Abstract
High ammonium release from chicken manure poses a significant limitation to aerobic digestion, impeding microbial processes and inhibiting biogas production. In this study, we conducted anaerobic digestion of a mixture consisting of chicken manure and corn straw as the fermented raw material. The inoculum used was obtained from the residue of previously fermented chicken manure. To assess the inhibitory effect, we varied the ammonia levels within the range of 750–4250 mg/L by introducing ammonium chloride. The efficiency of aerobic digestion was monitored through the measurement of volatile fatty acids (VFA), chemical oxygen demand (COD), total inorganic carbon (TOC), and methane yield. Our results indicated that elevated levels of ammonia nitrogen had a suppressive impact on methane release, and this decrease followed a linear relationship with the increasing ammonia nitrogen load. Moreover, the addition of ammonia led to a slower release, with the maximum daily ammonia concentration observed at 15 days compared to the 6th day at lower ammonia levels. Furthermore, on the 40th day of aerobic digestion, the cumulative methane production at 4250 mg/L was inhibited by 41% compared to the 750 mg/L condition. The patterns of VFA, inorganic carbon, and COD reduction were consistent across all ammonia levels, with VFA and TOC levels being highest at the highest ammonia concentration and lowest at the lowest ammonia concentration. The accumulation of VFA resulted in a decrease in pH and a decline in methanogenic activity. Additionally, high ammonia levels altered the relative abundance of methanogens. Acetoclastic methanogens (Methanosaeta) exhibited a decrease in abundance, while hydrogenotrophic methanogens (Methanosaeta, Methanoculleus) and methylotrophic methanogens (Candidatus Methanoplasma) demonstrated an increase in abundance. Overall, our findings highlight the inhibitory effects of high ammonia concentrations on biogas production, providing insights into the changes in microbial composition and activity during anaerobic fermentation.
1. Introduction
The disposal of food waste is problematic due to its high biodegradability, organic content, moisture content, and bulk density [1]. On the other hand, the high moisture and calorific value make food waste suitable for anaerobic digestion, which is by methanogenic bacteria [2]. Aerobic digestion is a natural or controlled breakdown of organic matter into biogas and fertilizer in a complicated cascade of microbially driven events, including hydrolysis, fermentation (including acetogenesis and acidogenesis), and methanogenesis [3].
Biogas, a form of biofuel, is produced in biodigesters, landfills, or wastewater treatment plants [4]. It primarily consists of methane and carbon dioxide, with small amounts of other gases such as hydrogen sulfide, nitrogen, oxygen, and trace amounts of water vapor. Methane typically makes up 50–70% of the gas mixture, while carbon dioxide accounts for approximately 30–50% [5]. The exact composition of biogas can vary depending on the feedstock used and the specific conditions of the anaerobic digestion process [6]. Various raw materials can be utilized for biogas production, including animal waste, crop residues, organic fractions of food and industrial waste, and wastewater sludge [4].
According to FAO [7], the global chicken population has more than doubled since 1990. In 2021, there were approximately 25.8-billion chickens, compared to 13.9 billion in 2000. This significant increase has led to a massive generation of chicken manure, reaching 267-million tons. Converting chicken manure into biofuel offers a sustainable solution for food disposal and energy generation [5]. However, chicken manure alone cannot be efficiently converted into biofuel. Therefore, it is often combined with plant materials such as straw and grass [8].
Biogas production has emerged as a promising approach for managing chicken manure waste. However, the high content of total ammoniacal nitrogen in chicken manure and other organic waste inhibits the activity of methanogenic bacteria and hampers biogas production [9,10]. Ammoniacal nitrogen comprises both free ammonia (NH3) and ammonium ion (NH4+), and both forms contribute to the inhibition of anaerobic digestion, with free ammonia being the stronger inhibitor. The reported threshold for ammonia concentration, beyond which stable biogas production is compromised, varies widely among studies due to differing operating conditions [11]. Most studies have identified a range of inhibitory ammonia concentrations, typically ranging from 0.7 to 15 g/L [12,13,14]. Yin et al. [10] reported a substantial reduction in methane generation (94%) and a significant increase in volatile fatty acid (VFA) accumulation (over 60-fold) during aerobic fermentation of chicken manure when the substrate and ammonium loads were increased (up to 8.5 g/L).
Research has shown that the co-digestion of livestock manure with non-degraded organic matter, particularly crop straw, enhances the utilization rate of waste/raw materials remaining in the biogas slurry [15,16,17,18].
The community of a manure digester consists of three groups of microbes: hydrolytic, acid-forming, and methanogenic bacteria. Hydrolytic bacteria break down proteins and polymeric carbohydrates into monomeric sugars and amino acids. Acid-forming bacteria are responsible for the production of VFAs, either directly (acetogenic), through the formation of acetate (homoacetogenic), or by converting larger VFAs into acetate and hydrogen gas (hydrogenogenic). Methanogens, which include acetoclastic and hydrogenotrophic methanogens, convert acetate and hydrogen gas into methane and carbon dioxide [18]. Among the microbial groups involved in anaerobic digestion, methanogens are considered more susceptible to inhibition by ammonia. An ammonia load can inhibit or alter the composition of the methanogenic community [19,20]. Utilizing tolerant methanogens can help overcome the inhibition and enhance methane production by more than 30% [14].
