Abstract
Grasslands and pastures are extensively studied due to their geographic variation, species richness, ecological functioning, and economic importance. They are vital components of land use in many parts of the world. The impact of grassland management on species diversity and species composition has also been widely discussed, but results have been contradictory. It is well known that the relationship between species richness and the sampled area is perhaps one of the most consistent rules in plant ecology. This relationship is particularly important in biodiversity studies as it helps to predict richness at larger scales. Additionally, species richness is also influenced by absolute plant abundance, spatial patterns, and the degree of species mixing. However, species richness also depends on absolute plant abundance, spatial patterns, and the degree of mixing species. To assess this relationship, we analyzed the impact of cattle grazing on species richness at a sampling scale in the Sierra of Zapaliname, a protected area in northern Mexico. Our results revealed that the increase in plant species concerning the sampling area significantly differed in the plots excluded from grazing from the control (grazed) plots, and these relationships are differently detected in the function of the scale. Despite the lack of differences in previous studies on species richness without considering the scale, once the scale is incorporated, differences arise among both treatments. As indicated in previous studies, grazing exclusion can lead to a decrease in species richness, but we suggest that some areas of the pasture could be excluded from grazing for longer periods, as long as it is compatible with the economic needs of the local inhabitants, to investigate changes and promote diversity, especially for plant species associated with areas excluded from grazing.
1. Introduction
The primary goal of conservation managers around the world is to maintain biodiversity [1,2]. In the case of plant communities subjected to long-term effects of herbivore grazing, it has been suggested that maintaining low to medium grazing intensities could enhance plant diversity [3,4]. However, research results are conflicting and depend on grazing management [5], environmental conditions [6], climate gradient [7], sampling scale [8], site productivity [9], and grazing intensity [10], among others.
Grasslands and pastures have been extensively studied due to their geographic variation, species richness, ecological functioning, and economic importance [11,12]. They are vital components of land use in many parts of the world [13]. The impact of grassland management has also been widely discussed, but the results have been contradictory. For instance, ungulate grazing has been reported to increase [14,15] or decrease [16] species richness. Depending on the successional state of the grassland and pasture, environmental conditions will have a higher impact on species composition than on species relationships. However, there are several mechanisms explaining species richness and coexistence in pastures and grasslands such as biomass, weather conditions, and ecological competition abilities between species [17].
Pastures in North America have a combination of tall and short grasses from southern Canada to central Mexico [18]. Semiarid pastures in Mexico are classified as shortgrass prairies, forming a part of a broader plant community that extends its distribution across a vast expanse, ranging from the northern reaches of Alberta down to the southern reaches of Arizona, New Mexico, Texas, and northern Mexico. This expansive geographical range underscores the adaptability of this plant community to a diverse array of climatic and ecological conditions, while the classification as shortgrass prairies signifies a distinctive ecological profile characterized by hardy, drought-tolerant vegetation. The presence of this community across such a range highlights its ecological resilience and its ability to thrive in regions with limited water availability and fluctuating environmental conditions. This ecological significance, spanning multiple regions and encompassing a spectrum of ecosystems, accentuates the importance of understanding its dynamics and management strategies in order to ensure its conservation and sustainable utilization. [19]. The genus Bouteloua comprising 29 species and 13 subspecies, is a dominant component of these ecosystems in Mexico [20]. Overgrazing by livestock is a widespread issue in northern Mexico [21,22]. It is recommended to establish recovery periods to enhance biomass production and promote the growth of highly palatable plants for livestock while managing negative impacts on shrub species. Similar outcomes to those observed in Mexico due to overgrazing have been reported in South Africa [23]. The consequences of overgrazing include a decline in rangeland condition, a reduction in palatable forages, and changes in plant species composition [24]. Rangeland management should consider the perceptual evidence of changes in soil and vegetation patterns, as well as socioeconomic factors such as land tenure and forms of organization [25].
The relationship between species richness and sampled area is a consistent rule in plant ecology [26] and has particular importance in biodiversity studies as it enables predictions of richness at larger scales [27]. However, species richness is also influenced by absolute plant abundance, spatial patterns, and the degree of species mixing [28].
Due to this, it is crucial to consider the scale at which biodiversity should be analyzed and assessed [29], as long as there are variations across different scales, and studying it at multiple scales would provide a more accurate representation of the overall patterns and drivers of biodiversity in pastures [30].
Scale is important because biodiversity is influenced by various factors operating at different spatial and temporal scales [31]. Local-scale factors, such as habitat structure and management practices, can have a significant impact on the composition and abundance of species within a small area [32]. On the other hand, landscape-scale factors, such as land use patterns and connectivity, can influence the movement and dispersal of plant species across larger areas [33]. Moreover, regional and global-scale processes, such as climate change and species invasions, can have far-reaching consequences for pasture biodiversity [34].
By considering multiple scales, researchers can gain insights into the interactions between these different factors and their effects on biodiversity [35]. This approach allows for a more holistic understanding of the mechanisms shaping biodiversity patterns in pastures. Furthermore, considering scale helps to identify the appropriate spatial and temporal scales at which conservation and management interventions should be implemented to maximize their effectiveness [36].
In this study, we hypothesized that grazing enhances diversity through microenvironmental disturbance caused by livestock. An additional hypothesis was that the sampling scale (from 0.01 m2 to 100 m2) alters plant diversity on control and grazing-excluded plots. By supplying pertinent information based on empirical findings, these results will equip range managers with the knowledge necessary to make informed decisions, fostering a proactive and adaptive approach to the sustainable management of the ecosystem.
2. Materials and Methods
2.1. Study Area
The study site is located in southeastern Coahuila State, which serves as a transition zone between the Chihuahuan Desert and the Sierra Madre Oriental physiographic province (25°13′57.48″–25°14′ 57.25″ N and 100°56′ 44.62″–101°01′5.17″ W). The study area lies within the Sierra de Zapaliname natural protected area (Figure 1).
The climate is arid to semiarid and falls under the BSKw classification of semiarid temperate weather (a cold steppe climate with dry winters, characterized by limited rainfall, relatively moderate temperatures, and a vegetation pattern dominated by grasslands and pastures), with precipitation occurring mainly in summer [37]. The study plots were established at elevations ranging from 2102 to 2268 masl. The site features calcareous rocks and deep, well-drained soils. The average annual temperature is 16.9 °C, and the average annual precipitation is 498 mm. The plant community in the area is dominated by Bouteloua curtipendula, B. dactyloides, B. gracilis, B. uniflora, Aristida havardii, A. pansa, and Muhlenbergia phleoides [38]. The woody species scattered in the area are Alloberberis trifoliolata, Buddleja scordioides, Gymnosperma glutinosum, Mimosa biuncifera, and Prosopis glandulosa.
