Abstract
This study evaluated the chemical properties of phosphocompost extracts and their effectiveness in inducing tomato seedlings resistance to Meloidogyne javanica. Phosphocomposts: Sugar beet phosphocompost (PC-SB: CP2), green waste phosphocompost (PC-GW: CP3), and olive mill waste phosphocompost (PC-OMW: CP4), were utilized to produce compost water extracts at concentrations of 1:5, 1:10, 1:20, and 1:100 g:mL and then applied as soil drenches for tomato seedlings one-week post-inoculation. The CP2 extract applied at a 1:5 dilution led to marked improvements in growth parameters, with plant height increasing by over 52.2%, shoot fresh biomass rising by approximately 52.44%, and shoot dry biomass showing a gain of 62.21%. Root biomass also rose by 33%. Chlorophyll a increased with CP4 at 1:5 and 1:100 (41.05% and 37.32%), chlorophyll b increased with CP3 at 1:5 and 1:10 (22.34% and 7.59%), while carotenes showed no variation. Polyphenols rose by 86.45–91.01% with CP2 from 1:5 to 1:20, and flavonoids increased by 64.90% with CP4 at 1:10. CP2 diminished the ultimate M. javanica population and reproduction factor by 171.43%, while CP4 at 1:20 decreased egg masses by 151.94%. The root gall index showed no variation. The chemical composition of phosphocomposts revealed that the strategic incorporation of diverse organic improvers (10%) in phosphocomposts yielded distinct nutrient signatures, with sugar beet waste enhancing PO43− (12.91 mg/L) and secondary macronutrients, green waste optimizing NO3− (69.91 mg/L) and SO42− (62.70 mg/L) availability, and olive mill waste producing superior micronutrient concentrations alongside dominant Ca (24.21 mg/L), K (392.50 mg/L), and P (9.17 mg/L) levels. Overall, the results underscore the potential of phosphocompost extracts as a viable, low-cost, and eco-friendly alternative to synthetic nematicides, offering a sustainable and resilient approach to M. javanica control while enhancing tomato plant growth.
1. Introduction
The 21st century presents previously unheard-of difficulties for global agriculture. Arable land has been drastically reduced by global warming, climate change, and rapid urbanization [1], and crop pests and illnesses are becoming more common, endangering worldwide food security. Many agricultural soils have been extensively deteriorated by years of intensive chemical usage, despite efforts to maintain native grasslands and enhance soil quality [2]. Our ability to feed the world’s expanding population is seriously threatened by these interrelated problems [3], which call for creative and sustainable agricultural approaches [4].
The tomato crop (Solanum lycopersicum L.) exemplifies both the opportunities and challenges of smart farming technologies, ranking as the world’s second most important vegetable crop after potato [5]. Global tomato production has surged over the past three decades, reaching 186.82 million tons across 5.05 million hectares in more than 165 countries by 2021 [6]. Morocco, which produces 1.31 million tons of tomatoes annually, has become a significant contributor to this global output [7]. In 2021, Morocco’s tomato business generated USD 327.758 million [8]. However, this crop faces a formidable threat from Meloidogyne spp., referred to as root-knot nematodes (RKNs) [9,10,11,12,13]. RKNs are minute parasites that live in the soil. They infiltrate plant roots and create intricate feeding sites, called giant cells and knots. The morphology and physiology of the root system are profoundly compromised by the deleterious effects of parasitic infestation, resulting in wilting, stunted development, and yield losses [14,15]. These pests are acknowledged as significant risks to tomato farming globally, even if the precise global impact of RKNs on tomato production varies based on variables including climate, soil type, and management approaches [10,13]. Historically, farmers have relied mostly on chemical treatments, which are associated with environmental and health concerns, notwithstanding their partial effectiveness [16]. Reduced biodiversity, chemical residues in food, and unsustainable reliance on non-renewable resources are examples of their adverse impacts [17]. Agricultural research is now focused on more cost-effective and eco-friendlier sustainable pest management techniques to attenuate these impacts.
In this vein, composting has become a viable remedy. In addition to recycling organic waste into nutrient-rich soil amendments, this biotechnology has several environmental advantages such as reducing greenhouse gas emissions, carbon sequestration, and waste disposal [18,19,20,21]. The application of compost has several advantages, including enhancing the inherent disease-suppressive qualities of the soil, improving soil health, and providing environmental benefits [22]. Recent relevant studies have demonstrate that compost is beneficial for controlling a variety of plant diseases, thereby reducing agricultural losses [16,23,24,25]. The varied microbial populations of compost are primarily responsible for its effectiveness [26]. According to Mehta et al. [27], these microbial consortia use direct antagonistic mechanisms against plant diseases such as resource competition, antibiotic compound synthesis, and hyperparasitism. Additionally, as described in a recent review by Bouchtaoui et al. [22], compost activates metabolic defense pathways that improve resistance to infections. This multifaceted strategy highlights the potential of compost as a long-term solution for managing plant diseases and boosting the agricultural output. Otherwise, recent research has reported that phosphocomposting further enhances microbial activity by improving the nutrient balance compared to conventional composting, owing to the inclusion of phosphate mineral sources (such as phosphate rock or its derivatives) [4,28,29]. Furthermore, phosphate-solubilizing microbiota, encompassing prokaryotic and eukaryotic organisms found in phosphocomposts, can enhance the bioavailable phosphorus concentrations within the rhizospheric matrix by mobilizing soluble phosphatic compounds, such as tricalcium phosphate, which improves phosphorus bioavailability [30,31,32]. Despite the demonstrated efficacy of phosphate-enriched compost in several agricultural applications [33,34], its capacity for pathogenic nematode management and the promotion of plant development, especially via aqueous extracts, is yet to be investigated.
Phosphocompost extracts, which are nutrient-rich solutions containing beneficial microorganisms [35], have gained significant attention for their potential to expand compost utilization within the framework of a circular bio-based economy that prioritizes environmental sustainability and resource efficiency [35,36,37]. These extracts are becoming increasingly important in modern agricultural and horticultural practices owing to their ability to promote plant growth and enhance resistance to a range of soil-borne diseases [35]. Notably, compost extracts highlight their promise in improving plant development and providing protection against RKNs, when applied as foliar sprays or soil drenches [22]. Additionally, compost extracts offer stronger, sustainably cost-effective alternatives to traditional methods. By requiring smaller quantities of compost to produce a highly concentrated liquid rich in water-soluble nutrients and beneficial microconsortia, they not only enhance plant health and the fertility of soil but also eliminate toxic compounds. This makes compost extracts a safer and more efficient substitute to chemical nematicides. In light of this goal, this investigative effort was initiated to assess the efficacy of innovatively phosphocompost extracts obtained from novel formulations against Meloidogyne javanica and using tomato as the host to evaluate their efficacy in promoting tomato growth and physiological functions under infection while hindering the proliferation of M. javanica. This will help in addressing a significant gap within the scientific and practical communities.
2. Material and Methods
2.1. Phosphocompost Source
The compost formulations used in this study were recently developed by Haouas et al. (2021) [4], and their compositions are detailed below:
CP2: 20% Phosphate Sludge + 70% Food Waste + 10% Sugar Beet Waste
CP3: 20% Phosphate Sludge + 70% Food Waste + 10% Green Waste
CP4: 20% Phosphate Sludge + 70% Food Waste + 10% Olive Mill Waste
2.2. Phosphocompost Chemical Composition
Available elemental concentrations in phosphocompost extracts were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES; Thermo iCAP 6500 DUO), while anion analysis was performed via ion chromatography (Metrohm, Switzerland).
2.3. Preparation of M. javanica Inoculum
Newly hatched M. javanica second-stage juveniles (J2s) came from a single egg mass and were multiplied in tomato roots under controlled greenhouse conditions at the Biotechnology Research Unit, INRA-Rabat. After a 90-day period of incubation, eggs were obtained from the roots via the technique of Hussey and Baker [38]. The collected eggs were cultured at 25 °C for four days to enable hatching.
2.4. Preparation of Phosphocompost Extracts
To prepare the phosphocompost extracts, raw phosphocompost was blended with distilled water at different proportions. Four distinct ratios were used: 1:5, 1:10, 1:20, and 1:100 (g:mL), which were designated as treatments T1, T2, T3, and T4, respectively.