The main focus of this research is to optimize the capacity of aerobic digestion by identifying the operational parameters that maximize methane production for different feedstocks. Ammonia release during the digestion of chicken manure reflects the efficiency of aerobic digestion. Our objective is to assess the inhibitory effect of different levels of ammonia nitrogen on methane production using chicken manure and corn straw as feedstocks. We will examine the abundance of methanogens and evaluate how varying concentrations of ammonia nitrogen affect VFA, chemical oxygen demand (COD), and methane production.
2. Materials and Methods
2.1. Test Material
In the experiment, a mixture of chicken manure and corn straw was utilized as the fermentation raw material. The inoculum for the experiment was selected from the residue of chicken manure that had been fermented for over 60 days under normal temperatures. The chicken manure was obtained from Taigu Honghao Breeding Cooperative in Jinzhong City, Shanxi Province. The corn stalks and inoculum were sourced from biogas digesters at the Dongyang Experimental Demonstration Base of Shanxi Agricultural University. The weeds were removed from the corn stalk, and corn stalk media was sun-dried and crushed to reduce particle size to 3–5 mm. The physicochemical characteristics of the fermentation materials and inoculum are presented in Table 1.

Table 1.
Physicochemical properties of fermentation materials and inoculum.
2.2. Test Device
The mesophilic anaerobic digester used in this study was a 10-L fully mixed continuous stirred-tank reactor (CSTR), manufactured by the Biogas Technology Laboratory of Shanxi Organic Dry Farming Agriculture Research Institute at Shanxi Agricultural University, as depicted in Figure 1. The device consists of three main components: a gas production unit, a gas collection unit, and a liquid collection unit.
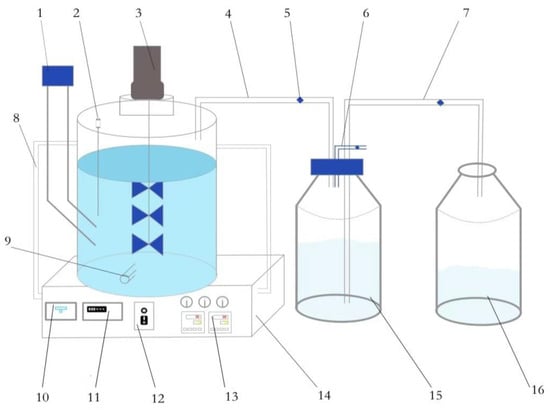
Figure 1.
Schematic diagram of anaerobic fermentation generator (10 L). 1: feed inlet; 2: thermometer; 3: mixing motor; 4: exhaust pipe; 5: switch knob; 6: air connection pipe; 7: drainpipe; 8: circulating water pipe; 9: discharge pipe; 10: power switch; 11: display meter; 12: mixing controller; 13: temperature controller; 14: water bath; 15: gas collection bottle; 16: water collection bottle.
The gas production unit comprises a liquid-reaction vessel and a parameter-control device. The gas collection unit is primarily composed of a gas collection bottle, an exhaust pipe, and a gas-connection pipe. The liquid-collection unit consists of a water-collection bottle and a drain pipe. The liquid-reaction vessel in the gas-production unit includes a stirring motor, a thermometer, an inlet, an outlet, and a water bath heating zone in the interlayer.
The parameter-control device comprises a stirring-motor controller, a water bath temperature controller, a water bath pot, a circulating water pipe, and other components. The fermentation liquid is introduced into the reaction device through the inlet, and the reaction temperature is regulated by the water bath. The biogas produced during the reaction enters the gas-collecting bottle, which is filled with water, through the exhaust pipe. Following the principle of the drainage gas-collection method, as the air pressure inside the gas-collection bottle increases, the water from the bottle flows into the water-collection bottle via the drain pipe. The volume of water in the water-collection bottle is then measured to determine the quantity of biogas produced.
To analyze the composition and content of the biogas, a gas-collection bag is connected to the gas pipe. The switch clip is opened to allow the gas to enter the collection bag. Subsequently, the bottle is connected to a biogas-component analyzer to determine the gas composition.
2.3. Experimental Design
The volatile solid (VS) fermentation mass ratio was set as a mixture of chicken manure and corn stalk in a ratio of 6:4, and a total of 8000 g of material were used. We selected this ratio based on the findings from our previous research, where we analyzed various ratios (9:1, 8:2, 7:3, 6:4, and 5:5) of chicken manure and corn stalk and found that the gas-production efficiency, daily gas production, cumulative gas production, and total methane production were highest at the ratio of 6:4 (unpublished results).
The mass concentration of the fermentation material was 8% (in terms of VS), and the inoculum accounted for 30% of the mass concentration of the fermentation material. We conducted the fermentation at a medium temperature (35 ± 1 ℃) for 40 days. The batch-fermentation method of one-time feeding and discharging was adopted.
To create different ammonia nitrogen concentrations in the fermentation-feed liquid, we used NH4Cl solution as the inorganic nitrogen source. The initial mass concentration of ammonia nitrogen in the reaction feed liquid was 746.32 mg/L. We prepared six different ammonia nitrogen concentrations in the fermentation feed liquid as follows: T1 = 750 mg/L, T2 = 1500 mg/L, T3 = 2250 mg/L, T4 = 3000 mg/L, T5 = 3500 mg/L, and T6 = 4250 mg/L. T1 served as the control (CK). The rates were calculated according to formula (1):
In the formula, C represents the target mass concentration of ammonia nitrogen (mg/L); C0 represents the mass concentration of the prepared NH4Cl solution (mg/L); 14.01 represents the relative atomic mass of N; and 53.5 indicates the relative molecular mass of NH4Cl.