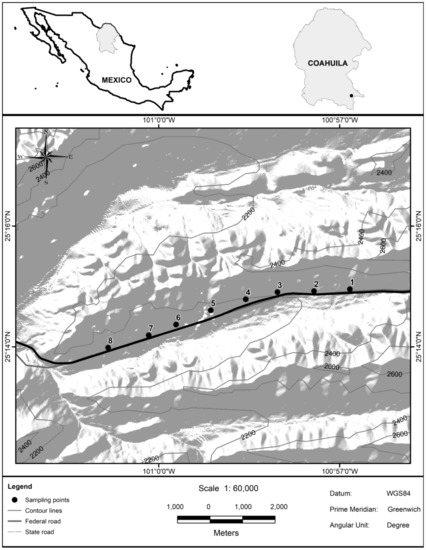
Figure 1.
Study site showing the sampling plots (black points for pair of plots) positioned through the pastures studied and the location in the protected area Sierra de Zapaliname, Coahuila State, Mexico [39].
Agricultural activities began in the late 19th century [40]. Cropland is largely devoted to wheat, corn, beans, and barley. Additionally, fruit trees were cultivated in the pasture areas and alluvial valleys. Currently, approximately 400 ha of pasture in the study site are grazed by cattle and horses, with a relatively constant population of 63 cattle heads and 37 equines. This total number of animals has remained constant in the last decade with a regular use of the pastures (personal communication).
Samplings were conducted between 2017 and 2021 during the humid period of the year (August). In 2016, the annual rainfall exceeded 500 mm. However, over the subsequent years, rainfall decreased to approximately 200 mm (much drier than the average). The average annual temperature remained relatively constant, with minimal fluctuations of less than 0.5 °C (Figure 2).

Figure 2.
Annual precipitation and average annual temperature in the area of Sierra of Zapaliname throughout the study period [39].
2.2. Sampling Design and Sample Collection
On March 2017, a systematic survey of the main pasture community in the natural protected area of Sierra Zapaliname was conducted. Along a transect, we established eight pairs of square plots (20 × 20 m2) approximately 1000 m apart from each other. One of the plots in each pair was excluded from livestock grazing, using barbed wire, while the other was used as a control. Within each plot, 10 × 10 m2 permanent plots were established, from which plant samples were collected. The control and grazing-excluded plots were separated by a minimum of 10 m. We used a global positioning system (GPS; Etrex, Garmin Ltd., Olathe, KS, USA) to register plot position and elevation.
To examine the effects of scale, we assessed vegetation at various sampling scales. We followed the procedure of Peet et al. [41,42] and recorded all vascular plant species in the nested square quadrats at each corner of the plots. The quadrats’ areas were 0.01, 0.1, 1, and 10 m2. We also observed new plant species in the remaining 100 m2 plot. Cover percentage of each plant species in the 100 m2 plot was estimated using a 10-point scale (1 = trace, 2 = <1% cover, 3 = 1–2%, 4 = 2–5%, 5 = 5–10%, 6 = 10–25%, 7 = 25–50%, 8 = 50–75%, 9 = >75%, 10 = 100%). Latitude, longitude, altitude, and slope, were also recorded (Table 1).

Table 1.
Plot characteristics (“E” for exclusion plots and “C” for control plots). Aspect measured in degrees using as reference the north and slope in sexagesimal degrees. (*) Meters above sea level.
Plant specimens were collected, and their taxonomic identities were determined. Vouchers were deposited at the ANSM herbarium (Autonomous Agraria University Antonio Narro’s herbarium), and species names were confirmed using the checklist of vascular plants of the Sierra of Zapaliname [38].
2.3. Statistical Analysis
The use of power models in ecological research to describe species–area relationships has been established in various studies [26,43,44]. The equation S = cAz (where “S” is the number of species, “A” is the sampled area that can be related to the total amount of resources or primary productivity, “c” is a constant that represents the number of species that can be supported in a minimum area, and “z” is a scaling exponent that characterizes the relationship between area and species richness) is commonly used to understand the effects of scale on biodiversity and to compare biodiversity among different areas. In the present study, we tested the adjustment of the power function to our log–log data using the Pearson correlation coefficient (p < 0.05). The species–area relationships were examined using the number of species observed at each plot for each scale. For each plot, we obtained four data points for each scale except for the largest scale (100 m2), where only one data point existed. To estimate the parameters “c” and “z”, we employed logarithms to linearize the data. These parameters were estimated for each plot across different years.
In this study, we aimed to assess the impact of two factors, control vs. excluded and sampling year, on the “c” and “z” values of the plots. These effects were analyzed using the Generalized Linear Model (GLM) procedure in SAS (SAS Institute Inc., Cary, NC, USA). The control vs. excluded factor refers to whether the plots were subjected to grazing control or excluded from grazing. The sampling year factor indicates the specific year in which data were collected.
We treated the main effects (control vs. excluded and sampling year) as fixed effects, meaning that they were factors intentionally manipulated or recorded. Additionally, we included the pair of plots as random effects, accounting for the potential variability between specific plot pairs.
To ensure the validity of this analysis, the Breusch–Pagan test was conducted, which checks for homogeneity of variances. This test helps to determine if the assumption of equal variances across the different groups or treatments is met. We considered a significance level of p < 0.05 to determine the presence of any significant deviations from homogeneity.
Rarefaction analysis is a valuable tool in ecology as it accounts for variations in sample sizes and allows for fair comparisons of species richness between different sampling efforts, providing a more comprehensive understanding of biodiversity patterns and comparisons. We implemented rarefaction analyses and calculated the expected number of species as a function of sampling effort using the species presence in subplots (except for 100 m2 plots, where the entire plot was used). We calculated rarefaction curves for grazed and non-grazed plots at different scales (160 plots for 0.01, 0.1, 1, and 10 m2 scales; and 40 plots for the 100 m2 scale). The accumulation curve incorporated the 95% confidence interval and was represented together for each different scale. Basic statistical methods followed that of Legendre and Legendre [45] and were carried out in the Vegan R software (version 4.1.3) [46].
3. Results
The study area had consistent environmental features under similar management conditions throughout the transect, with minimal altitudinal changes of less than 150 m, uniform aspect, and slope ranging from 10 to 20 degrees as shown in Table 1.
