Abstract
Enhanced colony nutrition can support brood development, resulting in better physiological conditions and increased resilience in adult honey bees, particularly under stress. This study investigated the effects of colony nutrition and adult dietary supplementation with green propolis on bee health under fungicide exposure. Colonies were managed under food restriction or nutritional supplementation for 22 weeks. Newly emerged bees from each colony were then caged and fed protein diets consisting of honey-pollen patties contaminated or not with fungicide, and sucrose sugar syrup with or without aqueous green propolis extract. Bees from supplemented colonies showed greater body weight, higher hemolymph protein levels, and higher consumption of protein food after seven days in cages. Fungicide exposure reduced hemolymph protein levels, altered the expression of detoxification and immune-related genes, and significantly decreased bee survival. Interestingly, propolis supplementation alone changed gene expression patterns and slightly reduced longevity compared to bees not exposed to propolis or fungicide. However, under fungicide stress, bees that ingested propolis survived longer, indicating a protective effect. While colony nutritional supplementation clearly promotes honey bee resilience against fungicide exposure, feeding propolis also showed promising effects, though further studies are needed to determine an optimal dietary concentration.
1. Introduction
Honey bees (Apis mellifera) play a central role in global pollination services, particularly in agriculture, being responsible for approximately 50% of all insect-mediated crop pollination worldwide []. Even within diverse natural ecosystems harboring a wide variety of native bee species, honey bees remain the most frequent floral visitors, accounting for an average of 13% of all recorded flower visits across different ecological networks []. Managed honey bee colonies are highly abundant, with around 102 million hives maintained globally—an 85% increase since 1961 []. This underscores the species’ dominant role not only in sustaining agricultural productivity but also in maintaining ecosystems, as they consistently appear as the most frequent floral visitors across a broad range of plant communities [].
Though the number of bee colonies has increased over the last 65 years, human population growth has outpaced hive expansion by 70%, intensifying the global demand for honey bee colonies to support agricultural pollination services []. At the same time, colony productivity and survival are increasingly threatened by multiple environmental stressors, including habitat loss, pesticide exposure, pathogens, climate change, and poor nutrition. These are primary factors affecting colony health and development [,,,]. The ongoing expansion of modern agriculture further exacerbates this issue by reducing both the availability and diversity of pollen, thereby limiting bees’ sources of essential amino acids, lipids, and micronutrients []. The various stressors often act synergistically, accelerating colony decline and reducing pollination efficiency, with serious implications for global food security [].
Colony nutrition plays a pivotal role in shaping the overall health and resilience of honey bees. Adequate and diverse nutritional inputs during larval development are essential for the proper development of physiological systems, including the immune system and detoxification pathways, which directly influence bees’ ability to cope with biotic and abiotic stressors [,]. Nutritional inadequacies during the brood stage can impair development, reduce body protein reserves, and compromise immune and detoxification responses in adult bees [,,].
Emerging evidence suggests that the benefits of colony-level nutritional management during brood development extend into adulthood, affecting bees’ physiological capacity to respond to subsequent environmental challenges []. However, whether such nutritional interventions can specifically enhance resilience against fungicide exposure remains unknown, which is particularly concerning given the frequent presence of fungicide residues in pollen collected from crop fields []. Although generally considered less harmful to bees, fungicides are now recognized for their detrimental effects on essential physiological functions, such as immune response, detoxification pathways, and gut microbiota homeostasis []. Moreover, lax regulatory policies frequently permit fungicide application during bloom, thereby facilitating direct exposure of pollinators to contaminated nectar and pollen [,].
This risk is further amplified by systemic formulations, which enable widespread translocation throughout the plant tissues [], and by products that combine more than one active ingredient, aiming to enhance the fungicidal effect. An example is a widely marketed fungicide in Brazil that contains the active ingredients bixafen, prothioconazole, and trifloxystrobin, and is used on large-scale crops such as cotton, soybean, sunflower, and corn, which are visited by bees for nectar or pollen collection []. In 2023, 1913, 6151, and 4315 tons of these compounds, respectively, were sold in Brazil [], and they continue to be commercialized in several other countries [,,]. We were concerned that this three-component fungicide would have a greater effect on bees than the previous options.
Understanding and mitigating these environmental challenges is essential for sustaining both honey bee populations and the agricultural systems that depend on them. Though there are limited practical options available to minimize fungicide-related risks, dietary interventions as mitigating factors are gaining attention. Among these, propolis, a resinous product rich in flavonoids and phenolic acids, has emerged as a promising supplement due to its documented antioxidant and immunomodulatory properties, potentially counteracting pesticide-induced stress in bees [,]. Recent studies suggest that propolis can bolster detoxification pathways and enhance resilience in pesticide-exposed bees, highlighting its potential as a natural mitigation tool, warranting further investigation [].
This study aimed to determine whether improved colony nutrition enhances brood development and adult bee resilience to pesticide stress, and whether adding propolis to a protein diet during adulthood mitigates the adverse effects of fungicide exposure on honey bee health
2. Materials and Methods
This study was carried out in Dracena, São Paulo state, Brazil (21°27′33″ S; 51°33′22″ W; 392 m altitude), from August 2022 to February 2023. In the first part of the experiment, we subjected honey bee colonies to contrasting food availability management, thereby obtaining bees that developed under two distinct nutritional conditions during the brood stage. In the second part of the study, newly emerged adult bees from these colonies were collected and housed in small cages (20 bees per cage) in an incubator. They were offered a protein-based paste prepared with sunflower pollen, which originated from plants that were treated or not with fungicide. Additionally, sucrose syrup was provided to these caged adult bees as a carbohydrate source, supplemented or not with propolis.
2.1. Nutritional Management of Honey Bee Colonies and Bee Pollen Collection
Eight colonies of Africanized honey bees (Apis mellifera), housed in standard Langstroth hives with 10 frames each, were submitted to two distinct dietary management protocols over a 22-week period. Alongside the natural food resources that the bees collected from the environment, four colonies received a dietary supplementation (S), consisting of 100 g of protein paste per week. This paste was prepared by mixing three parts of multifloral bee pollen with one part of wildflower honey. Additionally, these colonies were provided with 500 mL of sucrose syrup, made by dissolving equal parts (w/w) of crystallized cane sugar and water, offered twice weekly. For the second treatment, food availability for the colonies was restricted (R) by pollen traps at the entrances of the hives. No dietary supplements were provided to these four colonies.
All experimental colonies were headed by naturally mated queens and were standardized to contain six frames with brood and four frames with stored food (honey and pollen) at the start of the study. The 22-week duration was chosen to ensure that the worker bees used in subsequent evaluations had fully developed within their respective nutritional conditions during their larval stages, eliminating the influence of food resources accumulated before the start of the colony food management period.
Throughout the 22 weeks of dietary management, bee pollen collected by the traps installed in the R group colonies was gathered daily. After each collection, the total bee pollen yield per hive was weighed using a Shimadzu electronic scale with a 2200 g capacity and an accuracy of ±0.03 g.
To estimate the actual amount of natural protein feed available to the R colonies, the efficiency of the bee pollen traps was assessed. For this, an observer remained near each trap for a 30 min observation period, conducted between 8:00 and 10:00 AM on three separate days. During this time, the number of bees returning to the hive with pollen loads in their corbiculae was recorded. Immediately following the observation period, the number of bee pollen pellets retained in the trap drawer was counted. This procedure was performed in triplicate for each hive. The collected data was used to calculate the efficiency of the bee pollen traps [].