The phosphocompost and water mixtures were kept under continuous stirring for a 24-h period to ensure thorough mixing. Following this agitation phase, the mixtures were filtered to obtain the final extracts. These freshly prepared extracts were then directly utilized in the subsequent experimental procedures, as detailed in the forthcoming sections.
2.5. Preparation of Tomato Plants
Tomato seeds (S. lycopersicum cv. Campbell 33) were planted in cell trays containing peat, and each seed was placed in an individual cell. The cell trays were then positioned in the greenhouse under regulated conditions, including a 16-h light and 8-h dark cycle, along with 75% humidity. Seedlings were meticulously watered twice daily. After the seedlings produced two true leaves, they were used in the greenhouse experiment.
2.6. Greenhouse Experiment and Design
At the two-true-leaf stage of growth, tomato seedlings were transferred to pots measuring 20 cm in height and 15 cm in diameter. The pots contained a mixture of 2.5 kg sterile soil and peat in a 3:1 weight ratio. A day after transplanting, the tomato plants were infected with 150 J2s of M. javanica per 100 g of soil.
One week post-inoculation, plants received 300 mL of phosphocompost extracts at various dilutions (1:5, 1:10, 1:20, and 1:100 g:mL). Control plants (T0) without compost extract received 300 mL of distilled water. The one-week infection delay before treatment aimed to mimic real-world conditions where nematode infestations are frequently identified after root invasion has commenced and treatments are administered post-infection in order to evaluate the efficacy of phosphocompost extracts in limiting nematode proliferation and enhancing plant resilience in practical, post-infestation situations. The experiment followed a CRD with three replicates per phosphocompost extract concentration and was conducted twice.
The infected and treated plants were incubated in the greenhouse under regulated conditions of 16-h light and 8-h dark periods with 75% humidity. The compost extracts were administered in a single application, and plants received irrigation as required during the 90-day incubation, aligning with the standard physiological maturity timeline for tomato crops grown in greenhouses. This extended period facilitated a thorough assessment of plant development, physiological characteristics, and nematode population dynamics spanning the complete crop cycle.
2.7. Growth of Tomato Plants Infected with M. javanica Under Phosphocompost Extract Treatments
Then, 90-days post-inoculation and phosphocompost extract treatments, the tomato plants were evaluated for morphological responses. This assessment involved measuring plant height, shoot fresh and dry weights, root length, and root weight to measure the influence of phosphocompost extract treatments on tomato morphological development under nematode infection. The gall index, which is an indicator of nematode infection severity, was assessed using the Taylor method [39]. This approach uses a scale from 0 to 5, with 0 signifying no galls and 1 denoting the appearance of 1-2 galls on roots. The scores of 2, 3, 4, and 5 indicate the existence of 3–10, 11–30, 31–100, and surpassing 100 galls, respectively.
2.8. Final Population and Reproduction of M. javanica Under Phosphocompost Extract Treatments
To measure M. javanica final population density in the soil, the contents of each pot were thoroughly mixed, and a representative subsample of 100 g was collected. The nematodes were extracted from the soil samples using the Baermann funnel technique, with three replicate extractions performed for each sample. The nematode abundance was quantified and reported as the mean number of J2s per 100 g of soil. The RF was calculated to evaluate the proliferation capacity of M. javanica under the given experimental conditions. The RF was calculated using the formula proposed by Taylor [39], as follows:
RF = Final population/Initial population
The population growth rate (PGR) of M. javanica was calculated using the following formula:
where CPf: final population in the control treatment and TPf: final population in the treated plants
PGR = (CPf − TPf)/CPf
2.9. Photosynthetic Pigments
To evaluate the quantities of photosynthetic pigments, mature tomato leaves (1 g) from the middle to the top sections of plants cultivated under different phosphocompost extract concentrations were triturated in a mortar with 8 mL of methanol (90:10 v/v) for 1 min. The mixture was centrifuged at 6440× g at 4 °C for 5 min, as described by Machado et al. [40]. The supernatant was collected to quantify the content of chlorophyll a, chlorophyll b, and carotene, following the methodology described by Lichtenthaler and Buschmann [41]. Three replicates were performed for each compost concentration. Chlorophyll and carotene contents were quantified using the equations established by Lichtenthaler and Buschmann [41].
Chlorophyll a (μg/mL) = 16.82 × A665.2 − 9.28 × A652.4;
Chlorophyll b (μg/mL) = 36.92 × A652.4 − 16.54 × A665.2;
Carotenes (μg/mL) = (1000 × A470 − 1.91 × Chl a − 95.15 × Chl b)/225,
2.10. Secondary Metabolites
To evaluate the total phenolic compounds (TPCs) and total phenolic contents (TFCs), 1 g of mature tomato leaves was pulverized in a mortar using 90% methanol. The solution was then centrifuged at 6400× g at 4 °C for 5 min. The resultant supernatant was used in future tests to determine TPC and TFC concentrations in tomato leaves.
The quantitative evaluation of phenolic compounds in tomato leaves was performed using a colorimetric assay based on redox reactions, following a protocol modified from Singleton et al. [42]. In this method, 200 µL of plant extract from each replicate was combined with 1 mL of Folin–Ciocalteu phenol reagent (pre-diluted 1:10, v/v) and 0.8 mL of sodium carbonate solution (7.5%, w/v) [42]. The phenolic content in the tomato foliage samples was quantified and presented as milligrams of gallic acid equivalents (GAEs) per gram of fresh tissue. These values were determined by comparing the optical density readings to a standard curve with a strong linear correlation (R2 = 0.999). To ensure the reliability of the results, the analyses were carried out in triplicate for each treatment group. Furthermore, the entire assay procedure was repeated to confirm the consistency and reproducibility of the findings.
The TFC was quantified using the methodology outlined by Santas et al. [43], which entails the creation of a compound with aluminum chloride (AlCl3). In this process, 1 mL of sample suspension was combined with 1 mL of 2% AlCl3 in a methanolic solution. The mixture was then incubated at ambient temperature for 10 min and the absorbance was recorded at 410 nm relative to a blank sample. The TFC was determined by a quercetin calibration curve (R2 = 0.998), with findings reported as mg quercetin equivalent (QE) per gram of FW. All measurements were conducted in triplicate to ensure dependability, and the experiment was repeated twice.
2.11. Statistical Analysis
Statistical evaluation encompassed both morphological and physiological plant traits, and nematode population dynamics and reproductive M. javanica metrics. Data analyses were conducted using R software (v4.4.1), with all measurements performed in triplicate and each experimental setup independently repeated to ensure reproducibility. Where necessary, data transformations were applied to meet assumptions of normality and variance homogeneity. One-way analysis of variance (ANOVA) was employed to detect significant differences among compost extract concentrations and treatment groups. Subsequent pairwise comparisons were performed using Fisher’s least significant difference (LSD) test at a 5% significance threshold (p < 0.05). For variables violating parametric assumptions—specifically the gall index—a non-parametric Kruskal–Wallis test was implemented. Graphical representations of the data were generated using OriginPro 2024 (OriginLab Corp., Northampton, MA, USA).
3. Results
3.1. Compost Chemical Composition
The analysis of the macronutrients in the phosphocomposts revealed that they contain substantial levels of primary (P and K) and secondary macronutrients (Mg, S, Ca, and Na) (Table 1). CP2 had the highest concentrations of Mg (7.04 mg/L), S (34.72 mg/L), and Na (128.20 mg/L), whereas CP4 exhibited the greatest levels of Ca, K, and P, measuring 24.21, 392.50, and 9.17 mg/L, respectively. Among all the treatments, CP3 showed the lowest concentrations, with the exception of calcium, which exhibited a relatively high concentration of 15.38 mg/L.

Table 1.
Macronutrient composition of phosphocomposts.
Table 2 indicates that CP4 exhibited a higher availability of micronutrients compared to CP2 and CP3, with concentrations of Fe, B, Zn, Cu, and Ni measured at 2.44, 0.60, 0.15, 0.12, and 0.09 mg/L, respectively. CP2 exhibited the highest concentration of Mn at 0.07 mg/L, whereas CP3 was characterized by a predominant Mo concentration of 0.06 mg/L.

Table 2.
Micronutrient composition of phosphocomposts.