A comprehensive survey of the study area yielded 161 plant species (Appendix A). Out of these species, only three were identified as introduced, namely Asphodelus fistulosus, Malva parviflora, and Tribulus terrestris. However, these introduced species did not exhibit dominance over the control and grazing-excluded plots. Based on the classification of functional groups, the prevailing group was forbs, with 106 species, followed by grasses with 30 species, and then shrubs and cacti with 13 and 10 species, respectively, dominating these pastures. Out of the total species count, only 60 were deemed palatable. One fern was also found during the studied period (Ophioglossum engelmannii). The richness of species in these pastures not only supports local biodiversity, but also sustains important ecological processes, including pollination, nutrient cycling, and wildlife movement.
The estimated power function (Table 2) for all plots along the five years of sampling was significant in all cases (p < 0.001, the adjustment to the log–log), meaning that the z and c constants estimated can be reliable. When comparing these constants with the fixed factors of treatment and year, and the random factor pair of plots, for the constant c, we found significant differences for management, but not for year, with higher values in the case of the grazing-excluded plots (F1,80 = 13.11, p < 0.001), while these results were not significant for the year effect (F4,80 = 2.11, p > 0.05; Figure 3a). In the case of the constant z, similar results were observed and we found significant differences in the case of management, with higher values in the excluded plots (F1,80 = 13.82, p < 0.001), while these results were not affected by year (F4,80 = 2.20, p > 0.05; Figure 3b).

Table 2.
Power function constants for each plot at different years (tre = treatment; co: control; ex: grazing excluded).
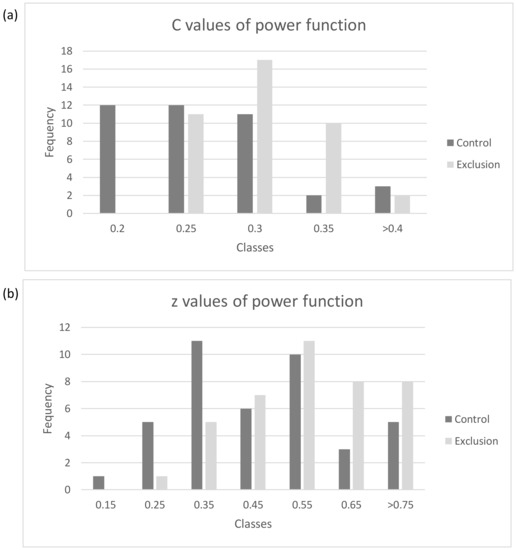
Figure 3.
Histogram with the power functions’ constants values for (a) c and (b) z of the Arrhenius equation.
The rarefaction estimated curves offered different results for the studied scales (Figure 4). Control plots revealed a rarefaction curve over the curve of the ungrazed plot at the scales of 1 dm2 and 10,000 dm2, being only significant in the first case over more than 100 accumulated plots. In 10 and 100 dm2, the estimated curves for excluded plots were over the accumulated curve of control plots, but only significant over 100 accumulated. In the case of the accumulation curve of 1000 dm2 plots, there were no differences (p > 0.05).

Figure 4.
Species accumulation curves at different scales; grey zones are 95% confident intervals for both curves, black for excluded and blue for control; (a) 1 dm2, (b) 10 dm2, (c) 100 dm2, (d) 1000 dm2 and (e) 10,000 dm2.
4. Discussion
The study examined the effects of five years of cattle grazing exclusion in a native pasture in northern Mexico. Previous studies on this site [39] revealed no significant differences in species richness, evenness, and soil nutrients between the grazed and ungrazed areas. However, some species showed a higher prevalence in the ungrazed compared to the grazed plots. Moreover, grazing exclusion of the rangeland led to the expansion of grasses, while forbs increased in the grazed areas, but only for a few species. These findings suggest that medium-term grazing exclusion did not significantly affect soil nutrient content, but promoted grass growth.
The analysis of the coefficient power functions c and z revealed that grazing management affected the accumulation of species along with the increase in sampling area as well as the overall species richness in this ecosystem. However, year did not affect the vegetation, which means that management variability is the most important factor driving diversity. Based on this power function, grazing will affect these diversity parameters. However, variation over the years did not have an effect, being considered important in other studies [7,47]. This hypothesis is supported by the relatively constant weather conditions of the study period.
Higher values of c (Figure 2a) for the exclusion plots indicated a higher species richness as the intercept with the logS–logA space. In practical terms, the higher intercept of c in the logS–logA graph for exclusion plots signifies a stronger sensitivity to area, indicating that relatively small changes in sampling area can lead to more pronounced shifts in the species richness. In the case of the z values, we also found higher values for the slope, which means a higher increase in richness concerning the area [26]. Consequently, higher z values emphasize the significance of landscape heterogeneity in fostering species coexistence and accentuate the need to consider both area and habitat diversity in conservation and management strategies. In previous studies on this site, general richness analyses revealed non-significant differences [39], so the consideration of the scale should be incorporated in the analyses to detect the impact of management on the plots [41].
When comparing the rarefaction curves of control and excluded subplots at different scales, we observed significant differences at different levels. Specifically, at small scales (1 dm2), the control plots had a higher species richness accumulation over 100 subplots. However, at 100 dm2, the richness was higher in the grazed plots, with significant differences over 100 plots. These differences in the curves can indicate different levels of richness, as previously suggested [48], and reveals that grazing has a stronger effect at smaller scales. Grazing by goats can create microhabitats by consuming dominant plants, which promotes diversity and coexistence among species by reducing competition for light and nutrients [49,50]. However, this effect disappears at larger scales (10,000 dm2) where environmental conditions become the main factor affecting species richness [7], suggesting that grazing exclusion effects are significant only at short study scales. It is important to note that while the impacts of scale might be more evident in smaller areas, larger areas are not immune to scale-related effects. Understanding the scale at which ecological processes operate is crucial for effective management and conservation, regardless of the size of the area under consideration.
The study of plant biodiversity in pastures is crucial for understanding the ecological dynamics of these ecosystems. However, it is important to recognize the importance of scale in such studies [51,52]. The scale at which biodiversity is measured can greatly influence our understanding of the relationships between plant communities, environmental factors, and management practices [53]. Therefore, researchers need to carefully consider the appropriate spatial and temporal scales when studying plant biodiversity in pastures. In our case, we have a particular pasture with a specific animal density for different species, but we obtained consistent results with regard to the impact of the scale. It has been demonstrated that varying degrees of herbivory across different spatial scales can lead to a heterogeneous distribution of plant species, enhancing overall biodiversity within grazing landscapes [6]. Moreover, the study of Bakker [54] underscores how the variable consumption patterns of herbivores contribute to niche differentiation among plant species, thereby influencing the assembly and composition of plant communities. The intricate relationship between animal variability and its ecological ramifications underscores the need for a comprehensive understanding of these interactions to effectively manage and conserve pastures’ biodiversity and species composition.
