Abstract
Biostimulants are becoming more prevalent in the production of forage and turfgrasses. Many can be classified as natural biostimulants, including humic acids (HA), fulvic acids (FA), protein hydrolysates (PHs) and seaweed extracts (SWE), in addition to chitosan, silicon, inorganic compounds, beneficial fungi, bacteria and synthetic biostimulants. The article reviews recent research on the effects of biostimulants in the cultivation of forage grasses (perennial ryegrass, annual ryegrass, Festulolium, Kentucky bluegrass, annual bluegrass, orchard grass and timothy-grass) and turfgrasses (perennial ryegrass, Kentucky bluegrass, tall fescue, red fescue and creeping bentgrass). Literature analysis suggests that biostimulants enhance the quality of grasses, augment their tolerance to environmental stresses, facilitate nutrient uptake and improve the visual aspect of grasses. While biostimulants cannot replace fertilisers, they can significantly improve crop effectiveness in utilising the nutrients present in the fertilisers. This paper also briefly describes the legal and regulatory status of biostimulants with a focus on the EU and PL.
1. Introduction
The main challenge facing the global agricultural sector is to increase productivity without exerting a negative impact on the environment. According to estimates, the global population will reach 9.7 billion by 2050, and crop production will increase by 60–100% to meet the growing demand for food [1]. Nutrient-poor soils pose a widespread problem in agricultural production, and nutrient deficiencies increase plants’ susceptibility to pathogens and decrease yields. Fertilisers and pesticides are used to increase yields and guarantee continuous food supplies [2]. Sustainable intensification of agriculture is needed to address the projected increase in food demand. The European Green Deal is a comprehensive strategy to transition towards a more sustainable model of economic growth. The overarching objectives of this strategy are to prevent climate change and promote the sustainable use of natural resources. European countries have committed to reducing their pesticide use, minimizing nutrient loss and limiting the use of mineral fertilisers. Chemical fertilisers enhance crop production, but fertiliser overuse decreases soil quality, contributes to air and water pollution and leads to greenhouse gas emissions. Improper use of artificial fertilisers and pesticides can exert a negative impact on the environment and pose a threat for humans and animals [3]. Synthetic fertilisers also contribute to the eutrophication of water bodies and the formation of dead zones devoid of living organisms [4]. Effective strategies are needed to prevent excess accumulation of mineral nitrogen (N) in soil [5]. There are reports suggesting that it is possible to replace, at least in part, the application of nitrogen fertilisation with the application of biostimulants to forage grasses [6]. The use of N fertilisers should also be reduced to cut nitrogen oxide emissions [7]. Agricultural chemicals increase productivity, but they also decrease farming incomes [8].
Grasses are a widespread group of plants found in all climatic and geographical zones. Their diversity is reflected in the various functions they perform in the natural environment and human economic activities.
Pasture provides affordable, nutritious and tasty food for livestock. With the appropriate botanical composition, grassland fodder can be the sole feed in the diet of animals, including those with high productivity. The basis of ruminant nutrition should be grassland fodder since the profitability of livestock production is mainly determined by the proportion of pasture grass, hay or haylage in the feed ration.
The increasing development of urban and infrastructural areas during recent years has led to a rise in demand for lawn grasses. Named from the French term ‘gazon’, meaning grass, lawn grasses are flexible and have high adaptability to diverse soil types and varying weather conditions, making them versatile and widely used. Perennial ryegrass (Lolium perenne L.) is one of the top species of lawn grasses used in cool temperature areas across the world. Perennial ryegrass is utilized in various mixtures such as amenity lawns, football turf, turf production and in reclamation.
Fertilisation is one of the factors that significantly impact the appearance of turf and the productivity of grassland. The cultivation of turf and fodder grasses requires fertilisers that provide a range of essential nutrients for their growth and development. Primary turfgrass’s primary nutrient, nitrogen, helps maintain green colour and adequate density and enables recovery from stresses like drought or disease. The primary goal of fertilising turfgrasses is to maintain high turf density, uniformity, adequate colour and disease resistance. The aim of fertilisation is to apply the lowest possible dose. Our goal should be to apply as little fertiliser as possible to achieve a healthy turf, while still meeting the aesthetic and functional standards of the area in question.
Based on the objectives of the ‘From Field to Fork’ strategy, the European Union should reduce fertiliser use by 20% by 2030. Therefore, new efficient methods are being researched to decrease the intensification of fertilisation. Grass supported by biostimulants is a feasible solution that aligns with the European Green Deal strategy.
In Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019, a biostimulant is defined as “a product stimulating plant nutrition processes independently of the product’s nutrient content with of product the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (a) nutrient use efficiency; (b) tolerance to abiotic stress; (c) quality traits; (d) availability of confined nutrients in soil or rhizosphere”. Biostimulants are compounds that promote plant growth and development, enhance resistance to stress, improve yields and enhance the quality of agricultural products. The effectiveness of biostimulants relies on their composition. Biostimulants comprise various organic and inorganic compounds that directly impact plant metabolism. Biostimulants are eco-friendly additives, and they find widespread use in sustainable agriculture. Nevertheless, the outcomes of biostimulants on grasses have not been adequately researched to this day.
Biostimulants can improve global food security and help address the challenges associated with population growth and climate change [9]. Biostimulants improve crop yields and productivity without any changes in fertilisation and irrigation regimes, and they increase the nutritional value and the content of protein and carbohydrates in plants. Biostimulants enhance yields under unfavourable environmental conditions, and they can be incorporated into sustainable agricultural practices to meet the growing demand for food. Biostimulants for sustainable farming can also be derived from agricultural wastes. However, the efficacy of biostimulants in grass production has not been widely investigated to date [10,11].
The aim of this review article was to identify the main types of biostimulants in the cultivation of forage grasses and turfgrasses and to evaluate their efficacy in grass production. Biostimulants are defined in the first section of the article. The composition of biostimulants, their mechanism of action and plant responses to biostimulants are described in the following subsequent sections, separately for forage grasses and turfgrasses. It should be noted that very few articles have reviewed the literature on the use of biostimulants in the production of forage grasses and turfgrasses.
2. Biostimulants
2.1. Biostimulant Regulation
Biostimulants are environmentally friendly, innovative substances that can help improve food security for the world’s growing population [10]. They are an important part of agricultural technologies in intensive crop farming [11]. However, despite these advantages, biostimulants are rarely incorporated in conventional agricultural regimes. Farmers have limited knowledge about the benefits delivered by biostimulants, and they are reluctant to apply these preparations in fear of increased production costs, lower crop quality and yields and reduced profits. A wide range of biostimulants are available on the market, and the selection of a product that can maximize yields in a given plant species can also pose a problem. Further research is needed to formulate preparations that deliver a wide range of functions, are easy to apply and can be safely combined with other growth-promoting agents [4].
In Poland, the production and use of fertilisers, excluding conformity assessment procedures for placing fertilising products on the EU market, which are set forth by Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019, are regulated by the following legislative acts:
- ∗
- Act of 10 July 2007 on fertilisers and fertilisation (Journal of Laws, 2021, item 76) (Dz. U. z 2021 r. poz. 76);
- ∗
- Regulation of the Minister of Agriculture and Rural Development of 18 June 2008 enforcing selected provisions of the Act on fertilisers and fertilisation (Journal of Laws, 2008, No. 119, item 765; 2009, No. 224, item 1804);
- ∗
- Regulation of the Minister of the Economy of 8 September 2010 on packaging mineral fertilisers, fertiliser labelling, testing mineral fertilisers and types of lime fertilisers (Journal of Laws, 2010, No. 183, item 1229).
The following products and substances that improve plant growth are listed in Article 2, points 7–10a, of the Act of 10 July 2007 on fertilisers and fertilisation [12]:
- Substances that improve soil properties—substances that are applied to soil to improve its properties, as well as chemical, physical, physicochemical and biological parameters and are not classified as fertilising products in the EU;
- Growth promoters—organic or mineral substances or mixtures thereof that promote plant development and other life processes in plants, which are not classified as fertilising products in the EU, excluding growth regulators defined as plant protection products in plant protection regulations;
- Agricultural substrates—growth substrates other than soil, including substrates for plant cultivation, which are not classified as fertilising products in the EU;
- Substances promoting plant growth—substances that improve soil properties, growth promoters and agricultural substrates;
- Microbiological fertilisers—products composed exclusively of microorganisms, including dead or inactivated microorganisms, microbial consortia, microbial growth substrates and their metabolites, as well as harmless residual components from such substrates that enhance soil biological activity or stimulate metabolic processes in plants and fungi, which are applied for the sole purpose of improving their plants’ and fungi’s ability to effectively utilize nutrients, increasing resistance to abiotic stress, improving qualitative parameters and promoting the assimilation of soil nutrients that are not readily available to plants.
There is no legally binding definition of a biostimulant in Poland. Biostimulants are not registered as a separate group of products, and they may be classified in one of the three categories: fertilisers, plant protection products and other categories of products. In Europe, the European Biostimulants Industry Council (EBIC) defined plant biostimulants as follows: “Plant biostimulants contain substance(s) and/or micro-organisms whose function when applied to plants or the rhizosphere is to stimulate natural processes to enhance/benefit nutrient uptake, nutrient efficiency, tolerance to abiotic stress and crop quality (Table 1). Biostimulants have no direct action against pests, and therefore do not fall within the regulatory framework of pesticides” [13].

Table 1.
Common benefits of biostimulants reported by EBIC [14].
2.2. Biostimulants’ Definitions and Classifications
Biostimulants play a significant role in agriculture by enhancing physiological and biochemical processes in plants and promoting plant adaptation to adverse environmental conditions such as drought, salinity, high temperature and nutrient depletion. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 [15] identifies two types of plant biostimulants: microbial and non-microbial [16]. In the above regulation, a plant biostimulant is defined as “a product stimulating plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (a) nutrient use efficiency; (b) tolerance to abiotic stress; (c) quality traits; (d) availability of confined nutrients in soil or rhizosphere”. The following definition was proposed by Yakhin et al. [17]: “a biostimulant is a formulated product of biological origin that improves plant productivity as a consequence of the novel or emergent properties of the complex constituents, and not as a sole consequence of the presence of known essential plant nutrients, plant growth regulators, or plant protective compounds”. This definition contributes very important information by indicating that a product’s biological function can be influenced by particles or combinations of particles whose mechanism of action has not been fully elucidated. The above suggests a particle’s mechanism of action is more important than its mode of action. If a given particle is responsible for a biostimulant’s biological function, the preparation should be classified based on the discovered function. It is also debatable whether preparations whose composition and mechanism action have not been fully determined should be registered as biostimulants. To some extent, biostimulants are first defined by what they are not, drawing a line between biostimulants and other widely used categories of plant substances: fertilisers and pesticides. Secondly, it has been shown that the positive effects attributed to chemical biostimulants—growth promotion, modulation of development and quality traits, increased tolerance to environmental stress—can also be provided by bacteria and fungi. Biostimulants exert a specific influence on plant growth, but their ingredients interact with often unpredictable effects. What we know about one biostimulant or plant species is not directly transferable to another biostimulant or plant species, as biostimulant effects are clearly species- and product-specific. A combination of several preparations can be more effective than the application of individual compounds [18]. For example, Khan et al. [19] observed that there is insufficient scientific evidence to confirm the presence of growth factors in seaweed or the mechanisms by which they affect plant growth. The mechanisms initiated by biostimulants remain unknown and are difficult to identify because most of these substances contain plant extracts, seaweed extracts (SWE) and amino acids. Biostimulants’ effects could result from synergistic interactions between ingredients [20].
Biostimulants enhance natural processes in plants that increase nutrient uptake and nutrient use efficiency; improve crop quality and tolerance to abiotic stress; maximize yields and crop vitality and reduce susceptibility to stress, pests and other threats, including climate change (Table 2). Biostimulants can help farmers adapt their production systems to climate variability, contribute to sustainable food production and promote the implementation of environmentally friendly farming models that are resilient and flexible [21]. Biostimulants synthesize compounds that inhibit pathogen growth, induce the production of allelochemicals and modify the composition of plant secretions to fight pests and pathogens. As a result, biostimulants effectively eliminate microbial pathogens [22].
Biostimulants are biologically active substances which contain hormones, enzymes, proteins, amino acids, mineral elements and other compounds that are applied in small concentrations to enhance plant metabolism and growth. These preparations regulate life processes in cells, organs and entire organisms [23,24]. Biostimulants have different mechanisms of action than fertilisers, and they are applied in small doses to stimulate plant growth. Low concentrations of nanoparticles (NPs) have biostimulatory effects and enhance plant growth. Nanoparticles and nanomolecules induce changes in the surface charge density of cells and are responsible for non-specific nano–bio-interactions [3].
Many products are marketed as plant biostimulants despite the fact that their composition and efficacy have not been scientifically validated. An unknown number of unregistered preparations are available in Poland. Registered preparations are researched and tested by specialised bodies, and the use of products sold without registration carries the risk that they are not only ineffective, but also unsafe [25]. Biostimulants have significant growth-promoting potential, and there is considerable empirical evidence to indicate that they deliver benefits in plant production. Growing levels of environmental awareness and legal regulations have contributed to the growth in the biostimulant sector. However, the metabolic responses of plants to biostimulants have not been sufficiently researched to date [9,26].
Biostimulants offer a promising and environmentally friendly alternative to mineral fertilisers. They contribute to agricultural sustainability and the evolution of biotechnology in the 21st century [27]. Improving tolerance to biotic and abiotic stresses, a major obstacle to the production of high quality crops and global food security, is a major benefit in agriculture. Improvement of soil fertility, promotion of plant growth and suppression of phytopathogens are the goals of the bioformulation industry, leading to the development of an eco-friendly environment. Bioformulations offer an environmentally sustainable approach to improving crop production and health. They are a major contributor to making the 21st century the age of biotechnology [28].
2.3. Biostimulants Categories
Plant biostimulants (PBs) contain substances and/or microorganisms that are applied to plants or the rhizosphere to stimulate natural processes, enhance nutrient use efficiency, improve tolerance to abiotic stress and improve crop quality, regardless of their nutrient content. Products containing a combination of such substances and/or microorganisms are also marketed as biostimulants [29,30]. Biostimulants are formulated with the use of high-precision tools, and they are applied to soil, plants or seeds to modify physiological processes, enhance plant growth and development, improve nutrient uptake and minimize susceptibility to stress. Biostimulants contain SWE, plant extracts, protein hydrolysates (PHs), humic acids (HA), fulvic acid (FA) and other substances. Solid formulations are applied directly to soil, whereas liquid biostimulants are sprayed onto leaves. In the past, biostimulants were applied only in organic farms, but, at present, they are also used in conventional and integrated crop production systems [31,32].
Nine categories of substances that act as biostimulants have been defined in [16]:
- Humic substances;
- Complex organic materials (obtained from agricultural, industrial and urban waste, sewage sludge extracts, compost and manure);
- Beneficial chemical elements (Al, Co, Na, Se and Si);
- Inorganic salts, including phosphite;
- SWE (brown, red and green macroalgae);
- Chitin and chitosan derivatives;
- Antiperspirants (kaolin and polyacrylamide);
- Free amino acids and N-containing substances (peptides, polyamines and betaines);
- Plant growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi (AMF) and Trichoderma spp.
In turn, Du Jardin [29,33] identified seven categories of biostimulants:
- HAs and FA;
- PHs;
- SWE;
- Chitosan;
- Inorganic compounds;
- Beneficial fungi;
- Beneficial bacteria.
The first world congress on the use of biostimulants in agriculture was held in Strasbourg in November 2012. On the basis of agricultural applications, biostimulants can be categorized into different types. The most commonly used biostimulants in the production of forage grasses and turfgrasses include natural preparations containing humic acids (HAs), fulvic acid (FA), protein hydrolysates (PHs), seaweed extracts (SWE), chitosan, inorganic compounds and beneficial fungi and bacteria as the active ingredients (Figure 1). Grasses are also supplied with synthetic biostimulants containing nutrients and phenolic compounds. These categories are briefly introduced in the next section and described in more detail in the accompanying papers in this special issue on plant biostimulants in forage and turfgrass production.
