Abstract
Leaf hydraulic conductance (KLeaf) is a measure of the efficiency of water transport through the leaf, which determines physiological parameters such as stomatal conductance, photosynthesis and transpiration rates. One key anatomical structure that supports KLeaf is leaf venation, which could be subject to evolutionary pressure in dry environments. In this context, it is useful to assess these traits in species from arid climates such as S. peruvianum and S. chilense, in order to determine their hydraulic strategy and potential aptitude for the improvement of domestic tomato (S. lycopersicum). In this work, we measured KLeaf, vein density, together with leaf water isotope composition (δ18O, δ2H) and leaf carbon isotope composition (δ13C), from which we derived proxies for outside-vein hydraulic resistance (Rox) and intrinsic water use efficiency (WUEi), respectively. The two wild species showed contrasting hydraulic strategies, with S. chilense performing as a water-spender, whereas S. peruvianum showed a water-saving strategy. Interestingly, S. lycopersicum was rather conservative, and showed the highest WUEi. The low water transport capacity of S. peruvianum was not explained by vein density traits, but was related with the effective pathlength L, an isotope-derived proxy for Rox. The low WUEi of S. peruvianum suggest strong photosynthetic limitations. Our results show a wide diversity in water-use strategies in the genus, encouraging a detailed characterization of wild relatives. From a methodological point of view, we provide evidence supporting the use of water isotopes to assess changes in mesophyll hydraulic conductance, not attributable to vein density.
1. Introduction
Water availability is the most important factor limiting the photosynthesis and growth of plants, especially in arid and semi-arid zones [1,2]. The regulation of water use is often considered to be primarily dominated by interactions between xylem architecture and stomatal behavior [1,2] and this may covary to optimize water use [3,4]. In this regard, plants with a conservative strategy (water savers) would reduce water use, but at the expense of a limited carbon gain; conversely, water spenders can keep higher growth rates, but require a higher water transport capacity [1,2,3]. In this regard, leaves constitute the main bottleneck for whole-plant hydraulic capacity, accounting for ca. 30% of the whole-plant resistance to water movement, and leaf hydraulic conductance (KLeaf) becomes a key limiting factor for water use and photosynthesis across a wide range of species [3,4,5].
The main water path through the leaves includes a complex system of serial and parallel xylem veins. In dicotyledons, venation consists of three major vein orders, and up to five minor vein orders, and the hydraulic limitation of a given vein order depends inversely on its density (and redundancy), relative to the other vein orders [3,6]. Hence, a denser vein system, and a larger proportion of major veins, would allow higher water transport capacity, and thus higher KLeaf [3,6]. In this sense, a higher major and minor vein density has been reported for drought-tolerant species, because this provides alternative pathways for water flow around embolized veins [7,8]. Alternatively, the proportion between major and minor vein density has been proposed as an indicator of hydraulic vulnerability [6]. However, the association between leaf venation and Kleaf across species is not always consistent and depends on the scale of the comparison [5,7,9].
Besides the xylem pathway, water also needs to move outside veins, through the bundle sheath and the mesophyll, and this pathway accounts for 30–80% of the total leaf hydraulic resistance to water flow [3,6,9]. So far, estimates of the xylem and outside-xylem components of KLeaf (Kx and Kox, respectively) have relied on either modeling approaches or tedious and invasive vein-cutting experiments [6,9]. In this context, the isotopic composition of leaf water has been proposed as an alternative approach to estimate Kox [10,11,12]. Briefly, during transpiration, leaf water is enriched in the heavier isotopes 18O and 2H, and this can be modeled from environmental drivers such as leaf temperature and relative humidity [13,14,15]. However, actual enrichment of the whole leaf lamina is lower than modeled values for the sites of evaporation due to the mass flow of non-enriched water, driven by transpiration, causing the so-called Péclet effect [13,14]. The magnitude of this effect is proportional to the transpiration rate, and the effective pathlength (L, in m), which accounts for the length and tortuosity of the water pathway from the xylem to the evaporative surfaces [13,16]. Consequently, this parameter, determined empirically, can provide a non-manipulative proxy for the outside-vein hydraulic resistance (i.e., the inverse of Kox). Up to now, this has been tested in a limited number of species, with contradictory results [11,12,17,18]; therefore, additional assays are needed to define the limitations of this approach.