The scale refers to the spatial extent and resolution at which biodiversity data are collected and analyzed, while temporal variability refers to changes in biodiversity over time. Considering scale is important because plant diversity can exhibit variations across different spatial scales, such as local, landscape, and regional scales, which are influenced by factors like habitat structure, land use patterns, and climate change [55,56]. Additionally, temporal variability in biodiversity is influenced by factors like seasonal changes, natural disturbances, and human activities, and understanding these fluctuations is vital for long-term monitoring and adaptive management strategies [57,58]. By considering both scale and temporal variability, researchers and land managers can gain a comprehensive understanding of the factors shaping plant biodiversity in pastures and develop informed conservation strategies that account for spatial and temporal dynamics. Managing recommendations can arise from these results.
5. Conclusions
These findings suggest that after five years of cattle and equine grazing exclusion, there were no significant differences in species richness concerning the scale over five years, but management revealed significant differences with a higher number of plant species and a higher increase in plant species accumulation along the scale in the case of the grazing-excluded plots. Therefore, our results indicate that medium-term grazing as the one occurring in the study area will have an impact on species richness as long as the scale is considered. As indicated in previous studies, grazing exclusion can lead to a decrease in species richness, but these data suggest that some areas of the pasture could be excluded from grazing for longer periods, as long as it is compatible with the economic needs of the local habitants, to investigate changes and promote diversity, especially for species associated with exclusion areas. However, it is imperative to strike a balance between conservation goals and the livelihoods of local communities. An effective implementation of grazing exclusion requires the careful consideration of traditional land use practices and the economic realities of those dependent on pasture resources. Collaborative efforts involving ecologists, community members, and policymakers can facilitate the establishment of well-defined exclusion zones that align with both ecological restoration objectives and the socioeconomic needs of the region.
Author Contributions
Conceptualization, J.R.A., J.A.E.-D. and M.M.; software, J.R.A., J.A.E.-D., C.G.-M. and M.M.; validation, J.R.A. and J.A.E.-D.; formal analysis, J.R.A., J.A.E.-D. and M.M.; investigation, J.R.A. and M.M.; data curation, J.R.A., J.A.E.-D. and A.C.-A.; writing—original draft preparation, J.R.A. and J.A.E.-D.; writing—review and editing, C.G.-M., J.A.E.-D. and M.M.; visualization, J.A.E.-D. and M.M.; supervision, J.R.A. and J.A.E.-D.; project administration, A.C.-A. and M.M.; funding acquisition, J.R.A., A.C.-A. and J.A.E.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
We wish to thank the staff of the Zapaliname protected area for supporting this research, especially Sergio C Marines Gómez. We also thank Leticia Jiménez, and Rocío Martinez for their assistance during field data collection. Many thanks to the Universidad de La Laguna in Tenerife, Spain, for their invaluable support during the preparation of this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Species family, scientific name, status, functional form, and palatability found in this study.
Table A1.
Species family, scientific name, status, functional form, and palatability found in this study.
| Family | Scientific Name | Status | Functional Form | Palatability |
|---|---|---|---|---|
| Euphorbiaceae | Acalypha monostachya Cav. | Native | Forb | Non-palatable |
| Euphorbiaceae | Acalypha phleoides Cav. | Native | Forb | Non-palatable |
| Poaceae | Achnatherum eminens (Cav.) Barkworth | Native | Grasses | Palatable |
| Agavaceae | Agave asperrima Jacobi | Native | Shrub | Palatable |
| Nyctaginaceae | Allionia incarnata L. | Native | Forb | Non-palatable |
| Amaranthaceae | Alternanthera repens (L.) J.F. Gmel. | Native | Forb | Non-palatable |
| Amaranthaceae | Amaranthus blitoides S. Watson | Native | Forb | Palatable |
| Amaranthaceae | Amaranthus hybridus L. | Native | Forb | Palatable |
| Asteraceae | Ambrosia confertiflora DC. | Native | Forb | Non-palatable |
| Malvaceae | Anoda cristata (L.) Schltdl. | Native | Forb | Palatable |
| Euphorbiaceae | Argythamnia neomexicana Müll. Arg. | Native | Forb | Non-palatable |
| Poaceae | Aristida adscensionis L. | Native | Grasses | Non-palatable |
| Poaceae | Aristida curvifolia E. Fourn. | Native | Grasses | Non-palatable |
| Poaceae | Aristida divaricata Humb. and Bonpl. ex Willd. | Native | Grasses | Palatable |
| Poaceae | Aristida havardii Vasey | Native | Grasses | Palatable |
| Poaceae | Aristida pansa Wooton and Standl. | Native | Grasses | Palatable |
| Poaceae | Aristida purpurea Nutt. | Native | Grasses | Palatable |
| Asphodelaceae | Asphodelus fistulosus L. | Introduced | Forb | Non-palatable |
| Fabaceae | Astragalus hypoleucus S. Schauer | Native | Forb | Non-palatable |
| Asteraceae | Baccharis pteronioides DC. | Native | Forb | Non-palatable |
| Asteraceae | Baccharis salicifolia (Ruiz and Pav.) Pers. | Native | Forb | Non-palatable |
| Asteraceae | Bahia absinthifolia Benth. | Native | Forb | Non-palatable |