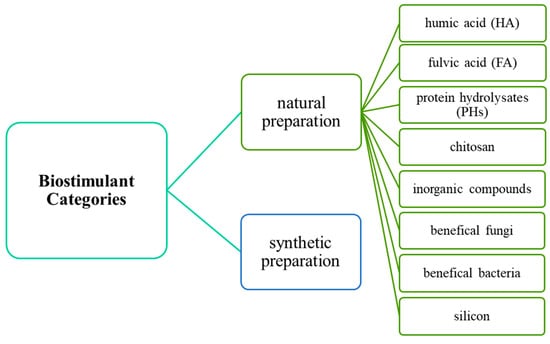
Figure 1.
Different biostimulant classes [15,32,33].
Biostimulants can be also classified based on the method of application (soil, foliar), origin (plant-based, animal-based) and the production process (hydrolysis, fermentation, extraction). Natural soil biostimulants enhance the growth of beneficial soil microorganisms that increase nutrient uptake by plants. The use of natural biostimulants that do not exert harmful impacts on the environment is particularly important due to the ongoing processes of soil degradation and air pollution [4].
The effectiveness of biostimulants is determined by their composition. Biostimulants contain various organic and mineral compounds that are used as metabolites by plants (Table 2). There is considerable scientific evidence to indicate that biostimulants exert a positive influence on crops, but their impact is more likely to be determined by crop species and variety than by the applied dose [34]. However, the efficacy of biostimulants in the production of forage grasses has not been thoroughly investigated to date [35].

Table 2.
Examples of different active ingredients used for commercial biostimulants.
Table 2.
Examples of different active ingredients used for commercial biostimulants.
| Biostimulant | Active Ingredients |
|---|---|
| Liqhumus and Fulvagra | active HA which is extracted from leonardite by alkaline extraction with KOH [36] |
| Fulvagra | mostly FA, which is removed from groundwater from aquifers beneath a peat layer during the treatment of drinking water [36] |
| Humus-Active Papka | N—0.2, P—1.3, K—4.6, Ca—3.0, Mg—0.5 g kg, Mn—15, Fe—500, Zn—3, Cu—1 mg kg; active humus and useful microorganisms [37,38,39] |
| Algex | extract of Ascophyllum nodosum seaweed containing vitamins, amino acids, phytohormones (auxins, cytokinins, gibberellins), polysaccharides, betaine, macronutrients and micronutrients, including N—8%, P—3.6%, K—7%, B—0.036%, Zn—0.025%, Cu—0.009%, Fe—0.016%, Mn—0.036% and Mo—0.0036% [6] |
| Bio-Algeen S90 | extract of Ascophyllum nodosum containing 90 groups of chemical compounds, such as amino acids, vitamins, alginic acid and other active ingredients of marine algae. N—0.2, P2O5—0.06, K2O—0.96, CaO—3.1, MgO—2.1 g kg, B—16.0, Fe—6.3, Cu—0.2, Mn—0.6, Zn—1.0 mg kg, Mo, Se, Co [40] |
| Kelpak SL | extract of Ecklonia maxima seaweed containing natural plant hormones: auxins (11 mg × L−1) and cytokinins (0.03 mg × L−1) [21,40,41] |
| CPR | a blend of natural sea plant extracts, micronutrients and surfactants containing 4% nitrogen (N), 1% K2O, 0.53% magnesium, 1% sulfur, 2% iron (Fe), 0.25% manganese (Mn) and 0.2% zinc (Zn) [42] |
| TurfVigor | contains 0.014% patented microbial strains (Bacillus spp. and Paenbacillus spp.), kelp extract, macro- and micronutrients: 9% N, 3%P2O5, 6% K2O, 0.6% Fe, 0.05% Mn and 0.05% Zn [42] |
| Bactofil-A and Bactofil-B | contain various combinations of Azospirillum brasilense, Azospirillum lipoferum, Azotobacter vinelandii, Bacillusmegaterium, Bacillus polymyxa, Bacillus circulans, Bacillussubtilis, Pseudomonas fluorescens, Streptomyces and plant growth hormones [43] |
| Blatt Boden-Foliar | a microbial solution that consists mainly of lactic acid bacteria: Lactobacillus casei (5 × 109 cfu mL−1) and Lactobacillus plantarum (5 × 109 cfu mL−1); photosynthetic bacteria: Rhodopseudomonas palustris (5 × 109 cfu mL−1); yeasts: Saccharomyces cerevisiae (5 × 103 cfu mL−1) and sugarcane molasses [44] |
| UGmax | N—1.2, P—0.2, K—2.9, Mg—0.1 g kg, Na—0.2, Mn—0.3 mg kg, lactic acid bacteria, photosynthetic bacteria, Azotobacter spp., Pseudomonas spp., yeasts, actinomycetes [37,38,39] |
| Eko-Użyźniacz | N—0.6, P—0.3, K—0.7 g kg, endomycorrhizal fungi, bacteria, earthworm fibrinolytic enzymes [37,39] |
| Asahi SL (Atoniki and Chaloperone) | 0.3% para-nitrophenol sodium salt, 0.2% ortho-nitrophenol sodium salt, 0.1% 5-nitroguaiacol sodium salt [6,40] |
| Tytanit (Mg-Tytanit) | 8.5 g Ti per1 dm3 (0.8% m/m) in the form of Ti-ascorbate [21,45,46] |
| AGRO-SORB Folium | a growth stimulant containing 18 biologically active free amino acids (L-alpha) obtained via enzymatic hydrolysis (minimum 9.3% w/w or 100 g per 1000 mL): aspartic acid—0.450%, serine—0.321%, glutamate acid—1.814%, glycine—2.743%, histidine—0.208%, arginine—0.131%, threonine—0.323%, alanine—0.524%, proline—0.347%, cysteine—0.435%, tyrosine—0.174%, valine—0.551%, methionine—0.349%, lysine—0.661%, methionine—0.308%, leucine—0.180%, phenylalanine—0.218% and tryptophan—0.05% [47] |
| Optysil | contains silicon (16.8% SiO2) and 2% chelated iron [48,49] |
| Herbagreen | contains silicon (17% SiO2), calcium (36.7% CaO), magnesium (2.4% MgO), iron (3.4% Fe2O3), titanium (0.5% TiO2) and other ingredients [48] |
| Stymjod | concentrated liquid fertiliser produced with the use of the cold plasma nanotechnology. The product contains an optimal ratio of macronutrients (N—6.3%; P—4.58%; K—6.42%; Mg—1.69%; S—1.6%) and micronutrients (B—0.46%; Cu—0.17%; Fe—0.14%; Mn—0.16%; Mo—0.028%; Zn—0.42%) for plants, as well as humic and amino acids that increase plant resistance to unfavourable environmental conditions [50,51] |
Biostimulants are widely used in agriculture as seed dressing agents and foliar sprays during the growing season and harvest. Biostimulants exert varied effects by triggering N metabolism and the release of phosphorus (P) from soil, stimulating soil microbial activity and root growth, promoting plant metabolism, stimulating germination, enhancing photosynthesis and nutrient uptake from soil and increasing agricultural productivity.
3. The Use of Biostimulants in the Production of Forage Grasses
In the European Union (EU-27), permanent grasslands occupy an area of around 60 million hectares [52] and account for 33% of total agricultural area in the EU [53]. Grasslands cover more than 45% of the Earth’s land surface and constitute a source of food for livestock and indigenous herbivores on nearly one-third of the Earth’s ice-free terrestrial surface [54,55]. In these ecosystems, most grasses are perennial species of the family Poaceae, which includes around 10,000 species and 700 genera [56]. Grasses are a source of food for both wild and domesticated animals. They have medicinal and health-promoting properties, and they easily adapt to various climatic zones and habitats. Grass species form communities in savannas, steppes, prairies and pampas, as well as in lowland, highland and arctic meadows [57].
In Poland, the area of permanent grasslands has decreased considerably in recent years. At present, permanent grasslands cover around 20% of farmland and occupy an area of 3,127,800 ha (2019), including 2,764,000 ha for meadows and 363,800 ha for pastures. Permanent grasslands are the most environmentally friendly approach to agricultural land use. In addition to supplying forage crops, grasslands also play important non-productive roles, including climatic, hydrological, protective, phytosanitary, health-promoting, landscaping and aesthetic. The biodiversity of these ecosystems is the main factor that contributes to their high natural and agricultural value [58,59]. Meadows and pastures play a critical role in the global carbon cycle, effectively manage grasslands capture and store atmospheric carbon [53]. Using biostimulants in forage grasses can have a number of positive effects on grass development and forage value (Figure 2, Table 3).
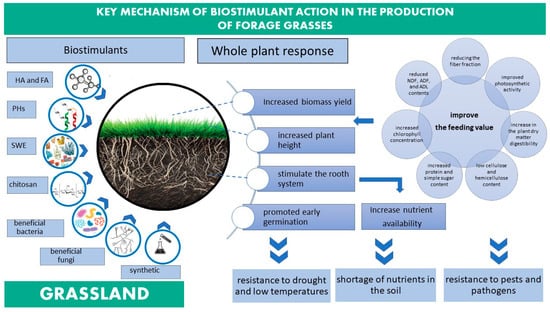
Figure 2.
Key mechanisms targeted by biostimulants in forage grasses.

Table 3.
Summary of species, biostimulants and effects in forage grass production.
3.1. Natural Biostimulants
3.1.1. Humic and Fulvic Acids
The interactions between these compounds lead to the formation of humic substances and humic complexes in soil. Crop yields can be improved by optimizing the interactions between soil microbiota and plant roots. For this reason, humic substances (soluble fractions of HA and FA) exert diverse yet globally positive effects on plant growth.
Humic substances are increasingly used as agricultural biostimulants due to their potential beneficial influence on nutrient uptake and crop yields [36]. Humic substances exert biostimulatory effects by inducing structural and physiological changes in plant roots and shoots which are responsible for the uptake, assimilation and distribution of nutrients. Humic substances can also alter primary and secondary metabolic processes associated with tolerance to abiotic stress, plant growth and resistance. Humic substances are biological activators of plant growth, and their effectiveness is strongly determined by their chemical properties, including hydrophobicity, conformational elasticity and functional group. The potential use of growth-promoting humic substances from waste materials has been recognized in several European projects, including BIO-FERTIL (Poland), BIOFECTOR (Germany) and HUMIC-XL (The Netherlands) [3].
The nutritional value of forage grasses is largely determined by the content of structural and non-structural carbohydrates and lignin. Structural carbohydrates and lignin decrease the digestibility and energy value of feed, whereas non-structural carbohydrates improve the nutritional value and palatability of forage plants and determine their applicability and processing suitability [77]. In a study by Wiśniewska-Kadżajan and Jankowski [38], slurry combined with soil conditioners and fertilisers (UGmax and Humus Active) increased hemicellulose content and decreased the content of non-structural carbohydrates in Festulolium braunii K. Richt. A. Camus. Using humic acid-based fertiliser Humus Active in combination with slurry increased hemicellulose content by around 23%. In contrast, the content of non-structural carbohydrates decreased by approximately 35% compared to plants treated with slurry alone. The authors of the study highlighted that the content of cellulose and hemicellulose in the grass varied significantly, depending on the growing season. This variation was, without a doubt, linked to the weather conditions during grass growth. The studies conducted by Ciepiela [78] and Golińska and Kozłowski [79] confirmed comparable seasonal changes in the compound content of grasses.
One of the greatest advantages of HA is that they increase the water-holding capacity of light soils and minimize the risk of drought. Humic substances also improve plant nutrient availability, stimulate the development of the root system and increase the abundance of beneficial microorganisms in soil [60]. According to White et al. [32], humic substances applied at concentrations as low as 0.01% stimulated the roots of annual meadow grass (Poa annua L.) to increase the internalization of bacteria into root cells. Humic substances promoted vertical root growth and root hair elongation. The biostimulating effects of humic substances may be due partly to their rhizophagy- and endophytism-enhancing properties. In contrast, in a study by Regelink and Koopmans [36], humic Liqhumus and fulvic acids Fulvagra biostimulants had no effect on the growth of rough meadow-grass (Poa trivialis) or N and P uptake by this species. The authors explained that the lack of effect of the tested humic substances is due to the fact that the doses of humic substances applied are low compared to the amount of soil organic carbon already present in the soil, meaning that the humic substances are adsorbed on the reactive mineral surface of the soil and, therefore, may not be able to interact with plant roots as would be the case if applied in a hydroponic or soilless substrate, and that the effect of humic substance addition on ortho-P availability can only be expected at high P loads (i.e., 0.4–0.6 mol mol−1), whereas the soil in the pot experiment had lower P loadings (i.e., 0.09 and 0.29 mol mol−1).
Humic substances have a diverse range of mechanisms by which they influence plants. They enhance soil water retention capacity, which mitigates the risk of drought. Additionally, they improve nutrient availability, promote root system development and increase the abundance of beneficial soil microorganisms. Empirical research has indicated that low concentrations of humic substances can stimulate root growth and affect their capacity for bacterial internalisation. However, the effect of these substances may vary depending on the type of humic compound and soil conditions.
3.1.2. Seaweed Extracts
Seaweed (macroalgae) and algae extracts have been used for centuries to improve soil properties and enhance the quality and productivity of agricultural crops and turfgrasses. Both whole seaweeds (mulch) and their components (compost) have been applied as organic fertilisers to increase soil fertility and promote crop growth [68]. Research has shown that commercial SWE improves root growth, plant health, biomass yields and quality and genetic pathways in plants [19,80].
Macroalgae are classified into three main groups based on their pigmentation: green (Chlorophyta), brown (Ochrophyta) and red (Rhodophyta). Many seaweed species (mainly brown algae such as Sargassum spp. and Turbinaria spp.) are applied as biofertilisers [19,81]. Brown algae extracts stimulate plant growth and increase tolerance to abiotic stresses such as high salinity, extreme temperature, nutrient deficiency and drought. Seaweed extracts contain complex polysaccharides, fatty acids, vitamins, phytohormones and minerals [82].
Bio-Algeen S90 is a natural extract from Ascophyllum nodosum brown seaweed that contains around 90 groups of chemical compounds, including amino acids, vitamins, alginic acid and other biologically active components. In a study by Biernacki et al. [83], Bio-Algeen S90 natural extract from Ascophyllum nodosum applied with mineral fertilisers increased yields in mixed stands of forage grasses. When biostimulants were used in conjunction with mineral phosphorus and potassium, the yield increased by up to 40%.
Ecklonia maxima is a species of brown algae that is typically found along the southern Atlantic coast of Africa. Kelpak is the brand name of a biostimulant containing Ecklonia maxima extract. In the work of Sosnowski [41], Kelpak extract from Ecklonia maxima increased biomass yield, number of shoots and leaf length in Festulolium braunii. The greenness index of leaves in plots sprayed with the highest concentration (60%) of Ecklonia maxima extract Kelpak was characterized by significantly higher SPAD values. Using seaweed extracts at a 20% concentration resulted in an over 17% reduction in this parameter in comparison to the control treatment. The authors observed that preparations have a powerful effect on plant hormones, thereby confirming their effectiveness in practice. Furthermore, this is dependent on the method of application and the type and variety of plant.