The positive association between Kleaf and maximum stomatal conductance is generally explained by the need to adjust water supply to water demand [5,19]. In turn, the association with photosynthesis and productivity would be mainly a consequence of stomatal constraints [4,5], reinforced by a covariation between Kox and mesophyll conductance for CO2 [11,12,20,21]. Hence, the coordination of KLeaf with photosynthesis generally implies a negative association between productivity and the ratio between photosynthesis and stomatal conductance (intrinsic water-use efficiency, WUEi). However, the sign of this association depends on the leading term in the quotient, with two main scenarios, as defined in Fardusi et al. [22]. In the first scenario, higher WUEi results from an increase in photosynthesis, independent from stomatal conductance, and we would expect a positive relationship with plant productivity [22,23]. In the second scenario, higher WUEi is mainly driven by stomatal limitations, and we would expect a negative association with growth and photosynthesis [24,25]. Although the general trend between Kleaf and photosynthesis fits with the second scenario, it has been observed, for example, that tropical rainforest species (adapted to wet soils and low atmospheric water demand) show higher potential photosynthesis than temperate trees for a given Kleaf [19,26]. However, it is unclear how species adaptation to different combinations of soil dryness and atmospheric demand affects the coordinated response of Kleaf and WUEi [3,18,27].
In summary, despite the existence of general trends across multiple species, the link between vein traits, KLeaf and WUEi is not straight-forward, differing across genera and species with distinct evolutionary history [4,7,8,18]. In this context, the tomato and its wild relatives constitute a model for the study of plants’ adaptation to drought and other environmental constraints [28,29,30,31]; however, the water use strategy of tomato wild relatives has not yet been assessed from a leaf hydraulics perspective. The Lycopersicon clade comprises the domesticated tomato (Solanum lycopersicum L.) and 12 closely related species, with a broad geographic and ecological distribution along western South America, including deserts, tropical rainforests and highlands [29,32,33]. In this work, we focused on two wild relatives (Solanum peruvianum L. and Solanum chilense Dunal) that can be found in arid and saline habitats (e.g., in the Atacama desert) but display different geographic and microhabitat preferences [29,30,32,33]. S. peruvianum has a wide distribution but is more common in the coastal areas of Perú, up to 2500 m.a.s.l., often in fog oases known as lomas, and occasionally growing as a weed in irrigated crops [29,30,32]. S. chilense is mainly found in northern Chile; it also appears in the lomas, but spreads to more continental areas and higher altitudes (up to 3500 m.a.s.l.), growing in dry river beds and ravines with stony soils, surviving in hyper-arid environments by deep roots [30,32,33]. To characterize the water use strategy of these two species, we combined measurements of leaf hydraulic conductance, leaf venation and the isotopic enrichment of leaf water, using a commercial tomato cultivar as a reference. We hypothesized that the wild relatives, more drought-adapted than the domesticated tomato, would show a conservative water-use strategy, associated with high minor vein density (high redundance), but low major vein density and leaf conductance. However, this tendency would be more exacerbated in S. chilense, which can thrive in drier areas. As a second aim, we assessed the association between WUEi and physiological and anatomical traits related to water transport. In this regard, following the hypothesized conservative strategy, we anticipated a higher WUEi in the two wild relatives than in the domesticated tomato. Finally, from a methodological point of view, we aimed to test the potential of the isotope-derived parameter L as a proxy for outside-vein hydraulic resistance, assessing its association with water-transport traits.