The trace element values of the compost samples are illustrated in Table 3. The Si element had the highest concentrations, which were between 2.60 and 4.39 mg/L in CP2 and CP4. Al was detected in all composts with the concentration ranging between 0.83 and 0.95 mg/L (for CP2 and CP4). V steadily increased from CP2 (0.09 mg/L) to CP4 (0.39 mg/L). Rb was present at variable concentrations (0.03–0.13 mg/L) across all composts. The remaining trace elements, including As, Be, Bi, Cd, Co, La, Li, Pb, Sb, Se, and thallium (Tl) were mostly below the detection threshold (<0.01 mg/L) in the majority of composts, with the exception of As (0.02 mg/L) and Tl (0.01 mg/L) in CP4.

Table 3.
Trace element composition of phosphocomposts.
Table 4 shows the anionic composition of phosphocompost samples. CP2 showed the highest amount of Cl− (187.64 mg/L) and PO43− (12.91 mg/L) while exhibiting very low F− and NO3− contents (below 0.02 mg/L and 1.0 mg/L, respectively). CP3 exhibited a high content of NO3− (69.91 mg/L) and SO42− (62.70 mg/L) but it had a lower PO43− content (2.20 mg/L) compared to the other composts. CP4 showed a greater concentration of F− (2.02 mg/L), a medium level of PO43− (6.79 mg/L), and lower contents of Cl− and NO3−, showing 55.61 mg/L and 2.23 mg/L, respectively. Br− showed a decreasing trend from CP2 to CP4. NO2− was not detectable in any of the three phosphocomposts.

Table 4.
Anion composition of phosphocomposts.
3.2. Comparison of Phosphocompost Extracts’ Effect on Plant Development and Pathogen Control in Tomato
3.2.1. Effect of Phosphocompost Extracts on the Morphological Development of Tomato Plants Under M. javanica Infection
Figure 1 depicts the effects of various phosphocompost extract compositions and concentrations (1:5, 1:10, 1:20, and 1:100 g:mL) on the morphological growth of tomato plants infected with M. javanica.
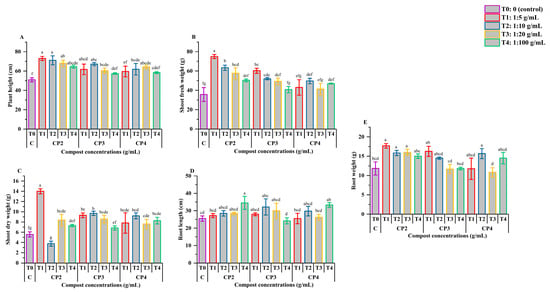
Figure 1.
Effect of phosphocompost extract composition and concentrations on morphological development, including root weight (A), root length (B), shoot dry weight (C), shoot fresh weight (D), and plant height (E) in tomato plants infected with Meloidogyne javanica. Data are presented as mean ± standard error (SE). Bars followed by the same lowercase letters indicate no significant difference according to ANOVA followed by Fisher’s least significant difference (LSD) test.
As illustrated in Figure 1A, the root weight exhibited minimal responses to both the nematode infection and the application of compost extracts. However, significant effects (p < 0.05) were observed with CP2 at 1:5, 1:10, and 1:20, CP3 at 1:5, and CP4 at 1:10 compared to the control, with improvements ranging from 25.26% to 33.05%. In contrast, root length (Figure 1B) was only mildly influenced by the infection and compost extract treatments. CP2 at 1:100 and CP4 at 1:100 demonstrated notable increases (p < 0.05) of 26.08% and 23.49%, respectively, compared to the control, although no significant difference was found between these two treatments (p > 0.05). The remaining treatments showed no statistically significant effects when compared to the control (p > 0.05).
The most substantial increase in shoot dry biomass (Figure 1C) was induced by CP2 at a 1:5 dilution, surpassing the control and other treatments by 60.21%, with the difference being statistically significant (p < 0.05). Apart from CP2 at 1:5, none of the treatments led to statistically significant enhancements in shoot fresh weight compared to the control (p > 0.05). The most pronounced effect on shoot fresh biomass was observed with CP2 at 1:5, resulting in a 52.44% increase, which was significantly different from both the control and all other treatment groups (p < 0.05) (Figure 1D). Treatments such as CP2 at 1:10, 1:20, and CP3 at 1:5 did not significantly differ from the control values (p > 0.05), suggesting limited effectiveness at these concentrations.
Plant height (Figure 1E) exhibited variable responses to the compost extract treatments. The most notable increase, approximately 52.44% compared to the control (p < 0.05), was observed with CP2 at 1:5 and 1:10. These two concentrations did not significantly differ from each other or from CP2 at 1:20 and 1:100, CP3 at 1:5 and 1:10, and CP4 at 1:20 (p > 0.05), indicating a plateau effect at these levels. A significant increase in height was observed for most treatments compared to the control, with the exception of CP3 at 1:100 and CP4 at both 1:5 and 1:100, which showed no significant difference (p > 0.05).
3.2.2. Effect of Phosphocompost Extracts on Photosynthetic Activity of Tomato Plants Under M. javanica Infection
Figure 2 shows the effect of M. javanica infection and phosphocompost treatments on the photosynthetic pigment profiles in tomato leaves. For chlorophyll a (Figure 2A), notable increases were observed under CP4 at 1:5 and 1:100, with pigment concentrations increasing from 0.89 μg/mL in the control to 1.52 and 1.42 μg/mL, respectively. These enhancements were statistically significant compared to the control (p < 0.05), although they did not significantly differ from most other treatments, with the exception of CP2 at 1:10. None of the other treatment groups induced significant changes in chlorophyll a levels when compared to the control.
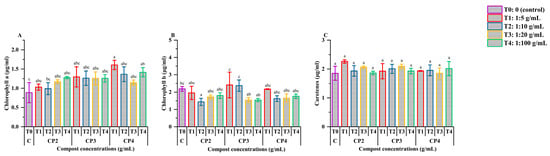
Figure 2.
Effect of phosphocompost extract composition and concentration on the development of photosynthetic pigments, including chlorophyll a (A), chlorophyll b (B), and carotenes (C), in tomato plants infected with Meloidogyne javanica. Data are presented as mean ± standard error (SE). Bars followed by the same lowercase letters indicate no significant difference according to ANOVA followed by Fisher’s least significant difference (LSD) test.
In the case of chlorophyll b (Figure 2B), the highest concentrations were found in plants treated with CP3 at 1:5 and 1:10 (2.82 and 2.37 μg/mL, respectively); however, these values were not statistically different from the control (2.19 μg/mL). In contrast, CP2 at 1:10 resulted in a significant decrease in chlorophyll b content, reaching 1.44 μg/mL, which was statistically different from the control (p < 0.05).
Carotenoid levels (Figure 2C) remained relatively stable across all treatments, with no statistically significant differences observed compared to the control values (p > 0.05).
3.2.3. Effect of Phosphocompost Extracts on Secondary Metabolites Synthesis of Tomato Plants Under M. javanica Infection
Figure 3 depicts the influence of M. javanica infection and phosphocompost treatments on the production of secondary metabolites, namely total phenolic content (TPC) and total flavonoid content (TFC). In the case of TPC (Figure 3A), CP2 treatments demonstrated a strong stimulatory effect, particularly at 1:10, 1:20, and 1:100 dilutions, where TPC levels reached 5841.31, 5271.51, and 3875.50 μg GAE/g FW, respectively. These values were significantly higher than the control (525.07 μg GAE/g FW), with statistical support (p < 0.05). However, the increase observed at 1:20 was not statistically different from that of CP4 at 1:5 (p > 0.05). The majority of other treatments did not result in significant changes in TPC compared to the control (p > 0.05), except for CP3 at 1:10 and CP4 at 1:10 and 1:20, which showed elevated values supported by p < 0.05.
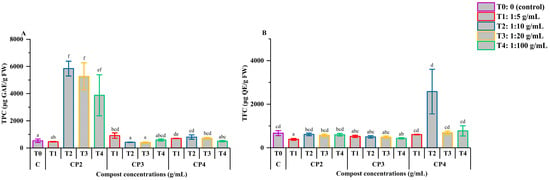
Figure 3.
Effect of phosphocompost extracts on the synthesis of secondary metabolites, including TPC (A) and TFC (B), in tomato plants infected with Meloidogyne javanica. Data are presented as mean ± standard error (SE). Bars followed by the same lowercase letters indicate no significant difference according to ANOVA followed by Fisher’s least significant difference (LSD) test.