Godlewska and Ciepiela [60] found differences in the protein and carbohydrate content of the grass species and cultivars (Dactylis glomerata L., cv. Amila and Tukan; Festulolium braunii, cv. Felopa and Agula) studied in relation to the application of Ecklonia maxima extract. The highest levels were observed in Festulolium braunii cv. Felopa cultivar. In a study by Godlewska and Ciepiela [60], the protein and sugar contents of the grass also depended on the cutting. The number of protein compounds was significantly higher in plants from the third cut, and the number of monosaccharides was highest in plants from the first cut. The authors suggest that the significantly higher levels of sugars found in the tested plants harvested in mid- and late summer may have been caused by an increase in plant respiration, which makes use of sugars, and tends to be more intense at higher temperatures. In another study, Ciepiela [61] observed an increased content of non-structural carbohydrates in Festulolium braunii and orchard grass after seaweed extract application. Ciepiela [61] reported that Kelpak SL decreased the content of cellulose, hemicellulose and lignin and increased the content of non-structural carbohydrates in Festulolium braunii and orchard grass.
In a different study [84], the authors found that the tested biostimulant also reduced the content of neutral detergent fibre (NDF) and acid detergent fibre (ADF) in the studied grass species (Dactylis glomerata L., cv. Amila and Tukan and Festulolium braunii A. Camus cv. Felopa and Agula). Matysiak [62] suggested that the impact of seaweed extract may be linked to the plant’s genotype and dependent on the individual cultivar characteristics. According to the author’s observations, the tested species and cultivars of grasses differed significantly in terms of the components studied. Festulolium braunii cultivar Agula had the highest nutritional value. Grass yields and concentrations of the examined components varied significantly during the growing season. The initial cut produced the highest biomass yield and ADF content in the tested grasses, while the third cut resulted in the highest nitrogen content. Kelpak SL significantly increased the content of protein compounds and simple sugars, as well as the carbohydrate-to-protein ratio in Festulolium braunii and orchard grass (Dactylis glomerata L.). The analysed biostimulant also reduced the content of neutral detergent fibre (NDF) and acid detergent fibre (ADF) in the studied grass species. Ciepiela [61] reported that Kelpak SL decreased the content of cellulose, hemicellulose and lignin and increased the content of non-structural carbohydrates in Festulolium braunii and orchard grass.
In another study, Godlewska and Ciepiela [63] found that Ecklonia maxima extract significantly increased the content of Zn, Cu, Fe and Mn in Festulolium braunii and orchard grass. According to the cited authors, N fertilisation significantly influenced micronutrient levels in both grass species. The content of Zn, Cu and Fe (but not Mn) decreased with a rise in the N rate. The seaweed extract increased N content on a dry matter basis and improved yields in both grass species [85]. Godlewska and Ciepiela [45] also reported that Ecklonia maxima extract enhanced the chemical composition of perennial ryegrass (Lolium perenne L.) and improved the quality of the produced forage by reducing the content of structural carbohydrates and lignin. Godlewska and Ciepiela [6] observed an improvement in the dry matter digestibility of annual ryegrass (Lolium multiflorum L.) supplied with the extract of Ascophyllum nodosum. In a study by Ciepiela [35], Ascophyllum nodosum extract also increased annual ryegrass yields.
The effect of biostimulants on plants can be modified by weather conditions. In a study by Szczepanek [11], weather influenced the response of maize plants to the applied dose of Ecklonia maxima extract and the duration of treatment. The tested biostimulant improved grain yields in years characterized by rain deficit in the stem elongation stage. Ratajczak et al. [86] also concluded that biostimulants, including Ecklonia maxima extract, can be used to enhance yields and the physiological status of plants exposed to abiotic stress (such as low temperature) and to improve crop productivity, especially in organic farming. The application of SWE promoted growth, development and photosynthetic activity in maize.
The use of biostimulants, such as natural extracts derived from algae like Ecklonia maxima, has been researched for its beneficial impacts on plant growth and yield. These extracts have demonstrated the ability to augment biomass yield, shoot count and leaf length in several types of grass species. Additionally, they can heighten essential micronutrient concentrations, particularly for zinc, copper, iron and manganese. Biostimulants can also enhance the regulation of plant hormones and affect plant physiology, depending on the application methods and types of plant. Their ability to improve crop productivity, particularly in harsh conditions, offers potential for sustainable agriculture.
3.1.3. Effective Microorganisms
Terrestrial plants associate with root microbiota that differ from the complex microorganisms in the surrounding soil. The microbiota colonizing the rhizosphere (soil surrounding the roots) and the endophytic compartment (within the root) promote plant growth, productivity and phytoremediation [87]. Bacteria, yeasts and fungi are used in the production of biostimulants [17]. Biostimulants increase the microbial activity of soil, plant nutrient availability and stimulate plant growth [43].
The phytomicrobiome delivers numerous benefits by stimulating plant growth and nutrient uptake, increasing soil fertility and releasing and modulating extracellular metabolites such as hormones, antimicrobial compounds and signalling molecules that protect plants against phytopathogens. The use of effective microorganisms (EM) in farming creates new prospects for sustainable agronomy practices [27].
Various microbial biostimulants are available on the market. According to the manufacturers, these products enhance plant growth, increase yields and improve soil conditions. Biostimulants supply beneficial microbial inoculants to the soil and stimulate the activity of soil microorganisms. Suspension fertilisers are usually applied to the soil in very low concentrations. In many cases, the microbial composition of biofertilisers is not described, which prevents a reliable assessment of these products. The effectiveness of biostimulants containing beneficial microorganisms has not been scientifically confirmed to date.
Bacteria of the genus Azospirillum are among the most widely researched PGPR. These facultative endophytes occur in the soil and are able to colonize external and internal plant tissues [88]. Effective microorganisms are widely used as biofertilisers [43]. This group of products also includes Bactofil-A and Bactofil-B (Agrinova GmbH), which, according to the producer’s specification, contain N-fixing and P-mobilizing bacteria, in particular, Bacillus subtilis, natural humates and SWE (Ascophyllum nodosum). According to the manufacturer, Bactofil-A and Bactofil-B mobilize plant nutrients, fix atmospheric N, enhance soil microbial activity and increase plant resistance to pathogens [43]. However, Schenck [43] found that EM-based products Bactofil-A and Bactofil-B reduced the composition and abundance of soil microorganisms in the cultivation of perennial ryegrass without exerting any effects on plant growth. The cited author concluded that these products deliver opposite effects to those postulated by the manufacturer. In turn, Olszewska [44] reported that Blatt Boden-Foliar and Blatt Boden-Multical biostimulants applied to the leaves improved the chemical composition of perennial ryegrass. The foliar application of Blatt Boden-Foliar and Blatt Boden-Multical increased the content of CP, P and chlorophyll in perennial ryegrass leaves, whereas it decreased the accumulation of CF, K and Ca in plants. According to the cited author, these products have important practical implications because they reduce fertiliser use and minimize adverse environmental impacts in the production of high-quality forage grasses. In turn, in a study by Biari et al. [89], maize plants inoculated with EM assimilated more nitrogen, phosphorus, potassium, iron, zinc, manganese and copper, which increased grain yields.
In addition to the chemical composition of forage grasses, fodder quality is also affected by the ratio of soluble carbohydrates to total protein. An increase in this ratio improves the nutritional value of plant biomass. The use of UGmax biostimulant in the production of annual ryegrass expanded the carbohydrate-to-protein ratio, increased crude ash content, improved organic matter digestibility and increased dry matter yield [64]. Jankowski et al. [39] also reported an increase in dry matter yield, crude protein content and the concentrations of soluble carbohydrates in orchard grass and perennial ryegrass in response to the application of UGMax and mineral fertilisers. Dry matter yield and crude protein content peaked when perennial ryegrass was supplied with Eko-Użyźniacz biostimulant in combination with mineral fertilisers. Truba at al. [37] observed an increase in the cellulose and hemicellulose content of perennial ryegrass and orchard grass after the application of UGmax. However, in a study by Sosnowski [68], UGmax had no effect on the nutritional value of perennial ryegrass, meadow fescue or orchard grass in all years of the study. The analysed biostimulant did not induce significant differences in NDF and ADF content, dry matter intake or digestibility. According to Sosnowski’s research [65,65,66,67], grasses grown on objects fertilised with minerals and additionally supported with a soil fertiliser have the highest yield of biomass on the ground. The yield of grasses on similar research was, on average, over 37% higher than the control (Festulolium braunii, Dactylis glomerata, Festuca pratensis and Lolium perenne). However, there is a natural yield increase of 20% in the normal control with Lolium multiflorum when NPK fertilisation with UGmax is used. The reaction of different grass species to the biostimulator varied, as the author highlighted.
In the work of Lopes et al. [69], EM (Burkholderia pyrrocinia rhizobacteria and Pseudomonas fluorescens) improved shade tolerance in Brachiaria brizantha by increasing chlorophyll content and stimulating the production of osmoregulating compounds such as carbohydrates and proteins. Biostimulants promoted root development, increased plant height, leaf area and biomass yields. According to the cited authors, biostimulants can minimize the adverse effects of shading, thus contributing to the growth and survival of forage grasses in integrated crop–livestock–forestry systems. Effective microorganisms also exerted positive effects on spring wheat in the work of Piskier [90]. In the cited study, an EM biopreparation applied to the soil and leaves increased grain yields by up to 23% by improving yield components and the biometric parameters of wheat plants.
Effective microorganisms (EMs) comprising Bacillus subtilis, humates and seaweed extract (SWE) have potential as biofertilisers to enhance microbial activity, mobilize nutrients and fix nitrogen. However, contrasting results have been observed. Biostimulants such as UGmax modify the chemical composition of forage grasses, improving their nutrient content and soluble carbohydrate-to-protein ratio. EM biopreparations contribute to shade tolerance in Brachiaria brizantha and enhance growth parameters in spring wheat. These findings indicate the varied impact of biostimulants on grasses, affecting nutrient assimilation, quality improvement and shade tolerance.
3.1.4. Chitosan
Chitosan is an aminopolysaccharide composed of N-acetyl glucosamine and D-glucosamine linked by β(1–4) glycosidic bonds, and it is derived from chitin through rapid alkaline deacetylation [3]. Chitin and chitosan are naturally occurring compounds that are used in agriculture to control plant diseases. These compounds exhibit toxic effects and inhibit fungal growth and development. They have also been shown to protect plants against viruses, bacteria and pests. Chitin and chitosan fragments trigger defence mechanisms in host plants in response to microbial infections, including the accumulation of phytoalexins, pathogen-related proteins, protease inhibitors, lignin synthesis and callose formation [91]. Chitosan stimulates photosynthesis and stomatal closure through ABA synthesis; it stimulates antioxidant enzymes via nitric oxide and hydrogen peroxide signalling pathways and induces the production of organic acids, sugars, amino acids and other metabolites that participate in osmotic regulation, stress signalling and energy metabolism under exposure to environmental stressors. Chitosan is an antitranspirant compound that is applied to leaves to reduce water use and protect plants against stressors. Due to its beneficial effects, chitosan is used in sustainable agriculture to combat the negative effects of climate change. Chitosan also forms complexes with heavy metals, and it is applied for the phytoremediation and bioremediation of soil [92], e.g., in the production of grasses. In a study by Kamari et al. [70], chitosan decreased heavy metal concentrations in soil sown with perennial ryegrass.
Biostimulants containing chitosan promoted early germination and elongation of leaves in perennial ryegrass [93]. In a study by Agbodjato et al. [71], chitosan improved seed germination and promoted the growth of maize plants in the vegetative stage. According to the cited authors, chitosan combined with rhizobacteria exerted more beneficial effects than chitosan alone. These results validate the observation that a combination of two biostimulants can be more effective than a single product. Chitosan stimulates various enzymes that scavenge reactive oxygen species such as superoxide dismutase (SOD), catalase and peroxidase. The signal transduction pathways from chitosan, which elicits its responses, involves hydrogen peroxide and nitric oxide signals; chitosan can also directly control gene expression by interacting with chromatin. This compound has been used as a biostimulant to enhance plant growth, increase tolerance to abiotic stresses and induce plant resistance to pathogens. However, these responses are highly complex, and they are conditioned by various chitosan-based structures and concentrations, as well as by plant species and stage of development [94]. According to Yin et al. [95], oligochitosan is a potential plant vaccine, and its effectiveness is comparable with vaccines that have been developed for human and animal use [96].
Chitosan is a deacetylated derivative of chitin which has antifungal properties and triggers resistance to fungal pathogens in plants [97,98]. However, in the work of Hofgaard et al. [93], a chitosan biostimulant applied to perennial ryegrass did not increase its resistance to pink snow mould.
The chitosan mechanism of action on grass involves several key aspects. Firstly, chitosan forms complexes with heavy metals, aiding in the purification of soil from these pollutants. Secondly, chitosan stimulates early germination and leaf elongation, thereby accelerating grass development. Thirdly, by activating enzymes such as superoxide dismutase (SOD), catalase and peroxidase, chitosan neutralises reactive oxygen species, protecting plants from oxidative stress. Additionally, chitosan induces signals related to hydrogen peroxide and nitrogen oxide and can directly affect gene expression through interaction with chromatin. These diverse chitosan mechanisms promote plant growth, enhance tolerance to abiotic stress and induce resistance to pathogens.
3.1.5. Silvit
Silvit is a natural growth biostimulant which contains plant-available silicon. This product inhibits tissue penetration by pests (by increasing cell wall resistance to pathogenic enzymes), increases tolerance to low temperature and drought, intensifies photosynthesis under limited light access and minimizes the adverse effects of salinity on plant growth [99].
Radkowski et. al. [74] have shown that silage produced from silicon-fertilised sward have a higher total protein content, nutritional value of protein and energy compared to that produced from non-fertilised swards. According to the authors, the trend towards higher protein content can be attributed to the higher proportion of legumes in the botanical composition of the sward and the higher protein content of these plants. Borawska-Jarmułowicz et al. [48] reported that silicon fertilisers (Optimal, Herbagreen) increased the crude protein content of mixed grass stands with a high share of Festulolium braunii and red clover during periods of drought. The nutritional value of mixtures was found to differ over the years when subjected to silicon fertilisation, and this variation was dependent on the botanical composition. Under low rainfall conditions, a significant proportion of F. braunii combined with T. pratense, which contributed to roughly 20% of the proportion, resulted in a higher content of crude protein. The authors concluded that these results could be utilized for fertilising temporary grassland to improve its nutritional value, particularly crude protein content, while also reducing fibre levels in the sward. Mastalerczuk et al. [73] also observed the beneficial effects of using silicon in organic farming. This included improved yield of grass and clover swards, as well as higher crude protein content and better organic matter digestibility.
In a study by Radkowski et al. [74], the milk of cows fed ensiled grass from a pasture fertilised with a biostimulant was characterized by a higher content of dry matter, protein and fat, as well as higher overall and microbiological quality. Milk yields and milk protein, dry matter and fat levels were highest in the group of cows fed ensiled plants fertilised with a higher rate of silicon.
The mechanism of silicon on grasses is multifactorial. Silicon may enhance plant metabolism, leading to greater production of nutrients. Furthermore, it provides support for plant defence, aiding plants in combating drought and disease. The augmented protein content in grasses could stem from enhanced cell structures that manage nitrogen storage and increased assimilation of minerals, including nitrogen. The yield and botanical composition of grasses may benefit from silicon due to improved soil structure and greater efficiency in nutrient utilisation. When fed to cows in the form of ensiled grass, silicon may enhance milk composition by promoting better nutritional performance of the grasses.
3.2. Synthetic Biostimulants
Nutrients and Phenolic Acids
Titanium (Ti) is a growth stimulator, and Ti ions are easily available to plants. Even small quantities of Ti enhance plant growth and increase stress resistance [94]. The biostimulant sold under the commercial name of Tytanit contains 8.5 g dm−3 of chelated Ti. Titanium belongs to a group of elements with the lowest phytoaccumulation index, and it stimulates the activity of select enzymes, including catalase, peroxidase, lipoxidase and nitrate reductase [100].