Each treatment was repeated three times. Biogas production was measured daily, and methane content was measured every 2 days. Various indicators, including the pH value, volatile fatty acid (VFA) content, total inorganic carbonate (TIC) content, ammonia nitrogen (NH4+-N) concentration, chemical oxygen demand (COD), and other physical and chemical indicators of the fermentation liquid were measured every 5 d. For this, about 30 mL of liquid were collected from the discharge pipe, and after centrifugation, 10 mL of supernatant were used to determine physicochemical indicators. The remaining liquid was mixed with 10 mL of water and then re-entered the generator through the feed inlet to keep the fermentation liquid volume unchanged. At the end of the 40-day reaction, we measured the microbial community and abundance by collecting an appropriate amount of fermentation feed liquid.
2.4. Indicator Determination and Method
2.4.1. Measurement of Performance Indicators of Fermentation Materials
(1) Determination of dry matter (TS): Samples of chicken manure, corn stalks, and inoculum were weighed, placed in an oven at a temperature of 105 °C, dried until reaching constant weight, and reweighed to calculate the dry matter based on the difference in weight.
(2) Determination of volatile solids (VS): The dried samples mentioned above were placed in a muffle furnace at a high temperature (550 °C) to burn away organic components, and the resulting ash was used to calculate the volatile solids (VS) based on the difference in weight [21].
2.4.2. Determination of Performance Indicators of Fermentation-Feed Liquid
A small amount of sample solution was extracted before the reaction and every 5 days during the reaction and then centrifuged at 5000 r/min for 15 min. After centrifugation, the supernatant was measured, and the excess sample solution was poured into the generator. The pH value, alkalinity, ammonia nitrogen (NH4+-N) mass concentration, chemical oxygen demand (COD), volatile fatty acid (VFA) content, and total inorganic carbonate (TIC) content were determined.
(1) The pH value was measured using a PHS- 3C acidity meter (Leici, Shanghai, China).
(2) The total inorganic carbonate content (TIC) was determined by neutralization titration (calculated as CaCO3).
(3) The concentration of NH4+-N was determined by the distillation–neutralization titration method according to the Chinese Standard (HJ537-2009) [22], and calculation was performed using formula (2):
where ρN represents the mass concentration of ammonia nitrogen in the water sample, expressed as nitrogen (mg/L); V represents the volume of undiluted water sample (mL); Va represents the volume of HCl standard titrant consumed for titrating the water sample (mL); Vb represents the volume of HCl standard titration solution consumed by titrating the blank (mL); c represents the concentration of HCl standard titration solution (mol/L); and 14.01 represents the relative atomic mass of nitrogen.
(4) Chemical oxygen demand (COD) was measured with reference to the standard GB/T11914-1989 [23]. The COD in water samples was calculated as Equation (3):
where ρ represents COD (mg/L); c represents the concentration of ferrous ammonium sulfate standard solution (mol/L); V0 represents the volume of the ferrous ammonium sulfate standard titration solution consumed by the titration blank (mL); V1 represents the volume of ferrous ammonium sulfate standard titrant consumed by the sample (mL); V2 represents the volume of undiluted water sample (mL); and 8 denotes the molar mass of 1/2 O (g/mol).
(5) The content of volatile organic acids (VFAs) was determined by gas chromatography (7890B gas chromatograph, Agilent) after centrifugation with hydrogen flame detector (FID), polar chromatographic column HP–FFAP (30 m × 0.25 mm × 0.25 μm), nitrogen as the carrier gas (flow rate 40 mL/min), and an injection volume of 1.0 μL. The temperature of the inlet and the detector was 250 °C, and the temperature program was used according to the detection needs with an initial temperature of 70 °C, stabilized for 3.5 min, and then raised to 180 °C according to the temperature increase of 20 °C/min and stabilized at 180 °C for 5 min.
(6) Determination of microbial community and abundance: DNA extraction was performed using TGuide S96 magnetic soil DNA extraction kit (Tiangen Biotech, Beijing, China). The 16SV3 and V4 of methanogenic archaea were amplified using double-ended primers Arch349F (5′-GYGCASCAGKCGMGAAW-3′) and Arch806R (5′-GGACTACVSGGGTATCTAAT-3′). The amplified and purified samples were subjected to high-throughput sequencing through the Illumina Novaseq sequencing platform to analyze the community of methanogenic microorganisms.
2.4.3. Determination of Daily Methane Production and Cumulative Methane Production
The daily biogas production was determined by measuring the volume of water collected in the water collection bottle daily. Gas produced by each test group was collected from a gas-collection bag daily, and methane production was measured using the 7890B gas chromatograph (Santa Clara, Agilent, United States). The chromatography conditions were as follows: HP–INNOWAX chromatographic column with a temperature range of 40–240 °C and dimensions of 60 m × 530 μm × 1 μm. The carrier gas used was hydrogen with a flow rate of 5 mL/min, a pressure of 7.2443 psi, an average linear velocity of 35.701 cm/s, and a retention time of 2.8011 min. The FID detector operated at a temperature of 300 °C, with an airflow rate of 400 mL/min and a hydrogen gas flow rate of 30 mL/min, while the gas-flow rate was set at 25 mL/min. The TCD detector temperature was set to 250 °C, the reference flow was 40 mL/min, and the tail-gas flow (H2) was 2 mL/min.
The methane content in the biogas was calculated by formula (4) and cumulative methane production was calculated by formula (5):
In the formula, Q is the cumulative methane production, Vn represents the methane production of the nth d of the anaerobic reaction (mL); Mn represents the mass of water in the water-collection bottle on the nth day of anaerobic reaction (g); ρwater represents the density of water at standard atmospheric pressure of about 1 g/cm3; and Kn is the methane content in the biogas in the nth day of anaerobic reaction (%).
