1. Introduction
The olive (
Olea europaea L.) is classified as a fruit species and, more precisely, as a stone fruit due to its morphological characteristics [
1]. Although it differs from most other fruits in that its mesocarp tissue accumulates oil, making it primarily cultivated for oil production, it still shares essential agricultural and horticultural practices with other fruit tree species [
2].
Traditionally, olives, as well as many other fruit crops, were harvested by hand. The manual approach allowed a careful selection and minimal damage to the fruit, but was very labour intensive and time consuming [
3]. In recent years, however, a growing shortage of seasonal labour, combined with the need to reduce production costs and increase operational efficiency, has led to the widespread use of mechanical harvesting [
4]. However, the latter is not without its drawbacks, as it can cause physical damage to branches, roots, and fruit, potentially affecting both tree health and fruit quality [
3].
When olives are damaged, the injured areas tend to oxidise rapidly [
5]. This is primarily due to the activation of oxidative enzymes such as polyphenol oxidase and peroxidase which, in the presence of atmospheric oxygen, cause enzymatic browning through the oxidation of phenolic compounds [
6]. Olives are particularly susceptible to this process because their fruits contain significantly higher contents of phenolic compounds than most other fruit species [
7]. The most abundant phenolics in olives are oleuropein, hydroxytyrosol, tyrosol, and verbascoside [
8]. Many of these compounds, especially oleuropein, are responsible for the intense bitterness of fresh olives, which make them unpalatable and unsuitable for raw consumption [
9].
In addition to their rich phenolic profile, olive fruit and olive oil are a good source of unsaturated fatty acids, particularly monounsaturated fatty acids, which further contribute to their nutritional value [
10]. The predominant fatty acid in olive oil is oleic acid (C18:1), which typically represents 55–83% of the total fatty acid content [
11]. This high proportion of oleic acid content is associated with improved lipid profiles, reduced inflammation, and lower risk of cardiovascular disease, making olive oil a key component of the Mediterranean diet [
12]. In addition to oleic acid, olive oil also contains amounts of polyunsaturated fatty acids, such as linoleic acid and linolenic acid, as well as saturated fatty acids such as palmitic acid [
13].
In order to preserve the many beneficial properties of olives—including their high nutritional value, distinctive aroma, and overall quality—it is essential not only to harvest the fruit at the optimum stage of ripeness and maintain proper agronomic care during the growing season but also to process them as soon as possible after harvest [
2]. Immediate processing is essential to minimise enzymatic degradation and oxidative changes that can impact negatively on both oil yield and quality [
14]. In practice, processing delays are common due to limited mill capacity. As a result, olives are often exposed to different storage conditions and in many cases, to high temperatures, which reduce the quality of the fruit and negatively affect the taste, aroma, and nutritional value of the final product.
Even brief postharvest storage can significantly alter the chemical composition and overall quality of olives, ultimately affecting the quality and sensory attributes of the resulting oil. Unlike many other fruit crops, olives are rarely stored for long periods after harvest, which has limited research into how storage conditions influence fruit quality. Careful attention must be paid to storage temperature, as very low temperatures (2–4 °C) can cause chilling injuries in the fruit, further reducing its quality [
4]. Most studies to date have focused on changes in phenolic compounds and fatty acids in olive oil [
7,
8,
10], and only a few have investigated the effects of cold storage on the fruit itself, often without detailed metabolic analyses [