Titanium is regarded as a beneficial element for plant growth. When applied to roots and leaves at low concentrations, Ti stimulates the activity of certain enzymes, increases chlorophyll content, stimulates photosynthesis, promotes nutrient uptake, improves stress tolerance and increases yields and crop quality. Commercial fertilisers containing Ti, including Tytanit and Mg-Tytanit, have been used as biostimulants to improve crop productions, but the mechanisms responsible for their stimulatory effects remain unclear. Titanium exerts beneficial effects on plants by interacting with other nutrients, mostly iron (Fe). Iron and Ti enter into synergistic and antagonistic interactions. In iron-deficient plants, Ti induces the expression of genes responsible for Fe acquisition, increases Fe uptake and utilization and improves plant growth. Some plants contain proteins that bind specifically or non-specifically with Ti. In plants with high Ti concentrations, Ti competes with Fe for ligands or proteins. Strong competition can lead to Ti phytotoxicity [101].
According to Malinowska and Wiśniewska-Kadżajan [100], the growth of Festulolium plants is enhanced when N fertilisers are combined with foliar application of Tytanit. In their opinion, Tytanit reduces production costs, delivers environmental benefits and increases the nutritional value of Festulolium plants. In a study by Malinowska et al. [102], Festulolium biomass yields peaked when plants were fertilised with mineral N and sprayed with 1% Tytanit in all growing seasons. This combined treatment also increased the protein content of grass. In the first and second year of the experiment, a single foliar treatment with 1% Tytatnit increased the content of soluble sugars in Festulolium braunii relative to grass fertilised with N only.
Ciepiela [35] reported an increase in the protein content of annual ryegrass supplied with Tytanit. The application of Tytanit significantly increased chlorophyll content in plant leaves and plant dry matter yield compared to the control. Radkowski [75], in turn also observed that foliar application of 0.08% Tytanit increased the leaf greenness index (SPAD values) in timothy-grass (Phleum pratense L.). According to Cigler et al. [76], Ti affects photosynthetic processes, as per their hypothesis. However, the primary effect of Ti fertilisation, manifested as an increase in leaf chlorophyll concentration (SPAD index above 20%), gives Timothy the initial growth advantage. As a result, stronger shoots with longer tillers, broader and longer leaves are formed. In conclusion, strong shoots with high chlorophyll concentrations produce more glucose, which translates into faster synthesis of biopolymers such as cellulose or starch in the seeds. This is a secondary effect of Ti fertilisation. The stronger plant shoots enable the formation of longer and broader inflorescences. Ti fertilisation accelerates all these processes. In conclusion, stronger plants produce larger and healthier seed kernels.
Stymjod is a nanotechnology-based growth regulator which contains highly available mineral and organic nutrients that promote plant ontogeny. In the study by Sosnowski et al. [50], Stymjod applied at a concentration of 4.5% significantly increased the number of shoots, leaf blades and dry weight of 100 leaf blades, which translated into more fresh and dry orchard grass plants. In the work of Sosnowski et al. [50], Stymjod increased the photosynthetic capacity and chlorophyll content of orchard grass plants. In addition, it was found that all concentrations of Stymjod increased the photosynthetic activity of the plants, resulting in an increase in the content of photosynthetic pigments and the value of the SPAD leaf greenness index. According to the authors, the use of Stymjod suggests the possibility of reducing mineral nitrogen doses without negative physiological consequences for plants.
Nano-Gro is another synthetic biostimulant; it is an organic preparation that which contains oligosaccharide granules saturated with Fe, Co, Al, Mg, Mn, Ni and Ag sulfates in nanomolar concentrations (10−9 mol). Jankowski et al. [103] found that Nano-Gro increased the germination energy of Festulolium braunii, perennial ryegrass and annual ryegrass. A greenhouse experiment involving grasses demonstrated that foliar application of phosphites improved root growth in perennial ryegrass. These results indicate that phosphites act as root biostimulants, rather than as pesticides or fertilisers [104]. According to the authors, Nano-Gro has been used in agriculture for cereals, oilseed rape, sugar beet, maize, vegetables and in greenhouses. However, there is a lack of information about the impact of this growth stimulator on various grass and legume species in the literature.
Formulations such as Titanite, Stymjod and Nano-Gro confer benefits to grasses through various mechanisms. The combination of Titanite and nitrogen fertilisers increases plant growth of Festulolium, reduces costs and enhances nutritional value. Stymjod promotes the growth of shoots and leaves, as well as the dry leaf mass, whilst improving the photosynthetic capacity and chlorophyll content. Additionally, Nano-Gro enhances the germination of grasses. These formulations can help to reduce nitrogen doses without harming the plants, which is crucial for ecological sustainability.
4. The Use of Biostimulants in the Production of Turfgrasses
Turfgrasses are grass species that are cut to a relatively low height (<10 cm) and are grown for ornamental, recreational and functional purposes. Many turfgrass species are also grown in meadows and pastures and are used in the production of livestock feed [104].
Around 50 grass species are grown for “turfgrass”, and all turfgrass species have their own varieties and specific growth requirements [105]. Based on their biology and ability to adapt to different climates, grasses can be divided into cool-season (Pooideae) and warm-season (Aristidoideae, Arundinoideae, Micrairoideae, Danthonioideae, Chloridoideae and Panicoideae) grasses [106]. Cool-season grasses, which are well adapted to moderate and subarctic climates, include perennial ryegrass (Lolium perenne L.), annual ryegrass (Lolium multiflorum L.), red fescue (Festuca rubra L.), tall fescue (Festuca arundinacea Schreb.), creeping bentgrass (Agrostis palustris Huds.), common bent (Agrostis capillaris L.) and Kentucky bluegrass (Poa pratensis L.) [107,108]. Kentucky bluegrass is the most popular turfgrass species in the northern USA. Mixtures of perennial ryegrass, Kentucky bluegrass and red fescue are widely used as football pitch grasses in Central Europe. Turfgrass species and varieties should be resistant to spring and fall frosts, as well as summer drought. These adverse weather conditions compromise plant vitality and exert a negative effect on the appearance of lawns [109,110].
Abiotic (non-living) and biotic (living) factors pose a health risk for turfgrasses (Figure 3), in particular in golf courses, where additional treatments are required [111]. Abiotic factors include extreme temperatures, excess rainfall or drought, nutrient deficit, high salinity, acidity, pollution, wind, ultraviolet radiation, chemical and mechanical damage and/or poor agronomic practices. Biotic factors involve plant pathogens (bacteria, fungi, viruses) that can compromise the health of turfgrasses. Fungal and bacterial infections decrease grass yields and cause significant harvest losses, and most turfgrass diseases are caused by fungi [4,29,105,112,113]. Harsh environmental conditions can inhibit plant growth, damage cell structures and lead to metabolic dysfunctions in plants [108].
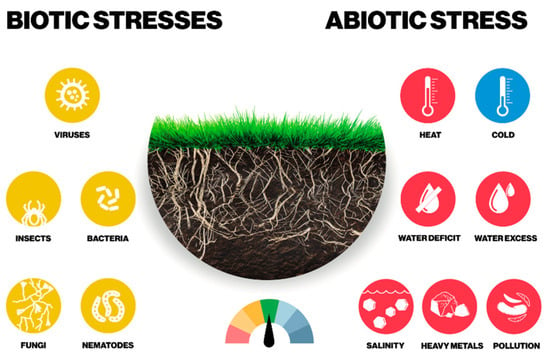
Figure 3.
Sources of abiotic and biotic stress in grasses.
Rapid urban growth and infrastructure development has increased the significance of grasses outside the farming sector. Lawns are important landscape components, and various grass species are used in recreational areas, sports facilities, home gardens and cemeteries [108,114,115,116]. Turfgrasses drive the urban ecology and are one of the most important indicators of economic growth and civilization. The biological characteristics of various grass species determine the productivity and quality of the turf and its applicability in landscapes, slopes and sports fields [117].
In addition to aesthetic and ornamental properties, turfgrasses also deliver functional benefits by maintaining the ecosystem balance and promoting the reclamation of polluted and overexploited natural areas in the urban landscape. Turfgrasses control soil erosion, improve soil quality, purify water, reduce noise and mitigate air pollution. In hot regions, well-kept turfs can decrease atmospheric temperature by sequestering carbon [117,118,119].
Fertilisation is one of the factors that significantly influence the appearance of grass lawns. Fertilisers applied in the production of sports turf contain nutrients that are essential for the growth and development of grass. Fertilisers increase turf density, enhance green colour, promote uniform grass coverage and increase resistance to disease without promoting excessive growth of the shoot apical meristem. The timing and rate of N fertilisation strongly affects the prevalence of turfgrass diseases [110,120]. Optimal N fertilisation decreases oxidative stress by maintaining osmoprotectants, stimulating antioxidant enzymes and sustaining metabolic activity at reduced tissue water potential, which increases resistance to drought. The availability and quality of irrigation water will decrease due to diminishing supply of surface water around the world [121]. Without additional irrigation, adequate water retention in soil will pose a growing problem in the production of turfgrasses [107].
The demand for turfgrasses is likely to increase in the future, which is why the management of plant-available nutrients, water and pest control is essential for maintaining healthy and aesthetically appealing landscapes [122]. In addition to a wide range of fertilisers to improve the appearance of the lawn, new biological agents are appearing on the lawn care market to stimulate the growth and development of the plant. Biostimulants have a positive effect on the metabolism of the lawn (Figure 4, Table 4). They stimulate vital processes and reduce the effects of unfavourable environmental conditions (drought, salinity, temperature fluctuations) and pathogens [123].
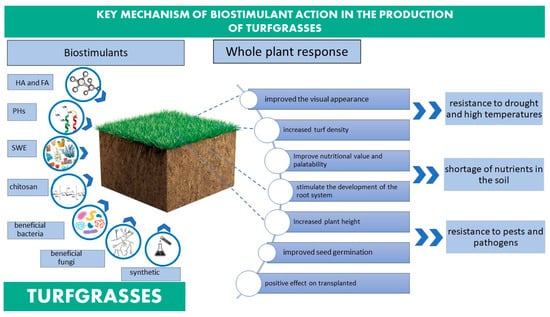
Figure 4.
Key mechanisms targeted by biostimulants in turfgrasses.

Table 4.
Summary of species, biostimulants and effects in turfgrass production.
4.1. Natural Biostimulants
4.1.1. Humic and Fulvic Acids
Various organic materials such as HA, SWE, organic matter and amino acids are used as fertilisation supplements in turfgrass cultivation. Humic substances and SWE are the leading organic ingredients in biostimulants for turfgrasses. These compounds contain phytohormones and osmoprotectants such as cytokinins, auxins, polyamines and betaines [124]. Humins promote the conversion of mineral compounds into plant-available forms. They stimulate seed germination and improve seed viability, and their effects are most prominent in the roots [147]. Humic and fulvic acids modulate primary and secondary metabolic processes in plants. Humic substances promote root growth and nutrient uptake and increase plant resistance to environmental stressors [148].
In recent years, many commercial products containing humic substances have been promoted as effective biostimulants for turfgrasses despite the fact that their efficacy has not been adequately documented [127]. Zhang et al. [124] observed that HA and SWE exerted positive effects on transplanted tall fescue. Seaweed extracts (Ascophyllum nodosum Jol.) and HA also improved the quality and heat resistance of transplanted tall fescue in another study by Zhang et al. [125]. The study found that foliar applications of SWE and HA, alone or together, increased SOD activity of field-grown creeping bentgrass. The authors clarified that the observed increases were associated with better turf quality, higher photochemical activity and lower incidence of dollar spot disease (Sclerotinia homoeocarpa F.T. Bennett). The overall physiological condition of the coppice was enhanced by the increased antioxidant superoxide dismutase (SOD) activity, independent of the nutrient application level. As per the authors, the favourable effects of these compounds on turfgrass production can be achieved with lesser but nutritionally appropriate fertilisation. The researchers suggest that the presence of hormones and compatible osmolytes in SWE and HA can upsurge both SOD and photochemical activities. These results closely correspond to those in other studies by Zhang et al. [126]. The authors showed that HA and SWE improved the physiology of creeping bentgrass cv. Southshore and increased root growth at both low and higher fertiliser levels. The authors suggested that natural biostimulants should be used to improve plant performance before and during summer abiotic stress, in addition to maintaining adequate levels of plant-available nutrients. Liu et al. [127] also found a similar effect while working with humic acids applied to creeping bentgrass grown hydroponically, as in this study. The researchers discovered that an application of high concentrations of humic acids (400 ppm) significantly improved photosynthetic rates and root mass regrowth.
Similarly, Mueller’s [128] study found that biostimulants containing seaweed extracts and humic acids could improve the tolerance of creeping bentgrass to heat and moisture stress. This study included biostimulant products that had wetting agents, while the other three products incorporated some seaweed extract or humic acid, which can explain the improvement in turfgrass quality. It was also observed that the biostimulants did not significantly alter the microbial community of the putting green in terms of enzyme activity or substrate utilization. The authors suggested that the positive response to biostimulants was not due to nutrition.
According to Mueller and Kussow [128], HA and SWE increased the resistance of creeping bentgrass to heat and water stress. In turn, Zhang et al. [125] observed that humic substances and SWE are useful supplements that can reduce fertiliser and fungicide use in the production of creeping bentgrass and demonstrated that HA and SWE enhanced the physiological function of creeping bentgrass cv. Southshore and improved root growth in both low and high fertilisation regimes. They suggested that, in addition to maintaining adequate levels of plant-available nutrients, natural biostimulants should be used before and during summer abiotic stresses to improve plant performance.
However, according to Daneshvar [129], foliar application of HA increased the uptake of selected nutrients, promoted root growth and improved drought tolerance in perennial ryegrass cv. Speedygreen. The results indicate that leaf P, K and Zn content, fresh and dry weight, chlorophyll content and fresh weight of roots were not affected by HA. All concentrations of HA were functionally adequate in improving the iron content. Regelink [36] made a similar observation when applying FA and HA to sandy and loamy soil with high and very low phosphorus content, respectively. This application had no impact on grass yield nor on total nitrogen and phosphorus uptake, regardless of nitrogen, phosphorus, potassium, sulfur and calcium fertilisation, as well as in unfertilised soil. The authors argued that the lack of effect of the tested HS was due to the fact that the applied doses of HS were low compared to the amount of Soil Organic Carbon (SOC) already present in the soil. HS adsorbs to reactive soil mineral surfaces and therefore may not be able to interact with plant roots as they would if applied in a hydroculture or soilless substrate. In addition, an effect of HS addition on ortho-P availability is only expected at high P loads (i.e., 0.4–0.6 mol mol−1), whereas the soils in the experiments had lower P loads (i.e., 0.09 and 0.29 mol mol−1).
Humins promote the conversion of mineral compounds into plant-available forms. They stimulate seed germination and improve seed viability, and their effects are most prominent in the roots [147]. This was confirmed in a study by Chen et al. [149], which reported that HA and FA improved seed germination, root initiation and total biomass of perennial ryegrass. The authors suggest that improved Fe and Zn nutrition can enhance a number of physiological processes related to plant growth due to Fe involvement in chlorophyll biosynthesis and cytochrome activity. The authors considered that the enhancement of plant growth in the nutrient solution and in the soil by HS is largely due to the maintenance of Fe and Zn at sufficient levels in the solution. They added that this effect depends on the pH levels and becomes more pronounced under high pH conditions. Krasa and Świerszcz [123] noted in another study that a biostimulant containing HA enhanced the vitality of a turfgrass mixture composed of perennial ryegrass and Kentucky bluegrass. Similar observations were made by Acuña et al. [130], who demonstrated that HA and complex organic materials exerted a positive effect on the performance of turfgrasses and identified differences in responses to the applied biostimulant between perennial ryegrass, creeping bentgrass and tall fescue. In the work of Erwin [131], HA significantly increased the mass and resistance of Kentucky bluegrass roots. In contrast, HA had no significant effect on the growth of ryegrass cv. Speedygreen in a study by Nikbakht et al. [150]. This fact can partly be explained by Van Dyke et al. [151]. According to a field study on creeping bentgrass, fulvic acid products did not affect turfgrass performance, as measured by phosphorus uptake and chlorophyll content. In all turfgrass species, Potente, an amino acid root stimulant, showed no significant differences in fresh and dry weight when compared to the control.