2.5. Data Processing
IBM SPSS Statistics (Version 21) were employed for data processing. Origin 2021 and Microsoft Excel were used for graphics drawing.
3. Results and Discussion
3.1. Effects of Different Ammonia Nitrogen Concentrations on Daily Methane Production
The results showed that methane production increased initially with the duration of fermentation and then started to decline (Figure 2). During the first 11 days of fermentation, the highest methane production was observed at the lowest ammonium nitrogen concentration (T1). As the ammonium nitrogen concentration increased from T1 to T6, methane production gradually decreased. Specifically, at T1, the highest methane production of 4.7 L/d was recorded on the 6th day of fermentation. It was observed that most of the methane was released within the first 15 days at T1, after which the rate of release gradually decreased. This suggests that the easily digestible materials were utilized during the initial 15 days under low ammonium nitrogen conditions, while the less digestible materials contributed to the slower release of methane [24,25].

Figure 2.
Effects of different ammonia nitrogen concentrations on methane production. Each value is a mean ± standard deviation of three replicates. T1 = 750 mg/L, T2 = 1500 mg/L, T3 = 2250 mg/L, T4 = 3000 mg/L, T5 = 3500 mg/L, and T6 = 4250 mg/L of ammonium nitrogen.
The maximum methane production was observed on different days of fermentation for each ammonia nitrogen concentration (T1, T2, T3, T4, T5, and T6), with the 6th, 7th, 9th, 12th, 14th, and 18th days showing the highest methane production, indicating a delayed release of methane with increasing ammonium concentration. Among the different concentrations, T1 exhibited the steepest positive slope, while T6 had the lowest positive slope. These results demonstrate the inhibitory effects of increasing ammonia nitrogen load on methane release, with a linear decrease in methane production corresponding to higher ammonia nitrogen concentrations. Methane production is primarily driven by microbial activity, particularly by methanogens. Although ammonia nitrogen is essential for microbial growth, high concentrations can impede methanogens. Previous studies have identified an inhibitory threshold for total ammonia nitrogen concentrations (including free ammonia and ammonium) above 1.7 g/L [8,26]. The reduced methane production can be attributed to the accumulation of volatile fatty acids (VFA), which leads to a decrease in pH. The acidic pH, in turn, inhibits the activity of methanogens, as they thrive in a near-neutral pH range [26]. Yin et al. [9] reported a 50% decline in methane production at pH 6 compared to alkaline pH conditions.
On the 1st day of aerobic digestion, the methane release ranged from 146–395.7 mL/d, while on the 40th day, methane release ranged from 53–72 mL/d, indicating that most of the methane was released within the 40 days.
3.2. Effects of Different Ammonia Nitrogen Concentrations on Cumulative Methane Production
The cumulative methane production per mass of volatile solids (VS) added increased with the duration of fermentation but decreased as the ammonium nitrogen concentration increased from T1 to T6 (Figure 3). During the first 20 days of fermentation, the highest methane production was observed at the lowest ammonium nitrogen concentration (T1). However, from Day 21 to Day 39 of fermentation, cumulative methane production at T2 surpassed that of T1. This indicates that higher ammonium concentration hindered the cumulative methane production.
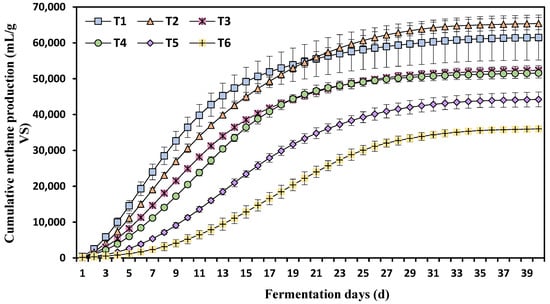
Figure 3.
Effects of different ammonia nitrogen concentrations on cumulative methane production. Each value is a mean ± standard deviation of three replicates. T1 = 750 mg/L, T2 = 1500 mg/L, T3 = 2250 mg/L, T4 = 3000 mg/L, T5 = 3500 mg/L, and T6 = 4250 mg/L of ammonium nitrogen.
3.3. The Effect of Different Concentrations of Ammonia Nitrogen on Volatile Fatty Acids
Volatile fatty acids (VFAs) are intermediate or end products generated during the hydrolysis and acidification of organic matter. They primarily include acetic acid, propionic acid, butyric acid, and other fatty acids with fewer than five carbon atoms [9]. VFAs produced during anaerobic digestion are ultimately converted into methane and carbon dioxide by methanogenic bacteria. VFA concentration is an important parameter that reflects the degradation status of organic matter during anaerobic fermentation. After 5 days of fermentation, VFA concentrations increased and gradually decreased until reaching stability around 25 to 30 days of fermentation (Figure 4).

Figure 4.
Effects of different ammonia nitrogen mass concentrations on the VFAs of the fermentation feed liquid. T1 = 750 mg/L, T2 = 1500 mg/L, T3 = 2250 mg/L, T4 = 3000 mg/L, T5 = 3500 mg/L, and T6 = 4250 mg/L of ammonium nitrogen.
The concentrations of VFAs were highest at the highest concentrations of ammonium nitrogen (T6) from 5 to 40 days of fermentation. Conversely, as ammonia nitrogen concentrations decreased, the VFA concentration in the media also decreased. T1 had the lowest VFA concentration, while T6 had the highest. The average initial nitrogen content in chicken manure was 2.013% (Table 1). During aerobic digestion, proteins and amino acids present in the manure are degraded, leading to the production of VFAs, particularly propionic and acetic acids.