For TFC (Figure 3B), most treatments yielded values similar to the control (670.53 μg QE/g FW), suggesting a limited impact on flavonoid accumulation (p > 0.05). However, there were notable exceptions: CP2 at 1:5 and CP3 at 1:100 led to reduced TFC levels of 383.94 and 434.76 μg QE/g FW, respectively, both showing statistically significant declines (p < 0.05). In contrast, CP4 at 1:10 significantly increased TFC, reaching 1910.37 μg QE/g FW (p < 0.05), indicating a treatment-specific enhancement in flavonoid biosynthesis.
3.2.4. Effect of Phosphocompost Extracts on the Development and Reproduction of M. javanica
Figure 4 illustrates the effect of phosphocompost extracts, applied at various dilution levels, on the density and reproductive performance of M. javanica. CP2 demonstrated the highest efficacy in suppressing nematode proliferation, with the lowest final juvenile counts observed at 1:5, 1:10, and 1:20 dilutions—resulting in 233.33, 233.33, and 266.67 J2s/100 g of soil, respectively (Figure 4A). These values were significantly lower than the untreated control (633.33 J2s/100 g), which represented a 2.16-fold increase from the initial inoculum (p < 0.05). In contrast, the corresponding CP2 treatments yielded final densities equivalent to 0.17, 0.17, and 0.33 times the initial population indicating a strong suppressive effect. While these reductions were statistically different from the control, they were not significantly different (p > 0.05) from most other compost extract treatments, except for CP4 at 1:100, which showed a higher juvenile count (p < 0.05).
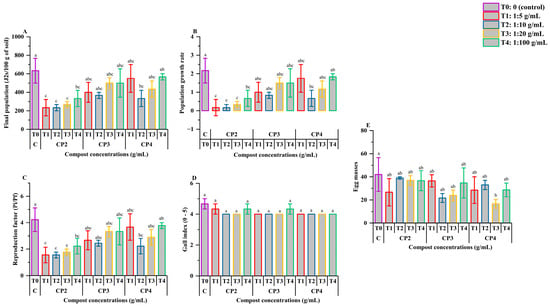
Figure 4.
Effect of phosphocompost extracts on the development of Meloidogyne javanica, including final population (A), population growth rate (B), reproduction factor (C), gall index (D), and egg masses (E) in tomato plants. Data are presented as mean ± standard error (SE). Bars followed by the same lowercase letters indicate no statistically significant difference according to ANOVA followed by Fisher’s least significant difference (LSD) test.
The nematode reproduction factor (Figure 4C) was also reduced under CP2 at 1:5, 1:10, and 1:20, with values of 1.17, 1.17, and 1.33, respectively, compared to 3.17 in the control group (p < 0.05). These treatments also significantly differed from CP4 at 1:100, which reached a value of 2.83. However, gall formation (Figure 4D) was not significantly affected by the treatments, with no observable differences between treatments or when compared to the control (p > 0.05).
In terms of egg mass production (Figure 4E), most treatments resulted in values similar to the control (42.00 egg masses; p > 0.05). However, CP4 at 1:20 yielded a significantly lower count (16.67 egg masses; p < 0.05). Despite this reduction, it did not significantly differ from the reductions caused by other compost treatments (p > 0.05).
3.3. Principal Component Analysis (PCA) of Phosphocompost Extract Effects on Tomato Growth Parameters and M. javanica Reproduction Metrics
The PCA (Figure 5) illustrates the influence of three phosphocompost extracts (CP2, CP3, and CP4) on the morphological and physiological growth parameters of tomato plants and the reproduction metrics of M. javanica. PC1, which explained 25.77% of the variance, primarily discriminated plant growth and biomass-related variables, such as plant height (PH), shoot fresh weight (SFW), shoot dry weight (SDW), and root weight (RW). On the other hand, PC2 (14.13% variance explained) encompassed variations in nematode reproductive metrics, including gall index (GI) and reproduction factor (RF), as well as physiological variables like carotenoids (Cc), chlorophyll a (Chla), and chlorophyll b (Chlb).
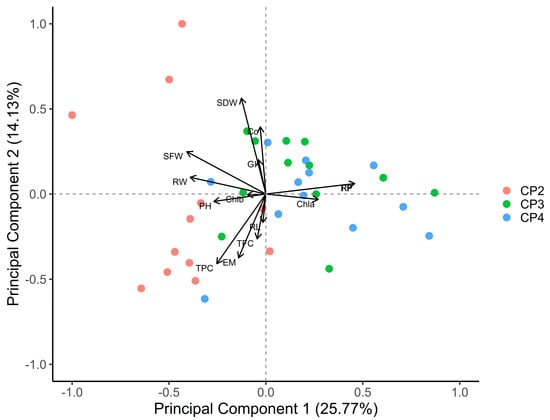
Figure 5.
PCA biplot illustration of the effects of phosphocompost extracts (CP2, CP3, and CP4) on tomato growth, including plant height (PH), shoot fresh weight (SFW), shoot dry weight (SDW), root weight (RW), root length (RL), and physiological parameters including chlorophyll a (Chla), chlorophyll b (Chlb), carotenoids (Cc), total phenolic compounds (TPC), and total flavonoid contents (TFC), and Meloidogyne javanica reproduction metrics including final population (FP), reproduction factor (RF), gall index (GI), and egg masses (EM).
CP2 is slightly associated with nematode reproductive variables (RF and GI) and strongly correlated with improved growth measures (e.g., SFW and RW) when concentrated on the negative axis of PC1. In contrast, CP4, located on the positive axis of PC1, was significantly correlated with elevated levels of nematode reproductive characteristics, such as final population (FP) and GI. CP3 made a minimal contribution to plant development and physiological traits, including Chla and Chlb, due to its balanced distribution.
The negative correlation between nematode reproduction parameters and TFC and TPC suggests that these secondary metabolites may have an inhibitory effect on M. javanica.
4. Discussion
4.1. Nutrient Profiling of Phosphocomposts
The addition of three different organic improvers (sugar beet waste, green waste, and olive mill waste) to uniform proportions of phosphate sludge (20%) and food waste (70%) in the phosphocompost formulations significantly influences their nutritional composition and agronomic viability. Specifically, the sugar beet waste in CP2 markedly increases the contents of Mg (7.04 mg/L), S (34.72 mg/L), and Na (128.20 mg/L), resulting in a unique nutritional profile distinguished by heightened secondary macronutrients. This compost produces the greatest concentrations of PO43− (12.91 mg/L) and Cl− (187.64 mg/L), indicating that sugar beet processing waste supplies both beneficial phosphorus and substantial salt levels that remain throughout the composting process. The organic acids generated during the breakdown of sugar beet waste [44,45] presumably contribute significantly to the solubilization of phosphorus from the sludge component [46].
The green waste in CP3 has a distinctly different nutritional profile, marked by an exceptionally high NO3− concentration (69.91 mg/L) and increased SO42− content (62.70 mg/L). The N-S dominance suggests that the green waste component likely included nitrogen-rich materials which experienced effective nitrification during composting [4]. Nonetheless, CP3 often demonstrates reduced micronutrient levels compared to the other composts, except for Mo (0.06 mg/L), indicating that green waste contributes less to micronutrient enrichment than sugar beet waste or olive mill waste.
The olive mill waste in CP4 produces the most balanced and micronutrient-dense compost, with predominant amounts of Ca (24.21 mg/L), K (392.50 mg/L), and P (9.17 mg/L) among macronutrients, while also displaying the greatest concentrations of Fe (2.44 mg/L), B (0.60 mg/L), Zn (0.15 mg/L), Cu (0.12 mg/L), and Ni (0.09 mg/L). This remarkable micronutrient composition corresponds with studies revealed that olive mill waste has substantial levels of critical metals and minerals for plants [47,48,49]. The elevated potassium level is notably indicative of olive processing residues, recognized for their potassium richness. Furthermore, the heightened F− concentration in CP4 (2.02 mg/L) seems to be a unique result of olive mill waste.
The trace element investigation indicates significant variations in metal mobilization patterns across the three amendments. The rising trend of V from CP2 (0.09 mg/L) to CP4 (0.39 mg/L) indicates that olive mill waste may facilitate V solubilization from the phosphate sludge component. Likewise, Si attains its peak concentration in CP4 (4.39 mg/L), indicating enhanced Si mobilization in the presence of olive mill waste. The identification of trace As (0.02 mg/L) and Tl (0.01 mg/L) alone in CP4 indicates that olive mill waste may marginally enhance the solubility of these elements, while their amounts are well below thresholds of agricultural concern.