| Poaceae | Bothriochloa barbinodis (Lag.) Herter | Native | Grasses | Palatable |
| Poaceae | Bouteloua curtipendula (Michx.) Torr. | Native | Grasses | Palatable |
| Poaceae | Bouteloua dactyloides (Nutt.) J.T. Columbus | Native | Grasses | Palatable |
| Poaceae | Bouteloua gracilis (Kunth) Lag. ex Griffiths | Native | Grasses | Palatable |
| Poaceae | Bouteloua hirsuta Lag. | Native | Grasses | Palatable |
| Rubiaceae | Bouvardia ternifolia (Cav.) Schltdl. | Native | Forb | Non-palatable |
| Poaceae | Bouteloua uniflora Vasey | Native | Grasses | Palatable |
| Asteraceae | Brickellia veronicifolia (Kunth) A. Gray | Native | Shrub | Non-palatable |
| Buddlejaceae | Buddleja scordioides Kunth | Native | Shrub | Palatable |
| Onagraceae | Calylophus berlandieri Spach | Native | Forb | Non-palatable |
| Onagraceae | Calylophus hartwegii (Benth.) P.H. Raven | Native | Forb | Non-palatable |
| Cyperaceae | Carex schiedeana Kuntze | Native | Forb | Palatable |
| Orobanchaceae | Castilleja sessiliflora Pursh | Native | Forb | Non-palatable |
| Solanaceae | Chamaesaracha coniodes (Moric. ex Dunal) Britton | Native | Forb | Non-palatable |
| Asteraceae | Chaetopappa ericoides (Torr.) G.L. Nesom | Native | Forb | Non-palatable |
| Amaranthaceae | Chenopodium foetidum Lam. | Native | Forb | Non-palatable |
| Rubiaceae | Clematis drummondii Torr. and A. Gray | Native | Forb | Non-palatable |
| Cactaceae | Corynopuntia schottii (Engelm.) F.M. Knuth | Native | Cacti | Non-palatable |
| Rubiaceae | Crusea diversifolia (Kunth) W.R. Anderson | Native | Forb | Non-palatable |
| Boraginaceae | Cryptantha mexicana (Brandegee) I.M. Johnst. | Native | Forb | Non-palatable |
| Cucurbitaceae | Cucurbita foetidissima Kunth | Native | Forb | Non-palatable |
| Cucurbitaceae | Cucurbita pepo L. | Native | Forb | Palatable |
| Cactaceae | Cylindropuntia imbricata (Haw.) F.M. Knuth | Native | Cacti | Non-palatable |
| Nyctaginaceae | Cyphomeris gypsophiloides (M. Martens and Galeotti) Standl. | Native | Forb | Non-palatable |
| Cyperaceae | Cyperus niger Ruiz and Pav. | Native | Forb | Palatable |
| Fabaceae | Dalea aurea Nutt. ex Pursh | Native | Forb | Palatable |
| Fabaceae | Dalea bicolor Humb. and Bonpl. ex Willd. | Native | Shrub | Palatable |
| Fabaceae | Dalea greggii A. Gray | Native | Shrub | Palatable |
| Fabaceae | Dalea laniceps Barneby | Native | Forb | Palatable |
| Fabaceae | Dalea pogonathera A. Gray | Native | Forb | Palatable |
| Fabaceae | Desmanthus painteri (Britton and Rose) Standl. | Native | Forb | Palatable |
| Convolvulaceae | Dichondra argentea Humb. and Bonpl. ex Willd. | Native | Forb | Non-palatable |
| Poaceae | Disakisperma dubium (Kunth) P.M. Peterson and N. Snow | Native | Grasses | Palatable |
| Caryophyllaceae | Drymaria anomala S. Watson | Native | Forb | Non-palatable |
| Asteraceae | Dyssodia acerosa DC. | Native | Forb | Non-palatable |
| Acanthaceae | Dyschoriste linearis (Torr. and A. Gray) Kuntze | Native | Forb | Non-palatable |
| Asteraceae | Dyssodia papposa (Vent.) Hitchc. | Native | Forb | Non-palatable |
| Asteraceae | Dyssodia pinnata (Cav.) B.L. Rob. | Native | Forb | Non-palatable |
| Asparagaceae | Echeandia flavescens (Schult. and Schult. f.) Cruden | Native | Forb | Non-palatable |
| Cactaceae | Echinocactus horizonthalonius Lem. | Native | Cacti | Non-palatable |
| Cactaceae | Echinocereus pectinatus (Scheidw.) Engelm. | Native | Cacti | Non-palatable |
| Cactaceae | Echinocereus reichenbachii (Terscheck ex Walp.) Haage | Native | Cacti | Non-palatable |
| Poaceae | Elymus elymoides (Raf.) Swezey | Native | Grasses | Palatable |
| Acanthaceae | Elytraria imbricata (Vahl) Pers. | Native | Forb | Non-palatable |
| Poaceae | Enneapogon desvauxii P. Beauv. | Native | Grasses | Non-palatable |
| Poaceae | Erioneuron avenaceum (Kunth) Tateoka | Native | Grasses | Palatable |
| Asteraceae | Erigeron pubescens Kunth | Native | Forb | Non-palatable |
| Euphorbiaceae | Euphorbia cinerascens Engelm. | Native | Forb | Non-palatable |
| Euphorbiaceae | Euphorbia dentata Michx. | Native | Forb | Non-palatable |
| Euphorbiaceae | Euphorbia exstipulata Engelm. | Native | Forb | Non-palatable |
| Euphorbiaceae | Euphorbia serrula Engelm. | Native | Forb | Non-palatable |
| Convolvulaceae | Evolvulus alsinoides (L.) L. | Native | Forb | Non-palatable |
| Convolvulaceae | Evolvulus sericeus Sw. | Native | Forb | Non-palatable |
| Asteraceae | Gaillardia pinnatifida Torr. | Native | Forb | Non-palatable |
| Onagraceae | Gaura coccinea Pursh | Native | Forb | Non-palatable |
| Polemoniaceae | Gilia incisa Benth. | Native | Forb | Non-palatable |
| Verbenaceae | Glandularia bipinnatifida (Nutt.) Nutt. | Native | Forb | Non-palatable |
| Asteraceae | Gymnosperma glutinosum (Spreng.) Less. | Native | Shrub | Non-palatable |
| Polygalaceae | Hebecarpa barbeyana (Chodat) J.R. Abbot | Native | Forb | Non-palatable |
| Rubiaceae | Hedyotis nigricans (Lam.) Fosberg | Native | Forb | Non-palatable |
| Rubiaceae | Hedyotis rubra (Cav.) A. Gray | Native | Forb | Non-palatable |
| Fabaceae | Hoffmannseggia watsonii (Fisher) Rose | Native | Forb | Palatable |
| Poaceae | Hopia obtusa (Kunth) Zuloaga and Morrone | Native | Grasses | Palatable |
| Violaceae | Hybanthus verbenaceus (Kunth) Loes. | Native | Forb | Non-palatable |
| Convolvulaceae | Ipomoea costellata Torr. | Native | Forb | Non-palatable |
| Convolvulaceae | Ipomoea purpurea (L.) Roth | Native | Forb | Palatable |
| Asteraceae | Laennecia coulteri (A. Gray) G.L. Nesom | Native | Forb | Non-palatable |