2.2. Assessment of Newly Emerged Bee Weight
At the end of the nutritional management phase, brood combs containing bees close to emergence were collected from all experimental colonies. These combs were used to create two distinct pools of newly emerged bees, corresponding to each nutritional treatment (S and R), sampling 100 newly emerged workers from each. The bees were first kept in a freezer (−20 °C) for one hour. Subsequently, subsamples of 10 bees were transferred into crucibles, with five replicates per treatment. The samples were then dried in a forced-air oven at 105 °C for 16 h []. The weight of each group of 10 bees was measured both before and after the drying process.
2.3. Preparation of Sugar Syrup and Determination of Propolis Concentration
The sugar syrup for the caged bees was prepared with two parts cane sugar and one part water (w/w). To determine the quantity of propolis to be added to the syrup, an oral toxicity test was conducted using syrups with different concentrations of an 11% (w/v) aqueous green propolis extract produced by Apis Flora Company. The tested concentrations of aqueous green propolis extract in the syrup were: 0, 6.25, 12.5, 25, and 50% (v/v %) [].
Forager honey bees from colonies not subjected to nutritional management were collected at the hive entrance and divided into groups of 20 individuals. Each group was housed in a transparent plastic cage with a volume of 250 mL, equipped with a perforated lid to allow gas exchange. A 1.5 mL Eppendorf tube was inserted through the lid, with a small hole pierced at the bottom using a needle to provide access to the syrup, which was offered ad libitum. Three replicate cages were set up for each treatment and kept in an incubator at a controlled temperature of 33 °C and relative humidity (RH) of 70%, where they remained for 96 h. Bee mortality was recorded daily.
Based on the mortality data, the median lethal concentration (LC50) was determined to be 54.4% (log LC50 = 1.699; probit = 1.782 × Log(Concentration) + 1.9053; coefficient of determination (R2) = 0.91; standard error (SE) = 0.132). Considering these results, the sugar syrup used in the experiment contained the lowest concentration (6.25%) of green propolis extract tested (P+), corresponding to 11.5% of the calculated LC50. For the sugar syrup control, no propolis extract was added (P−).
2.4. Sunflower Cultivation, Fungicide Treatment, Pollen Collection, and Preparation of Protein Feed
Sunflower plants (Helianthus annuus), cultivar CATI Multissol, were cultivated in a 3000 m2 field area at a density of 55,555 plants·ha−1. Both planting and topdressing fertilizations followed the current Brazilian recommendations for sunflower cultivation []. No pesticide applications were performed during crop growth. To obtain sunflower pollen, about 500 flower heads were covered with non-woven fabric bags before anthesis. Once most of the capitula had bloomed, the sunflower stems were cut, and the inflorescences were transported to the laboratory.
In the lab, the flower heads were unbagged and arranged on the floor of a spray testing facility, maintaining the same spacing used in the field. The area occupied by the inflorescences was measured to calculate the required amount of fungicide for application. The fungicide spray was applied following the label instructions for the Fox Xpro product [], recommended for controlling Alternaria helianthi (Pleosporaceae), an important fungal disease affecting sunflowers. The formulation of Fox Xpro contains 125 g/L of Bixafen, 175 g/L of Prothioconazole, and 150 g/L of Trifloxystrobin as active ingredients. The fungicide was applied at a rate of 0.5 L ha−1, using a spray volume of 144 L per hectare, with equipment calibrated at 200 kPa pressure, positioned 0.5 m above the flowers, and operated under calm (windless) conditions. After 15 min, allowing sufficient time for the spray to dry, the inflorescences were manually shaken over a plastic tray to dislodge the pollen.
In the case of the control group (no fungicide exposure), the pollen was harvested immediately after inflorescence collection, without any spray treatment. All collected pollen was stored in 50 mL plastic tubes at −80 °C until it was incorporated into the preparation of the bees’ protein diet paste.
The protein feed offered to the bees in the cages was prepared by mixing three parts of freshly collected sunflower pollen—originating from plants either treated (F+) or untreated (F−) with the target fungicide, with one part of wildflower honey (w/w).
2.5. Collection of Newly Emerged Bees and Food Intake Measurement
Brood combs containing bees close to emergence were collected from all experimental colonies, allowing the formation of representative bee pools of each dietary management (S and R). The brood combs were kept in an incubator for 24 h (33 °C, 70% RH). After emergence, groups of 20 bees from the same nutritional treatment were maintained under the same conditions as those used for determining the propolis concentration in the sucrose syrup, differing only in the provision of protein feed. A total of eight treatments were established, considering colony dietary managements (S and R), exposure of the flowers to fungicide (F+) or not (F−), and the inclusion of aqueous green propolis extract in the syrup (P+) or not (P−).
The amounts of carbohydrate and protein feed offered and consumed were recorded daily by weighing the empty feeders and the feeders containing food both before offering and after the consumption period. This procedure was carried out over seven days, during which the cages were maintained in an incubator (33 °C, 70% RH). For each treatment, three cages with 20 bees each were used.
2.6. Protein Quantification in Honey Bee Hemolymph
Newly emerged bees from both colony nutritional managements were separated into two groups: the first group was analyzed on the day of emergence (D0), and the second group was analyzed after seven days (D7), during which bees were fed experimental feed containing protein paste with sunflower pollen either contaminated or uncontaminated with fungicide, and sugar syrup with or without green propolis aqueous extract.
Pools of 10 µL hemolymph were collected from bees subjected to the same treatment by opening an orifice through antennal removal and applying gentle pressure to the thorax with the index finger []. Four biological replicates were performed for each treatment. Protein quantification was performed using the Bradford method (1976) [] in flat-bottom ELISA plates and processed on a Biochrom EZ Read 2000 Microplate Reader. Protein concentration was determined based on a previously established bovine serum albumin (BSA) standard curve [].
2.7. Gene Expression Analysis
2.7.1. Sample Collection and Preparation
To investigate the expression of key genes related to bee health following the seven-day feeding period in cages, the bees were euthanized by freezing and subsequently dissected. Dissections were performed under a stereo binocular microscope using fine-tipped scissors and entomological forceps. The heads, thoraxes, and intestines were removed and discarded, retaining only the abdomens without the digestive tract for analysis. Each sample was composed of six abdomens, and four biological replicates were collected for each treatment.
2.7.2. RNA Extraction and cDNA Synthesis
For total RNA extraction, approximately 100 mg of tissue (equivalent to six dissected abdomens) was homogenized in 1 mL of Trizol® LS reagent (Invitrogen™, Waltham, MA, USA), followed by the addition of 200 µL chloroform. Samples were centrifuged at 12,000× g for 15 min at 4 °C. The aqueous phase was carefully transferred to a new microtube, mixed with isopropanol, vortexed, and then centrifuged again at 12,000× g for 10 min at 4 °C. The resulting RNA pellet was washed three times with 100 µL ethanol, followed by centrifugation and supernatant removal. After air-drying for approximately 15 min, the RNA was resuspended in molecular-grade water (Sigma, St. Louis, MO, USA).
RNA concentration was determined in nanograms by measuring absorbance at 260 nm with a Nanodrop-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). To evaluate RNA integrity, a fraction of each sample was electrophoresed on a 1% agarose gel and stained with GelRed® (Sigma). Complementary DNA (cDNA) synthesis was performed using 1 μg of total RNA as a template, following reverse transcription with iScriptTM III Reverse Transcriptase (Life Technologies, Waltham, MA, USA). The synthesized cDNA was stored at −20 °C until further use.