Biostimulants containing humic acids and marine algae extracts have been shown to have a positive impact on grasses. They stimulate antioxidant enzymes, photochemical processes, root growth and stress resistance. Technical terms are explained when first used. Due to the presence of hormones and osmolytes, they enhance the quality of the grass and its ability to defend against disease. The mechanism of action is to aid nutrient uptake and stimulate root development. However, the effectiveness varies depending on the grass species and soil conditions. These findings demonstrate the need for further research to maximize the potential of biostimulants in enhancing grass growth and health.
4.1.2. Seaweed Extracts
Seaweed extracts contain phytohormones, vitamins, amino acids and minerals. Ascophyllum nodosum algae are plant growth regulators which increase the antioxidant activity of SOD in the production of turfgrasses. Increased antioxidant activity in plants reduces oxidative stress [152]. Seaweed extracts are biodegradable, non-toxic and environmentally friendly fertilisers, and they are widely used in organic and integrated farming. Algae enhance plant and seedling growth and stimulate the development of the root system. They increase nutrient uptake and resistance to diseases and pests and stimulate plant defence responses against biotic and abiotic stresses. Seaweed extracts induce various biochemical pathways in plants, stimulate the secretion of root flavonoids and promote microbial colonization [153]. They are applied mainly to improve photosynthetic capacity, plant growth and nutrient use efficiency [154].
In a study by Guillard [132], SWE had no significant influence on the value of the normalized difference vegetative index (NDVI) in frequently mowed perennial turfgrasses (Kentucky bluegrass and tall fescue). Very high temperatures were not observed during the study, but water stress was periodically noted and further research was recommended to determine the effect of SWE on turfgrass.
According to Xu et al. [42], summer decline in turf quality and the growth of cool-season grass species pose significant challenges in turfgrass management. The aim of the cited study was to determine whether foliar application of two SWE biostimulants (TurfVigor and CPR) would mitigate the decline in the growth of creeping bentgrass in the summer and to analyse the impact of the tested biostimulants on leaf senescence and root growth. Both biostimulants significantly improved the visual quality of turfgrass and enhanced shoot and root growth. The study demonstrated that properly applied biostimulants can improve the summer performance of creeping bentgrass. In the work of Talar-Krasa [133], Bio-Algeen S90 significantly decreased the prevalence of fungal diseases in perennial ryegrass and Kentucky bluegrass and increased turf density.
Seaweed extracts can have a positive impact on turf due to their high levels of phytohormones, vitamins, amino acids and minerals. Especially beneficial are extracts from the Ascophyllum nodosum algae, which can regulate growth, increase antioxidant activity and reduce oxidative stress. When used as an organic fertiliser, they can promote plant growth and bolster resistance. Mechanisms of action comprise increased nutrient uptake and root development, bolstered defence against pests and diseases and heightened stress response, activation of biochemical pathways and promotion of microbial growth. Seaweed extracts advance photosynthesis, plant growth and nutrient utilisation, with distinct benefits for the quality of grasses during summer and for species that thrive in cooler climates, as demonstrated by research. The effectiveness varies according to the plant species and the environmental conditions in which they are cultivated.
4.1.3. Protein Hydrolysates and Free Amino Acids
Protein-based fertilisers can be divided into PHs containing a mixture of peptides and amino acids and products containing individual amino acids. Both plant and animal sources of proteins are used in the production of commercial biostimulants. These components are derived by chemical, thermal or enzymatic hydrolysis [155]. Protein hydrolysates stimulate plants by enhancing carbon and N metabolism and increasing N assimilation [156].
In Commission Regulation (EU) No. 142/2011 (Annex I, point 14), hydrolysed proteins are defined as “polypeptides, peptides, and amino acids, and mixtures thereof, obtained by the hydrolysis of animal by-products”. Protein hydrolysates also contain other compounds with biostimulatory effects, including lipids, carbohydrates, phenols, minerals, phytohormones and other organic compounds. Protein hydrolysates derived from agricultural by-products offer a sustainable solution to waste management and contribute to environmentally friendly production. They exert positive effects on plants by increasing root and shoot biomass, improving productivity and increasing plant yields and nutrient uptake [3].
Biostimulants containing PHs improve crop nutrient status and reduce the use of chemical fertilisers. Agricultural by-products are a valuable source of protein and can be recycled into PH-based biostimulants. Protein hydrolysates contain nutrients with functional and/or bioactive properties that can be used in medicine, nutrition, food production and agriculture. The properties of PH-based biostimulants constitute an interesting topic of research [3].
According to Bahugana [3], PHs are an effective tool in sustainable agriculture because they promote plant growth and development. However, further research is needed to identify ingredients that are responsible for the mechanism of action of PHs. The method of application and the optimal dose should be determined for every PH-based biostimulant. Little is known about PHs’ influence on the communities of soil-dwelling microorganisms in the rhizosphere, which is why enzyme sources and hydrolysis conditions should be optimized in the production process.
Preparations containing amino acids also increase plant resistance to adverse environmental conditions. Amino acids and peptides improve plant tolerance to heavy metal contamination. Proline synthesis is induced in plants in soils with high concentrations of heavy metal ions. Proline is an osmoregulatory agent which maintains homeostasis in an organism’s water content under exposure to heavy metals [156]. According to Mertz [157], amino acids are more effective in promoting the growth of turfgrasses than mineral fertilisers, but their catabolism in plants has not been thoroughly investigated to date. Many turfgrass fertilisers contain amino acids, but some amino acids have better fertilising properties than others. Three branched-chain amino acids (BCAA) are commonly recognized as effective sources of N for plant growth: leucin (L), isoleucine (IL) and valine (V). These compounds are nonpolar and hydrophobic, which makes them particularly effective in increasing nutrient uptake by plants.
Radkowski et al. [47] analysed the effectiveness of the AGRO-SORB® Folium biostimulant containing amino acids in the production of a turfgrass mixture composed of perennial ryegrass, tall fescue and red fescue. Plants supplied with the highest rate of the analysed biostimulant received the highest scores for appearance and functionality. Grasses fertilised with biostimulant containing amino acids AGRO-SORB® Folium were more resistant to disease. In plots supplied with the analysed biostimulant, the prevalence of Fusarium infections decreased by 16%, whereas the prevalence of net blotch was reduced by 20% relative to control. Higher rates of the applied biostimulant also increased the content of N, K, Mg, Na and Mn in the turfgrass mix. According to the cited authors, the effectiveness of the tested biostimulant increased with the applied dose. In their opinion, turfgrass appearance can be improved by increasing the rate of amino acid fertiliser application. Kim et al. [134] assessed the effect of functional liquid fertilisers containing saponin (SLF) and amino acids (ALF) on creeping bentgrass. The tested products stimulated N and K uptake and enhanced grass quality by increasing the number of shoots and root length. The cited authors concluded that functional liquid fertilisers such as SLF and AFL can effectively replace compound fertilisers because they increase N availability and absorption and improve the quality of creeping bentgrass turfs.
Biostimulants containing amino acids enjoy growing popularity because they improve the quality of specialty crops. However, the mechanisms by which these biostimulants are absorbed by plants and utilized in cell metabolism have not been fully elucidated. McCoy [135] found that BCAA was more effective than urea in increasing the number of shoots in creeping bentgrass. The results of the study demonstrate that creeping bentgrass leaves absorb glutamate quickly to produce GABA and proline. As per the author, glutamate is primarily absorbed without any alteration, and the conversion into other forms of nitrogen is a secondary process. The research presents evidence that amino acids applied as exogenous sources to turfgrass leaves can be rapidly absorbed and serve as stable sources of precursor molecules for incorporation into the plant’s metabolism.
Amino acids enhance turfgrass resilience of turfgrass to harsh conditions by regulating water balance with proline and boosting tolerance to heavy metals, among other benefits. This supports the plant’s health and appearance, increases nutrient and nitrogen uptake and improves turf quality. Additionally, these acids are rapidly absorbed by grass leaves, providing a continuous supply of metabolic precursors.
4.1.4. Fungi
A mutualistic symbiosis between plants and microorganisms can increase plant tolerance to environmental stressors [158]. The presence of Epichloë fungal endophytes in host plant tissues increased chlorophyll content, photosystem II efficiency and the accumulation of heavy metal ions in aerial plant parts, and it was influenced by the genotype of the host plant. Fungal endophytes colonize intercellular spaces in host plants and often increase the production of secondary metabolites. They protect plants against fungal pathogens and increase their resistance to abiotic stresses such as drought. By increasing the production of antioxidants, Epichloë spp. neutralize the negative consequences of reactive oxygen species and improve drought tolerance [159]. According to Pańka [114], endophytes of the genus Epichloë play a significant role in plant growth and can be used as biological protectants to improve the functional properties of various grass biotypes. They concluded that endophytes enhance the growth and resilience of turfgrass, increase its resistance to water deficit and salinity, promote nutrient use efficiency and improve overall plant health [160].
Festuca rubra subsp. pruinosa is a host of Epichloë festucae fungal endophytes. In perennial ryegrass inoculated with Festuca rubra subsp. pruinosa, a Diaporthe strain significantly increased leaf biomass under normal irrigation and salinity. These results suggest that the core mycobiome of Festuca rubra subsp. pruinosa can play an important role in host plant adaptation and can be useful for improving grass production [161]. According to Stingl [136], effective microbial products should deliver long-lasting effects, should be easy to apply and should be non-toxic for living organisms and the environment. According to the cited author, endophytic microorganisms have the greatest potential for controlling turfgrass pathogens and pests. Endophytes can colonize plant tissues without inducing visible symptoms of biotic stress. Species of the genus Epichloë (Clavicipitaceae and Hypocreales) colonize grasses in winter and grow in aerial plant parts by forming systemic and usually asymptomatic associations that lead to defensive mutualism [158].
Arbuscular–mycorrhizal symbiosis delivers numerous benefits for host plants, including higher tolerance to abiotic and biotic stress as well as improved growth and nutrient use efficiency. In a study by Pelletier et al. (Festuca rubra L.), Kentucky bluegrass, red fescue and perennial ryegrass inoculated with Glomus intraradices were established more quickly than turfgrass inoculated with Glomus etunicatum. In the work of Nikbakht [150], colonization by AMF increased the dry and fresh matter yield of perennial ryegrass cv. Speedygreen. According to the authors, mycorrhizal inoculation greatly improved the visual appearance of grass. Glomus intraradices colonized more roots than Glomus mosseae, and the authors concluded that perennial ryegrass has a greater preference for G. intraradices than G. mosseae. In turn, Charesta and Dalpe [137] reported higher biomass yields in creeping bentgrass and Kentucky bluegrass colonized by Glomus mosseae. Nikbakht [150] suggested that AM inoculation improves the assimilation of selected nutrients, promotes root growth in perennial ryegrass, reduces the demand for fertilisers and increases tolerance to drought.
According to Xia [122], little is known about microbial species colonizing the rhizosphere and endosphere of turfgrasses. They identified the dominant bacterial and fungal species in the rhizosphere and endosphere of turfgrasses, including P. veronii, J. lividum and Pseudogymnoascus spp. These microorganisms are probably involved in the biocontrol, biotransformation and nutrient uptake in plants. Allan-Perkins [162] found that Proteobacteria spp. were the predominant microorganisms in soil samples from a golf course, and they noted that soil management practices significantly influenced fungal abundance, diversity and pathogens. The cited study was the first report which relied on metagenomics to characterize microbial communities in three management areas of golf courses, including organically managed courses.
The mechanism of fungi’s action on turf is an advantageous symbiosis that enhances the plants’ resistance to severe conditions. Endophytic fungi, such as Epichloë, increase turf productivity through the production of secondary metabolites and antiparasitic defence. Arbuscular mycorrhiza (AMF) promotes growth and access to nutrients, improving tolerance to stresses. These processes contribute positively to the overall condition and health of the turf.
4.1.5. Chitosan
Chitosan is a linear cationic polysaccharide composed of randomly distributed N-acetyl glucosamine and D-glucosamine linked by β(1–4) glycosidic bonds. Chitosan is derived by deacetylation of chitosan, which occurs naturally in crustacean exoskeletons and fungal cell walls. Depending on its source, the molecular weight of chitosan ranges from 1 × 104 Da to 1.5 × 106 Da [72].
Chitin is the second-most abundant polysaccharide on earth after cellulose, and this biodegradable, biocompatible and non-toxic compound has numerous practical applications [163,164,165]. This naturally occurring compound has a high physiological potential, and it has attracted growing interest in recent years. In plants, chitosan influences physiological responses such as resistance and defence mechanisms involving various enzymes, including phenylalanine ammonia lyase, polyphenol oxidase and tyrosine ammonia lyase, as well as antioxidant enzymes such as SOD, catalase and peroxidase that protect plants against environmental stressors. Recent research has shown that chitosan induces tolerance to biotic and abiotic stresses in plants and promotes the formation of barriers that enhance plant productivity [166].
In a study by Li et al. [139], chitosan applied to the leaves of creeping bentgrass improved heat tolerance and enhanced growth under high temperature conditions. Chitosan promoted shoot and root growth and improved turfgrass quality. According to the cited authors, chitosan mitigates the decline in the rate of photosynthesis and promotes cell membrane stability. According to Acemi [140], chitosan enhanced the density of turfs composed of perennial ryegrass, red fescue and Kentucky bluegrass. However, constant or excessive chitosan treatments could increase leaf elongation, thus contributing to nutritional deficiency and higher mowing costs.
Yoon and Kim [141] reported significant differences in chlorophyll content and NDVI values in turfgrasses supplied with SWE and chitosan preparations. In Kentucky bluegrass and creeping bentgrass, chlorophyll content and NDVI increased after foliar application of chitosan and SWE. Olszewska [44] also found that effective microorganisms applied to the leaves improved the chemical composition of perennial ryegrass. According to the cited authors, biostimulants can improve turfgrass management in golf courses in the fall.
Chitosan strengthens defence mechanisms, increases tolerance to environmental stresses and improves growth in grasses. However, excessive use can cause problems such as leaf elongation or higher maintenance costs. When combined with effective micro-organisms, chitosan upgrades the quality of grasses, benefiting grass management, especially on golf courses during autumn.
4.2. Synthetic Biostimulants
Nutrients
In heavily used lawns and meadows, grass blades/tissues are damaged due to friction, tearing and compression, which increases their susceptibility to pests and diseases. Silicon stimulates plant growth and development, and it decreases the risk of pathogenic infections and pest infestation. Silicon increases water absorption in plants, thus improving productivity and plant quality in periods of drought.
In a study by Chai et al. [142], the application of Si under salinity stress promoted the growth of Kentucky bluegrass and improved its quality. According to the cited authors, Si induces the transfer of K+ from roots to shoots, but inhibits the absorption and transfer of Na+, which may improve turf growth and quality when Si is applied under saline conditions. This observation is particularly helpful for maintaining lawns in the proximity of roads. Silicon alleviates the severity of many diseases transmitted by seeds, soil and leaves. When applied to leaf blades, Si is polymerized on the surface and forms a protective chemical and physical barrier. In turn, when applied to the soil, Si is absorbed by the roots and participates in plant defence mechanisms. Silicon stimulates phenylpropanoid and terpenoid biosynthesis pathways in plants, accelerates the transcription of protective genes and stimulates defence enzymes. Silicon also enhances photosynthesis and the antioxidant defence system in plants [143]. Amin et al. [165] reported that Si applied to drought-stressed maize improved plant growth and yields by increasing the rate of photosynthesis and reducing transpiration. Silicon can act at the molecular level by regulating the expression of genes involved in defence responses in plants [166].