The anion composition elucidates the unique contributions of each modification. The disparity in NO3− concentrations between CP3 (69.91 mg/L) and CP4 (2.23 mg/L) underscores the enhanced nitrogen mineralization in green waste relative to olive mill waste [4]. Olive mill waste seems to increase the F− availability, as shown by CP4’s elevated F− content (2.02 mg/L). The values of PO43− exhibit a hierarchy of CP2 > CP4 > CP3, indicating that both sugar beet waste and olive mill waste enhance phosphorus availability more efficiently than green waste.
4.2. Effects of Phosphocompost Extracts on Tomato Morphological Growth
Tomato production is currently facing multiple challenges, with M. javanica. infection being a significant concern. This study addressed the aforementioned issue by implementing a sustainable and eco-friendly substitute, specifically phosphocompost extract for the mitigation of M. javanica-induced pathogenicity on tomato crop. The results of this investigation demonstrated the efficacy of compost extracts in promoting tomato growth while simultaneously providing control against M. javanica. This aligns with the prior research indicating the effectiveness of different phosphocompost extracts in promoting plant growth during nematode infection. For instance, Tikoria et al. [50] demonstrated that vermicompost extracts from neem and cattle dung significantly enhance the morphological traits and growth of M. incognita infected-tomato plants. Similarly, Xiao et al. [13] demonstrated that compost and vermicompost derived from cow manure enhanced tomato growth under M. incognita infection. Additionally, Rostami et al. [16] showed that compost and vermicompost extracts from arugula effectively enhanced tomato growth in the presence of M. javanica. Xu et al. [51] found that applying compost extracts derived from pig manure and rice straw directly increased the root fresh weight of Meloidogyne spp. infected-tomato plants.
Among the evaluated extracts, CP2, especially at the 1:5 (g:mL), displayed the highest efficacy in boosting tomato growth. The enhanced performance can be attributed to the incorporation of sugar beet waste with the composted materials, which has demonstrated improvements in soil physicochemical properties, such as the content in organic matter and nutrient mobilization [52]. The incorporation of compost made from sugar beet waste has been proved to improve soil fertility via the increase in enzyme activity and nutrient availability, which support early vegetative growth and overall plant health [52,53]. The effectiveness of CP2 may also be attributed to the proliferation of specific microbiota capable of solubilizing phosphate and other essential nutrients stimulated by sugar beet waste as a carbon source [4], thereby offering them for plant absorption and boosting the morphological growth of tomato plants.
4.3. Effect of Phosphocompost Extracts on Physiological Development of Tomato
The negative effect of M. javanica infection on photosynthetic pigments was significant, consistent with the earlier studies that have demonstrated that Meloidogyne spp. infection stresses tomato plants, which leads to reduced chlorophyll content [54,55,56]. The observed reduction is likely attributable to the destabilization of chloroplast membranes, resulting from the accumulation of hydrogen peroxide and other reactive oxygen species [57]. The phosphocompost extract treatments demonstrated different levels of effectiveness in alleviating this effect. CP4 remarkably increased chlorophyll a content; however, this increase was not a concentration-dependent effect. Recently, Faraloni [58] reported that the application of olive mill waste compost to Tanacetum balsamita significantly improved its photosynthetic quantum yield and electron transport efficiency. The application of olive mill waste also increases the levels of RuBisCO and photosynthetic pigments in tobacco plants, indicating a potential enhancement in photosynthetic capacity [59]. CP3 at 1:5 and 1:10 restored chlorophyll b levels to those observed in the control group. The incorporation of humic acid and biochar to composted green waste has been proved to enhance the overall chlorophyll levels, especially chlorophyll b [1]. Furthermore, the combination of green compost and Chlorella microalgae mitigated the impacts of drought stress and importantly, increased the content in chlorophyll b in plants [25]. Carotene levels were consistently elevated across all treatments, showing no significant differences across the treatments or relative to the control. This indicates that carotene accumulation may represent a general stress response to Meloidogyne infection rather than being specifically triggered by compost extract treatment, as no significant differences were noted among the treatments or the control. These findings support those of Tikoria et al. [50], who reported increases in chlorophyll a and b levels following compost extract treatment, whereas carotene levels showed no significant change. This increase in chlorophyll content can be linked to the higher nitrogen levels found in the leaves of plants treated with compost extracts [50].
The responses of TPC and TFC to different phosphocompost extracts reveal complex interactions between extract composition, concentration, and plant secondary metabolites. CP2 demonstrated a distinct concentration-dependent effect on TPC synthesis, with increases noted at lower dilutions (1:10, 1:20, and 1:100), whereas a decrease was observed at the highest concentration (1:5). This pattern suggests a hormetic response, potentially related to sugar beet waste components, characterized by a biphasic dose–response relationship, where low doses produce stimulatory effects, and high doses lead to inhibitory outcomes [60,61,62]. Additionally, compost made from sugar beet waste has a more substantial impact on the concentration of polyphenols in plants than flavonoids owing to its richness in phenolic precursors in addition to its capability of encouraging microbial activity, and enriching the soil with minerals that are conducive to polyphenol biosynthesis [63,64]. In contrast, CP4 significantly enhanced TFC at a 1:10 concentration, whereas the other treatments exhibited no notable changes in TFC. This demonstrates a specific dose-dependent activation of flavonoid biosynthesis, likely due to the polyphenol-rich content of olive mill waste [65], which may act as a precursor or stimulator of flavonoid production.
The observed lack of TFC response across treatments, juxtaposed with the variable TPC outcomes, suggests that these extracts primarily affect non-flavonoid phenolic compounds. Previous studies have indicated that compost may enhance the phenolic content, but it may not similarly affect flavonoids and could potentially reduce their levels [66,67]. The observed disparity may result from the unique nutrient composition of the compost or the metabolic response of the plant to various stressors or nutrient availability [66,67]. Yusof et al. [67] reported that vermicompost application enhanced the stability of phenolic compounds while minimally affecting flavonoid expression in Clinacanthus nutans (Acanthaceae), underscoring the importance of the compost type and nutrient composition in influencing the levels of various bioactive compounds.
4.4. Effect of Phosphocompost Extracts on M. javanica Reproduction
The findings regarding M. javanica reproduction and multiplication indicated that phosphocompost extracts, particularly CP2, exhibited dose-dependent effects on M. javanica population management at 1:5, 1:10, and 1:20. CP2 markedly decreased the final nematode population, population growth rate, and reproduction capacity relative to those of the control, indicating its potential efficacy as a nematode management strategy. This finding was corroborated by Akhtar and Alam [68], who indicated that sugar beet by-products can regulate nematode populations by enhancing the activity of predators, resulting in diminished nematode populations and improved crop yields.
Nonetheless, the minimal effect on the egg masses and gall index across the majority of treatments suggests that although these phosphocompost extracts may reduce overall nematode populations, they do not effectively inhibit initial root invasion or fully disrupt the nematode life cycle. This observation corresponds to the findings of Nico et al. [69], who indicated that while nematode populations may be reduced, certain composts do not have a significant impact on root galling. The observed discrepancy may be due to the timing and method of compost application, which may not align with the critical stages of nematode infection and gall formation [69].
Although the compost extracts exhibited promising effects, a limitation of this study is the reliance on a single end-point evaluation conducted 90 days post-inoculation. This timing was purposefully chosen to align with the typical physiological maturity period of tomato crops, which generally spans 90 to 120 days in greenhouse settings. This allowed for a comprehensive evaluation of the long-term plant performance and cumulative nematode suppression. However, the absence of earlier sampling points may have hindered our ability to detect the immediate protective effects of the extracts during the initial stages of nematode invasion. Future research should consider incorporating multiple assessment time points, such as 30 and 60 days, to better capture the temporal dynamics of extract efficacy. Additionally, conducting long-term trial across different seasons and environmental conditions would help confirm the reproducibility and scalability of the results for sustainable nematode management.