2.7.3. Target Genes and Primer Sequences
Gene expression was assessed using real-time quantitative PCR (qPCR), with primers specific to target genes related to honey bee health, as well as a reference gene. Table 1 lists all genes evaluated in this study, along with primer sequences and their respective references.

Table 1.
Reference gene and target genes associated with honey bee health evaluated in this study, along with their respective primer sequences and literature references.
2.7.4. Quantitative PCR and Data Analysis
Gene expression levels were quantified using real-time qPCR (RT-qPCR), and relative expression was calculated following the 2−ΔΔCt method [,]. Reactions were conducted in 96-well plates, using the iTaqTM Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s protocol.
Each 20 µL reaction contained 10 µL of 2× SYBR Green Master Mix, 8 µL of primers at a final concentration of 200 nM, and 2 µL of cDNA template. Amplification was performed on an iCycler iQ™ Real-Time Detection System (Bio-Rad Laboratories), using the following thermal cycling conditions: an initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 °C for 30 s. Following amplification, a melting curve analysis was conducted to confirm the specificity of the amplified products.
The cycle threshold (Ct) values were determined during the exponential phase of amplification, based on fluorescence detection. Gene expression levels were normalized to the reference gene RPL32 []. PCR efficiency and relative expression ratios for each target gene were calculated following the Pfaffl method [].
2.8. Longevity Test
The longevity assay involved a total of 480 bees, equally divided between the two nutritional management groups (S and R). Newly emerged bees were distributed in groups of 20 individuals per cage, totaling 24 plastic cages, with three replicates (cages) per treatment. All cages received the experimental feeds, which were provided ad libitum. Mortality was monitored daily, with the number of dead bees recorded until all individuals had died. The cages were kept in an incubator (33 °C and 70% RH).
2.9. Statistical Analyses
The experiment followed a completely randomized design in a split-plot arrangement. The main experimental unit was the bee colony, which was subjected to one of two levels of nutritional management. After emergence, newly emerged adult bees from each colony were distributed into cages for dietary treatments. These cages represented the subplots, in which bees received diets containing or not fungicide in the protein patty and/or green propolis extract in the sugar syrup. Pollen collection by food-restricted (R) colonies was recorded weekly, and pollen trap efficiency was determined from six measurements per colony, with data variability assessed using the standard error of the mean. Adult bee weight at emergence—collected prior to fungicide exposure and propolis ingestion—was analyzed by ANOVA, with colony nutrition as the single fixed factor. When significant effects were detected (p < 0.05), means were compared using Tukey’s test.
Variables related to protein and sugar syrup consumption, hemolymph protein content, and gene expression were analyzed using a split-plot mixed linear model. The main plot factor was colony nutrition (S or R), and the subplot factor was adult bee diet, with four combinations based on fungicide (F+ or F−) and propolis (P+ or P−) presence, yielding eight treatment groups: S_F−P−, S_F+P−, S_F+P+, S_F−P+, R_F−P−, R_F+P−, R_F+P+, and R_F−P+. Treatment effects and interactions were evaluated using SAS software 9.4 M7 [], with adjusted means compared by Tukey’s test (α = 0.05). To validate the assumptions of normality and homogeneity of variances required by ANOVA and mixed models, residuals were tested using the Shapiro–Wilk test.
Bee longevity was analyzed using a mixed-effects Cox regression model, with colony nutrition, fungicide exposure, and propolis ingestion as fixed effects and cage as a random effect, using R software [].
3. Results
3.1. Colony Feeding Management Period
A mean of 61.5 ± 12.1 g of bee-collected pollen was harvested weekly from each colony subjected to the restricted feeding management (R), resulting in a mean cumulative reduction of 1617.7 g of pollen availability per colony over the 22-week period. During the same period, each colony in the supplemented group (S), which did not have pollen traps installed, received 2200 g of protein patty and 22 L of sugar syrup, in addition to unrestricted access to all pollen and nectar collected from the field.
The efficiency of the pollen traps installed on the R colonies was 67.6 ± 2.0%. Based on this efficiency and the amount of pollen collected, it was estimated that forager bees from the R colonies effectively transported between 23.7 and 35.2 g of pollen into their hives per week, totaling between 521.2 and 775.3 g over the entire nutritional management period.
3.2. Influence of Colony Nutritional Management on the Emergence Weight of Honey Bees
After the 22-week colony nutritional management period, newly emerged bees from the S group exhibited higher live weight (p = 0.041) and dry weight (p = 0.006) compared to those from the R group (Table 2). No significant difference (p > 0.20) was observed in the dry weight to live weight ratio between the two colony nutritional management groups (Table 2).

Table 2.
Mean live weight, dry weight in mg, and dry weight/live weight ratio of newly emerged bees obtained from colonies with supplemented or restricted food availability.
3.3. Consumption of Experimental Feeds in Cages by Bees That Developed as Brood in Colonies Subjected to Nutritional Managements
For protein patty consumption, a significant interaction was observed (p < 0.001) between the colony’s nutritional management during brood development and the type of feed offered to adult bees in the cages (Table 3). When comparing colony nutritional managements, bees from the supplemented group (S) consumed more protein feed when offered protein patties without fungicide and with syrup free of propolis (F−P−) compared to bees from the restricted group (R). For the other feeds offered, no significant differences in consumption were found between bees from the different colony nutritional backgrounds.

Table 3.
Daily mean consumption of protein patty (mg), containing pollen with (F+) or without (F−) fungicide, and sucrose syrup with (P+) or without (P−) green propolis extract, by honey bees originating from colonies subjected to either nutritional supplementation or food restriction.
Considering only bees from the S colonies, the highest protein consumption occurred with the F−P− feed, followed by the feed that differed only by the inclusion of propolis in the syrup (F−P+) (Table 3). For this group, consumption was lower when the protein patty contained fungicide, regardless of the inclusion of propolis in the sugar syrup (F+P+ and F+P−). For bees from the R colonies, the highest protein consumption occurred with patties without fungicide, whether the syrup contained propolis or not (F−P+ and F−P−). The consumption of the F−P− feed did not differ significantly from that of the feed containing both fungicide and propolis (F+P+) (Table 3).
The estimated syrup consumption per bee was 9.51 ± 0.37 µg/day. No significant effect was observed due to the previous colony nutritional management or the type of feed offered to the bees, regardless of the inclusion or not of fungicide and propolis.
3.4. Protein Content in the Hemolymph of Newly Emerged Bees and After Seven Days of Consumption of the Experimental Feeds
At emergence, considering only the effect of colony nutritional management, hemolymph protein content was higher (p < 0.001) in bees from supplemented colonies (S) (8.94 ± 0.19 μg/μL) compared to bees from restricted colonies (R) (6.76 ± 0.20 μg/μL). For bees fed the experimental feeds for seven days post-emergence, hemolymph protein content was not significantly influenced (p > 0.05) by colony nutritional management during brood development (Table 4).

Table 4.
Hemolymph protein content (μg/μL) after a seven-day period during which newly emerged honey bees from colonies with supplemented or restricted feeding consumed protein paste containing pollen with (F+) or without (F−) fungicide, and sugar syrup with (P+) or without (P−) green propolis extract.
Regarding the experimental feeds, hemolymph protein content was highest (p < 0.001) when bees consumed feed without fungicide and propolis (F−P−), followed by feeds containing propolis without fungicide (F−P+), propolis with fungicide (F+P+), and lowest when consuming feed with fungicide but without propolis (F+P-) (Table 4).