| Polemoniaceae | Loeselia greggii S. Watson | Native | Forb | Non-palatable |
| Malvaceae | Malva parviflora L. | Introduced | Forb | Palatable |
| Cactaceae | Mammillaria heyderi Muehlenpf. | Native | Cacti | Non-palatable |
| Scrophulariaceae | Mecardonia vandellioides (Kunth) Pennell | Native | Forb | Non-palatable |
| Oleaceae | Menodora coulteri A. Gray | Native | Forb | Palatable |
| Fabaceae | Mimosa aculeaticarpa Ortega | Native | Shrub | Palatable |
| Fabaceae | Mimosa subinermis (S. Watson) B.L. Turner | Native | Forb | Palatable |
| Nyctaginaceae | Mirabilis oblongifolia (A. Gray) Heimerl | Native | Forb | Non-palatable |
| Poaceae | Muhlenbergia arenicola Buckley | Native | Grasses | Palatable |
| Poaceae | Muhlenbergia depauperata Scribn. | Native | Grasses | Non-palatable |
| Poaceae | Muhlenbergia phleoides (Kunth) J.T. Columbus | Native | Grasses | Palatable |
| Poaceae | Muhlenbergia repens (J. Presl) Hitchc. | Native | Grasses | Palatable |
| Poaceae | Muhlenbergia rigida (Kunth) Kunth | Native | Grasses | Palatable |
| Poaceae | Muhlenbergia torreyi (Kunth) Hitchc. ex Bush | Native | Grasses | Palatable |
| Poaceae | Muhlenbergia villiflora Hitchc. | Native | Grasses | Palatable |
| Poaceae | Munroa pulchella (Kunth) L.D. Amarilla | Native | Grasses | Palatable |
| Poaceae | Nassella leucotricha (Trin. and Rupr.) R.W. Pohl | Native | Grasses | Palatable |
| Poaceae | Nassella tenuissima (Trin.) Barkworth | Native | Grasses | Palatable |
| Brassicaceae | Nerisyrenia linearifolia (S. Watson) Greene | Native | Forb | Non-palatable |
| Nostocaceae | Nostoc commune Vaucher ex Bornet and Flahault | Native | Bacteria | Non-palatable |
| Onagraceae | Oenothera berlandieri (Spach) Spach ex D. Dietr. | Native | Forb | Non-palatable |
| Ophioglossaceae | Ophioglossum engelmannii Prantl | Native | Fern | Non-palatable |
| Cactaceae | Opuntia engelmannii Salm-Dyck | Native | Cacti | Palatable |
| Cactaceae | Opuntia lindheimeri Engelm. | Native | Cacti | Palatable |
| Cactaceae | Opuntia stenopetala Engelm. | Native | Cacti | Palatable |
| Poaceae | Panicum hallii Vasey | Native | Grasses | Palatable |
| Asteraceae | Parthenium confertum A. Gray | Native | Forb | Non-palatable |
| Asteraceae | Parthenium incanum Kunth | Native | Shrub | Palatable |
| Plantaginaceae | Penstemon barbatus (Cav.) Roth | Native | Forb | Non-palatable |
| Montiaceae | Phemeranthus aurantiacus (Engelm.) Kiger | Native | Forb | Non-palatable |
| Brassicaceae | Physaria argyraea (A. Gray) O’Kane and Al-Shehbaz | Native | Forb | Non-palatable |
| Brassicaceae | Physaria fendleri (A. Gray) O’Kane and Al-Shehbaz | Native | Forb | Non-palatable |
| Solanaceae | Physalis hederifolia A. Gray | Native | Forb | Non-palatable |
| Phyllanthaceae | Phyllanthus polygonoides Nutt. ex Spreng. | Native | Forb | Non-palatable |
| Polygalaceae | Polygala dolichocarpa S.F. Blake | Native | Forb | Non-palatable |
| Fabaceae | Pomaria canescens (Fisher) B.B. Simpson | Native | Forb | Palatable |
| Portulacaceae | Portulaca pilosa L. | Native | Forb | Non-palatable |
| Fabaceae | Prosopis glandulosa Torr. | Native | Shrub | Palatable |
| Asteraceae | Pseudognaphalium luteoalbum (L.) Hilliard and B.L. Burtt | Native | Forb | Non-palatable |
| Asteraceae | Pseudognaphalium roseum (Kunth) Anderb. | Native | Forb | Non-palatable |
| Polygalaceae | Rhinotropis lindheimeri (A. Gray) J.R. Abbott | Native | Forb | Non-palatable |
| Anacardiaceae | Rhus microphylla Engelm. | Native | Shrub | Non-palatable |
| Anacardiaceae | Rhus virens Lindh. ex A. Gray | Native | Shrub | Non-palatable |
| Fabaceae | Rhynchosia senna Gillies ex Hook. and Arn. | Native | Forb | Palatable |
| Lamiaceae | Salvia ballotiflora Benth. | Native | Shrub | Non-palatable |
| Lamiaceae | Salvia reflexa Hornem. | Native | Forb | Palatable |
| Asteraceae | Sanvitalia ocymoides DC. | Native | Forb | Non-palatable |
| Apocynaceae | Sarcostemma crispum Benth. | Native | Forb | Non-palatable |
| Fabaceae | Senna demissa (Rose) H.S. Irwin and Barneby | Native | Forb | Palatable |
| Malvaceae | Sida abutifolia Mill. | Native | Forb | Palatable |
| Malvaceae | Sida spinosa L. | Native | Forb | Palatable |
| Acanthaceae | Siphonoglossa pilosella (Nees) Torr. | Native | Forb | Non-palatable |
| Solanaceae | Solanum elaeagnifolium Cav. | Native | Forb | Palatable |
| Malvaceae | Sphaeralcea angustifolia (Cav.) G. Don | Native | Forb | Palatable |
| Malvaceae | Sphaeralcea hastulata A. Gray | Native | Forb | Palatable |
| Asteraceae | Stevia tomentosa Kunth | Native | Forb | Non-palatable |
| Brassicaceae | Synthlipsis greggii A. Gray | Native | Forb | Non-palatable |
| Rutaceae | Thamnosma texana (A. Gray) Torr. | Native | Forb | Non-palatable |
| Asteraceae | Thelesperma simplicifolium (A. Gray) A. Gray | Native | Forb | Non-palatable |
| Asteraceae | Thymophylla pentachaeta (DC.) Small | Native | Forb | Non-palatable |
| Asteraceae | Thymophylla setifolia Lag. | Native | Forb | Non-palatable |
| Boraginaceae | Tiquilia canescens (A. DC.) A.T. Richardson | Native | Forb | Non-palatable |
| Asteraceae | Townsendia mexicana A. Gray | Native | Forb | Non-palatable |
| Zygophyllaceae | Tribulus terrestris L. | Introduced | Forb | Non-palatable |
| Cactaceae | Turbinicarpus beguinii (N.P. Taylor) Mosco and Zanov. | Native | Cacti | Non-palatable |
| Poaceae | Urochloa meziana (Hitchc.) Morrone and Zuloaga | Native | Grasses | Palatable |