2. Materials and Methods
2.1. Plant Material
Seeds of S. lycopersicum (var. ‘Moneymaker’), S. peruvianum (Acc. Q958) and S. chilense (Acc. Q966) were provided by Germplasm Bank Network of the Instituto de Investigaciones Agropecuarias (INIA) under a standard material transfer agreement and an Institutional INIA Policy for ABS of plant genetic material following international agreement signed by Chile (see Appendix A, Table A1 for details). The seeds were germinated in a growth chamber during one month, with photoperiod 18/6 h light-dark, at 25/18 °C and 60% relative humidity. Later, the plantlets were relocated to the greenhouse. Leaves were obtained from plants grown in greenhouse under optimal conditions at the end of October (S. chilense (SC), n = 5; S. peruvianum (SP), n = 10; S. lycopersicum (SL) n = 6). Collected leaves were stored at ambient temperature in glass flasks filled with distilled water and covered with black nylon until KLeaf measure.
2.2. Leaf Hydraulics
Leaf hydraulic conductance was determined by the Evaporative Flux Method [5]. Prior to the measurement, the leaf was hydrated overnight, in dark conditions to avoid water loss by transpiration. Leaves were placed at the same height as the water reservoir to ensure that the water movement was driven by transpiration, instead of being forced by positive pressure due to gravity. Leaves were illuminated with 1000 µmol m−2s−1 PAR (module CI-301 LA, CID Bioscience Inc., Camas, OR, USA), maintaining a stable leaf temperature between 23 °C and 28 °C. Each leaf was connected for ca. 20 min to reach steady-state conditions. Then, the weight change of the water reservoir was recorded every 30 s during 15 min to determine water flow (E, in g s−1, then converted to mmol s−1). At the end of the protocol, the leaf was removed from the system and stored in a sealed plastic bag for 20 min to prevent water loss, and leaf water potential (ΨLeaf, in Mpa) was determined with a Scholander chamber. Total leaf hydraulic conductance (K) was first determined as the ratio between E and the water potential gradient from the water reservoir to the leaf (Ψsource − ΨLeaf), assuming Ψsource = 0 Mpa. Area-adjusted leaf hydraulic conductance (KLeaf) was then calculated by dividing K by leaf area. Following [5], KLeaf was normalized at a temperature of 20 °C to account for temperature-dependent changes in water viscosity. Finally, total leaf conductance to water vapor (gw) was estimated from measured flow and the water vapor gradient from the intercellular space to ambient air, in molar fraction (wi-wa, in mol H2O mol air−1). The molar fraction gradient was calculated from air temperature and relative humidity (monitored in situ during the experiment with a thermo-hygrometer), leaf temperature (measured with a calibrated K-type thermocouple) and mean atmospheric pressure of 99.5 kPa, based on data from a nearby agrometeorological station (Santa Rosa Experimental Station, INIA-Quilamapu, 36°32′ S; 71°54′ W; 195 m a.s.l.).
2.3. Leaf Water Distillation and Isotope Composition
After measuring KLeaf, the midrib was removed for each leaflet and the leaf lamina stored in sealed glass vials and kept at −80 °C until cryogenic distillation. The leaf lamina water was extracted by cryogenic vacuum distillation, as described in Palacio et al. [34]. The samples (inside of glass vials) were submerged in a water bath at 80 °C to facilitate the evaporation of leaf lamina water and connected to the vacuum pressure distilling system at −760 in Hg (−0.88 MPa), where the water condenses in glass collector submerged in liquid N. Samples were stored in sealed glass vials at 4 °C until analysis. As a reference value for source water, we took two aliquots from the distilled water used to determine KLeaf. Isotope composition of water (δ18O, δ2H) was determined by infra-red laser spectroscopy (CRDS) at the Serveis Científico-Tècnics of Universitat de Lleida (Lleida, Spain), using a water isotopes analyzer Picarro L2120-I (Picarro Inc., Sunnyvale, CA, USA), coupled to a high-precision vaporizer (A0211). The possible spectral interference caused by organic contaminants was corrected following [35].