3.5. Expression of Genes Important for Honey Bee Health
The expression of antioxidant and detoxification system genes in seven-day-old bees that had been fed diets without fungicide and propolis did not differ between individuals from the two colony nutritional managements (Figure 1). The expression of the cat gene increased when bees were fed diets containing fungicide and/or propolis, regardless of colony nutritional management, when compared to their respective controls. When exposed to fungicide and no propolis, the increase in cat expression was greater in bees from colonies under restricted feeding. Conversely, when only propolis extract was added to the diet (without fungicide), higher cat expression was observed in bees from supplemented colonies.
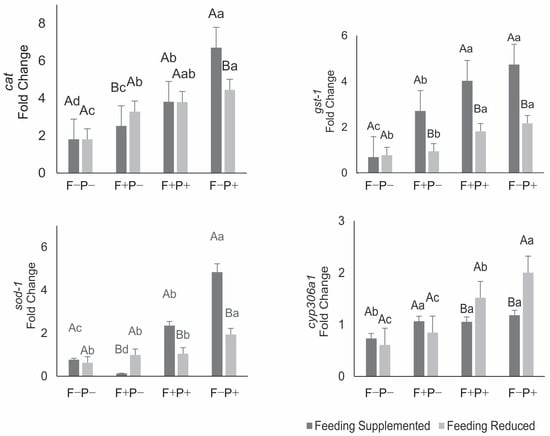
Figure 1.
Expression of antioxidant and detoxification genes in the fat body of seven-day-old honey bees from colonies under supplemented or restricted feeding and fed with a protein patty with (F+) or without (F−) fungicide, and sucrose syrup with (P+) or without (P−) aqueous propolis extract. Data represent means of four biological replicates per treatment, each analyzed in triplicate. For each gene, means followed by the same capital or lowercase letter do not differ significantly (Tukey test, p > 0.05). Capital letters indicate comparisons between nutritional managements under the same diet; lowercase letters indicate comparisons among bee diets within the same colony nutritional management.
Fungicide exposure, with or without propolis in the syrup, induced increased gst-1 gene expression, except for bees fed protein paste containing fungicide-treated pollen and syrup without propolis. Propolis in the syrup promoted gst-1 upregulation, especially in bees from supplemented colonies, with its isolated effect even surpassing that of fungicide alone (F−P+) (Figure 1).
The expression of the sod-1 gene showed distinct patterns between bees from the two nutritional management groups (Figure 1). In bees from colonies supplemented during brood development, sod-1 expression decreased when fed fungicide-contaminated protein feed and syrup without propolis as adults. Propolis in the diet, with or without fungicide, induced upregulation of this gene, with a stronger effect observed in the absence of fungicide. In bees from nutritionally restricted colonies, fungicide-containing diets, with or without propolis, did not significantly alter sod-1 expression; however, propolis alone (without fungicide) increased sod-1 expression in these bees.
Consumption of fungicide-containing and propolis-free diets by bees from supplemented colonies led to upregulation of cyp306a1. In bees developed in restricted colonies, the same diet did not affect cyp306a1 expression. Diets containing fungicide and propolis (F+P+) or without fungicide but with propolis (F−P+) increased cyp306a1 expression in bees from both nutritional backgrounds. When fungicide and/or propolis were included in the adult bee diets, gene expression was always higher in individuals from supplemented colonies (Figure 1).
The expression of immune system genes and storage protein genes did not differ between bees that developed as brood in colonies under either of the two nutritional managements and that, during the first seven days of adulthood, received a diet free of both fungicide and propolis (Figure 2). When bees were fed a diet containing fungicide but no propolis, there was downregulation of all these genes—except for hym and hex70b in bees from restricted colonies (R), which did not differ from the control group (F−−P−). Diets containing both fungicide and propolis caused reduced expression of all immune system genes and storage proteins, except for def1 (S), vg (S and R), and hex70b (R), whose expression did not differ from the control (Figure 2). Propolis ingestion in the absence of fungicide induced upregulation of all immune system and storage protein genes except for aba, api, and hym in bees from supplemented colonies (S) (Figure 2).
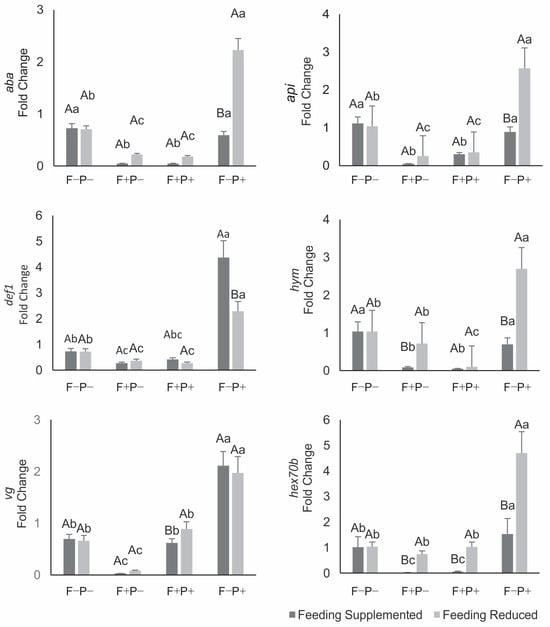
Figure 2.
Expression in the fat body of vg, hex70b and immune system antimicrobial peptide genes in the fat body of seven-day-old honey bees from colonies under supplemented or restricted feeding, and fed with a protein patty with (F+) or without (F−) fungicide, and sucrose syrup with (P+) or without (P−) aqueous propolis extract. Data represent means of four biological replicates per treatment, each analyzed in triplicate. For each gene, means followed by the same capital or lowercase letter do not differ significantly (Tukey test, p > 0.05). Capital letters indicate comparisons between previous nutritional managements in bees fed the same diet; lowercase letters indicate comparisons among bee diets within the same colony nutritional management.
3.6. Bee Longevity
Longevity of adult worker bees was influenced by colony dietary management, exposure to commercial fungicide, and ingestion of propolis (Table 5). Bees from colonies maintained under restricted diets (R) exhibited shorter lifespans than those from supplemented colonies (S), with a 3.8-fold higher hazard of death (p < 0.001). Likewise, exposure to fungicide-treated sunflower pollen (F+) significantly increased bee mortality risk compared to non-exposed bees (F−P−), when bees ingested no propolis (5.08-fold increase, p < 0.001). Additionally, propolis ingestion itself (F−P+ vs. F−P−) also raised mortality risk (2.88-fold, p < 0.001), even in the absence of fungicide exposure (Table 5).

Table 5.
Comparative assessment of nutritional management of honey bee colonies (S for supplemented and R for restricted feed), fungicide exposure (F+ for pollen from sunflowers treated with commercial fungicide and F− for untreated sunflower pollen that was used as protein food for the bees), and propolis supplementation (P+ and P− for sucrose syrup with and without propolis extract, respectively).
When comparing bees from different dietary managements within the same fungicide and propolis treatments, nutritional restriction further increased mortality risk. Specifically, bees from restricted colonies had a higher hazard of death compared to supplemented colonies both in the F+P− treatment (hazard ratio = 0.35, p < 0.001), in the F+P+ treatment (hazard ratio = 0.51, p = 0.011), and in the F−P+ treatment (hazard ratio = 0.21, p < 0.001) (Table 3). Although the random effect of cage was included in the model, it showed minimal variance (σ2 = 0.003; SD = 0.052), suggesting that the experimental cage did not substantially influence bee survival.