In a study by Radkowski et al. [49], the Optysil biostimulant improved the visual appearance of a turfgrass mixture composed of perennial ryegrass, annual ryegrass, tall fescue and red fescue. In plots supplied with silica, the prevalence of Fusarium patch decreased by around 12%, and the prevalence of leaf blotch was reduced by around 18% relative to control. Higher rates of the silicon supplement significantly increased the content of N, P, K, Ca, Mg, Na, Cu and Fe in plants. Pruyne et al. [145] reported that silicates applied to perennial ryegrass increased turf density.
According to Zhang et al. [144], silicate-based biostimulants should not be applied to suppress brown patch in tall fescue and creeping bentgrass. In these grass species, silicates can exacerbate symptoms of brown patch by contributing to nutrient imbalance. Nanayakkara et al. [146] reported that perennial ryegrass accumulated Si (the Si content of plants was determined at up to 4% on a dry matter basis) and concluded that soil type, source of Si and the rate of Si application should be considered when selecting a Si fertilisation treatment.
The mechanism of action of silicon on turf entails multiple key aspects. When grown in saline conditions, silicon regulates the transfer of K+ and Na+ ions, which promotes turf growth and enhances its quality. Additionally, silicon functions as a safeguard, diminishing the occurrence of seed-, soil- and leaf-borne diseases. Its application to the foliage generates a protective layer, while absorption by the roots triggers the plant’s defence mechanisms. Silicon stimulates the production of defence compounds, accelerates photosynthesis and enhances antioxidants. However, in certain species of grass, silicon has been found to exacerbate disease symptoms, making its careful selection a crucial aspect in any fertilisation strategy.
5. Conclusions
Climate change exerts a significant influence on crop production. Therefore, further research is needed to develop new solutions that maximize plant growth and crop yields. The yield potential of plants can be fully harnessed by minimizing stress. Biostimulants are highly effective in promoting plant growth and crop yields. In Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019, a biostimulant is defined as “a product stimulating plant nutrition processes independently of the product’s nutrient content with the sole aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (a) nutrient use efficiency; (b) tolerance to abiotic stress; (c) quality traits; (d) availability of confined nutrients in soil or rhizosphere”. Natural preparations containing HA, FA, PHs, SWE, chitosan, silicon, inorganic compounds and beneficial fungi and bacteria, as well as synthetic compounds, are used in the production of forage grasses and turfgrasses. Grasses are also supplied with synthetic biostimulants containing nutrients and phenolic compounds.
In the analysed studies, HA increased the content of non-structural carbohydrates in forage grasses. This effect was exacerbated through the use of natural fertilisers. However, HA exerted varied effects on the root system of rough bluegrass. There is evidence to indicate that SWE improve the quality of forage grasses by reducing the content of structural carbohydrates and lignin in biomass. Seaweed extracts were also found to increase biomass yields and the leaf greenness index (SPAD). The beneficial effects of SWE were exacerbated in combination with a nitrogen fertiliser, which is a very important observation. Similarly as for SWE, the efficacy of microbial biostimulants was enhanced in combination with mineral fertilisers. In turn, chitosan-based biostimulants promoted early seed germination in fodder grass and reduced heavy metal concentrations in soil. Synthetic biostimulants delivered similar effects.
There is evidence to suggest that biostimulants containing HA improve resistance to adverse environmental conditions in turfgrasses by promoting root growth. In turn, biostimulants containing SWE improved the appearance of turfgrasses, stimulated shoot and root growth in creeping bentgrass and decreased the prevalence of fungal diseases. Products containing PHs and free amino acids deserve special attention in the group of biostimulants applied in the production of turfgrasses. According to research, these biostimulants enhance the uptake of fertiliser nutrients and significantly increase nutrient use efficiency in grasses. Biostimulants containing beneficial fungi delivered similar effects. Silicon-based natural biostimulants are increasingly used in the production of turfgrasses. When applied at the optimal rate, these products can enhance the appearance of lawns and improve their resistance to pests and pathogens. The reviewed studies indicate that silicon-based biostimulants promote the growth and quality of turf subjected to salinity stress.
In the analysed studies, HA increased the content of non-structural carbohydrates in Festulolium and stimulated root growth in annual bluegrass. This effect was exacerbated through the use of natural fertilisers. However, HA exerted varied effects on the root system of rough bluegrass. Some researchers found that HA stimulated root growth, whereas, in other studies, HA exerted inhibitory effects on the root system in this grass species.
There is evidence to indicate that SWE improve the quality of perennial ryegrass and annual ryegrass fodder by reducing the content of structural carbohydrates and lignin in biomass. Seaweed extracts were also found to increase biomass yields and the leaf greenness index (SPAD) in Festulolium braunii. Seaweed extracts enhanced the nutritional value of Festulolium braunii and orchard grass by decreasing the content of structural carbohydrates (cellulose and hemicellulose), lignin, NDF and ADF in plant tissues. In the above grass species, the application of SWE increased the content of protein compounds and simple sugars and improved yields. The beneficial effects of SWE were exacerbated in combination with a nitrogen fertiliser, which is a very important observation. However, some researchers found that seaweed-based biostimulants can decrease the feed value of Festulolium and orchard grass.
Recent research has shown that biostimulants increase the content of dry matter and total protein in perennial ryegrass and orchard grass. Similarly as for SWE, the efficacy of microbial biostimulants was enhanced in combination with mineral fertilisers. These observations suggest that preparations containing EM can increase shade tolerance in forage grasses, thus improving their resistance to environmental stressors. In turn, chitosan-based biostimulants promoted early seed germination in perennial ryegrass and reduced heavy metal concentrations in soil. Synthetic biostimulants delivered similar effects. An organic biostimulant (Nano-Gro) increased germination energy in forage grasses (Festulolium braunii, perennial ryegrass and annual ryegrass). In some studies, chlorophyll concentration in forage grasses (annual ryegrass, orchard grass and timothy-grass) increased in response to synthetic biostimulants. These preparations also increased the content of soluble sugars in Festulolium braunii and the content of protein in annual ryegrass.
There is evidence to suggest that biostimulants containing HA improve resistance to adverse environmental conditions in turfgrasses (tall fescue, creeping bentgrass and perennial ryegrass) by promoting root growth. In turn, biostimulants containing SWE improved the appearance of turfgrasses, stimulated shoot and root growth in creeping bentgrass and decreased the prevalence of fungal diseases in perennial ryegrass and Kentucky bluegrass.
Products containing PHs and free amino acids deserve special attention in the group of biostimulants applied in the production of turfgrasses. According to research, these biostimulants enhance the uptake of fertiliser nutrients and significantly increase nutrient use efficiency in grasses. Biostimulants containing beneficial fungi delivered similar effects. In one of the reviewed articles, mycorrhizal inoculation greatly improved the visual appearance of perennial ryegrass. In turn, chitosan improved heat tolerance in creeping bentgrass. This natural biostimulant also enhanced turf density.
Silicon-based synthetic biostimulants are increasingly used in the production of turfgrasses. When applied at the optimal rate, these products can enhance the appearance of lawns and improve their resistance to pests and pathogens. The reviewed studies indicate that silicon-based biostimulants promote the growth and quality of turf subjected to salinity stress.
In summary, biostimulants can increase nutrient uptake and improve nutrient use efficiency in grasses. Biostimulants are not fertilisers, but they can enhance the effects delivered by fertilisers. At the same time, biostimulants can significantly decrease the demand for mineral fertilisers and plant protection products. Biostimulants also increase resistance to pests and pathogens, and they promote plant growth under stress conditions. Biostimulants improve the quality of biomass in forage grasses and enhance the visual appearance of turfgrasses. However, despite these advantages, biostimulants are rarely incorporated in conventional agricultural regimes. Farmers have limited knowledge about the benefits delivered by biostimulants, and they are reluctant to apply these preparations in fear of increased production costs, lower crop quality and yields and reduced profits. A wide range of biostimulants are available on the market, and the selection of a product that will maximize yields in a given plant species could also pose a problem. Further research is needed to formulate preparations that deliver a wide range of functions, are easy to apply and can be safely combined with other growth-promoting agents.
Despite the fact that beneficial effects biostimulants on plant growth have been extensively researched, their mechanism of action has not been fully elucidated in grasses. There is a general scarcity of research on the effects of biostimulants applied in combination with other preparations. The optimal dose should be determined individually for each grass species because biostimulants can exert toxic effects when applied in excessive amounts. Further field research is needed to determine the potential of biostimulants in a real-world setting.
Future research should focus on the synergistic effects of different types of biostimulants described in this review article. In theory, the combination of microbial immune modifiers, SWE and humic substances could generate more repeatable benefits in crop production. The profitability and risks associated with alternative nutrient sources should also be explored. The influence of biostimulants on forage crops and the soil microbiome has not been researched extensively to date. New solutions and formulations combining minerals with novel biostimulants are needed to optimize soil conditions and maximize crop yields.
Author Contributions
Conceptualization, E.M.-W. and M.O.; methodology, E.M.-W. and M.O.; formal analysis, E.M.-W. and M.O.; writing—original draft preparation, E.M.-W.; writing—review and editing, E.M.-W.; visualization, E.M.-W.; supervision, M.O.; project administration, E.M.-W. and M.O. All authors have read and agreed to the published version of the manuscript.
Funding
The results presented in this paper were obtained as part of a comprehensive study financed by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry, Department of Agrotechnology and Agribusiness (grant No. 30.610.013-110). Project financially supported by the Minister of Education and Science under the program entitled ‘Regional Initiative of Excellence’ for the years 2019–2023, Project No. 010/RID/2018/19, amount of funding 12,000,000 PLN.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Popp, J.; Kovács, S.; Oláh, J.; Divéki, Z.; Balázs, E. Bioeconomy: Biomass and Biomass-Based Energy Supply and Demand. New Biotechnol. 2021, 60, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Sharma, S.; Rai, A.; Bhardwaj, R.; Sahoo, S.K.; Pandey, A.; Yadav, B. Advance Technology for Biostimulants in Agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–412. ISBN 978-0-323-85581-5. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Żarczyński, P.J.; Sienkiewicz, S.; Wierzbowska, J.; Mackiewicz-Walec, E.; Jankowski, K.J.; Krzebietke, S.J. Effect of Land Protectionon the Content of Mineral Nitrogen in Soli. Fresen. Environ. Bull. 2019, 28, 4506–4513. [Google Scholar]
- Godlewska, A.; Ciepiela, G.A. Italian Ryegrass (Lolium multiflorum Lam.) Fiber Fraction Content and Dry Matter Digestibility Following Biostimulant Application against the Background of Varied Nitrogen Regime. Agronomy 2020, 11, 39. [Google Scholar] [CrossRef]
- Murawska, B.; Gabrowska, M.; Spychaj-Fabisiak, E.; Wszelaczynska, E.; Chmielewski, J. Production and Environmental Aspects of the Application of Biostimulators Asahi SL, Kelpak SL and Stimulator Tytanit with Limited Doses of Nitrogen. Ochr. Srodowiska Zasobów Nat. 2017, 28, 10–15. [Google Scholar] [CrossRef][Green Version]
- Lau, S.-E.; Teo, W.F.A.; Teoh, E.Y.; Tan, B.C. Microbiome Engineering and Plant Biostimulants for Sustainable Crop Improvement and Mitigation of Biotic and Abiotic Stresses. Discov. Food 2022, 2, 9. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef]
- Tang, X.; Tian, Q. Sources and Applications of Biostimulants. IOP Conf. Ser. Earth Environ. Sci. 2022, 1035, 012007. [Google Scholar] [CrossRef]
- Szczepanek, M.; Ochmian, I.; Wszelaczyńska, E.; Pobereżny, J.; Keutgen, A.J.; Szczepanek, M.; Wilczewski, E. Effect of Biostimulants and Storage on the Content of Macroelements in Storage Roots of Carrot. J. Elem. 2015, 20, 1021–1031. [Google Scholar] [CrossRef]
- Ustawa z Dnia 10 Lipca 2007 r. o Nawozach i Nawożeniu 2007. (Dz. U. z 2021 r., poz. 76 z późn. zm.). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20071471033 (accessed on 1 April 2023).
- European Biostimulants Industry Council (EBIC). Plant Biostimulants Contribute to Climate-Smart Agriculture. Available online: https://biostimulants.eu/issue/plant-biostimulants-contribute-to-climate-smart-agriculture (accessed on 5 March 2023).
- European Biostimulants Industry Council (EBIC). What Are Biostimulants? 2012. Available online: http://www.biostimulants.eu/about/what-are-biostimulants (accessed on 1 September 2022).
- European Parliament and European Council. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. In Official Journal of the European Union; European Union: Brussels, Belgium, 2019. [Google Scholar]
- García-Sánchez, F.; Simón-Grao, S.; Navarro-Pérez, V.; Alfosea-Simón, M. Scientific Advances in Biostimulation Reported in the 5th Biostimulant World Congress. Horticulturae 2022, 8, 665. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants Research in Some Horticultural Plant Species—A Review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Ertani, A.; Nardi, S.; Altissimo, A. Review: Long-term research activity on the biostimulant properties of natural origin compounds. Acta Hortic. 2013, 1009, 181–188. [Google Scholar] [CrossRef]
- European Biostimulants Industry Council (EBIC). Plant nutrition & soil fertility: Key to delivering Europe’s Green Deal objectives. Available online: https://biostimulants.eu/highlights/plant-nutrition-soil-fertility-key-to-delivering-europes-green-deal-objectives (accessed on 15 June 2023).