4.5. Principal Component Analysis (PCA) of Phosphocompost Extract Effects on Tomato Growth Parameters and M. javanica Reproduction Metrics
The differential impacts of phosphocompost extracts on M. javanica reproduction and tomato plant performance are emphasized by the PCA results, which identify CP2 as the most promising extract for effective management of RKNs. The strong correlation between CP2 and the growth parameters of tomato, such as SFW and RW, and its limited correlation with nematode reproduction metrics, indicate that this extract effectively promotes plant growth while reducing nematode infestation. This effect may be attributed to the increased levels of phenolic and flavonoid compounds in CP2, as it contains sugar beet waste [64], which have been associated with the suppression of nematode activity, as shown with the PCA. Conversely, CP4, despite its favorable impact on specific physiological traits such as Chla, appears to facilitate nematode reproduction, as demonstrated by its significant correlation with GI, FP, and RF. This discovery suggests that there may be potential trade-offs linked with the employment of CP4, as it may have the capacity to exacerbate the infestations of nematodes in susceptible crops. Although its efficacy in controlling the density of nematodes was less pronounced than that of CP2, CP3 could be a viable intermediate option owing to its balanced impact across multiple parameters. These results underscore the necessity of customizing compost formulations to improve the performance of plant and attenuate the risk of pathogen proliferation. CP2 displays the greatest effectiveness as a prospective bionematicide for sustainable tomato cultivation under nematode stress.
Overall, CP2 demonstrated the most favorable outcomes, exhibiting significant positive effects on various essential plant growth parameters and TPC, while effectively reducing nematode populations and reproduction rates. In contrast, CP4 specifically elevated TFC, suggesting that the origin of various compost extracts may elicit different physiological responses in plants. The differences between CP2 and CP4 highlight the significance of compost source and composition in influencing their effectiveness against nematodes and their effects on plant health. The superior effectiveness of CP2 in boosting plant growth and reducing nematode populations may be attributed to its distinct chemical profile. CP2 contained the highest concentrations of Mg (7.04 mg/L), S (34.72 mg/L), and Na (128.20 mg/L), along with elevated PO43− levels (12.91 mg/L). Magnesium and phosphorus are key elements involved in chlorophyll synthesis and energy transfer [70,71], supporting robust vegetative growth, while sulfur plays a role in the synthesis of defense-related secondary metabolites [72]. Sodium may contribute to enhanced osmotic regulation and stress tolerance [73]. These combined effects likely explain the pronounced increases in shoot fresh weight, root development, and polyphenol accumulation observed with CP2. In contrast, CP4, which was rich in Ca (24.21 mg/L) and K (392.50 mg/L), was more effective at enhancing chlorophyll content and flavonoids, but showed less consistent suppression of nematode reproduction. These findings suggest that nutrient composition, particularly micronutrient and anion balance, plays a pivotal role in defining both plant and nematode responses to compost extracts. The distinct advantages of each extract indicate the possibility of customizing applications based on the primary goals of plant protection and growth enhancement.
Compared to synthetic chemical nematicides, phosphocompost extracts offer a low-cost organic material and secure substitute for managing M. javanica in tomato crops. While chemical nematicides such as fluopyram and fluopimomide have shown efficacy—reducing nematode reproduction by over 50–82% [73,74]—their action is primarily limited to nematode control. They do not contribute to plant growth promotion or soil improvement and may pose risks such as phytotoxicity, environmental contamination, and resistance development. In contrast, phosphocompost extracts like CP2 not only suppressed nematode populations at comparable or superior levels but also improved plant growth, enhanced nutrient uptake, and stimulated the accumulation of defense-related compounds such as polyphenols. Furthermore, these organic formulations may promote beneficial microbial communities and introduce antagonistic organisms into the rhizosphere, contributing to long-term soil health and resilience. Their multifaceted benefits align well with integrated pest management (IPM) and agroecological principles, supporting their use as a sustainable solution for smallholder and organic farming systems.
5. Conclusions
Root-knot nematodes, particularly M. javanica, pose persistent and economically damaging threats to tomato production by severely impairing the development of plant growth and biomass. In light of the increasing regulatory pressure against chemical nematicides, the development of effective and eco-friendly substitutes has become a pressing priority, especially in intensive production systems, where nematode outbreaks are common.
This investigation proved that phosphocompost water extracts, specifically CP2 at a 1:5 g:mL ratio, can significantly enhance plant performance while suppressing nematode development. CP2-treated plants exhibited a consistent rise in plant development metric traits and TPC, indicating the activation of defense-related pathways. Concurrently, the nematode density in the soil was reduced dramatically in comparison to the untreated controls, confirming a strong suppressive effect on the reproduction of nematode.
However, the limited effect of the extract on root egg mass and galling suggests that while it disrupts the multiplication nematode in the soil, it is less effective in preventing early root penetration. This is likely due to the timing of post-inoculation application, by which point the juveniles had already entered the root system. This limitation highlights the need for early application, ideally prior to nematode exposure, to maximize protective efficacy.
Although assessments were conducted at 90 days to coincide with the standard physiological maturity of tomato (90–120 days), future studies should include earlier time points to better capture the immediate defensive attributes of phosphocompost extracts against initial nematode invasion and validate their efficacy under different seasonal and environmental conditions; additionally, long-term studies and evaluation on different crops are needed. Future studies should also explore the optimal application timing, formulation adjustments, and synergistic use with other organic or biological inputs to fully harness their potential within sustainable tomato production systems. Overall, these findings indicate that phosphocompost extracts are promising dual-function agents that enhance plant vigor, while biologically suppressing nematodes. Their low cost, scalability, and dual roles in biostimulation and biocontrol make them strong candidates for integration into environmentally responsible pest control strategies.
Author Contributions
E.M.B.: conceptualization, writing—original draft, writing—review and editing, data curation, and analysis; A.H.: resources; M.F.: writing—review and editing; A.B.: writing—review and editing; A.A.D.: writing—review and editing; A.O.A.: writing—review and editing; K.K.: writing—review and editing; A.Z.: writing—review and editing; D.I.: writing—review and editing; K.A.: writing—review and editing; A.S.: conceptualization, writing—review and editing, supervision, and validation; F.M.: conceptualization, writing—review and editing, supervision, and validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. The study did not involve human participants or vertebrate animals. It involved greenhouse trials evaluating compost extracts efficacy against the plant-parasitic nematode Meloidogyne javanica on tomato.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ongoing analyses and potential use in future publications.
Acknowledgments
The authors would like to thank the National Institute for Agricultural Research (INRA) of Rabat, Morocco; the Faculty of Sciences, Mohammed V University in Rabat, Morocco; OCP, Morocco; the Mohammed VI Polytechnic University, Morocco; the National Center of Scientific and Technical Research CNRST, Morocco; the Ministry of Higher Education, Morocco; and Scientific Research and Professional Training of Morocco (MESRSFC) for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, L.; Sun, X.Y.; Tian, Y.; Gong, X.Q. Biochar and humic acid amendments improve the quality of composted green waste as a growth medium for the ornamental plant Calathea insignis. Sci. Hortic. 2014, 176, 70–78. [Google Scholar] [CrossRef]
- Henwood, W.D. Toward a strategy for the conservation and protection of the world’s temperate grasslands. Great Plains Res. 2010, 20, 121–134. [Google Scholar]
- Ren, H.; Hu, J.; Hu, Y.; Yang, G.; Zhang, Y. Divergence of compost extract and bio-organic manure effects on lucerne plant and soil. PeerJ 2017, 5, e3775. [Google Scholar] [CrossRef]
- Haouas, A.; El Modafar, C.; Douira, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; Moukhli, A.; Amir, S. Evaluation of the nutrients cycle, humification process, and agronomic efficiency of organic wastes composting enriched with phosphate sludge. J. Clean. Prod. 2021, 302, 127051. [Google Scholar] [CrossRef]
- González-Hernández, A.I.; Gómez-Sánchez, M.Á.; Pérez-Sánchez, R.; Morales-Corts, M.R. Garden waste compost tea: A horticultural alternative to promote plant growth and root traits in tomato (Solanum lycopersicum L.) plants. Horticulturae 2023, 9, 1127. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Faostat: Agriculture Data. 2021. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 20 October 2024).
- STATISTA. Production of tomatoes in Morocco from 2010 to 2021. 2024. Available online: https://www.statista.com/statistics/1302586/production-of-tomatoes-in-morocco/ (accessed on 13 October 2024).
- FAOSTAT. Food and Agriculture Data. 2024. Available online: https://www.fao.org/faostat/en/#home (accessed on 13 October 2024).