15,
16].
The aim of this study was to investigate how different storage conditions—room temperature and cold storage—affect changes in key quality parameters, individual phenolic compounds, and fatty acid composition in mechanically harvested fruits of the olive cultivar ‘Leccino’. We hypothesize that cold storage is more effective in preserving the quality of olives than is storage at room temperature, as it slows down the metabolism and degradation processes.
2. Materials and Methods
2.1. Experimental Setup and Plant Material
The experiment was conducted during the 2021 and 2022 growing seasons in a certified organic olive orchard situated in Izola, Slovenia (45° 32′ 12.98′′ N; 13° 39′ 42.98′′ E). The orchard comprised 20-year-old grafted olive trees of the ‘Leccino’ cultivar, trained in an open-vase system. In both years, olives were harvested at full maturity from the same trees and consistently from a well-lit, sun-exposed part of the canopy, using a pneumatic olive rake (Campagnola Olistar Tuono, Campagnola, Italy). After harvesting, 5 kg of olives were placed in net bags and stored under cold and room storage conditions. In the
cold storage conditions, the temperature was maintained at 5 °C ± 0.5 °C and the relative humidity at 87.2% ± 3%. In contrast, the
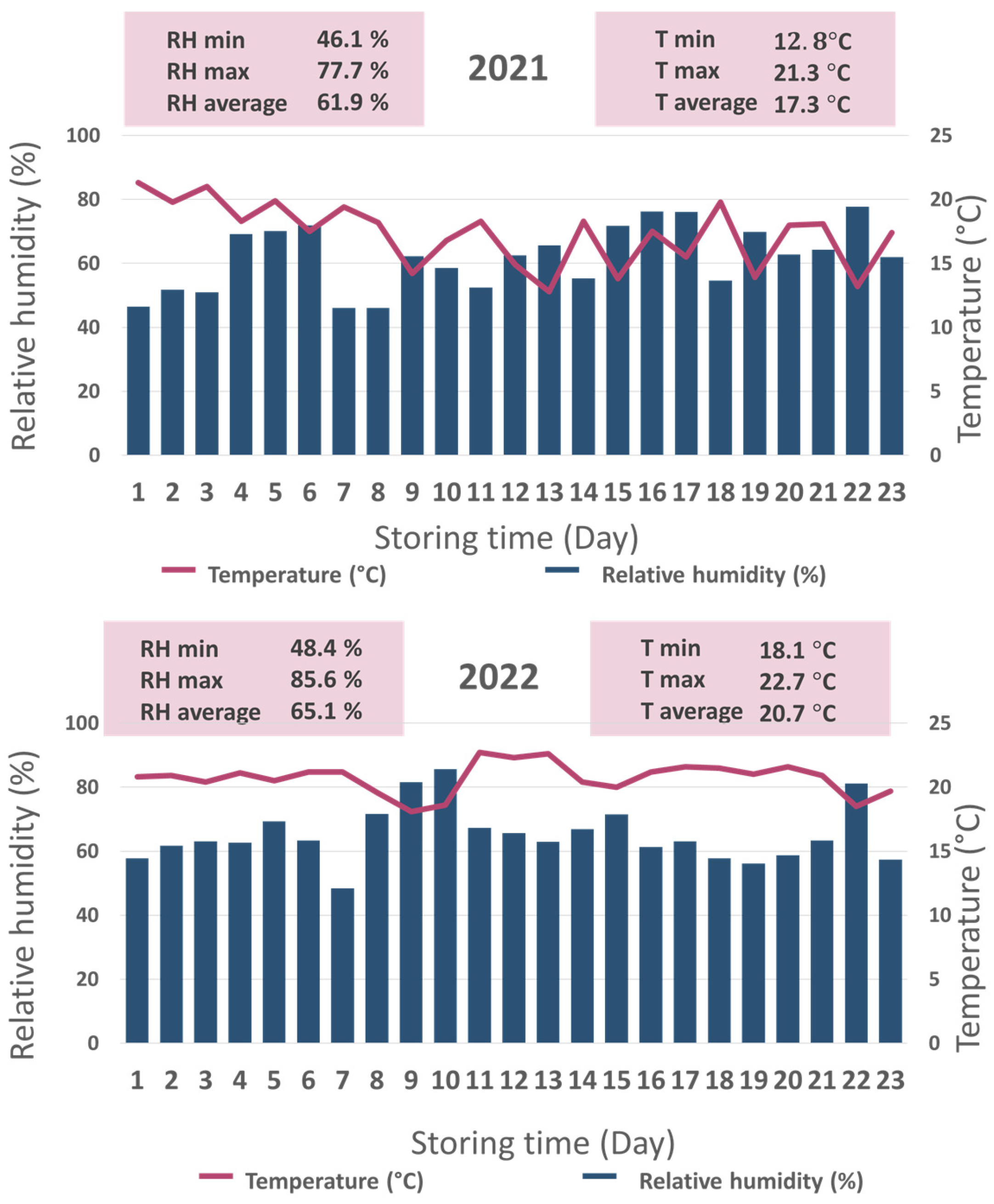
room storage conditions involved fluctuating temperatures ranging from 12.8 °C to 21.3 °C in 2021 and from 18.1 °C to 22.7 °C in 2022, with relative humidity from 46.1% to 77.3% in 2021 and from 48.4% to 65.1% in 2022. Storage conditions were monitored using a Voltcraft DL-121 TH data logger (Hirschau, Germany) every day at 12:00 PM. Detailed results are present in
Figure 1.
Immediately after harvest (
D0) and after 3 (
D3), 11 (
D11), and 23 (
D23) days of storage, 25 fruits were randomly sampled from the 5 kg bags for evaluation of fruit weight, colour, maturity index, and firmness. Fruit firmness was measured using a penetrometer (model FT 011, QA Supplies, Norfolk, VA, USA) equipped with a 3.0 mm diameter cylindrical probe. Fruit colour was measured using a CR-300 Chroma colorimeter (Minolta Co., Osaka, Japan). Colour values were recorded according to the CIE (Commission Internationale de l’Éclairage) colour space parameters, where L* represents the lightness on a scale from 0 (black) to 100 (white), C* represents the intensity of the colour, and h° represents the hue angle: 0–90° is red towards yellow, 90–180° is yellow towards green, 180°–270° is green towards blue, and 270–360° is blue towards red. The maturity index (MI) included eight classification groups (0–7). Fruits are visually evaluated according to the colour of the skin and the colour of the flesh, and each olive is assigned to one of the following classes: 0—skin is deep green; 1—skin is yellow-green; 2—skin shows reddish to purple spots on less than half of the surface; 3—skin shows reddish to purple coloration on more than half of the surface; 4—skin is completely black, while the flesh is still white next to the stone; 5—skin is black and the flesh has turned purple up to one-third from the skin toward the stone; 6—skin is black, and the flesh is purple up to two-thirds from the skin toward the stone; 7—both skin and flesh are completely purple up to the stone. To determine the maturity index, the number of fruits in each class is recorded and multiplied by the class value (from 0 to 7). The sum of these products is then divided by the total number of fruits in the sample, giving an average score that represents the overall ripening stage of the olives. This method was determined according to Guzmán et al. [
17]. The results of the maturity index are presented in
Table S1.
For further analysis, stones were removed, and the pulps were immediately frozen in liquid nitrogen. The frozen tissue was then ground using an A11 basic analytical mill (IKA, Staufen im Breisgau, Germany) and stored at −20 °C until further analysis.