These results indicate that high ammonia nitrogen levels lead to increased accumulation of VFAs, either by slowing down the anaerobic digestion of manure through methanogen inhibition [27] and/or by enhancing the hydrolysis of organic matter into VFAs [9]. The inhibited activity of methanogens due to high ammonia nitrogen results in higher production of VFAs compared to their consumption [26]. The addition of high nitrogen alters the composition and concentration of VFAs by lowering the pH of the medium, as lower pH levels favor VFA accumulation [9]. Yin et al. [28] also reported that methanogen activity is 50% inhibited at pH 6. Therefore, at low pH levels, aerobic digestion is disrupted and slowed down due to reduced methanogen activity, leading to increased VFA accumulation. The accumulation of VFAs has been observed to place the process under greater stress, increasing the risk of process failure and reducing methane release, particularly under high organic load conditions [8]. In the present study, the elevated VFA levels resulting from increased ammonia nitrogen align with the reduced methane production.
3.4. Effects of Different Ammonia Nitrogen Mass Concentrations on Total Inorganic Carbonate
Total inorganic carbonate (TIC) was increased after 5 d of fermentation and then started a sharp decrease until 25 d of fermentation before nearly becoming stable (Figure 5). TIC was increased with the increase of ammonia nitrogen, and the least TIC was reported at T1 (750 mg/L ammonia nitrogen).
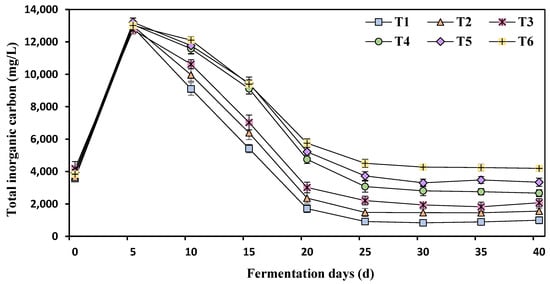
Figure 5.
Effects of different ammonia nitrogen mass concentrations on the TIC of the fermentation feed liquid. T1 = 750 mg/L, T2 = 1500 mg/L, T3 = 2250 mg/L, T4 = 3000 mg/L, T5 = 3500 mg/L, and T6 = 4250 mg/L of ammonium nitrogen.
TIC is a crucial parameter that indicates the buffering capacity of a system as it helps to mitigate the presence of acidic substances and maintain system stability. TIC mainly exists in the form of dissolved inorganic carbon, including carbonate (CO32−), carbon dioxide (CO2), carbonic acid (H2CO3), and bicarbonate (HCO3−), as well as particulate inorganic carbon like CaCO3. In systems with high ammonia levels, the abundance of inorganic carbon is elevated, resulting in enhanced buffering capacity. This is particularly important for counteracting low pH conditions (acidification) and minimizing the conversion of ammonia nitrogen into biogas [29]. Following stabilization, the TIC range observed was within the normal range of 835–1710 [16].
3.5. Kinetic Changes in the pH Values at Different Ammonia Nitrogen Concentrations
The pH exhibited a significant decline from its initial value after 5 days of fermentation (Figure 6). Subsequently, between Days 10 and 20 of fermentation, the pH began to rise again, followed by a slower decline. The ammonia nitrogen concentration had a noticeable impact on the pH levels. The pH was most alkaline at T1 (750 mg/L ammonia nitrogen) and gradually became acidic as the ammonia nitrogen concentration increased from T1 (750 mg/L) to T6 (4250 mg/L). pH plays a vital role in regulating metabolic processes and has a direct influence on methane and VFA production [30]. Ammonia nitrogen has a basic nature, existing in the form of NH4+ at neutral pH. The rapid decline in pH observed on the 5th day of aerobic digestion is attributed to the accumulation of VFAs [9,26].
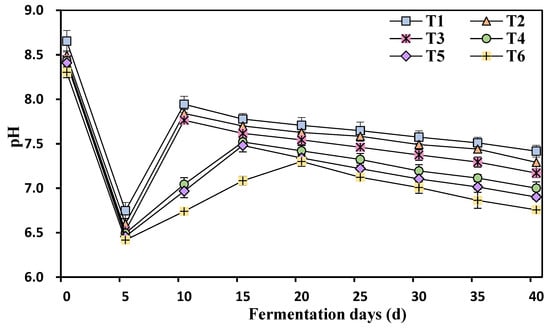
Figure 6.
Effects of different ammonia nitrogen mass concentrations on the pH value of the fermentation feed liquid. T1 = 750 mg/L, T2 = 1500 mg/L, T3 = 2250 mg/L, T4 = 3000 mg/L, T5 = 3500 mg/L, and T6 = 4250 mg/L of ammonium nitrogen.
3.6. Effects of Different Ammonia Nitrogen Mass Concentrations on COD
The COD initially increased from its initial value to the 5th day of fermentation, reaching its maximum level (Figure 7). Between Days 5 and 20 of fermentation, the COD value exhibited a sharp decline, followed by stabilization or a very slight decrease. The ammonia nitrogen concentration had an impact on the COD value. The highest COD value was observed at T1 (750 mg/L ammonia nitrogen), and as the ammonia nitrogen concentration increased from T1 to T6 (4250 mg/L), the COD value started to decrease. COD serves as an indicator of oxygen demand in anaerobic digestion and measures the organic matter present in the absence of oxygen [31]. Overloading leads to the rapid hydrolysis of chicken manure and the accumulation of excessive VFAs [25]. Increasing ammonium concentration reduces COD by diminishing methanogenic activity [32].