| Fabaceae | Vachellia glandulifera (S. Watson) Seigler and Ebinger | Native | Shrub | Non-palatable |
| Asteraceae | Verbesina hypomalaca B.L. Rob. and Greenm. | Native | Forb | Non-palatable |
| Verbenaceae | Verbena neomexicana (A. Gray) Small | Native | Forb | Non-palatable |
| Asteraceae | Viguiera dentata (Cav.) Spreng. | Native | Forb | Non-palatable |
| Asteraceae | Xanthisma spinulosum (Pursh) D.R. Morgan and R.L. Hartm. | Native | Forb | Non-palatable |
| Asteraceae | Zinnia acerosa (DC.) A. Gray | Native | Forb | Non-palatable |
References
- Olff, H.; Ritchie, M.E. Effects of herbivores on grassland plant diversity. Trends Ecol. Evol. 1998, 13, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, O.; Mossman, H.L.; Suggitt, A.J.; Curtis, R.J.; Maclean, I.M.D. Using in situ management to conserve biodiversity under climate change. J. Appl. Ecol. 2016, 53, 885–894. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, S.J. Ecology of a grazing ecosystem: The Serengeti. Ecol. Monogr. 1985, 53, 291–320. [Google Scholar] [CrossRef]
- Tallowin, J.R.B.; Rook, A.J.; Rutter, S.M. Impact of grazing management on biodiversity of grasslands. Anim. Sci. 2005, 81, 193–198. [Google Scholar] [CrossRef]
- Crawley, M.J. (Ed.) Plant-herbivore dynamics. In Plant Ecology, 2nd ed.; Blackwell Science: Oxford, UK, 1997; pp. 401–474. [Google Scholar]
- Milchunas, D.G.; Sala, O.E.; Lauenroth, W.K. A generalized model of the effects of grazing by large herbivores on grasslands community structure. Am. Nat. 1998, 132, 87–106. [Google Scholar] [CrossRef]
- De Bello, F.; Lepš, J.; Sebastià, M.T. Grazing effects on species-area relationship: Variation along a climatic gradient in NE Spain. J. Veg. Sci. 2007, 18, 25–34. [Google Scholar] [CrossRef]
- Canals, R.M.; Sebastià, M.T. Analyzing mechanisms regulating diversity in rangelands through comparative studies: A case in the south-western Pyrennees. Biodiv. Conserv. 2000, 9, 965–984. [Google Scholar] [CrossRef]
- Austrheim, G.; Eriksson, O. Plant species diversity and grazing in the Scandinavian mountains- patterns and processes at different spatial scales. Ecography 2001, 24, 683–695. [Google Scholar] [CrossRef]
- Pakeman, R.J. Consistency of plant species and trait responses to grazing along a productivity gradient: A meta-analysis. J. Ecol. 2004, 92, 893–905. [Google Scholar] [CrossRef]
- Krahulec, F. Species coexistence in temperate grasslands. Folia Geobot. Phytotax. 1995, 30, 113–117. [Google Scholar] [CrossRef]
- Perkins, L.B.; Ahlering, M.; Larson, D.L. Looking to the future: Key points for sustainable management of northern Great Plains grasslands. Restor. Ecol. 2019, 27, 1212–1219. [Google Scholar] [CrossRef]
- Tracy, B.F.; Sanderson, M.A. Patterns of plant species richness in pasture lands of the northeast United States. Plant Ecol. 2000, 149, 169–180. [Google Scholar] [CrossRef]
- Perevolotsky, A.; Seligman, N.G. Role of Grazing in Mediterranean Rangeland Ecosystems. Inversion of a paradigm. Biosci. 1998, 48, 1007–1017. [Google Scholar] [CrossRef]
- Grace, J.B. The factors controlling species density in herbaceous plant communities: An assessment. PPEES 1999, 2, 1–28. [Google Scholar] [CrossRef]
- Perelman, S.B.; León, R.J.; Bussacca, J.P. Floristic changes related to grazing intensity in a Patagonian shrub-steppe. Ecography 1997, 20, 400–406. [Google Scholar] [CrossRef]
- Palmer, M.W. Variation in species richness—Towards a unification of hypotheses. Folia Geobot. Phytotax. 1994, 29, 511–530. [Google Scholar] [CrossRef]
- Coupland, R.T. (Ed.) Natural temperate grasslands. In Grassland Ecosystems of the World; Cambridge University Press: Cambridge, UK, 1979; pp. 41–111. [Google Scholar]
- Shreve, F. Grassland and related vegetation in northern Mexico. Madroño 1942, 6, 190–198. [Google Scholar]
- Valdés-Reyna, J.; Villaseñor, J.L.; Encina-Domínguez, J.A.; Ortiz, E. The grass family (Poaceae) in Coahuila, Mexico: Diversity and distribution. Bot. Sci. 2015, 93, 119–129. [Google Scholar] [CrossRef]
- Estrada-Castillón, E.; Scott-Morales, L.; Villarreal-Quintanilla, J.A.; Jurado-Ybarra, E.; Cotera-Correa, M.; Cantú-Ayala, C.; García-Pérez, J. Clasificación de los pastizales halófilos del noreste de México asociados con perrito de las praderas (Cynomys mexicanus): Diversidad y endemismo de especies. Rev. Mex. Biodiv. 2010, 81, 401–416. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Medina, G.; Amador, M.D. Carga Animal del Pastizal Mediano Abierto en Zacatecas; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Zacatecas, Mexico, 2007; pp. 1–37. [Google Scholar]
- Van Coller, H.; Siebert, F.; Scogings, P.F.; Ellis, S. Herbaceous responses to herbivory, fire and rainfall variability differ between grasses and forbs. S. Afr. J. Bot. 2018, 119, 94–103. [Google Scholar] [CrossRef]
- Gusha, J.; Mugabe, P.H. Unpalatable and wiry grasses are the dominant grass species in semi-arid communal rangelands in Zimbabwe. Int. J. Dev. Sustain. 2013, 2, 1075–1083. [Google Scholar]
- Manzano, M.G.; Navar, J.; Pando, M.M.; Martínez, A. Overgrazing and desertification in northern Mexico: Highlights on north eastern region. Ann. Arid. Zone 2000, 39, 285–304. [Google Scholar]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: New York, NY, USA, 1995. [Google Scholar]
- Kunin, W.E. Extrapolating species abundance across spatial scales. Science 1998, 281, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.J.; Harral, J.E. Scale Dependence in Plant Biodiversity. Science 2001, 291, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Pascual, U.; Stenseke, M.; Martín-López, B.; Watson, R.T.; Molnár, Z.; Hill, R.; Chan, K.M.; Baste, I.A.; Brauman, K.A.; et al. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2019. [Google Scholar]