2.2. Analysis of Phenolic Compounds
Phenolic compounds were extracted according to the protocol previously described by Burin et al. [
15] in four replicates for each treatment. The extracts were stored in vials at −20 °C until analysis. Phenolic compounds were quantified by high-performance liquid chromatography (HPLC; Thermo Scientific, San Jose, CA, USA) equipped with a Gemini C18 column (150 × 4.6 mm, 3 μm; Phenomenex, Torrance, CA, USA) and a diode array detector (DAD) set at 280 nm. The HPLC conditions followed the protocol previously described by Smrke et al. [
18]. Identification of phenolics was performed by mass spectrometry (LTQ XL™; Thermo Scientific, Waltham, MA, USA) by comparison of the fragmentation patterns in negative ion mode scanning from
m/
z 115−1500 with the literature data, their retention times, and external standards. Results were expressed as mg/kg fresh weight (FW). Compounds for which no standards were available were expressed as equivalents of the most similar standard (see
Section 2.4 and
Table S2).
2.3. Analysis of Fatty Acids
To analyse the fatty acid (FA) profile, a modified transesterification protocol was performed according to the methods of Vidrih et al. (2021) [
19]. Approximately 10 mg of frozen powdered olive fruit tissue was placed in a vial, followed by the addition of 100 µL of heptadecanoic acid, C17:0 (internal standard), which was prepared by dissolving 0.1 g of internal standard, 4.8 mL of methanol, and 2.4 mL of hexane. Then, 300 mL of dichloromethane and 3 mL of 0.5 M sodium hydroxide were added. The vials were sealed and incubated at 90 °C for 50 min. Subsequently, 1 mL of 10% sulphuric acid/methanol was added, and the mixture was incubated at the same temperature for another 10 min. Then, 3 mL of 10% sodium chloride/H
2O and 4 mL of hexane were added. The mixture was vortexed and centrifuged at 1500 rpm for 5 min. The non-polar phase was carefully transferred to glass vials, and 1 µL was injected into the gas chromatograph (GC-MS QP2020; (Shimadzu Corporation, Kyoto, Japan) connected to an electron ionisation (EI) detector and a single quadrupole mass spectrometer. The sample was injected into a ZB-FAME capillary column (30 m × 0.5 µm × 0.2 µm, Phenomenex, CA, USA) with a 1:800 split ratio at a flow rate of 1 mL min
−1. The column temperature was set to start at 100 °C (for 2 min), then increased at a rate of 5 °C min
−1 to 260 °C, where it was maintained for 5 min. The interface temperature between the GC column and the MS detector was set at 240 °C. Fatty acid methyl esters (FAMEs) were identified using a standard mixture of 37 C4-C24 FAMEs supplied by Supelco (Sigma-Aldrich, St. Louis, MI, USA). All analyses were performed in four replicates, and the results were expressed as mg per 100 g fresh weight (FW), considering the internal standards’ and individual fatty acids’ peak areas and specific coefficients (ratio between molecular weights of each individual fatty acid and its methyl ester). The relative values, expressed as percentages, were calculated from the absolute values, with the sum of all measured components taken as 100%.
2.4. Chemicals and Standards
The standards used for the quantification of phenolic compounds were 3-hydroxytyrosol (Sigma-Aldrich Chemi GmbH, Steinheim, Germany) for hydroxytyrosol, hydroxytyrosol glucoside, oleoside-11-methyl ester, oleoside and tyrosol; oleuropein (Sigma-Aldrich Chemi GmbH, Steinheim, Germany) for oleuropein, oleuropein aglicone, demethyloleuropein and demethyloleuropein glucoside; chlorogenic acid (Sigma-Aldrich Chemi GmbH, Steinheim, Germany) for elanolic acid; and verbascoside (HWI Group, Rülzheim, Germany) for verbascoside.
For phenolic extraction, >99.9% HPLC methanol, formic acid (Sigma-Aldrich Chemi GmbH, Steinheim, Germany), and double-distilled water purified with a Milli-Q system (Millipore, Bedford, MA, USA) were used. The HPLC mobile phases were >99.9% acetonitrile (HPLC-MS), formic acid (Sigma-Aldrich Chemi GmbH, Steinheim, Germany), and double-distilled water.
Hexane (Merck-Alcaloid, Darmstadt, Germany), heptadecanoic acid—C17:0, >99.9% HPLC methanol, sodium chloride, and sodium hydroxide (Sigma-Aldrich Chemi GmbH, Steinheim, Germany), as well as dichloromethane and sulphuric acid (Honeywell, Offenbach, Germany), were used for fatty acid analyses.
2.5. Data and Statistical Analysis
Statistical analyses were performed using R Commander (version 4.0.5, 31 March 2021). Differences between treatments were assessed using the Student’s t-test for independent samples, with the significance level set at p ≤ 0.05. Statistically significant differences within the same year are indicated by different letters. Statistical differences between years at the same storage period and under the same storage conditions are presented with symbols the *, **, ***, and NS, corresponding to p-values of 0.01–0.05, 0.05–0.001, <0.001, and no statistical difference, respectively. Graphs were generated in Microsoft Excel (Office Professional Plus 2020).
Hierarchical clustering of fruit colour was carried out using PAST software (version 4.17), applying the Euclidean distance similarity index and the UPGMA clustering method (Figure 3).
Principal component analysis (PCA) was used to explore grouping patterns among phenolic compounds and fatty acids, and was conducted using the R Commander interface in R (Figure 6).