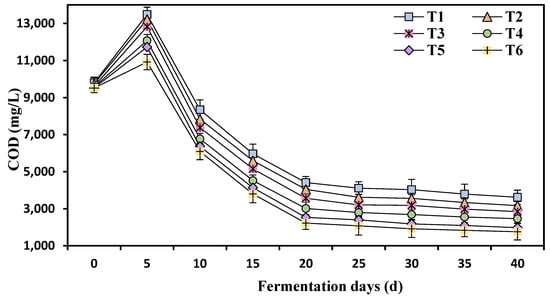
Figure 7.
Effects of different ammonia nitrogen mass concentrations on chemical oxygen demand. T1 = 750 mg/L, T2 = 1500 mg/L, T3 = 2250 mg/L, T4 = 3000 mg/L, T5 = 3500 mg/L, and T6 = 4250 mg/L of ammonium nitrogen.
3.7. Variation in the Relative Abundance of Microbial Communities under Different Concentrations of Ammonia Nitrogen
The effect of different ammonia loads was observed on methanogen abundance at the genus level. Results showed substantial changes in the relative abundance of methanogens (Figure 8). The abundance of Methanosaeta and LNR_A2_18 were decreased by increasing the concentration of ammonia nitrogen. Meanwhile, the abundance of Methanosarcina, Methanoculleus, and uncultured bacteria increased with the ammonia level.
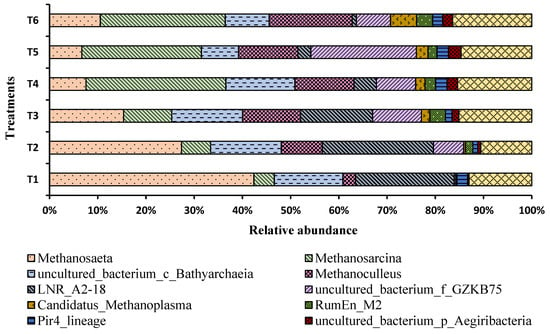
Figure 8.
Relative abundance of methanogens (genus level) in each treatment. T1 = 750 mg/L, T2 = 1500 mg/L, T3 = 2250 mg/L, T4 = 3000 mg/L, T5 = 3500 mg/L, and T6 = 4250 mg/L of ammonium nitrogen.
The Methanosarcina genotype belongs to the Class I methanogens of Methanosarcinales and is indicative of high ammonium levels and unstable fermentation [14]. Methanosarcina is a mixotrophic methanogen and perhaps the only known anaerobic methanogen that utilizes all three recognized metabolic pathways for methanogenesis. Methanosarcina spp. can utilize not only acetate but also CO2, H2, and methanol as substrates [33]. Consequently, Methanosarcina species exhibit relatively higher tolerance to high ammonia concentrations compared to other methanogens and can withstand levels of up to 7000 mg ammonia/L [34]. Methanosarcina spp. Demonstrates a higher yield and growth rates than Methanosaeta under high ammonia conditions [20,35].
Under conditions of low acetate availability, methane production can occur through the activity of hydrogenotrophic methanogens utilizing CO2 and H2. The relative abundance of Methanoculleus increased with higher ammonia nitrogen concentrations. Methanoculleus is a hydrogenotrophic methanogen that converts CO2 and H2 into methane [36,37]. In anaerobic environments with low ammonia nitrogen levels (<1000 mg/L), where ammonia inhibition is absent, methane formation typically occurs through acetoclastic methanogens utilizing acetate (67%) and hydrogenotrophic methanogens utilizing H2 (33%) [38]. However, under conditions of ammonia inhibition, there is a shift in the dominant methanogenesis from acetoclastic to hydrogenotrophic [39] and syntrophic [40,41] pathways. Studies have reported that the proportion of methane generated through syntrophic acetate oxidation coupled with hydrogenotrophic methanogenesis (SAO–HM) increased from 9–23% to 68–75% when total ammonia nitrogen (TAN) concentrations increased from 0.2 to 1.6 g/L to 3.6–6.1 g/L, respectively [40].
Bathyarchaeia (or Bathyarchaeota) is considered one of the most abundant microbial groups in anaerobic environments. In our study, it was found to be the third-most abundant archaeal species under low ammonia conditions, but its abundance decreased as ammonia levels increased to ≤ 3500 mg/L [42]. This research suggests that Bathyarchaeia may play a role in methane metabolism, although its exact function remains unclear. Previous studies have indicated the capacity and involvement of Bathyarchaeia spp. in methylotrophic acetogenesis [43,44].
On the other hand, Candidatus Methanoplasma is known to produce methane through the methylotrophic pathway, utilizing methanol with a hydrogen-dependent reduction [45,46]. The relative abundance of Methanoplasma was found to increase with higher ammonia concentrations [45]. Previous studies have also reported positive correlations between the relative abundance of Methanoplasma and concentrations of TAN and VFA [45], which is consistent with our current findings (Figure 6).
4. Conclusions
At a low ammonia nitrogen concentration (750 mg/L), minimal levels of volatile fatty acids (VFA) and total inorganic nitrogen were detected after 25 days of aerobic digestion, while the highest daily methane production (4726 mg/L) occurred on the 6th day. These findings indicate the effective removal of ammonia. As the ammonia concentrations increased (from 1500 to 4250 mg/L), there was a gradual increase in VFA and total inorganic carbon, which was accompanied by a decrease in pH on a specific day and a decrease in chemical oxygen demand (COD). The ammonia nitrogen level also influenced the relative abundance of methanogens, with a decrease in acetoclastic methanogens and an increase in hydrogenotropic methanogens, indicating a shift in the composition of methanogens. These results suggest that reducing ammonia nitrogen levels can enhance methane production during the aerobic digestion of chicken manure.