- Silva, M.S.R.D.A.d.; Santos, B.D.M.S.D.; Silva, C.S.R.D.A.D.; Antunes, L.F.D.S.; dos Santos, R.M.; Santos, C.H.B.; Rigobelo, E.C. Humic Substances in Combination with Plant Growth-Promoting Bacteria as an Alternative for Sustainable Agriculture. Front. Microbiol. 2021, 12, 719653. [Google Scholar] [CrossRef]
- Jankowski, K.; Dubis, B. Biostymulatory w Polowej Produkcji Roślinnej, Biostymulatory w Nowoczesnej Uprawie Roślin; Publikator Wieś Jutra: Warszawa, Poland, 2008. [Google Scholar]
- Joshi, N.; Parewa, H.P.; Joshi, S.; Sharma, J.K.; Shukla, U.N.; Paliwal, A.; Gupta, V. Use of Microbial Biostimulants in Organic Farming. In Advances in Organic Farming; Elsevier: Amsterdam, The Netherlands, 2021; pp. 59–73. ISBN 978-0-12-822358-1. [Google Scholar]
- Matyjaszczyk, E. The Introduction of Biostimulants on the Polish Market. The Present Situation and Legal Requirements Wprowadzanie Biostymulatorów Do Obrotu Handlowego w Polsce. Sytuacja Bieżąca i Uwarunkowania Prawne. Chem. Rev. 2015, 1, 215–218. [Google Scholar] [CrossRef]
- Talar-Krasa, M.; Wolski, K.; Biernacik, M. Biostimulants and Possibilities of Their Usage in Grassland. Grassl. Sci. 2019, 65, 205–209. [Google Scholar] [CrossRef]
- Mojumdar, A.; Behera, H.T.; Das, S.; Ray, L. Microbe-Based Plant Biostimulants and Their Formulations for Growth Promotion and Stress Tolerance in Plants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 213–230. ISBN 978-0-323-85163-3. [Google Scholar]
- Aamir, M.; Rai, K.K.; Zehra, A.; Dubey, M.K.; Kumar, S.; Shukla, V.; Upadhyay, R.S. Microbial Bioformulation-Based Plant Biostimulants: A Plausible Approach toward next Generation of Sustainable Agriculture. In Microbial Endophytes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–225. ISBN 978-0-12-819654-0. [Google Scholar]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The Role of Biostimulants and Bioeffectors as Alleviators of Abiotic Stress in Crop Plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kaur, M.; Kaur, J. Role of Biostimulants in Agriculture. In Biostimulants: Exploring Sources and Applications; Plant Life and Environment Dynamics; Ramawat, N., Bhardwaj, V., Eds.; Springer Nature: Singapore, 2022; pp. 239–262. ISBN 9789811670794. [Google Scholar]
- White, J.F.; Chang, X.; Kingsley, K.L.; Zhang, Q.; Chiaranunt, P.; Micci, A.; Velazquez, F.; Elmore, M.; Crane, S.; Li, S.; et al. Endophytic Bacteria in Grass Crop Growth Promotion and Biostimulation. Grass Res. 2021, 1, 5. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant Activity of Silicon in Horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Sultana, V.; Ehteshamul-Haque, S.; Ara, J.; Athar, M. Comparative Efficacy of Brown, Green and Red Seaweeds in the Control of Root Infecting Fungi and Okra. Int. J. Environ. Sci. Technol. 2005, 2, 129–132. [Google Scholar] [CrossRef]
- Ciepiela, G.A. The Effect of Biostimulants Derived from Various Materials on the Yield and Selected Organic Components of Italian Rye Grass (Lolium multiflorum Lam.) against the Background of Nitrogen Regime. Appl. Ecol. Env. Res. 2019, 17, 12407–12418. [Google Scholar] [CrossRef]
- Regelink, I.C.; Koopmans, G.F. Effects of Biostimulants and Fertilization on Nutrient Uptake by Grass and Composition of Soil Pore Water Versus 0.01 M CaCl2 Soil Extracts. Commun. Soil Sci. Plant Anal. 2021, 52, 2516–2532. [Google Scholar] [CrossRef]
- Truba, M.; Wiśniewska-Kadżajan, B.; Sosnowski, J.; Malinowska, E.; Jankowski, K.; Makarewicz, A. The Effect of Soil Conditioners on Cellulose, Hemicellulose, and the Adl Fibre Fraction Concentration in Dactylis glomerata and Lolium perenne. J. Ecol. Eng. 2017, 18, 107–112. [Google Scholar] [CrossRef][Green Version]
- Wiśniewska–Kadżajan, B.; Jankowski, K. Effect of Slurry Used with Soil Conditioners and Fertilizers on Structural, Non-structural Carbohydrate and Lignin Content. Agron. J. 2021, 113, 2812–2820. [Google Scholar] [CrossRef]
- Jankowski, K.; Truba, M.; Wiśniewska-Kadżajan, B.; Sosnowski, J.; Malinowska, E. The Effect of Soil Conditioners on Yield of Dry Matter, Protein, and Sugar in Grass. Acta Agroph. 2018, 25, 45–58. [Google Scholar] [CrossRef]
- Głosek-Sobieraj, M.; Cwalina-Ambroziak, B.; Wierzbowska, J.; Waśkiewicz, A. The Influence of Biostimulants on the Contentof P, K, Ca, Mg, and Na in the Skin and Fleshof Potato Tubers. Pol. J. Environ. Stud. 2019, 28, 1693–1700. [Google Scholar] [CrossRef]
- Sosnowski, J.; Jankowski, K.; Wiśniewska-Kadźajan, B. Effect of Growth Regulator Kelpak SL on the Formation of Aboveground Biomass of Festulolium Braunii (K. Richt.) A. Camus. Acta Agrobot. 2013, 66, 149–154. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Huang, B. Responses of Creeping Bentgrass to Trinexapac-Ethyl and Biostimulants under Summer Stress. HortScience 2010, 45, 125–131. [Google Scholar] [CrossRef]
- Schenck zu Schweinsberg-Mickan, M.; Müller, T. Impact of Effective Microorganisms and Other Biofertilizers on Soil Microbial Characteristics, Organic-matter Decomposition, and Plant Growth. Z. Pflanzenernähr. Bodenk. 2009, 172, 704–712. [Google Scholar] [CrossRef]
- Olszewska, M. Effects of Cultivar, Nitrogen Rate and Biostimulant Application on the Chemical Composition of Perennial Ryegrass (Lolium perenne L.) Biomass. Agronomy 2022, 12, 826. [Google Scholar] [CrossRef]
- Godlewska, A.; Ciepiela, G.A. Carbohydrate and Lignin Contents in Perennial Ryegrass (Lolium perenne L.) Treated with Sea Bamboo (Ecklonia maxima) Extract against the Background of Nitrogen Fertilisation Regime. Appl. Ecol. Env. Res. 2020, 18, 6087–6097. [Google Scholar] [CrossRef]
- Kováčik, P.; Wierzbowska, J.; Kováčik, P.; Šimanský, V.; Renčo, M. Impact of Foliar Application of the Biostimulator Mg-Titanit on the Formation of Winter Oilseed Rape Phytomass and Its Titanium Content. J. Elem. 2016, 21, 1235–1251. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I.; Bocianowski, J.; Sladkovska, T.; Wolski, K. The Effect of Foliar Application of an Amino Acid-Based Biostimulant on Lawn Functional Value. Agronomy 2020, 10, 1656. [Google Scholar] [CrossRef]
- Borawska-Jarmułowicz, B.; Mastalerczuk, G.; Janicka, M.; Wróbel, B. Effect of Silicon-Containing Fertilizers on the Nutritional Value of Grass–Legume Mixtures on Temporary Grasslands. Agriculture 2022, 12, 145. [Google Scholar] [CrossRef]
- Radkowski, A.; Wolski, K.; Radkowski, A.; Radkowska, I. Effect of Silicon Foliar Application on the Functional Value of Lawns. J. Elem. 2018, 23, 1257–1270. [Google Scholar] [CrossRef]
- Sosnowski, J.; Jankowski, K.; Truba, M.; Novák, J.; Zdun, E.; Skrzyczyńska, J. Morpho-Physiological Effects of Stymjod Foliar Application on Dactylis glomerata L. Adv. Search 2020, 18, 1036–1045. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P.; Kafkas, N.E.; Yesil, B.; Cieśliński, S. Foliar Application of Some Macronutrients and Micronutrients Improves Yield and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae 2022, 8, 664. [Google Scholar] [CrossRef]
- EUROSTAT Utilised Agricultural Area by Categories. Available online: https://data.europa.eu/data/datasets/lxlnp2kccsysb9o90hmlg?locale=en (accessed on 28 February 2019).
- Novák, J.; Jankowski, K.; Sosnowski, J.; Malinowska, E.; Wiśniewska-Kadżajan, B. Influence of Plant Species and Grasslands Quality on Sequestration of Soil Organic Carbon. Ekológia 2020, 39, 289–300. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J. Soil Respiration and Human Effects on Global Grasslands. Glob. Planet. Chang. 2009, 67, 20–28. [Google Scholar] [CrossRef]
- Cobon, D.H.; Baethgen, W.E.; Landman, W.; Williams, A.; van Garderen, E.A.; Johnston, P.; Malherbe, J.; Maluleke, P.; Kgakatsi, I.B.; Davis, P. Agroclimatology in Grasslands. In Agronomy Monographs; Hatfield, J.L., Sivakumar, M.V.K., Prueger, J.H., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Inc.: Madison, WI, USA, 2018; pp. 369–423. ISBN 978-0-89118-358-7. [Google Scholar]
- Finot, V.L.; Barrera, J.A.; Marticorena, C.; Rojas, G. Systematic Diversity of the Family Poaceae (Gramineae) in Chile. In The Dynamical Processes of Biodiversity: Case Studies of Evolution and Spatial Distribution; Books on Demand: Paris, France, 2011. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Impact of Various Grass Species on Soil Bacteriobiome. Diversity 2020, 12, 212. [Google Scholar] [CrossRef]
- Gabryszuk, M.; Barszczewski, J.; Wróbel, B. Journal of Water and Land DevelopmentJournal of Water and Land Development. J. Water Land Dev. 2021, 243–249. [Google Scholar] [CrossRef]
- Grzegorczyk, S. The Roleof Grassland Ecosystems in Environmental Management. Zesz. Probl. Post. Nauk Rol 2016, 586, 19–32. [Google Scholar]
- Pikuła, D. Rola Substancji Humusowych Oraz Innowacyjne Produkty Zwiększające Ich Zawartość w Glebie. ZESZYT 2016, 48, 81–93. [Google Scholar] [CrossRef]
- Ciepiela, G.A. Zawartość węglowodanów strukturalnych i niestrukturalnych oraz ligniny w Dactylis glomerata L. i Festulolium braunii (K. Richt.) A. Camus zasilanych biostymulatorem Kelpak SL i azotem. Nauka Przyr. Technol. 2014, 8, 2. [Google Scholar]
- Matysiak, K. Kelpak—A Natural Regulator of Plant Growth and Devel-Opment. In Selected Ecological Issues in Modern Agriculture. Ind. Inst. Agric. Poznań 2005, 375, 188–193. (In Polish) [Google Scholar]
- Godlewska, A.; Ciepiela, G.A.; Godlewska, A. Effect of the Biostimulant Kelpak SL on the Content of Some Microelements in Two Grass Species. J. Elem. 2016, 21, 373–381. [Google Scholar] [CrossRef]
- Sosnowski, J.; Jankowski, K. Wpływ Użyźniacza Glebowego Na Skład Chemiczny i Strawność Lolium multiflorum Lam. Nauka Przyroda Techn. 2013, 7, 15. [Google Scholar]
- Sosnowski, J. Kształtowanie Się Biomasy Nadziemnej Lolium multiflorum Lam. Pod Wpływem Użyźniacza Glebowego. Agron. Sci. 2012, 67, 24–32. [Google Scholar] [CrossRef]
- Sosnowski, J. Wartośc Produkcyjna, Energetyczna i Pokarmowa Festulolium Braunii (K. Richt.) A. Camus Zasilnaej Mikrobiologicznie i Mineralnie. Fragm. Agron 2012, 29, 115–122. [Google Scholar]
- Sosnowski, J. Reaction of Dactylis glomerata L., Festuca pratensis Huds. and Loliumk perenne L. Ti Microbiological Fertilizer and Mineral Fertilization. Acta Sci. Pol. Agric. 2012, 11, 91–98. [Google Scholar]
- Sosnowski, J. Wpływ Użyźniacza Glebowego Stosowanego w Uprawie Lolium perenne L., Dactylis glomerata L. I Festuca pratensis Huds. Na Względną Wartość Pokarmową (RFV) Paszy. Fragm. Agron. 2012, 29, 136–143. [Google Scholar]
- Lopes, M.J.S.; Dias-Filho, M.B.; Castro, T.H.R.; Gurgel, E.S.; Silva, G.B. Efficiency of Biostimulants for Alleviating Shade Effects on Forage Grass. J. Agric. Stud. 2021, 9, 14–30. [Google Scholar] [CrossRef]
- Kamari, A.; Pulford, I.D.; Hargreaves, J.S.J. Accumulation in Lolium perenneand Brassica Napusas Affected by Application of Chitosans. Int. J. Phytoremediation 2012, 14, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Agbodjato, N.A.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic Effects of Plant Growth Promoting Rhizobacteria and Chitosan on In Vitro Seeds Germination, Greenhouse Growth, and Nutrient Uptake of Maize (Zea mays L.). Biotechnol. Res. Int. 2016, 2016, 7830182. [Google Scholar] [CrossRef] [PubMed]
- Hofgaard, I.S.; Ergon, A.; Wanner, L.A.; Tronsmo, A.M. The Effect of Chitosan and Bion on Resistance to Pink Snow Mould in Perennial Ryegrass and Winter Wheat. J. Phytopathol. 2005, 153, 108–119. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Dąbrowski, P.; Szara, E.; Perzanowska, A.; Wróbel, B. Can the Application the Silicon Improve the Productivity and Nutritional Value of Grass–Clover Sward in Conditions of Rainfall Shortage in Organic Management? Agronomy 2020, 10, 1007. [Google Scholar] [CrossRef]
- Sosin-Bzducha, E.; Radkowski, A.; Radkowska, I.; Sosin-Bzducha, E. Effects of Silicon Foliar Fertilization of Meadow Plants on the Nutritional Value of Silage Fed to Dairy Cows. J. Elem. 2017, 22, 1311–1322. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I.; Lemek, T. Effects of Foliar Application of Titanium on Seed Yield in Timothy (Phleum pratense L.). Ecol. Chem. Eng. S 2015, 22, 691–701. [Google Scholar] [CrossRef]
- Cigler, P.; Olejnickova, J.; Hruby, M.; Csefalvay, L.; Peterka, J.; Kuzel, S. Interactions between Iron and Titanium Metabolism in Spinach: A Chlorophyll Fluorescence Study in Hydropony. J. Plant Physiol. 2010, 167, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Ciepiela, G.; Godlewska, A. The effect of growth regulator on structural and non-structural carbohydrates and lignin content in selected grass species and cultivars. J. Ecol. Eng. 2015, 16, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ciepiela, G.A. Reaction of Some Grass Species on the Nitrogenfertilization Applied in Urea Solution and in Ammonium Nitrate; University of Podlasie: Siedlce, Poland, 2004. (In Polish) [Google Scholar]
- Golińska, B.; Kozłowski, S. Variation in the Occurrenceof Organic and Mineral Compounds InPhalaris Arundinacea. Ann. Univ. Mariae Curie-Sklodowska 2006, 61, 353–360, (In Polish with English Abstract). [Google Scholar]
- Sangha, J.S.; Kelloway, S.; Critchley, A.T.; Prithiviraj, B. Seaweeds (Macroalgae) and Their Extracts as Contributors of Plant Productivity and Quality. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 189–219. ISBN 978-0-12-408062-1. [Google Scholar]
- Hong, D.D.; Hien, H.M.; Son, P.N. Seaweeds from Vietnam Used for Functional Food, Medicine and Biofertilizer. J. Appl. Phycol. 2007, 19, 817–826. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Biernacik, M.; Wolski, K.; Biernacik, M.; Talar-Krasa, M.; Leshchenko, O.; Świerszcz, S. Effect of the Application of a Biostimulant and Mineral Fertilization on the Mineral Element Concentration in the Sward of Forage Mixtures Cultivated on Light Soil. J. Elem. 2018, 24, 385–397. [Google Scholar] [CrossRef]
- Ciepiela, G.A.; Godlewska, A. Changes in Protein Compounds and Monosaccharides in Select Grass Species Following Application of a Seaweed Extract. Pol. J. Environ. 2014, 23, 35–41. [Google Scholar]
- Godlewska, A.; Ciepiela, G.A. The Effect of Growth Regulator on Dry Matter Yield and Some Chemical Components in Selected Grass Species and Cultivars. Soil Sci. Plant Nutr. 2016, 62, 297–302. [Google Scholar] [CrossRef]