3. Results
3.1. Storage Conditions and Quality Parameters During Storage
The storage environment under room conditions was not constant and varied across days. Measurements of temperature and relative humidity were obtained at 12:00 PM each day during both study years (
Figure 1). The results indicated that both relative humidity and temperature were mostly lower in 2021 compared to 2022.
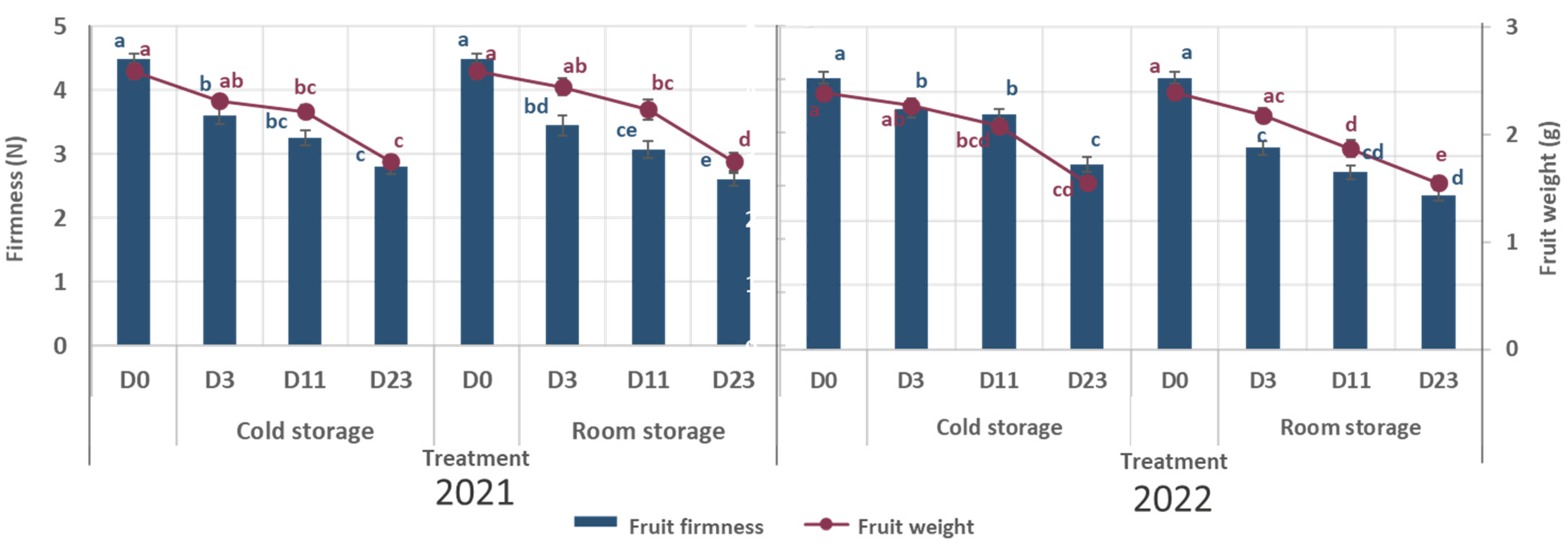
During storage, fruit weight decreased under all storage conditions in both years (
Figure 2;
Table S1). In 2021, fruit weight decreased by 19% under cold storage and by 33% under room storage. Similarly, in 2022, weight loss reached 18% under cold storage and 35% under room storage. The results show that fruit weight decreased similarly under both storage conditions up to day 11 of storage. However, by day 23, fruit weight was lower under room storage compared to cold storage. In particular, fruit harvested in 2022 was lighter at the beginning of storage and showed a more rapid weight decrease under room storage compared to the results for fruit harvested in 2021 (
Table S1).
Similar to weight loss, fruit firmness also decreased during storage under both storage conditions in both years (
Figure 2;
Table S1). In 2021, firmness decreased by 37% under cold storage and by 42% under room storage. In 2022, the loss of firmness was 32% and 43% for cold and room storage, respectively. In both years, a higher loss of firmness was observed under room storage conditions. Although fruits harvested in 2021 were significantly firmer at harvest than those harvested in 2022, no statistically significant differences in firmness between years were observed at later storage dates under the same storage conditions (
Table S1). However, the maturity index showed a constant increase during storage under both storage conditions in both years (
Table S1). Under cold storage conditions, the maturity index increased by 22% in 2021 and by 5% in 2022. The greatest increase was observed under room storage conditions, with a rise of 28% in 2021 and 11% in 2022.
At harvest, the fruit of both years had a very dark purple colour, as indicated by the colour parameter values (
Table S1). Comparing changes in individual colour parameters (L*, C*, h°) between storage conditions, only minimal variations were observed during storage. However, hierarchical clustering of the colour data provided additional insights (see
Figure 3). In 2021, no clear differences between storage conditions were observed, as samples clustered primarily according to storage duration rather than storage type (group 1–3). In 2021, after 3 days, the colour of the fruit stored under both conditions remained almost unchanged (group 3). In 2022, the clustering results were consistent with those of the previous year, again indicating no significant colour differences between fruit stored for 3 days under different conditions (group 4). However, the colour parameters of fruit stored for 11 days under room storage conditions were more similar to those of fruit stored for 23 days in cold storage (group 5), than to those stored for 11 days under cold conditions.