- Jiang, G.; Liu, Y.; Liu, C.; Yang, G.; Liu, Q. Scale effects of soil moisture spatial heterogeneity on grassland biodiversity in an arid region. Sci. Total Environ. 2020, 739, 139923. [Google Scholar]
- Fahrig, L. Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Gilbert, B.; Bennett, J.R.; Fukami, T. Assembly history dictates ecosystem functioning: Evidence from wood decomposer communities. Ecol. Lett. 2010, 13, 675–684. [Google Scholar]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef] [PubMed]
- Dornelas, M.; Gotelli, N.J.; McGill, B.; Shimadzu, H.; Moyes, F.; Sievers, C.; Magurran, A.E. Assemblage time series reveal biodiversity change but not systematic loss. Science 2014, 344, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Luck, G.W. A review of the relationships between human population density and biodiversity. Biol. Rev. 2007, 82, 607–645. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Fischer, J.; Felton, A.; Crane, M.; Michael, D.; Macgregor, C. Biodiversity monitoring in the era of climate change: A review of methods for detection of species shifts. Clim. Change Responses 2012, 1, 5. [Google Scholar]
- Anon. Programa de Manejo de la Zona Sujeta a Conservación Ecológica “Sierra de Zapalinamé”; Secretaría de Desarrollo Social, Gobierno del Estado de Coahuila: Saltillo, Mexico, 1998; 179p. [Google Scholar]
- Encina-Domínguez, J.A. Riqueza Florística y Comunidades Vegetales de la Sierra de Zapalinamé, Saltillo, Coahuila, México. Ph.D. Thesis, Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Mexico, 2017; 145p. [Google Scholar]
- Arévalo, J.R.; González-Montelongo, C.; Encina-Domínguez, J.A.; García, E.; Mellado, M. Changes in richness and species composition after five years of grazing exclusion in an endemic pasture of northern Mexico. Land 2022, 11, 1962. [Google Scholar] [CrossRef]
- Favret-Tondato, R.C. Apropiación de los recursos naturales y producción en el territorio de la sierra de Zapalinamé. In Guía Para Conocer y Valorar el Área Protegida de la Sierra de Zapalinamé; Arizpe-Narro, A., Ed.; Elemento Cero Ediciones: Saltillo, Mexico, 2013; pp. 89–101. [Google Scholar]
- Wiens, J.A. Spatial scaling in ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Peet, R.K.; Wentworth, T.R.; White, P.S. A flexible, multipurpose method for recording vegetation composition and structure. Castanea 1998, 63, 262–274. [Google Scholar]
- Arrhenius, O. Species and area. J. Ecol. 1921, 9, 95–99. [Google Scholar] [CrossRef]
- Scheiner, S.M. Six types of species-area curves. Glob. Ecol. Biogeogr. 2003, 12, 441–447. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998; p. 853. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Wagner, H. Vegan: Community Ecology Package. R Package Version 4.1-3. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 3 October 2022).
- Fernández-Lugo, S.; de Nascimento, L.; Mellado, M.; Arévalo, J.R. Grazing effects on species richness depends on scale: A 5 year study in Tenerife pastures (Canary Islands). Plant Ecol. 2011, 212, 423–432. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. B Biol. Sci. 1994, 345, 101–118. [Google Scholar]
- Al-Mufti, M.M.; Sydes, C.L.; Furness, S.B.; Grime, J.P.; Band, S.R. A Quantitative analysis of shoot phenology and dominance in herbaceous vegetation. J. Ecol. 1977, 65, 759–791. [Google Scholar] [CrossRef]
- Spiegelberger, T.; Matthies, D.; Muller-Scharer, H.; Schaffner, U. Scale-dependent effects of land use on plant species richness of mountain grassland in the European Alps. Ecography 2006, 29, 541–548. [Google Scholar] [CrossRef]
- Loucougaray, G.; Farruggia, C.; Hoste, H. Effects of two contrasting livestock grazing systems on vegetation dynamics in heterogeneous mountain pastures. Appl. Veg. Sci. 2011, 14, 80–89. [Google Scholar]
- Kozak, J.; Duffy, L.K.; Weathers, K.C.; Giblin, A.E. Spatial scales of plant community composition variation in a temperate grassland. Ecosphere 2018, 9, e02156. [Google Scholar]
- Martín-López, B.; Iniesta-Arandia, I.; García-Llorente, M.; Palomo, I.; Casado-Arzuaga, I.; Amo, D.G.D.; Gómez-Baggethun, E.; Oteros-Rozas, E.; Palacios-Agundez, I.; Willaarts, B.; et al. Uncovering ecosystem service bundles through social Preferences. PLoS ONE 2012, 7, e38970. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.S.; Olff, H.; Gleichman, J.M. Herbivore Impact on Grassland Plant Diversity Depends on Habitat Productivity and Herbivore Size. Ecol. Lett. 2006, 9, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Leitão, R.P.; Zuanon, J.; Villéger, S.; Williams, S.E.; Baraloto, C.; Fortunel, C.; Mendonça, F.P.; Mouillot, D.; Magnusson, W.E. Rare species contribute disproportionately to the functional structure of species assemblages. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160084. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Turner, M.G.; Bakker, W.L.; Peterson, C.J. Factors influencing succession: Lessons from large, infrequent natural disturbances. Ecosystems 2004, 7, 511–523. [Google Scholar] [CrossRef]
- Fridley, J.D.; Vandermast, D.B.; Kuppinger, D.M.; Manthey, M.; Peet, R.K.; Sutherland, S. Co-occurrence-based assessment of habitat generalists and specialists: A new approach for the measurement of niche width. J. Ecol. 2007, 95, 707–722. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).