- Ratajczak, K.; Sulewska, H.; Panasiewicz, K.; Faligowska, A.; Szymańska, G. Phytostimulator Application after Cold Stress for Better Maize (Zea mays L.) Plant Recovery. Agriculture 2023, 13, 569. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the Core Arabidopsis Thaliana Root Microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, A.; Król, M.; Perzyński, A. Zmiany Aktywności Biologicznej Gleb Skażonych Wielopierścieniowymi Węglowodorami Aromatycznymi (WWA) i Olejem Napędowym (ON) w Ryzosferze Kukurydzy. Zesz. Probl. Post. Nauk Roln. 2009, 535, 111–120. [Google Scholar]
- Biari, A.; Gholami, A.; Rahmani, H.A. Growth Promotion and Enhanced Nutrient Uptake of Maize (Zea mays L.) by Application of Plant Growth Promoting Rhizobacteria in Arid Region of Iran. J. Biol. Sci. 2008, 8, 1015–1020. [Google Scholar] [CrossRef]
- Piskier, T. Reaction of Spring Wheat to the Application of Biostimulators and Soil Absorbents. J. Res. Appl. Agric. Eng. 2006, 51, 136–138. [Google Scholar]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of Chitosan on Plant Responses with Special Reference to Abiotic Stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ham, S.K.; Lee, J.P.; Hwang, Y.S.; Lee, K.S. Effects of Two Amino Acid Fertilizers on Growth of Creeping Bentgrass and Nitrogen Uptake. Weed Turfgrass Sci. 2014, 3, 246–252. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant Activity of Chitosan in Horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, X.; Du, Y. Oligochitosan: A Plant Diseases Vaccine—A Review. Carbohydr. Polym. 2010, 82, 1–8. [Google Scholar] [CrossRef]
- Kamari, A.; Pulford, I.D.; Hargreaves, J.S.J. Chitosan Assisted Phytoextraction of Heavy Metal from Lead/Zinc Tailings Using Lolium perenne—A Preliminary Study. In Proceedings of the 15th International Conference on Heavy Metals in the Environment, Gdansk, Poland, 19–23 September 2010. [Google Scholar]
- Amborabe, B.-E.; Bonmort, J.; Fleurat-Lessard, P.; Roblin, G. Early Events Induced by Chitosan on Plant Cells. J. Exp. Bot. 2008, 59, 2317–2324. [Google Scholar] [CrossRef]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef] [PubMed]
- Gugala, M.; Sikorska, A.; Findura, P.; Kapela, K.; Malaga-Tobola, U.; Zarzecka, K.; Domanski, L. Effect of Selected Plant Preparations Containing Biologically Active Compounds on Winter Rape (Brassica napus L.) Yielding. Appl. Ecol. Environ. Res. 2019, 17, 2779–2789. [Google Scholar] [CrossRef]
- Malinowska, E.; Wiśniewska-Kadżajan, B. Effects of Tytanit and Nitrogen on Cellulose and Hemicellulose Content of Festulolium Braunii and on Its Digestibility. Agronomy 2022, 12, 1547. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef]
- Malinowska, E.; Jankowski, K.; Kania, P.; Gałecka, M. The Effect on Tytanit Foliar Application on the Yield and Nutritional Value of Festulolium Braunii. Agronomy 2020, 10, 848. [Google Scholar] [CrossRef]
- Jankowski, K.; Deska, J.; Truba, M.; Jankowska, J. Impact on Nano-Gro Stimulator on the Seeds Germination and Growth Kinetics of Seedlings of Selected Grass and Legumes Species. Environ. Prot. Nat. Resour. 2013, 24, 23–26. [Google Scholar] [CrossRef][Green Version]
- Rossall, S.; Qing, C.; Paneri, M.; Bennett, M.; Swarup, R. A ‘Growing’ Role for Phosphites in Promoting Plant Growth and Development. Acta Hortic. 2016, 1148, 61–68. [Google Scholar] [CrossRef]
- Stackhouse, T.; Martinez-Espinoza, A.D.; Ali, M.E. Turfgrass Disease Diagnosis: Past, Present, and Future. Plants 2020, 9, 1544. [Google Scholar] [CrossRef]
- Huang, B. Grass Research for a Productive, Healthy and Sustainable Society. Grass Res. 2021, 1, 1. [Google Scholar] [CrossRef]
- Hatfield, J. Turfgrass and Climate Change. Agron. J. 2017, 109, 1708–1718. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, W.; Amombo, E.; Hu, L.; Kjorven, J.O.; Chen, L. Mechanisms of Environmental Stress Tolerance in Turfgrass. Agronomy 2020, 10, 522. [Google Scholar] [CrossRef]
- Grabowski, K.; Grzegorczyk, S.; Łachacz, A.; Olszewska, M. Functional Characteristics of a Grass Swardas per an Athletic Field at the Universityof Warmia and Mazury in Olsztyn. Pol. J. Environ. Stud. 2020, 29, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, B.; Vidrih, M.; Bohinc, T.; Trdan, S. Impact of Fertilisers on Five Turfgrass Mixtures for Football Pitches under Natural Conditions. Hort. Sci. 2021, 48, 190–204. [Google Scholar] [CrossRef]
- Stowell, L.J.; Gelernter, W.D. Diagnosis of Turfgrass Diseases. Annu. Rev. Phytopathol. 2001, 39, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Prus, P.; Szwarc, K.; Muhire, J.d.D. Achieving the European Green “Deal” of Sustainable Grass Forage Production and Landscaping Using Fungal Endophytes. Agriculture 2021, 11, 390. [Google Scholar] [CrossRef]
- de Castilho, R.M.M.; Freitas, R.C.; Santos, P.L.F.d. The Turfgrass in Landscape and Landscaping. Ornam. Hortic. 2020, 26, 499–515. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S. Salinity Tolerance Turfgrass: History and Prospects. Sci. World J. 2013, 2013, 409413. [Google Scholar] [CrossRef]
- Jiuxin, L.; Liebao, H. Progress and Challenges in China Turfgrass Abiotic Stress Resistance Research. Front. Plant Sci. 2022, 13, 922175. [Google Scholar] [CrossRef]
- Sithin, M. Role of Turfgrass in Urban Landscapes. J. Plant Dev. Sci. 2021, 13, 247–255. [Google Scholar]
- Godoy, L.J.G.d. Turfs and Turfgrasses in Brazil. Ornam. Hortic. 2020, 26, 326–327. [Google Scholar] [CrossRef]
- Głąb, T.; Szewczyk, W.; Gondek, K.; Mierzwa-Hersztek, M.; Palmowska, J.; Nęcka, K. Optimization of Turfgrass Fertigation Rate and Frequency. Agric. Water Manag. 2020, 234, 106107. [Google Scholar] [CrossRef]
- Serba, D.D.; Hejl, R.W.; Burayu, W.; Umeda, K.; Bushman, B.S.; Williams, C.F. Pertinent Water-Saving Management Strategies for Sustainable Turfgrass in the Desert U.S. Southwest. Sustainability 2022, 14, 12722. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Predominant Microbial Colonizers in the Root Endosphere and Rhizosphere of Turfgrass Systems: Pseudomonas Veronii, Janthinobacterium Lividum, and Pseudogymnoascus spp. Front. Microbiol. 2021, 12, 643904. [Google Scholar] [CrossRef] [PubMed]
- Talar-Krasa, M.; Świerszcz, S. The Influence of Using Biostimulant on Thevisual Quality of Turf. Episteme 2015, 26, 365–373. [Google Scholar]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Seaweed Extract, Humic Acid, and Propiconazole Improve Tall Fescue Sod Heat Tolerance and Posttransplant Quality. HortScience 2003, 38, 440–443. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Physiological Effects of Liquid Applications of a Seaweed Extract and a Humic Acid on Creeping Bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R.E.; Ervin, E.H.; Doak, S. Creeping Bentgrass Physiological Responses to Natural Plant Growth Regulators and Iron under Two Regimes. HortScience 2002, 37, 898–902. [Google Scholar] [CrossRef]
- Liu, C.; Cooper, R.J. Humic Substances Influence Creeping Bentgrass Growth. Golf. Course Manag. 2000, 68, 49–53. [Google Scholar]
- Mueller, S.R.; Kussow, W.R. Biostimulant Influences on Turfgrass Microbial Communities and Creeping Bentgrass Putting Green Quality. HortScience 2005, 40, 1904–1910. [Google Scholar] [CrossRef]
- Daneshvar Hakimi Maibodi, N.; Kafi, M.; Nikbakht, A.; Rejali, F. Effect of Foliar Applications of Humic Acid on Growth, Visual Quality, Nutrients Content and Root Parameters of Perennial Ryegrass (Lolium perenne L.). J. Plant Nutr. 2015, 38, 224–236. [Google Scholar] [CrossRef]
- Acuña, A.; Gardner, D.; Villalobos, L.; Danneberger, K. Effects of Plant Biostimulants on Seedling Root and Shoot Growth of Three Cool-season Turfgrass Species in a Controlled Environment. Int. Turfgrass Soc. Res. J. 2022, 14, 416–421. [Google Scholar] [CrossRef]
- Ervin, E.H.; Zhang, X.; Roberts, J. Improving Root Development with Foliar Humic Acid Applications during Kentucky Bluegrass Sod Establishment on Sand. Acta Hortic. 2008, 783, 317–322. [Google Scholar] [CrossRef]
- Guillard, K.; Inguagiato, J.C. Normalized Difference Vegetative Index Response of Nonirrigated Kentucky Bluegrass and Tall Fescue Lawn Turf Receiving Seaweed Extracts. HortScience 2017, 52, 1615–1620. [Google Scholar] [CrossRef]
- Talar-Krasa, M.; Wolski, K.; Radkowski, A.; Khachatryan, K.; Bujak, H.; Bocianowski, J. Effects of a Plasma Water and Biostimulant on Lawn Functional Value. Agronomy 2021, 11, 254. [Google Scholar] [CrossRef]
- Kim, J.; Ham, S.K.; Lee, J.; Hwang, Y.S. The Growth Effects of Creeping Bentgrass by Application of Liquid Fertilizer with Saponin and Liquid Fertilizer with Amino Acid. Asian J. Turfgrass Sci. 2012, 26, 54–59. [Google Scholar]
- McCoy, R.M.; Meyer, G.W.; Rhodes, D.; Murray, G.C.; Sors, T.G.; Widhalm, J.R. Exploratory Study on the Foliar Incorporation and Stability of Isotopically Labeled Amino Acids Applied to Turfgrass. Agronomy 2020, 10, 358. [Google Scholar] [CrossRef]
- Stingl, U.; Choi, C.J.; Dhillon, B.; Schiavon, M. The Lack of Knowledge on the Microbiome of Golf Turfgrasses Impedes the Development of Successful Microbial Products. Agronomy 2021, 12, 71. [Google Scholar] [CrossRef]
- Charest, C.; Clark, G.; Dalpe, Y. The Impact of Arbuscular Mycorrhizae and Phosphorus Status on Growth of Two Turfgrass Species. J. Turfgrass Manag. 1997, 2, 1–14. [Google Scholar] [CrossRef]
- Pelletier, S.; Dionne, J. Inoculation Rate of Arbuscular-mycorrhizal Fungi Glomus Intraradicesand and Glomus Etunicatum Affects Establishment of Landscape Turf with No Irrigation or Fertilizer Inputs. Crop Sci. 2004, 44, 335–338. [Google Scholar] [CrossRef]
- Li, Q.; Li, R.; He, F.; Yang, Z.; Yu, J. Growth and Physiological Effects of Chitosan on Heat Tolerance in Creeping Bentgrass (Agrostis stolonifera). Grass Res. 2022, 2, 6. [Google Scholar] [CrossRef]
- Acemi, A.; Tirli, D.; Yildiz, S.; Özen, F. Developmental Responses of Perennial Ryegrass, Red Fescue, and Kentucky Bluegrass to In Vitro Chitosan Treatments. Biotech Stud. 2021, 30, 63–70. [Google Scholar] [CrossRef]
- Yoon, O.; Kim, K. Effects of Chitosan on the Growth Responses of Kentucky Bluegrass (Poa pratensis L.). Korean Turfgrass Sci. 2007, 21, 163–176. [Google Scholar]
- Chai, Q.; Shao, X.; Zhang, J. Silicon Effects on Poa Pratensis Responses to Salinity. HortScience 2010, 45, 1876–1881. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Abiotic and Biotic Plant Stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef]
- Zhang, Q.; Fry, J.; Lowe, K.; Tisserat, N. Evaluation of Calcium Silicate for Brown Patch and Dollar Spot Suppression on Turfgrasses. Crop Sci. 2006, 46, 1635–1643. [Google Scholar] [CrossRef]
- Pruyne, D.T.; Schlossberg, M.J.; Uddin, W. Perennial Ryegrass Wear Resistance and Soil Amendment by Ca- and Mg-Silicates. Agronomy 2019, 9, 578. [Google Scholar] [CrossRef]
- Nanayakkara, U.N.; Uddin, W.; Datnoff, L.E. Application of Silicon Sources Increases Silicon Accumulation in Perennial Ryegrass Turf on Two Soil Types. Plant Soil 2008, 303, 83–94. [Google Scholar] [CrossRef]
- Akinci, S.; Büyükkeskin, T.; Eroğlu, A.; Erdoğan, B.E. The Effect of Humic Acid on Nutrient Composition in Broad Bean (Vicia faba L.) Roots. Not. Sci. Biol. 2009, 1, 81–87. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Chen, Y.; Clapp, C.E.; Magen, H. Mechanisms of Plant Growth Stimulation by Humic Substances: The Role of Organo-Iron Complexes. Soil Sci. Plant Nutr. 2004, 50, 1089–1095. [Google Scholar] [CrossRef]
- Nikbakht, A.; Pessarakli, M.; Daneshvar-Hakimi-Maibodi, N.; Kafi, M. Perennial Ryegrass Growth Responses to Mycorrhizal Infection and Humic Acid Treatments. Agron. J. 2014, 106, 585–595. [Google Scholar] [CrossRef]
- Van Dyke, A.; Johnson, P.G.; Grossl, P.R. Humic Substances Effect on Moisture Retention and Phosphorus Uptake in Intermountain West Putting Greens. Appl. Turfgrass Sci. 2008, 5, 1–9. [Google Scholar] [CrossRef]
- Fike, J.H.; Allen, V.G.; Schmidt, R.E.; Zhang, X.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Coelho, R.W.; Wester, D.B. Tasco-Forage: I. Influence of a Seaweed Extract on Antioxidant Activity in Tall Fescue and in Ruminants. J. Anim. Sci. 2001, 79, 1011. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, J.S. Seaweed Extract: Biostimulator of Plant Defense and Plant Productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Corsi, S.; Ruggeri, G.; Zamboni, A.; Bhakti, P.; Espen, L.; Ferrante, A.; Noseda, M.; Varanini, Z.; Scarafoni, A. A Bibliometric Analysis of the Scientific Literature on Biostimulants. Agronomy 2022, 12, 1257. [Google Scholar] [CrossRef]
- Pipiak, P.; Skwarek, M. The Use of Amino Acid Fertilizers in Agriculture. Technol. Jakość Wyr. 2020, 65, 144–157. [Google Scholar]
- Rutkowska, A. Biostymulatory w Nowoczesnej Uprawie Rośl. ZESZYT 2016, 48, 65–80. [Google Scholar] [CrossRef]
- Mertz, I.T.; Christians, N.E.; Thoms, A.W. Branched-Chain Amino Acids for Use as a Nitrogen Source on Creeping Bentgrass. HortScience 2019, 29, 833–837. [Google Scholar] [CrossRef]
- Żurek, G.; Wiewióra, B.; Rybka, K.; Prokopiuk, K. Different response of perennial ryegrass—Epichloë endophyte symbiota to the elevated concentration of heavy metals in soil. J. Appl. Genet. 2022, 63, 47–59. [Google Scholar] [CrossRef]
- Matzrafi, M.; Preston, C.; Brunharo, C.A. Review: Evolutionary Drivers of Agricultural Adaptation in Lolium spp. Pest Manag. Sci. 2021, 77, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, K.G.; Popay, A.J.; Hofmann, R.W.; Caradus, J.R. Epichloë—A Lifeline for Temperate Grasses under Combined Drought and Insect Pressure. Grass Res. 2021, 1, 7. [Google Scholar] [CrossRef]
- Pereira, E.; Vázquez de Aldana, B.R.; San Emeterio, L.; Zabalgogeazcoa, I. A Survey of Culturable Fungal Endophytes from Festuca rubra subsp. Pruinosa, a Grass from Marine Cliffs, Reveals a Core Microbiome. Front. Microbiol. 2019, 9, 3321. [Google Scholar] [CrossRef] [PubMed]
- Allan-Perkins, E.; Manter, D.K.; Jung, G. Soil Microbial Communities on Roughs, Fairways, and Putting Greens of Cool-Season Golf Courses. Crop Sci. 2019, 59, 1753–1767. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a Promising Natural Compound to Enhance Potential Physiological Responses in Plant: A Review. Ind. J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of Silicon Fertilization on Maize Performance Under Limited Water Supply. Silicon 2018, 10, 177–183. [Google Scholar] [CrossRef]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of Silicon on Plant–Pathogen Interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).