3.2. Individual Phenolic Compounds During Storage
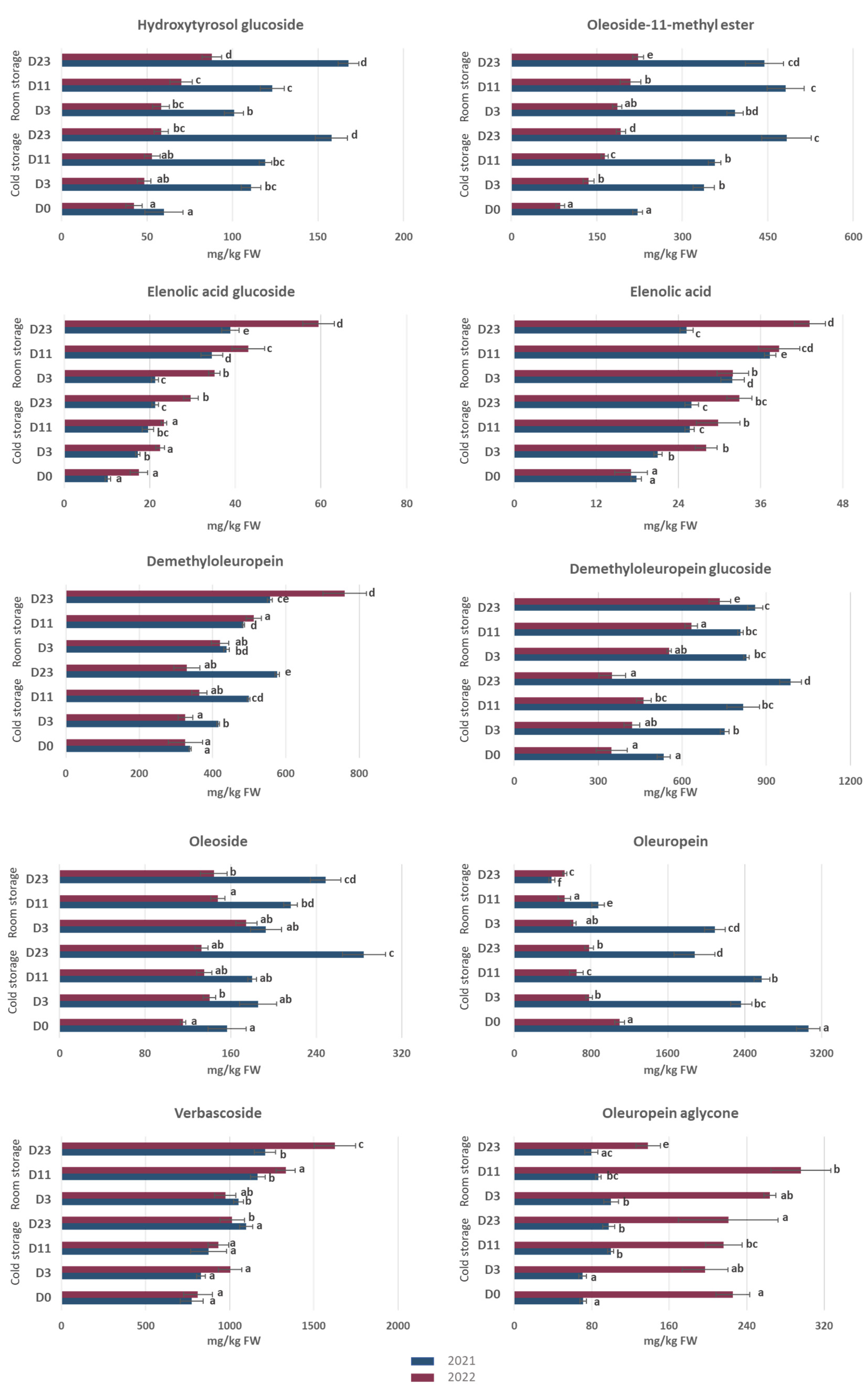
The contents of individual phenolic compounds varied significantly between years, storage condition, and storage duration (
Figure 4 and
Table S3). Based on their variation patterns during storage, the compounds can be classified into three general trends: (1) compounds that showed a progressive decrease, (2) compounds that increased over time, and (3) compounds that fluctuated with smaller variations throughout the storage period.
Oleuropein was the only phenolic compound that mostly decreased during storage in both years, particularly under room storage conditions. In 2021, it decreased by about 39% in cold storage and by 87% in room storage conditions. In 2022, the decrease was 29% under cold storage and 52% under room storage conditions.
Compounds that mostly increased over time were hydroxytyrosol glucoside, elenolic acid glucoside, elenolic acid, verbascoside, demethyloleuropein, demethyloleuropein glucoside, and oleoside-11-methyl ester.
In 2021, hydroxytyrosol glucoside content increased by approximately 164% under cold storage and 180% under room storage conditions over the entire storage period. In 2022, a statistically significant increase was observed only at D23 under cold storage, with a total rise of 37%. Under room temperature conditions, the content began increasing as early as D3, similar to the results for the previous year, ultimately reaching a 107% increase by the end of storage.
Elenolic acid glucoside showed an increasing trend in both years and for both storage conditions. In 2021, its content increased by about 108% under cold storage but increased by 280% under room storage conditions. In 2022, on the other hand, the content remained stable under cold storage until D23, after which it increased by 69%. Under room conditions, a continuous increase was observed throughout the storage period, reaching a total increase of 240%.
In 2021, the verbascoside content remained unchanged during cold storage. In 2022, it remained stable until D23, when it increased by 25%. Under room storage conditions in 2021, the content had already increased by 35% on D3 and then remained stable until the end of storage. In 2022, an increase was observed only after D23, reaching a total increase of 100%.