The highest survival was observed in bees from the supplemented diet colonies that were not exposed to fungicide and did not ingest propolis (15.90 ± 0.55 days), followed by bees from the same nutritional management that were not exposed to fungicide but received propolis (13.50 ± 0.29 days), and those exposed to fungicide with propolis ingestion (13.30 ± 0.33 days) (Figure 3). The shortest mean lifespan among bees from supplemented colonies occurred in the group exposed to fungicide without propolis ingestion (11.30 ± 0.40 days) (Figure 3).
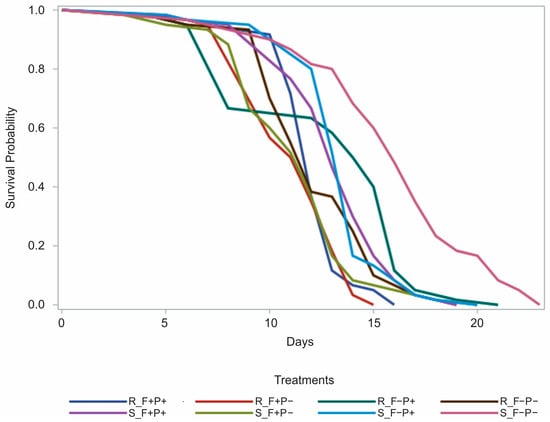
Figure 3.
Proportion of surviving bees that developed during the brood stage in colonies with supplemented (S) or restricted (R) feeding and were fed as adults with a protein patty with (F+) or without (F−) fungicide, and sucrose syrup with (P+) or without (P−) aqueous propolis extract.
Among bees that developed as brood in the restricted diet colonies, the greatest survival was recorded in the group fed with pollen not treated with fungicide but supplemented with sugar syrup with propolis (12.80 ± 0.53 days), followed by the group exposed to the fungicide but not to propolis (12.20 ± 0.38 days), followed by the group exposed to fungicide and propolis (12.00 ± 0.25 days), and finally the group exposed to fungicide without propolis (11.40 ± 0.28 days) (Figure 3).
Overall, the longest survival times were recorded in bees from supplemented colonies that were not exposed to the fungicide, while the shortest lifespans were observed in fungicide-exposed groups that did not receive propolis (11.30 ± 0.40 and 11.40 ± 0.28 days, for supplemented and restricted colonies, respectively). Propolis ingestion appeared to mitigate, at least partially, the negative impact of fungicide exposure, as bees from both nutritional managements that consumed propolis while exposed to the fungicide showed longer survival than their counterparts without propolis.
4. Discussion
Increased food availability during the brood stage, provided by colony supplementation, proved beneficial for adult honey bees that had developed in supplemented colonies. The first measurable indicator supporting this observation was the higher body weight at emergence among workers reared in well-nourished colonies compared to those from nutritionally restricted ones (Table 2). On average, bees from supplemented colonies were 5.67 mg heavier in fresh weight and 0.71 mg heavier in dry weight, representing increases of 6.3% and 4.9%, respectively. Another indicator of the benefits of colony food supplementation was the increased total protein content in the hemolymph of newly emerged bees, which was 32% higher compared to individuals from food-restricted colonies. These differences reflect enhanced larval growth and likely indicate improved physiological condition and resource accumulation.
The influence of colony pollen availability on the body weight of newly emerged bees was previously demonstrated by Scofield and Mattila [], who reported a 59% reduction in weight in bees reared under pollen restriction. Although artificial high-protein diets are commercially designated as pollen substitutes, they often fail to support full larval development [], reflecting their limitations [,]. In our study, protein patties consisted exclusively of honey and bee-collected pollen, which are natural food sources for bees.
Pollen’s nutritional complexity extends beyond proteins and amino acids, also providing lipids, carbohydrates, vitamins, minerals, and a wide array of secondary metabolites []. These compounds act synergistically to support development, immunity, and overall bee health, and may enhance resilience to environmental stressors, including pesticides []. This underlines the irreplaceable role of natural pollen in colony nutrition. When facing resource scarcity, honey bee colonies tend to reduce their population size to balance demand and supply. However, even with this adjustment, severe shortages may still impair brood development [,]. Such a scenario may have occurred in our experiment, considering that pollen traps were used continuously for 22 weeks in the food-restricted colonies.
Although newly emerged bees from supplemented colonies initially showed higher hemolymph protein levels, the difference between them and bees from food-restricted colonies was no longer evident after seven days of caged feeding with experimental diets lacking both fungicide and propolis (Table 4). During this period, the control diet included only honey and sunflower pollen—a protein source of known low nutritional quality [,], particularly deficient in methionine and tryptophan []. This pollen was selected specifically to minimize any compensatory effects during adult life. Given these limitations, sunflower pollen is unlikely to provide the level of dietary compensation that, according to Schilcher et al. (2022) [], may partially mitigate physiological deficits in bees resulting from suboptimal larval nutrition.
Hemolymph protein content in adult bees consuming diets free of both fungicide and propolis remained unaffected by larval nutritional history. However, dietary contamination with fungicide led to a 67.7% reduction in hemolymph protein levels for bees from both nutritional groups (Table 4). Interestingly, even the addition of propolis alone, without fungicide, reduced protein content by 25.9% (Table 4). The negative impact of the fungicide was mitigated when bees received propolis-supplemented syrup along with the contaminated diet, resulting in a smaller reduction in protein levels (55.3%) (Table 4). Fisher et al. [] also observed decreased hemolymph protein concentration in bees chronically exposed to the commercial fungicide Pristine (containing boscalid and pyraclostrobin). In contrast, DeGrandi-Hoffman et al. [], using the same fungicide, found no such effect in bees orally exposed in cages for 3 or 7 days. The commercial product used in our study included the following three active ingredients: bixafen, prothioconazole, and trifloxystrobin, which may have had additive effects contributing to the pronounced reduction in protein levels.
Propolis ingestion via syrup also led to lower hemolymph protein levels. Although a direct relationship between propolis intake and protein content in hemolymph has not been previously reported, propolis contains multiple phytochemicals whose detoxification involves enzyme-mediated metabolism. While bees do not appear to actively ingest propolis [], they may consume its components indirectly via nectar stored in propolized combs, where it is converted to honey. This is supported by evidence that many phytochemicals present in honey are not found in nectar, implicating propolis as a secondary source and revealing the bees’ biochemical capacity to process such compounds []. Green propolis is a high-value-added beekeeping product due to its medicinal properties. In Brazil, its main botanical source is the plant Baccharis dracunculifolia. The chemical composition of green propolis is complex and rich in phenolic compounds such as flavonoids and phenolic acids, including artepillin C, the main marker of this type of propolis. These compounds are known to exhibit antioxidant, anti-inflammatory, antimicrobial, and even antitumor activities. However, it is important to note that these effects have mainly been observed in studies conducted on other animal species or in in vitro models, rather than specifically in honey bees []. Although individual compounds may have beneficial effects on honey bee health, certain properties—such as antimicrobial activity—have only been observed when using whole propolis extracts, not isolated components. These interactions may enhance activity against bee pathogens, reinforcing the importance of considering the chemical complexity of propolis when evaluating its biological potential [].