- El Aimani, A.; Houari, A.; Laasli, S.E.; Mentag, R.; Iraqi, D.; Diria, G.; Khayi, S.; Lahlali, R.; Dababat, A.A.; Mokrini, F. Antagonistic potential of Moroccan entomopathogenic nematodes against root-knot nematodes, Meloidogyne javanica on tomato under greenhouse conditions. Sci. Rep. 2022, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Krif, G.; Mokrini, F.; Aissami, A.E.; Laasli, S.E.; Imren, M.; Özer, G.; Paulitz, T.; Lahlali, R.; Dababat, A.A. Diversity and management strategies of plant parasitic nematodes in Moroccan organic farming and their relationship with soil physico-chemical properties. Agriculture 2020, 10, 447. [Google Scholar] [CrossRef]
- Krif, G.; Lahlali, R.; El Aissami, A.; Laasli, S.E.; Mimouni, A.; Serderidis, S.; Picaud, T.; Moens, A.; Dababat, A.A.; Fahad, K.; et al. Efficacy of authentic bio-nematicides against the root-knot nematode, Meloidogyne javanica infecting tomato under greenhouse conditions. Physiol. Mol. Plant Pathol. 2022, 118, 101803. [Google Scholar] [CrossRef]
- Krif, G.; Lahlali, R.; El Aissami, A.; Laasli, S.E.; Mimouni, A.; Dababat, A.A.; Zoubi, B.; Mokrini, F. Potential effects of nematophagous fungi against Meloidogyne javanica infection of tomato plants under in vitro and in vivo conditions. J. Crop Health 2024, 76, 829–839. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, M.; Jiang, L.; Chen, X.; Griffiths, B.S.; Li, H.; Hu, F. Vermicompost increases defense against root-knot nematode (Meloidogyne incognita) in tomato plants. Appl. Soil Ecol. 2016, 105, 177–186. [Google Scholar] [CrossRef]
- Abad, P.; Favery, B.; Rosso, M.N.; Castagnone-Sereno, P. Root-knot nematode parasitism and host response: Molecular basis of a sophisticated interaction. Mol. Plant Pathol. 2003, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.C.; Dubreuil, G.; Quentin, M.; Perfus-Barbeoch, L.; Lecomte, P.; de Almeida Engler, J.; Abad, P.; Rosso, M.-N.; Favery, B. Root-knot nematodes manipulate plant cell functions during a compatible interaction. J. Plant Physiol. 2008, 165, 104–113. [Google Scholar] [CrossRef]
- Rostami, M.; Karegar, A.; Taghavi, S.M.; Ghasemi-Fasaei, R.; Ghorbani, A. Effective combination of arugula vermicompost, chitin and inhibitory bacteria for suppression of the root-knot nematode Meloidogyne javanica and explanation of their beneficial properties based on microbial analysis. PLoS ONE 2023, 18, e0289935. [Google Scholar] [CrossRef]
- Pimentel, D.; Hepperly, P.; Hanson, J.; Douds, D.; Seidel, R. Environmental, energetic, and economic comparisons of organic and conventional farming systems. BioScience 2005, 55, 573–582. [Google Scholar] [CrossRef]
- Azim, K.; Soudi, B.; Boukhari, S.; Perissol, C.; Roussos, S.; Thami Alami, I. Composting parameters and compost quality: A literature review. Org. Agric. 2018, 8, 141–158. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef]
- Mu, D.; Horowitz, N.; Casey, M.; Jones, K. Environmental and economic analysis of an in-vessel food waste composting system at Kean University in the U.S. Waste Manag. 2017, 59, 476–486. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Adamcová, D.; Winkler, J.; Koda, E.; Petrželová, L.; Maxianová, A. Alternative method of composting on a reclaimed municipal waste landfill in accordance with the circular economy: Benefits and risks. Sci. Total Environ. 2020, 723, 137971. [Google Scholar] [CrossRef]
- Bouchtaoui, E.M.; Haouas, A.; Dababat, A.A.; Lahlali, R.; Benali, A.; Fahr, M.; Smouni, A.; Azim, K.; Liu, Z.; Li, J.; et al. Exploring mechanisms of compost-mediated suppression of plant pathogens: A critical review. Appl. Soil Ecol. 2024, 203, 105644. [Google Scholar] [CrossRef]
- Bakker, C.; Popescu, I.; Schott, H.; Smith, M.L.; Avis, T.J. Compost teas provide reduction of grey mould (Botrytis cinerea Pers.) on tomato plants. Eur. J. Plant. Pathol. 2024, 169, 643–656. [Google Scholar] [CrossRef]
- Mohamed, O.Z.; Yassine, B.; Hilali Rania, E.; El Hassan, A.; Abdellatif, H.; Rachid, B. Evaluation of compost quality and bioprotection potential against Fusarium wilt of date palm. Waste Manag. 2020, 113, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, S.; Karmidarenjani, M.; Mirahmadinejad, E.A.; Robati, R. The effect of green compost processed organic fertilizer and Chlorella microalgae solution on chlorophyll a, chlorophyll b, carotenoid and proline content of tropaeolum majus under drought stress. Annu. Res. Rev. Biol. 2023, 38, 17–27. [Google Scholar] [CrossRef]
- De Corato, U.; Salimbeni, R.; De Pretis, A.; Patruno, L.; Avella, N.; Lacolla, G.; Cucci, G. Microbiota from ‘next-generation green compost’ improves suppressiveness of composted municipal-solid-waste to soil-borne plant pathogens. Biol. Control 2018, 124, 1–17. [Google Scholar] [CrossRef]
- Mehta, C.M.; Palni, U.; Franke-Whittle, I.H.; Sharma, A.K. Compost: Its role, mechanism and impact on reducing soil-borne plant diseases. Waste Manag. 2014, 34, 607–622. [Google Scholar] [CrossRef]
- Gumiere, T.; Rousseau, A.N.; da Costa, D.P.; Cassetari, A.; Cotta, S.R.; Andreote, F.D.; Gumiere, S.J.; Pavinato, P.S. Phosphorus source driving the soil microbial interactions and improving sugarcane development. Sci. Rep. 2019, 9, 4400. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, X.; Chen, T.; Yang, J.; Yang, J.; Liu, J.; Shi, X. Passivation of lead and cadmium and increase of the nutrient content during sewage sludge composting by phosphate amendments. Environ. Res. 2020, 185, 109431. [Google Scholar] [CrossRef]
- Sarr, P.S.; Tibiri, E.B.; Fukuda, M.; Zongo, A.N.; Compaore, E.; Nakamura, S. Phosphate-solubilizing fungi and alkaline phosphatase trigger the p solubilization during the co-composting of sorghum straw residues with Burkina Faso phosphate rock. Front. Environ. Sci. 2020, 8, 559195. [Google Scholar] [CrossRef]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fan, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zuo, H.; Li, J.; Ding, G.; Zhan, Y.; Zhang, L.; Wu, W.; Su, L.; Wei, Y. Insight into the mechanisms of insoluble phosphate transformation driven by the interactions of compound microbes during composting. Environ. Sci. Pollut. Res. 2021, 28, 32844–32855. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Tsang, D.C.W.; Li, G. Effects of external additives: Biochar, bentonite, phosphate, on co-composting for swine manure and corn straw. Chemosphere 2020, 248, 125927. [Google Scholar] [CrossRef]
- Mussarat, M.; Ali, H.; Muhammad, D.; Mian, I.A.; Khan, S.; Adnan, M.; Fahad, S.; Wahid, F.; Dawar, K.; Ali, S.; et al. Comparing the phosphorus use efficiency of pre-treated (organically) rock phosphate with soluble P fertilizers in maize under calcareous soils. PeerJ 2021, 9, e11452. [Google Scholar] [CrossRef] [PubMed]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; Egea-Gilabert, C. Application of directly brewed compost extract improves yield and quality in baby leaf lettuce grown hydroponically. Agronomy 2020, 10, 370. [Google Scholar] [CrossRef]
- Spaccini, R.; Cozzolino, V.; Di Meo, V.; Savy, D.; Drosos, M.; Piccolo, A. Bioactivity of humic substances and water extracts from compost made by ligno-cellulose wastes from biorefinery. Sci. Total Environ. 2019, 646, 792–800. [Google Scholar] [CrossRef]
- El Rasafi, T.; Haouas, A.; Tallou, A.; Chakouri, M.; Aallam, Y.; El Moukhtari, A.; Hamamouch, N.; Hamdali, H.; Oukarroum, A.; Farissi, M.; et al. Recent progress on emerging technologies for trace elements-contaminated soil remediation. Chemosphere 2023, 341, 140121. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Taylor, A.L. Introduction to Research on Plant Nematology. An FAO Guide to the Study and Control of Plant-Parasitic Nematodes; Food and Agricultural Organization of the United Nations: Rome, Italy, 1967; p. 133. [Google Scholar]
- Machado, R.M.A.; Alves-Pereira, I.; Lourenço, D.; Ferreira, R.M.A. Effect of organic compost and inorganic nitrogen fertigation on spinach growth, phytochemical accumulation and antioxidant activity. Heliyon 2020, 6, e05085. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 1987, 1, F4. 3.1–F4. 3.8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. Available online: https://www.sciencedirect.com/science/article/pii/S0076687999990171 (accessed on 20 September 2024).