In 2021, the demethyloleuropein content increased by 64% under room storage and by 70% under cold storage. In 2022, on the other hand, its content remained stable under cold storage, while an increase of 133% was observed under room storage only after D23.
In 2021, the demethyloleuropein glucoside content increased by 85% under cold storage conditions. Under room conditions, it increased by 55% after D3 and then remained stable until the end of the experiment. In 2022, under cold storage conditions, the content increased by 33% at D11 and then decreased to its initial content. Under room storage conditions, a significant increase of 111% was observed at D23.
In 2021, the content of oleoside-11-methyl ester increased by 118% under cold storage conditions, while under room storage, it increased by 101%. In 2022, a similar pattern was observed under both storage conditions, with increases of 124% under cold storage and 159% under room storage.
The last two measured compounds were oleoside and oleuropein aglycone, and their contents fluctuated during storage.
3.3. Fatty Acid Composition
The content of individual fatty acids varied depending on the storage treatment and duration, with notable differences observed between cold and room storage among the storage periods (
Figure 5;
Tables S4 and S5).
Palmitic, palmitoleic, stearic, and arachidic acids contents remained relatively stable over both years and storage conditions. No statistically significant differences were observed between treatments within each year.
Oleic acid showed a more dynamic response. In 2021, its content increased significantly by 27% under cold storage at D23. In 2022, a similar increase of 27% was observed under cold storage at D23, while a significant decrease of 17% occurred under room storage at D11.
The linoleic acid content increased by 25% in 2021 at D23 under cold storage, whereas it remained relatively stable under room storage. In 2022, the differences between treatments were less pronounced, with only cold storage conditions showing a significantly higher value at D23.
The content of linolenic acid increased during cold storage in both years, by 37% in 2021 and by 20% in 2022. Under room storage, it remained stable throughout the storage period in both years.
Eicosenoic acid showed the most pronounced variations. In 2021, its content increased by 65% at D11 under cold storage, followed by a decrease at D23. No significant changes were observed between the room storage treatments in both years. In 2022, cold storage again led to a significant increase of 104% at D23.
3.4. Multivariate Analysis of the Phenolic and Fatty Acids Profiles
Principal component analysis (PCA) was performed to evaluate the combined effects of storage conditions and duration on the composition of phenolic compounds and fatty acids in olive fruits stored under cold storage (CS) and room storage (RS) in 2021 and 2022 for a period of 23 days. The first two principal components (Dim1 and Dim2) explained 60.7% of the total variability in the data set (
Figure 6).
Year-to-year variation was evident, with samples from 2022 showing greater dispersion along Dim2 than those from 2021. Along Dim1, a clear separation between early sampling points (D0, D3) and later stages of storage (D11, D23) was observed, particularly under room storage conditions. Room storage (RS) samples from both years, especially those collected at D11 and D23, were positioned on the positive value of Dim1, reflecting more pronounced compositional changes over time. In contrast, cold storage (CS) samples, especially at earlier time points (D0, D3), clustered more tightly on the negative values of Dim1, indicating greater stability in the profile of phenolic compounds and fatty acids during storage.
4. Discussion
During storage, significant decreases in both fruit firmness and weight were observed, probably due to water loss through transpiration [
20]. This effect was particularly pronounced under room storage conditions, where higher temperatures and lower relative humidity accelerated the loss compared to that of fruit stored under cold conditions. As fruit loses moisture over time, its overall weight decreases, and the tissue becomes softer [
21]. In addition, even after harvest, various metabolic processes continue within the fruit, including enzymatic degradation of cell wall components, which compromises structural integrity and further reduces firmness [
22]. Similar trends have been reported in other fruit species, where higher storage temperatures have been shown to cause more rapid decreases in both weight and firmness [
23,
24,
25].
Our results showed that the fruits harvested in 2022 were lighter and showed a faster weight loss compared to those harvested in 2021. This can be explained by a study on peppers, which showed that smaller fruits have a relatively larger surface area in relation to their volume, accelerating moisture loss and leading to faster weight loss during storage compared to that of larger fruits [
26]. Moreover, the fruit harvested in 2022 was exposed to higher ambient storage temperatures compared to those of the previous year, which increased transpiration and water loss.
Fruit firmness measurements indicated that the fruits harvested in 2022 were softer at harvest. As it is well known that fruit firmness decreases with ripening [
3], this suggests that fruits in 2022 were harvested at a more advanced stage of ripening. This assumption is further supported by the ripening index, which was slightly higher at harvest in 2022 compared to that of the previous year, and it continued to increase during storage. Similar observations have been reported by other authors [
16].
The colour results showed that differences between storage conditions were minimal, as indicated by the basic colour parameters. However, hierarchical clustering in 2021 showed that the samples grouped primarily according to storage time. This suggests that storage temperature exhibited a limited effect on colour development in that year. In contrast, results from 2022 showed that fruit stored for 11 days at room temperature was more similar in colour to fruit stored for 23 days under cold conditions than to that of fruit stored for 11 days under cold conditions. This suggests that higher temperatures may have accelerated pigment development, leading to faster ripening under room storage conditions, which has already been found in apples [
27]. Furthermore, it is possible that the colour changes observed in 2022 were also influenced by the fact that the fruit was harvested at a more advanced stage of ripening and stored at higher room temperatures compared to the levels of the previous year, which has also been found in other fruit species [
28].