The amount of protein patty consumed during the first week of adult life—when diets were free of both fungicide and propolis—was 84.3% higher in bees from supplemented colonies than in those from restricted colonies (Table 3). In another study with colonies maintained under nutritional management for 24 weeks, diet consumption declined in bees from both groups (fed diets without fungicide and propolis); however, food intake in bees from restricted colonies was 38.3% lower than in bees from supplemented colonies []. Although emphasis is typically placed on protein content in nutritional supplements, the contribution of lipids and other micronutrients also plays a role in early adult metabolic function, which continues to the forager stage []. In the presence of dietary stressors, however, the ability of adult bees to adjust their food intake based on prior nutritional experience appears to be limited.
Fungicide contamination consistently reduced protein patty consumption, regardless of larval nutrition. An exception was noted among bees from food-restricted (R) colonies that received propolis-supplemented syrup; in this group, only the fungicide-contaminated patty without propolis reduced intake. Interestingly, for bees from supplemented (S) colonies, the inclusion of propolis alone (in the absence of fungicide) reduced food intake, possibly due to aversive effects or the physiological burden of processing its phytochemicals—particularly in bees that had already accumulated nutritional reserves during larval stages. When both fungicide and propolis were included, intake remained low but was slightly higher than in the fungicide-only group, suggesting a modest mitigating effect of propolis. These results suggest that early-life nutrition influences adult feeding behavior, but xenobiotics in the adult diet may override developmental effects.
Oral exposure to Pristine, administered in a protein-based diet containing pollen, sucrose, and Baker’s Drivert sugar, led to reduced food intake in honey bees []. In that study, the fungicide was diluted in water and mixed into the patties at a sublethal dose, which reflected the residue levels found in pollen collected by foragers visiting almond blossoms, where this fungicide is applied during bloom. Similarly, the fungicide used in our experiment is recommended for application on flowering crops frequently visited by bees, such as soybean, sunflower, and cotton []. In our design, however, pollen was obtained directly from plants following fungicide application, aiming to simulate the most realistic possible contamination scenario, based on both dosage and strict adherence to agronomic guidelines. This exposure strategy helps reduce uncertainty regarding spray practices and offers a revised perspective on what constitutes a field-relevant exposure level, by accounting for the full range of agronomic procedures involved in pesticide preparation and application [].
Sugar syrup consumption was not significantly affected by the inclusion of propolis extract. Nevertheless, propolis intake via the syrup altered protein patty consumption. Turcatto et al. [] found that propolis extract added directly to protein patties (without honey or bee pollen) increased consumption. The amount of supplementation differed; the propolis concentration used in our study was approximately 11 times greater than in theirs. The adverse effects observed from propolis supplementation in our study may be related to the relatively high intake levels, which negatively impacted food consumption, altered the expression of certain genes, and reduced longevity in bees not exposed to the fungicide through protein food. Nevertheless, our findings provide insights for future studies aiming to optimize the amount of propolis extract offered to adult bees.
Regarding the expression of the antioxidant and detoxification genes, a general upregulation was observed in bees fed diets containing fungicide, compared to the control group (Figure 1). A similar pattern was reported in forager bees exposed by contact to each of the three components of the commercial fungicide used in this study, when applied at a dose of 1 μg/bee. However, at a higher dose (7 μg/bee), a significant suppression of gene expression was observed []. These exposure levels were established based on a study that estimated the amount of each active ingredient that may be deposited on a bee’s body during fungicide spraying in treated crops []. In another study using the same experimental design with forager bees exposed to the three active ingredients by contact, a decrease in the activity of the enzymatic antioxidant glutathione peroxidase (GPx) was observed, while catalase (CAT) activity remained unaffected. The concentrations of GSH, GSSG, and the redox state of pyridine nucleotides (NAD(P)H) were not altered by fungicide exposure. Nonetheless, oxidative stress was evidenced by an increase in malondialdehyde (MDA) levels following exposure to both 1 and 7 μg/bee []. In our current study, the fungicide suppressed the expression of vitellogenin, hexamerin, and immune-related antimicrobial peptide genes. Exposure to specific components, such as bixafen and trifloxystrobin, which are known inhibitors of cellular energy production, may impair gene expression by limiting the energy available for cellular responses (Figure 2). This constraint can compromise bees’ ability to cope with toxic stress, potentially increasing their vulnerability to additional environmental challenges []. Overall, propolis consumption was associated with upregulation of the genes, which may be due to the relatively high dose used in this experiment.
The effects observed in the variables can be better understood by evaluating bee longevity in relation to the nutritional history of the source colony and the presence of fungicide and/or propolis in the adult diet. Food restriction at the colony level, as well as the consumption of diets containing fungicide, negatively affected bee survival (Table 5). Adequate nutrition during both larval and adult stages is essential to enhance survival [,,,]. Although propolis negatively affected the survival of bees not exposed to the fungicide, its consumption helped mitigate the harmful impact of fungicide exposure on longevity, particularly in individuals from food-restricted colonies. When supplementing only with the phytochemicals p-coumaric acid and quercetin, Liao et al. [] observed increased longevity in bees exposed to the fungicide propiconazole and the insecticide chlorantraniliprole.
Propolis is a bee-derived product that promotes colony health by preventing the proliferation of harmful microorganisms, supporting social immunity [], and contributing to increased bee longevity []. Because immune system activation requires high energy expenditure [], propolis use may reduce the need for such activation in bees []. However, the provision of propolis, particularly in environments with limited availability of its botanical precursors, requires further study to determine appropriate supplementation methods and safe, effective dosages that confer benefits without causing toxicity.
5. Conclusions
Colony nutritional management during brood development significantly shaped key physiological traits and feeding behavior in adult honey bees. Bees from supplemented colonies emerged with higher body weight and hemolymph protein levels and showed increased protein food intake under favorable dietary conditions. This improved nutritional background also enhanced their physiological resilience and survival when later exposed to dietary stressors during adulthood.
Fungicide-contaminated pollen fed during adulthood severely compromised hemolymph protein levels, altered the expression of detoxification and immune-related genes, and reduced bee survival. Notably, even in the absence of fungicide, ingestion of green propolis aqueous extract affected gene expression and was associated with reduced longevity compared to bees fed a control diet free of both fungicide and propolis, suggesting a physiological cost linked to stress pathway activation.
However, under fungicide stress, the effect of propolis changed. Bees consuming both fungicide and propolis survived longer than those exposed to fungicide without propolis, indicating that propolis bioactive compounds may mitigate pesticide toxicity, potentially through upregulation of detoxification and antioxidant defenses.
Overall, the interaction between larval nutritional history and adult diet was central to modulating physiological responses and survival. While propolis ingestion alone may negatively affect bee health under non-stress conditions, its protective role against pesticide stress highlights its potential as a dietary supplement under field-relevant exposure scenarios. These findings emphasize the multifactorial nature of stressors affecting honey bee health and reinforce the need for integrated colony management strategies to improve resilience.