- Santas, J.; Carbó, R.; Gordon, M.H.; Almajano, M.P. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008, 107, 1210–1216. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Schneider, R.; Hoss Lunelli, B.; Vaz Rossell, C.E.; Maciel Filho, R.; Venus, J. A Simple Biorefinery Concept to Produce 2G-Lactic Acid from Sugar Beet Pulp (SBP): A High-Value Target Approach to Valorize a Waste Stream. Molecules 2020, 25, 2113. [Google Scholar] [CrossRef]
- Micard, V.; Renard, C.M.G.C.; Thibault, J.F. Enzymatic saccharification of sugar-beet pulp. Enzym. Microb. Technol. 1996, 19, 162–170. [Google Scholar] [CrossRef]
- Pérez, C.; Boily, J.F.; Skoglund, N.; Jansson, S.; Fick, J. Phosphorus release from hydrothermally carbonized digested sewage sludge using organic acids. Waste Manag. 2022, 151, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Lyamlouli, K. Olive mill waste sludge: From permanent pollution to a highly beneficial organic biofertilizer: A critical review and future perspectives. Ecotoxicol. Environ. Saf. 2023, 259, 114997. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, F.; Chronopoulou, L.; Pizzichini, D.; Lionetti, V.; Fontana, C.; Aromolo, R.; Socciarelli, S.; Gambelli, L.; Bartolacci, B.; Finotti, E.; et al. Olive mill wastes: A source of bioactive molecules for plant growth and protection against pathogens. Biology 2020, 9, 450. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef] [PubMed]
- Tikoria, R.; Kaur, A.; Ohri, P. Potential of vermicompost extract in enhancing the biomass and bioactive components along with mitigation of Meloidogyne incognita-induced stress in tomato. Environ. Sci. Pollut. Res. 2022, 29, 56023–56036. [Google Scholar] [CrossRef]
- Xu, D.; Raza, W.; Yu, G.; Zhao, Q.; Shen, Q.; Huang, Q. Phytotoxicity analysis of extracts from compost and their ability to inhibit soil-borne pathogenic fungi and reduce root-knot nematodes. World J. Microbiol. Biotechnol. 2012, 28, 1193–1201. [Google Scholar] [CrossRef]
- Rodríguez, R.; Vassilev, N.; Azcón, R. Increases in growth and nutrient uptake of alfalfa grown in soil amended with microbially-treated sugar beet waste. Appl. Soil Ecol. 1999, 11, 9–15. [Google Scholar] [CrossRef]
- Medina, A.; Vassileva, M.; Barea, J.M.; Azcón, R. The growth-enhancement of clover by Aspergillus-treated sugar beet waste and Glomus mosseae inoculation in Zn contaminated soil. Appl. Soil Ecol. 2006, 33, 87–98. [Google Scholar] [CrossRef]
- Lu, P.; Davis, R.F.; Kemerait, R.C.; van Iersel, M.W.; Scherm, H. Physiological effects of Meloidogyne incognita infection on cotton genotypes with differing levels of resistance in the greenhouse. J. Nematol. 2014, 46, 352–359. [Google Scholar]
- Sikandar, A.; Wu, F.; He, H.; Ullah, R.M.K.; Wu, H. Growth, physiological, and biochemical variations in tomatoes after infection with different density levels of Meloidogyne enterolobii. Plants 2024, 13, 293. [Google Scholar] [CrossRef]
- Tikoria, R.; Kaur, A.; Ohri, P. Modulation of various phytoconstituents in tomato seedling growth and Meloidogyne incognita–induced stress alleviation by vermicompost application. Front. Environ. Sci. 2022, 10, 891195. [Google Scholar] [CrossRef]
- Liang, C.; Li, A.; Yu, H.; Li, W.; Liang, C.; Guo, S.; Zhang, R.; Chu, C. Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant. Sci. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Faraloni, C.; Giordano, C.; Arcidiaco, L.; Benelli, C.; Di Lonardo, S.; Anichini, M.; Stefani, F.; Petruccelli, R. Effective microorganisms and olive mill wastewater used as biostimulants to improve the performance of Tanacetum balsamita L., a medicinal plant. Appl. Sci. 2023, 13, 722. [Google Scholar] [CrossRef]
- Parrotta, L.; Campani, T.; Casini, S.; Romi, M.; Cai, G. Impact of raw and bioaugmented olive-mill wastewater and olive-mill solid waste on the content of photosynthetic molecules in tobacco plants. J. Agric. Food. Chem. 2016, 64, 5971–5984. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A Compelling platform for sophisticated plant science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Romero-Gomez, S.D.J.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Alvarez-Arquieta, L.D.L.; Torres-Pacheco, I. Plant hormesis management with biostimulants of biotic origin in agriculture. Front. Plant. Sci. 2017, 8, 1762. [Google Scholar] [CrossRef]
- Baryga, A.; Ziobro, R.; Gumul, D.; Rosicka-Kaczmarek, J.; Miśkiewicz, K. Physicochemical properties and evaluation of antioxidant potential of sugar beet pulp—Preliminary analysis for further use (future prospects). Agriculture 2023, 13, 1039. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Mihaylova, D.; Marangon, C.M.; Grigoletto, L.; Lante, A. Impact of sample pretreatment and extraction methods on the bioactive compounds of sugar beet (Beta vulgaris L.) leaves. Molecules 2022, 27, 8110. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Millner, P.D.; Meyer, S.L.F.; Roig, A. Potential of olive mill waste and compost as biobased pesticides against weeds, fungi, and nematodes. Sci. Total Environ. 2008, 399, 11–18. [Google Scholar] [CrossRef]
- Omar, N.F.; Hassan, S.A.; Yusoff, U.K.; Abdullah, N.A.P.; Wahab, P.E.M.; Sinniah, U.R. Phenolics, flavonoids, antioxidant activity and cyanogenic glycosides of organic and mineral-base fertilized cassava tubers. Molecules 2012, 17, 2378–2387. [Google Scholar] [CrossRef]
- Yusof, Z.; Ramasamy, S.; Mahmood, N.Z.; Yaacob, J.S. Vermicompost supplementation improves the stability of bioactive anthocyanin and phenolic compounds in Clinacanthus nutans lindau. Molecules 2018, 23, 1345. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Alam, M.M. Utilization of waste materials in nematode control: A review. Bioresour. Technol. 1993, 45, 1–7. [Google Scholar] [CrossRef]
- Nico, A.I.; Jiménez-Díaz, R.M.; Castillo, P. Control of root-knot nematodes by composted agro-industrial wastes in potting mixtures. Crop Prot. 2004, 23, 581–587. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Lai, N.W.; Guo, J.X.; Chen, L.S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in citrus sinensis seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef]
- Künstler, A.; Gullner, G.; Ádám, A.L.; Kolozsváriné Nagy, J.; Király, L. The versatile roles of sulfur-containing biomolecules in plant defense—A road to disease resistance. Plants 2020, 9, 1705. [Google Scholar] [CrossRef]
- Wu, G.Q.; Feng, R.J.; Liang, N.; Yuan, H.J.; Sun, W.B. Sodium chloride stimulates growth and alleviates sorbitol-induced osmotic stress in sugar beet seedlings. Plant Growth Regul. 2015, 75, 307–316. [Google Scholar] [CrossRef]
- Li, J.; Meng, Z.; Li, N.; Dong, B.; Ji, X.; Zhang, S.; Qiao, K. Evaluating a new non-fumigant nematicide fluopimomide for management of southern root-knot nematodes in tomato. Crop Prot. 2020, 129, 105040. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).