The differences between storage conditions were not only limited to quality parameters but were also evident in changes in the content of phenolic compounds. Among all phenolic compounds found in olives, oleuropein is considered the most abundant [
29] and it is known for its characteristic bitterness and strong antioxidant activity [
13]. Its content decreased under both storage conditions in both years, with a more pronounced decrease under room storage conditions. This accelerated degradation at higher temperatures may be related to the increased activity of enzymes such as beta-glucosidase and polyphenol oxidase, which are known to contribute to the degradation of oleuropein [
30]. More specifically, Zhang (2023) [
31] reported that the activity of polyphenol oxidase increases approximately 2–3-fold with every 10 °C rise in temperature until reaching an optimum, beyond which activity decreases due to enzyme denaturation. The initial oleuropein content was higher in 2021 compared to 2022. Its known that the content of oleuropein decreases during ripening [
13], which supports the conclusion that the fruit harvested in 2021 was at an earlier stage of ripening, as also confirmed by the corresponding quality parameter measurements. Furthermore, in 2022, fruits were exposed to higher storage temperatures under room conditions, which further accelerated the degradation process compared to that of the previous year.
Oleuropein was the only phenolic compound among those studied whose content consistently decreased during storage. It is known that oleuropein is enzymatically hydrolysed to oleuropein aglycon and then to hydroxytyrosol and elenolic acid, primarily through the action of β-glucosidase [
32].
The content of oleuropein aglycone increased during cold storage in 2021, whereas it first increased and then decreased under room conditions. The observed increase can clearly be attributed to the enzymatic degradation of oleuropein, whereas the subsequent decrease under room conditions may be due to further degradation of oleuropein aglycone itself, which is known to be a relatively unstable molecule [
33]. Moreover, the oleuropein aglycone content was higher in 2022 than in the previous year, indicating that the fruit harvested that year was at a more advanced stage of ripening, as oleuropein aglycone content is known to increase during ripening [
34]. However, in 2022 the content fluctuated more during storage than in 2021. This instability could be attributed to the higher initial content associated with riper fruit, leading to an earlier onset of its degradation during storage.
In both years, the content of hydroxytyrosol glucoside increased under both storage conditions. In 2021, no statistically significant differences were observed between the storage methods, whereas in 2022 the increase in hydroxytyrosol glucoside was more pronounced under room storage conditions. Since the fruits harvested in 2021 were at an earlier stage of ripening, they initially contained higher content of phenolic compounds, including oleuropein, which has also been reported by other authors [
35]. Phenolic compounds are known for their strong antioxidant activity, which helps to protect cells and molecules from oxidative stress [
36]. The higher initial content of phenolic compounds in 2021 may have provided greater protection against oxidation, potentially reducing the effect of storage temperature on hydroxytyrosol glucoside formation.
The content of elenolic acid, another degradation product of oleuropein, also increased during storage and was consistently higher under room storage conditions in both years. These results support the complementary relationship between elenolic acid and oleuropein, as previously reported by other authors [
35]. Our results also suggest that higher storage temperatures may have increased the activity of the enzymes responsible for oleuropein degradation, which has also been demonstrated by other authors under in vitro conditions [
31].
Similar to elenolic acid, the content of elenolic acid glucoside increased under both storage conditions in both years. More pronounced changes in the content of this compound were observed during storage at room temperature. A similar trend was also observed for oleoside-11-methyl ester. It is well known that mechanical harvesting causes varying degrees of tissue damage [
37], which increases the exposure of phenolic compounds to oxidation in damaged areas [
3]. In addition, such damaged areas are often colonised by microorganisms, further contributing to degradation processes [
38]. In combination with higher storage temperatures, these factors can accelerate the degradation of oleuropein, which may explain the higher contents of its downstream degradation products, such as oleoside-11-methyl ester and elenolic acid glucoside, especially under room storage conditions.
These results further emphasise the importance of fruit maturity at harvest, as it is well established that enzyme activity and gene expression in olives increase during ripening [
39,
40,
41]. The physiological state of the fruit at harvest, including baseline enzymatic activity, can significantly impact the biochemical transformations of individual phenolic compounds after harvest [
42]. Fruits that are less mature, such as those harvested in 2021, undergo slower enzymatic and metabolic activity during storage. This could explain the uniform phenolic behaviour observed across storage treatments in that year. Conversely, fruits harvested at a more advanced ripening stage, such as those obtained in 2022, exhibit greater metabolic activity, resulting in accelerated degradation or transformation processes influenced by storage conditions. Similar observations have been reported in other fruit species, where the physiological state of the fruit at harvest strongly affects phenolic metabolism during postharvest handling [
43].
Verbascoside is known to be a relatively stable molecule at low temperatures [
44], which aligns with our findings. Its content remained almost unchanged during cold storage in both years, suggesting that low temperatures help to preserve its stability and prevent metabolic degradation or transformation. In contrast, a different pattern was observed under room storage conditions. In 2021, an increase in verbascoside content was already noticeable by D3 of storage, whereas in 2022, a similar increase was only detected after D23. This interannual variation may be attributed to differences in the physiological state or maturity of the fruits at harvest. The observed increase in verbascoside under room storage conditions could result from the enhanced enzymatic activity that promotes the glycosylation and esterification of hydroxytyrosol or tyrosol with caffeic acid and sugar moieties, ultimately leading to the biosynthesis of verbascoside [
45]. Additionally, room storage conditions may accelerate enzymatic activity and thus promote more rapid biochemical transformations compared to those noted under cold storage.