Author Contributions
Y.M.M.F.: conceptualization, validation, investigation, resources, data curation. A.Y.K.: investigation, resources, data curation. T.A.d.L.F.: investigation, resources, data curation. C.R.d.A.G.: investigation, resources, data curation. T.R.R.A.: investigation, resources, data curation. M.F.T.: investigation, resources, data curation. S.M.K.: writing—original draft, writing—review and editing. R.d.O.O.: writing—original draft, writing—review and editing. D.D.J.: conceptualization, writing—original draft, writing—review and editing. J.D.B.: conceptualization, validation, writing—review and editing. D.N.: conceptualization, formal analysis, writing—original draft, writing—review and editing, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (process no. 2021/00702-1) to Daniel Nicodemo and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)-Finance Code 001 to Yara Martins Molina Ferraz.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.M.; Kremen, C.; M’Gonigle, L.K.; Rader, R.; et al. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 7414. [Google Scholar] [CrossRef]
- Hung, K.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef]
- Phiri, B.J.; Fèvre, D.; Hidano, A. Uptrend in global managed honey bee colonies and production based on a six-decade viewpoint, 1961–2017. Sci. Rep. 2022, 12, 21298. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.; Bartomeus, I.; Simpson, D.; Allen-Perkins, A.; Garibaldi, L.; Winfree, R. Wild insects and honey bees are equally important to crop yields in a global analysis. Glob. Ecol. Biogeogr. 2024, 33, e13843. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2021, 232, 8–27. [Google Scholar] [CrossRef]
- Aurell, D.; Bruckner, S.; Wilson, M.; Steinhauer, N.; Williams, G.R. A national survey of managed honey bee colony losses in the USA: Results from the Bee Informed Partnership for 2020–21 and 2021–22. J. Apic. Res. 2023, 63, 1–14. [Google Scholar] [CrossRef]
- Castle, D.; Alkassab, A.T.; Bischoff, G.; Steffan-Dewenter, I.; Pistorius, J. High nutritional status promotes vitality of honey bees and mitigates negative effects of pesticides. Sci. Total Environ. 2023, 806, 151280. [Google Scholar] [CrossRef]
- Rudelli, C.; Galuppi, R.; Cabbri, R.; Dalmonte, T.; Fontanesi, L.; Andreani, G.; Isani, G. Field application of an innovative approach to assess honeybee health and nutritional status. Animals 2024, 14, 2183. [Google Scholar] [CrossRef]
- Smart, M.D.; Otto, C.R.V.; Lundgren, J.G. Nutritional status of honey bee (Apis mellifera L.) workers across an agricultural land-use gradient. Sci. Rep. 2019, 9, 16252. [Google Scholar] [CrossRef]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.; Huang, E.; Huang, M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect Physiol. 2010, 56, 1184–1191. [Google Scholar] [CrossRef]
- Corona, M.; Branchiccela, B.; Alburaki, M.; Palmer-Young, E.C.; Madella, S.; Chen, Y.; Evans, J.D. Decoupling the effects of nutrition, age, and behavioral caste on honey bee physiology, immunity, and colony health. Front. Physiol. 2023, 14, 1149840. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, A.; Schmid, A.P.; Manzer, S.; Schulte, J.; Scheiner, R. Interaction of insecticides and fungicides in bees. Front. Insect Sci. 2022, 1, 808335. [Google Scholar] [CrossRef]
- Rondeau, S.; Raine, N.E. Fungicides and bees: A review of exposure and risk. Environ. Int. 2022, 165, 107311. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Impacts of pesticides on honey bees. In Beekeeping and Bee Conservation—Advances in Research; InTech: Rijeka, Croatia, 2016; pp. 77–97. [Google Scholar]
- Bayer. Fox® Xpro: Fungicida; Bayer, S.A.: São Paulo, Brasil, 2019; Available online: https://www.agro.bayer.com.br/d/fungicida-bcs-fox-xpro-br (accessed on 28 June 2025).
- IBAMA. Vendas de ingredientes ativos por unidade da federação. In Relatórios de Comercialização de Agrotóxicos; IBAMA: Brasília, Brazil, 2024. [Google Scholar]
- Ernst, G.; Agert, J.; Heinemann, O.; Hellpointner, E.; Gladbach, A. Realistic exposure of the fungicide bixafen in soil and its toxicity and risk to natural earthworm populations after multiyear use in cereal. Integr Environ Assess Manag 2022, 18, 734–747. [Google Scholar] [CrossRef]
- Feng, Y.; Qi, X.; Wang, X.; Liang, L.; Zuo, B. Residue Dissipation and Dietary Risk Assessment of Trifloxystrobin, Trifloxystrobin Acid, and Tebuconazole in Wheat under Field Conditions. Int. J. Environ. Anal. Chem. 2022, 102, 1598–1612. [Google Scholar] [CrossRef]
- López-Ballesteros, A.; Delaney, A.; Quirke, J.; Stout, J.C.; Saunders, M.; Carolan, J.C.; White, B.; Stanley, D.A. Assessing Availability of European Plant Protection Product Data: An Example Evaluating Basic Area Treated. PeerJ 2022, 10, e13586. [Google Scholar] [CrossRef] [PubMed]
- Turcatto, A.P.; Lourenço, A.P.; De Jong, D. Propolis consumption ramps up the immune response in honey bees infected with bacteria. Apidologie 2018, 49, 287–296. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Ewert, A.M.; Simone-Finstrom, M.; Read, Q.; Husseneder, C.; Ricigliano, V. Effects of ingested essential oils and propolis extracts on honey bee (Hymenoptera: Apidae) health and gut microbiota. J. Insect Sci. 2023, 23, iead087. [Google Scholar] [CrossRef]
- Kato, A.Y.; Freitas, T.A.L.; Gomes, C.R.A.; Alves, T.R.R.; Ferraz, Y.M.M.; Trivellato, M.F.; De Jong, D.; Biller, J.D.; Nicodemo, D. Bixafen, prothioconazole, and trifloxystrobin alone or in combination have a greater effect on health-related gene expression in honey bees from nutritionally deprived than from protein-supplemented colonies. Insects 2024, 15, 523. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- OECD. Test No. 213: Honeybees, Acute Oral Toxicity Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 1998. [Google Scholar]
- Cantarella, H.; Mattos, D., Jr.; Boaretto, R.M.; Quaggio, J.A.; Raij, B.V. Recomendações de Adubação e Calagem para o Estado de São Paulo, 2nd ed.; Instituto Agronômico: Campinas, Brazil, 2022. [Google Scholar]
- Borsuk, G.; Ptaszyńska, A.A.; Olszewski, K.; Domaciuk, M.; Krutmuang, P.; Paleolog, J. A new method for quick and easy hemolymph collection from Apidae adults. PLoS ONE 2017, 12, e0170487. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Cremonez, T.M.; De Jong, D.; Bitondi, M.M.G. Quantification of hemolymph proteins as a fast method for testing protein diets for honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 1998, 91, 1284–1289. [Google Scholar] [CrossRef]
- Lourenço, A.P.; Mackert, A.; Cristino, A.S.; Simões, Z.L.P. Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie 2008, 39, 372–385. [Google Scholar] [CrossRef]
- Corona, M.; Hughes, K.A.; Weaver, D.B.; Robinson, G.E. Gene expression patterns associated with queen honey bee longevity. Mech. Ageing Dev. 2005, 126, 1230–1238. [Google Scholar] [CrossRef]
- Chaimanee, V.; Evans, J.D.; Chen, Y.; Jackson, C.; Pettis, J.S. Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J. Insect Physiol. 2016, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Siede, R.; Meixner, M.D.; Büchler, R. Comparison of transcriptional changes of immune genes to experimental challenge in the honey bee (Apis mellifera). J. Apic. Res. 2012, 51, 320–328. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Moran, N.A.; Evans, J.D. Early gut colonizers shape parasite susceptibility and microbiota composition in honey bee workers. Proc. Natl. Acad. Sci. USA 2016, 113, 9345–9350. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, E.D.L.; Costa, J.J.D.N.; Passos, M.J.; Passos, J.R.D.S.; Hurk, R.V.D.; Silva, J.R.V. Real time PCR and importance of housekeeping genes for normalization and quantification of mRNA expression in different tissues. Braz. Arch. Biol. Technol. 2013, 56, 143–154. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS OnDemand for Academics, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2024; Available online: https://www.sas.com/pt_br/software/on-demand-for-academics.html (accessed on 21 June 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 25 June 2025).