Author Contributions
Conceptualization, Y.L. and J.Z. (Jingxuan Zhang); methodology, J.Z. (Jiaoning Zhu); formal analysis, J.Z. (Jiaoning Zhu) and Y.T.; investigation, X.S. and J.W.; writing—original draft preparation, S.A.; writing—review and editing, S.A. and L.G.; supervision, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
[Research Program Sponsored by State Key Laboratory of Integrative Sustainable Dryland Agriculture (in preparation), Shanxi Agricultural University (No. 202105D121008-1-2); Key Research and Development Project in Shanxi Province (No. 202102140601012)].
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Arthurson, V. Closing the Global Energy and Nutrient Cycles through Application of Biogas Residue to Agricultural Land—Potential Benefits and Drawbacks. Energies 2009, 2, 226–242. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical background and biotechnological applications. Amb Express 2018, 8, 1. [Google Scholar] [PubMed]
- IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth; IEA: Paris, France, 2002; Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth (accessed on 4 April 2023).
- Ulusoy, Y.; Ulukardesler, A.H.; Arslan, R.; Tekin, Y. Energy and emission benefits of chicken manure biogas production—A case study. Environ. Sci. Pollut. Res. Int. 2017, 28, 12351–12356. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas composition from agricultural sources and organic fraction of municipal solid waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- FAO. Global egg production from 1990 to 2021. In Statista. Available online: https://www.statista.com/statistics/263972/egg-production-worldwide-since-1990/ (accessed on 4 April 2023).
- Zhongming, Z.; Linong, L.; Xiaona, Y.; Wangqiang, Z.; Wei, L. Turning Chicken Poop and Weeds into Biofuel; ACS News Service Weekly PressPac: Verona, WI, USA, 2017. [Google Scholar]
- Bi, S.; Westerholm, M.; Qiao, W.; Xiong, L.; Mahdy, A.; Yin, D.; Song, Y.; Dong, R. Metabolic performance of anaerobic digestion of chicken manure under wet, high solid, and dry conditions. Bioresour. Technol. 2020, 296, 122342. [Google Scholar] [CrossRef]
- Yin, D.M.; Uwineza, C.; Sapmaz, T.; Mahboubi, A.; De Wever, H.; Qiao, W.; Taherzadeh, M.J. Volatile fatty acids (VFA) production and recovery from chicken manure using a high-solid anaerobic membrane bioreactor (AnMBR). Membranes 2022, 12, 1133. [Google Scholar] [CrossRef]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- El Hadj, T.B.; Astals, S.; Galí, A.; Mace, S.; Mata-Álvarez, J. Ammonia influence in anaerobic digestion of OFMSW. Water Sci. Technol. 2009, 59, 1153–1158. [Google Scholar] [CrossRef]
- Duan, H.; He, P.; Zhang, H.; Shao, L.; Lü, F. Metabolic Regulation of Mesophilic Methanosarcina barkeri to Ammonium Inhibition. Environ. Sci. Technol. 2022, 56, 8897–8907. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas production by co-digestion of goat manure with three crop residues. PLoS ONE 2013, 8, e66845. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Chen, C.; Liu, G.; He, Y.; Liu, X. Biogas production from co-digestion of corn stover and chicken manure under anaerobic wet, hemi-solid, and solid state conditions. Bioresour. Technol. 2013, 149, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Feng, L.; Shao, L.; Kou, W.; Liu, P.; Gao, P.; Dong, X.; Yu, M.; Wang, J.; Zhang, D. The Effect of digested manure on biogas productivity and microstructure evolution of corn stalks in anaerobic co-fermentation. BioMed Res. Int. 2018, 2018, 5214369. [Google Scholar] [CrossRef]
- Sterling Jr, M.C.; Lacey, R.E.; Engler, C.R.; Ricke, S.C. Effects of ammonia nitrogen on H2 and CH4 production during anaerobic digestion of dairy cattle manure. Bioresour. Technol. 2001, 77, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, A.; Nordberg, Å. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Kong, X.; Li, L.; Yuan, Z.; Dong, R.; Sun, Y. Effects of ammonia on propionate degradation and microbial community in digesters using propionate as a sole carbon source. J. Chem. Technol. Biotechnol. 2017, 92, 2538–2545. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association and Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- HJ537–2009; Chinese Standard. Water Quality-Determination of Ammonia Nitrogen-Distillation-Neutralization Titration. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2009. Available online: https://www.chinesestandard.net/PDF/English.aspx/HJ537-2009 (accessed on 4 April 2023).
- GB/T11914-89; Chinese Standard. Water Quality-Determination of the Chemical Oxygen Demand Dichromate Method. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2017. Available online: https://www.chinesestandard.net/PDF/BOOK.aspx/GBT11914-1989 (accessed on 4 April 2023).