The oleoside content increased during storage under both conditions and in both years. Among the studied compounds, oleoside was the only one for which no statistically significant differences were observed between cold and room storage conditions. This suggests that storage conditions may not decisively influence its dynamics, at least not to the same extent as for other phenolic compounds.
We also monitored changes in fatty acid content during storage, which remained significantly more stable than phenolic compounds. The levels of palmitic, palmitoleic, stearic, and arachidic acids remained stable, regardless of the storage conditions, in both years. Such results are expected because saturated fatty acids like palmitic, stearic, and arachidic acids are highly resistant to oxidation due to the absence of double bonds [
46]. Palmitoleic acid, as a monounsaturated fatty acid with only one double bond, despite being more reactive than saturated acids, remains relatively stable [
47]. In contrast, we observed increases in oleic, linoleic, linolenic, and eicosanoic acids, especially after D23 of cold storage, while their levels under room storage conditions remained mostly unchanged. This dynamic may reflect the influence of temperature on fruit metabolism and ripening rate. Cold storage slows down metabolic processes [
48] and enables the gradual accumulation of certain fatty acids as enzymatic pathways become less active. Conversely, higher temperatures accelerate metabolism, resulting in faster fruit ripening. Therefore, it could be possible that fatty acids are synthesised and degraded at the same time, which means that the amounts did not change much. Low temperatures can act as a mild stress factor, triggering biochemical adaptations in the fruit [
49]. These responses include increased activity of phospholipase D, lipase, and lipoxygenase [
50], affecting membrane lipid metabolism. Zhan et al. [
51] report that fruits stored at lower temperatures synthesize more unsaturated fatty acids, thereby improving the fluidity of their cell membranes and increasing their resistance to cold stress. However, caution must be exercised when storing at low temperatures, as various studies report that temperatures below 4 °C cause chilling injury [
4,
50]. However, an analysis of fatty acid changes during olive fruit ripening showed that accumulated thermal time affects the expression of genes involved in fatty acid biosynthesis, leading to changes in the contents of oleic and linoleic acids in cv. Arbequina, which is consistent with our results [
52]. Nevertheless, it should be noted that physiological processes in fruits still attached to the tree differ from those occurring during postharvest storage.
Although mechanical damage can trigger lipolytic processes, thereby increasing the content of free fatty acids [
53], the effects in this case were apparently not strong enough to cause any measurable changes in the contents of specific fatty acids.
The PCA analysis, which incorporated the results of phenolic compounds and fatty acids, clearly differentiated between samples based on the year of harvest. As was observed in the quality parameters, these differences are likely due to variations in the ripeness of the fruit at the time of harvesting. Fruit harvested in 2022 was more mature than that harvested in 2021, as reflected by greater variability among the 2022 samples in the PCA. This may also have been influenced by higher storage temperatures under room storage conditions in 2022 compared to those in 2021.
In addition to year-based separation, the PCA also distinguished samples according to storage duration. Samples analysed at the beginning of the storage period (D0 and D3) were clearly separated from those analysed at later stages (D11 and D23), indicating progressive metabolic changes in fruit composition during storage. This separation was particularly pronounced in samples stored at room temperature; by the end of the storage period, the results for these samples had considerably shifted away from those of the others. This further confirms that room temperature accelerates the degradation or transformation of phenolic compounds and fatty acids during storage.
5. Conclusions
In this 2-year study, we evaluated how different storage conditions affected quality parameters, phenolic compounds, and fatty acids in mechanically harvested ‘Leccino’ olive fruit. The olives were stored either under cold or room temperature conditions, with samples taken at harvest and after 3, 11, and 23 days of storage.
From the fruit quality parameters at harvest, it was observed that each year, the olives were harvested at different ripeness stages, which was also confirmed by the phenolic compound content results. The fruits harvested in 2022 were found to be riper, as indicated by a higher maturity index, lower firmness, and reduced oleuropein content compared to the results for the previous year.
Furthermore, clear differences in storage conditions were observed in both years. Fruit stored under room storage conditions lost weight and firmness more quickly due to increased evaporation and water loss compared to fruit stored at a lower temperature. Changes were also observed at the metabolic level. Oleuropein degraded faster under room storage conditions, resulting in an increase in its breakdown products (hydroxytyrosol glucoside, elenolic acid glucoside, elenolic acid, verbascoside, demethyloleuropein, demethyloleuropein glucoside, and oleoside-11-methyl ester). Higher temperatures are known to enhance the activity of enzymes such as beta-glucosidase and polyphenol oxidase, thereby accelerating fruit metabolism. Furthermore, the most marked changes were observed in fruit harvested in 2022 and stored under room conditions, which was also clearly demonstrated by the PCA analysis. These changes occurred due to their more advanced ripening stage at harvest and the fact that both temperature and relative humidity were higher in 2022 than in the previous year.
Among the metabolites studied, fatty acids proved to be more stable, showing minimal change during storage. Of the eight fatty acids examined, most unsaturated fatty acids (oleic, linoleic, linolenic, and eicosanoic acid) showed increased levels under cold storage, but only after 23 days of storage. The saturated fatty acids remained unchanged, suggesting a response to low temperatures during long-term storage, which may involve improved membrane fluidity as a resistance to cold stress.
Although fatty acids appear stable, significant changes in phenolic contents occurred during storage, which can negatively affect the quality of the fruit and potentially, that of the olive oil. Understanding the complex interplay between fruit ripeness and storage environment enables better control over fruit quality and functional properties during the postharvest period. To gain a deeper understanding of these changes, future research should also monitor enzymatic activity.