the Submitted Notice; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017. [Google Scholar]
- Environmental Protection Agency. Pesticide Product Registration; Receipt of Applications for New Active Ingredients. Fed. Regist. 2024, 89, 68156. [Google Scholar]
- Bugaeva, E.N.; Voronina, M.V.; Vasiliev, D.M.; Lukianova, A.A.; Landyshev, N.N.; Ignatov, A.N.; Miroshnikov, K.A. Use of a Specific Phage Cocktail for Soft Rot Control on Ware Potatoes: A Case Study. Viruses 2021, 13, 1095. [Google Scholar] [CrossRef] [PubMed]
- Korniienko, N.; Kharina, A.; Budzanivska, I.; Burketová, L.; Kalachova, T. Phages of Phytopathogenic Bacteria: High Potential, but Challenging Application. Plant Prot. Sci. 2022, 58, 89–91. [Google Scholar] [CrossRef]
- Snyder, A.B.; Perry, J.J.; Yousef, A.E. Developing and Optimizing Bacteriophage Treatment to Control Enterohemorrhagic Escherichia coli on Fresh Produce. Int. J. Food Microbiol. 2016, 236, 90–97. [Google Scholar] [CrossRef]
- Davies, T.; Cando-Dumancela, C.; Liddicoat, C.; Dresken, R.; Damen, R.H.; Edwards, R.A.; Ramesh, S.A.; Breed, M.F. Ecological Phage Therapy: Can Bacteriophages Help Rapidly Restore the Soil Microbiome? Ecol. Evol. 2024, 14, e70185. [Google Scholar] [CrossRef] [PubMed]
- Dong, L. Phage Soil Additives: A Frontier in Agricultural Health. Theor. Nat. Sci. 2024, 37, 103–108. [Google Scholar] [CrossRef]
- Kobra, N.; Jalil, K.; Youbert, G. Effects of Three Glomus Species As Biocontrol Agents Against Verticillium-Induced Wilt in Cotton. J. Plant Prot. Res. 2009, 49, 186–189. [Google Scholar] [CrossRef]
- Peter, O.; Imran, M.; Shaffique, S.; Kang, S.M.; Rolly, N.K.; Felistus, C.; Bilal, S.; Dan-Dan, Z.; Injamum-Ul-Hoque, M.; Kwon, E.H.; et al. Combined Application of Melatonin and Bacillus Sp. Strain IPR-4 Ameliorates Drought Stress Tolerance via Hormonal, Antioxidant, and Physiomolecular Signaling in Soybean. Front. Plant Sci. 2024, 15, 1274964. [Google Scholar] [CrossRef]
- Baliyan, N.; Dhiman, S.; Dheeman, S.; Vishnoi, V.K.; Kumar, S.; Maheshwari, D.K. Bacteriophage Cocktails as Antibacterial Agents in Crop Protection. Environ. Sustain. 2022, 5, 305–311. [Google Scholar] [CrossRef]
- Sauvageau, D.; Cooper, D.G. Two-Stage, Self-Cycling Process for the Production of Bacteriophages. Microb. Cell Factories 2010, 9, 81. [Google Scholar] [CrossRef]
- Malik, D.J.; Goncalves-Ribeiro, H.; GoldSchmitt, D.; Collin, J.; Belkhiri, A.; Fernandes, D.; Weichert, H.; Kirpichnikova, A. Advanced Manufacturing, Formulation and Microencapsulation of Therapeutic Phages. Clin. Infect. Dis. 2023, 77, S370–S383. [Google Scholar] [CrossRef]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Application of Bacteriophages in the Agro-Food Sector: A Long Way toward Approval. Front. Cell. Infect. Microbiol. 2018, 8, 296. [Google Scholar] [CrossRef]
- Meaden, S.; Koskella, B. Exploring the Risks of Phage Application in the Environment. Front. Microbiol. 2013, 4, 358. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Zhang, X.; Zhang, K.; Liu, W.; Zhang, R.; Zhang, Z. Enterobacter hormaechei in the Intestines of Housefly Larvae Promotes Host Growth by Inhibiting Harmful Intestinal Bacteria. Parasites Vectors 2021, 14, 598. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Banerjee, S.; Acharya, U.; Mitra, A.; Mallick, I.; Haldar, A.; Haldar, S.; Ghosh, A.; Ghosh, A. Evaluation of Plant Growth Promotion Properties and Induction of Antioxidative Defense Mechanism by Tea Rhizobacteria of Darjeeling, India. Sci. Rep. 2020, 10, 15536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).