- Moody, L.B.; Burns, R.T.; Bishop, G.; Sell, S.T.; Spajic, R. Using biochemical methane potential assays to aid in co-substrate selection for co-digestion. Appl. Eng. Agric. 2011, 27, 433–439. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Hobbs, S.R.; Landis, A.E.; Rittmann, B.E.; Young, M.N.; Parameswaran, P. Enhancing anaerobic digestion of food waste through biochemical methane potential assays at different substrate: Inoculum ratios. Waste Manag. 2018, 71, 612–617. [Google Scholar] [CrossRef]
- Tampio, E.A.; Lucia, B.; Vainio, M.M.; Kahala, M.M.; Rasi, S.E. Volatile fatty acids (VFAs) and methane from food waste and cow slurry: Comparison of biogas and VFA fermentation processes. GCB Bioenergy 2019, 11, 72–84. [Google Scholar] [CrossRef]
- Yin, D.M.; Westerholm, M.; Qiao, W.; Bi, S.J.; Wandera, S.M.; Fan, R.; Jiang, M.M.; Dong, R.J. An explanation of the methanogenic pathway for methane production in anaerobic digestion of nitrogen-rich materials under mesophilic and thermophilic conditions. Bioresour. Technol. 2018, 264, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bressani-Ribeiro, T.; Almeida, P.; Chernicharo, C.; Volcke, E. Inorganic carbon limitation during nitrogen conversions in sponge-bed trickling filters for mainstream treatment of anaerobic effluent. Water Res. 2021, 201, 117337. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.T.; Ahn, J.; Kim, J.; Lee, S.; Lee, I.; Kim, S.; Chang, S.; Chung, W. Volatile Fatty Acid Production from Food Waste Leachate Using Enriched Bacterial Culture and Soil Bacteria as Co-Digester. Sustainability 2021, 13, 9606. [Google Scholar] [CrossRef]
- Harnadek, C.M.W.; Guilford, N.G.; Edwards, E.A. Chemical Oxygen Demand Analysis of Anaerobic Digester Contents. STEM Fellowsh. J. 2015, 1, 2–5. [Google Scholar] [CrossRef]
- Sossa, K.; Alarcón-Vivero, M.; Aspé, E.; Urrutia, H. Effect of ammonia on the methanogenic activity of methylaminotrophic methane producing Archaea enriched biofilm. Anaerobe 2004, 10, 13–18. [Google Scholar] [CrossRef]
- Karakashev, D.; Batstone, D.J.; Trably, E.; Angelidaki, I. Acetate Oxidation Is the Dominant Methanogenic Pathway from Acetate in the Absence of Methanosaetaceae. Appl. Environ. Microbiol. 2006, 72, 5138–5141. [Google Scholar] [CrossRef]
- de Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef]
- Ferry, J. Fermentation of Acetate. In Methanogenesis. Ecology, Physiology, Biochemistry and Genetics; Ferry, J.G., Ed.; Chapman and Hall: New York, NY, USA, 1993; pp. 305–334. [Google Scholar]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- Yan, M.; Treu, L.; Zhu, X.; Tian, H.; Basile, A.; Fotidis, I.A.; Campanaro, S.; Angelidaki, I. Insights into ammonia adaptation and methanogenic precursor oxidation by genome-centric analysis. Environ. Sci. Technol. 2020, 54, 12568–12582. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Elizondo, S.; Parameswaran, P.; Delgado, A.G.; Maldonado, J.; Rittmann, B.E.; Krajmalnik-Brown, R. Archaea and Bacteria Acclimate to High Total Ammonia in a Methanogenic Reactor Treating Swine Waste. Archaea 2016, 2016, 4089684. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, Q.; Ma, Y.; Wang, X.; Peng, X. A mesophilic anaerobic digester for treating food waste: Process stability and microbial community analysis using pyrosequencing. Microb. Cell Factories 2016, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Mulat, D.G.; Jacobi, H.F.; Feilberg, A.; Adamsen, A.P.S.; Richnow, H.-H.; Nikolausz, M. Changing Feeding Regimes to Demonstrate Flexible Biogas Production: Effects on Process Performance, Microbial Community Structure, and Methanogenesis Pathways. Appl. Environ. Microbiol. 2016, 82, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.G.; Bendia, A.G.; Moreira, J.C.F.; Franco, D.C.; Signori, C.N.; Yu, T.; Wang, F.; Jovane, L.; Pellizari, V.H. Bathyarchaeia occurrence in rich methane sediments from a Brazilian ría. Estuar.Coast. Shelf Sci. 2021, 263, 07631. [Google Scholar] [CrossRef]
- Loh, H.Q.; Hervé, V.; Brune, A. Metabolic potential for reductive acetogenesis and a novel energy-converting [NiFe] hydrogenase in Bathyarchaeia from termite guts–a genome-centric analysis. Front. Microbiol. 2021, 11, 635786. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Y.; Zhu, P.; Yang, N.; Liang, L.; Yu, T.; Niu, M.; Konhauser, K.; Wang, F. Taxonomic and carbon metabolic diversification of Bathyarchaeia during its co-evolution history with the early Earth surface environment. BioRxiv 2022. [Google Scholar] [CrossRef]
- Lang, K.; Schuldes, J.; Klingl, A.; Poehlein, A.; Daniel, R.; Brune, A. New mode of energy metabolism in the seventh order of methanogens as revealed by comparative genome analysis of “Candidatus Methanoplasma termitum”. Appl. Environ. Microbiol. 2015, 81, 1338–1352. [Google Scholar] [CrossRef]
- Pyzik, A.; Ciezkowska, M.; Krawczyk, P.S.; Sobczak, A.; Drewniak, L.; Dziembowski, A.; Lipinski, L. Comparative analysis of deep sequenced methanogenic communities: Identification of microorganisms responsible for methane production. Microb. Cell Factories 2018, 17, 197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).