- Scofield, H.N.; Mattila, H.R. Honey bee workers that are pollen stressed as larvae become poor foragers and waggle dancers as adults. PLoS ONE 2015, 10, e0121731. [Google Scholar] [CrossRef]
- Nicholls, E.; Rossi, M.; Niven, J.E. Larval nutrition impacts survival to adulthood, body size and the allometric scaling of metabolic rate in adult honeybees. J. Exp. Biol. 2021, 224, jeb242393. [Google Scholar] [CrossRef]
- Noordyke, E.R.; Ellis, J.D. Reviewing the Efficacy of Pollen Substitutes as a Management Tool for Improving the Health and Productivity of Western Honey Bee (Apis mellifera) Colonies. Front. Sustain. Food Syst. 2021, 5, 772897. [Google Scholar] [CrossRef]
- Bryś, M.S.; Strachecka, A. The Key Role of Amino Acids in Pollen Quality and Honey Bee Physiology—A Review. Molecules 2024, 29, 2605. [Google Scholar] [CrossRef]
- Palmer-Young, E.C.; Farrell, I.W.; Adler, L.S.; Milano, N.J.; Egan, P.A.; Irwin, R.E.; Stevenson, P.C. Secondary metabolites from nectar and pollen: A resource for ecological and evolutionary studies. Ecology 2019, 100, e02621. [Google Scholar] [CrossRef] [PubMed]
- Ardalani, H.; Vidkjær, N.H.; Kryger, P.; Fiehn, O.; Fomsgaard, I.S. Metabolomics unveils the influence of dietary phytochemicals on residual pesticide concentrations in honey bees. Environ. Int. 2021, 152, 106503. [Google Scholar] [CrossRef]
- Torres, D.J.; Ricoy, U.M.; Roybal, S. Modeling honey bee populations. PLoS ONE 2015, 10, e0130966. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Williams, S.T.; Oliver, R. Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Vet. Res. 2022, 18, 52. [Google Scholar] [CrossRef]
- Schmidt, J.O.; Thoenes, S.C.; Levin, M.D. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. J. Econ. Entomol. 1987, 80, 176–183. [Google Scholar] [CrossRef]
- Radev, Z. Pollen protein content from different regions in Bulgaria suggests low variability. Bee World 2019, 96, 108–110. [Google Scholar] [CrossRef]
- Nicolson, S.W.; Human, H. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 2013, 44, 144–152. [Google Scholar] [CrossRef]
- Schilcher, F.; Hilsmann, L.; Ankenbrand, M.J.; Krischke, M.; Mueller, M.J.; Steffan-Dewenter, I.; Scheiner, R. Honeybees are buffered against undernourishment during larval stages. Front. Insect Sci. 2022, 2, 951317. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.; Chahal, K.; DeGrandi-Hoffman, G.; Smith, B.H.; Fewell, J.H.; Harrison, J.F. Exposure to a widely used mito-toxic fungicide negatively affects hemolymph protein and vitellogenin levels in honey bees (Apis mellifera). Environ. Toxicol. Pharmacol. 2025, 115, 104676. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Chen, Y.; DeJong, E.W.; Chambers, M.L.; Hidalgo, G. Effects of oral exposure to fungicides on honey bee nutrition and virus levels. J. Econ. Entomol. 2015, 108, 2518–2528. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Borba, R.S.; Wilson, M.; Spivak, M. Propolis Counteracts Some Threats to Honey Bee Health. Insects 2017, 8, 46. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Calla, B. Honey as a Functional Food for Apis mellifera. Annu. Rev. Entomol. 2021, 66, 185–208. [Google Scholar] [CrossRef]
- Guimarães, N.S.S.; Mello, J.C.; Paiva, J.S.; Bueno, P.C.P.; Berretta, A.A.; Torquato, R.J.; Nantes, I.L.; Rodrigues, T. Baccharis dracunculifolia, the main source of green propolis, exhibits potent antioxidant activity and prevents oxidative mitochondrial damage. Food Chem. Toxicol. 2012, 50, 1091–1097.59. [Google Scholar] [CrossRef] [PubMed]
- Mihai, C.M.; Mărghitaş, L.A.; Dezmirean, D.S.; Chirilă, F.; Moritz, R.F.; Schlüns, H. Interactions among flavonoids of propolis affect antibacterial activity against the honeybee pathogen Paenibacillus larvae. J. Invertebr. Pathol. 2012, 110, 68–7260. [Google Scholar] [CrossRef]
- Alves, T.R.R.; Trivellato, M.F.; Freitas, T.A.L.; Kato, A.Y.; Gomes, C.R.A.; Ferraz, Y.M.M.; Serafim, J.A.; De Jong, D.; Prado, E.P.; Vicente, E.F.; et al. Pollen contaminated with a triple-action fungicide induced oxidative stress and reduced longevity though with less impact on lifespan in honey bees from well fed colonies. Environ. Toxicol. Pharmacol. 2024, 112, 104587. [Google Scholar] [CrossRef]
- Freitas, T.A.L.; Kato, A.Y.; Gomes, C.R.A.; Alves, T.R.R.; Ferraz, Y.M.M.; Serafim, J.A.; Silva, M.A.G.; De Jong, D.; Prado, E.P.; Vicente, E.F.; et al. Contact exposure of honey bees and social stingless bees to fungicide sprayed on cotton and soybean in a controlled field simulation system. J. Appl. Entomol. 2024, 148, 861–869. [Google Scholar] [CrossRef]
- Freitas, T.A.L.; Alves, T.R.R.; Trivellato, M.F.; Kato, A.Y.; Gomes, C.R.A.; Ferraz, Y.M.M.; Vicente, E.F.; De Jong, D.; Miranda, C.A.; Mingatto, F.E.; et al. Food supplementation does not prevent oxidative stress in forager honey bees exposed to the fungicides bixafen, prothioconazole and trifloxystrobin. J. Apic. Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.W.; Zeng, Z.J.; Yan, W.Y. Nutrition affects longevity and gene expression in honey bee (Apis mellifera) workers. Apidologie 2014, 45, 618–625. [Google Scholar] [CrossRef]
- Liao, L.H.; Pearlstein, D.J.; Wu, W.Y.; Kelley, A.G.; Montag, W.M.; Hsieh, E.M.; Berenbaum, M.R. Increase in longevity and amelioration of pesticide toxicity by natural levels of dietary phytochemicals in the honey bee, Apis mellifera. PLoS ONE 2020, 15, e0243364. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M.; Warner, J.F.; Sommerfeldt, B.A.; Spivak, M. Social Fever or General Immune Response? Revisiting an Example of Social Immunity in Honey Bees. Insects 2020, 11, 528. [Google Scholar] [CrossRef]
- Nicodemo, D.; Malheiros, E.B.; De Jong, D.; Couto, R.H.N. Increased brood viability and longer lifespan of honeybees selected for propolis production. Apidologie 2014, 45, 269–275. [Google Scholar] [CrossRef]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.-L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Borba, R.S.; Klyczek, K.K.; Mogen, K.L.; Spivak, M. Seasonal benefits of a natural propolis envelope to honey bee immunity and colony health. J. Exp. Biol. 2015, 218, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).