2.4. Modeling Isotopic Enrichment of Leaf Water
Isotopic enrichment of leaf lamina relative to source water (Δ18O, Δ2H) was calculated from ΔL = (δL − δS)/(1 + δS), where δL and δS are the isotope composition (δ18O, δ2H) of leaf-lamina and source water, respectively. Isotopic enrichment at the sites of evaporation under steady state (Δe) were modeled using the Craig and Gordon model, as adapted to plant leaves by Dongman et al. [36].
where ε+ is the equilibrium fractionation between liquid water and vapor, εk is the kinetic fractionation of vapor diffusion from the leaf to the atmosphere, Δv is the isotopic enrichment of atmospheric water vapor and ea/ei is the ratio of ambient to intercellular vapor pressures. Fractionation factors were taken from the literature, as detailed elsewhere [10]. The steady-state isotopic enrichment of leaf lamina water (ΔLss) was calculated by correcting for the gradient from xylem water to enriched water at the sites of evaporation, the Péclet effect [13]:
where φ is the Péclet number, E is the leaf transpiration rate, L is the scaled effective path length (m) for water movement from the veins to site of evaporation, C is the molar concentration of water (5.56 103 mol m−3) and D is the tracer diffusivity (m2 s−1) of the heavy water isotopologue (H218O/HDO) in ‘light’ water (H2O), corrected by temperature, as described in [10]. The effective pathlength L under steady-state assumption (Lss) was determined by fitting Equation (2) to the Péclet number:
2.5. Leaf Carbon Isotope Composition
Carbon isotope composition (δ 13C) was determined from leaf dry matter, as a proxy for WUEi [13,22,37]. For that, the dry material remaining after water distillation was ground in mortar until obtaining a fine powder. Isotope ratios were determined using a combustion and gas preparation module (EA-GSL Elemental Analyzer, Sercon, UK) attached to an Isotope Ratio Mass Spectrometer (20–22 IRMS, Sercon, UK) at the Soil, Water and Forest Lab (LISAB, Universidad de Concepción, Chile).
2.6. Leaf Vein Density
In each measured leaf, the terminal leaflet was cut for leaf vein density analysis. The leaflet was diaphanized according to Scoffoni et al. [8]. The leaflets were treated with NaOH at 5% for five days at ambient temperature. After this period, the samples were rinsed with distilled water to remove the NaOH, remaining in vacuum for 10 min. Then, the leaflets were bleached with sodium hypochlorite at 50% and washed with distilled water several times. Later, the leaflets were dehydrated in alcohol baths with ascending concentrations (30, 50, 70 and 95%), remaining five minutes in each bath, then placed in a safranin 1% bath for 24 h. Excess safranin was removed under vacuum and the samples were rehydrated following the series of alcohol baths in descending order. Finally, 2.5 × 2.5 cm bleached leaflet sections were mounted in distilled water for the quantification of leaf venation. Pictures were taken under two objective lenses (0.67× for major veins and 3× for minor veins), taking measures in three leaflet subsectors (from base to tip). ImageJ (1.5r version, National Institutes of Health, Bethesda, Maryland, USA) was used to measure major vein density (MVD) in the image and the result of MVD was divided by the image area (15.61 × 11.66 mm). Minor vein density (mVD) was measured in the same way in a smaller image area (3.39 × 2.53 mm). Finally, the data obtained in each subsector were averaged to obtain one datum per leaflet. TotalVD was calculated as the sum of MVD and mVD.
2.7. Statistical Analysis
Data were analyzed by ANOVA, previously checking for the assumption of normality and homoscedasticity. For variables following these assumptions, we performed a One-Way ANOVA and applied the multiple comparison test of LSD Fisher, due to heterogeneous sample size. Conversely, for non-normal and heteroscedastic variables we used the Kruskal–Wallis (K–W) test, and the multiple comparison test of Dunn. The assessment of possible relations between variables was performed using two correlation analyses, Pearson and Spearman, both with significance level of p < 0.05. Multivariate association of leaf hydraulics and isotopic variables was performed by a Principal Component Analysis (PCA), using zero mean. Statistical analysis was performed in SigmaPlot 11.0 and PCA was performed in RStudio (version 1.2.1335) with vegan package. The full dataset is available in Supplementary Materials Table S1.
3. Results
3.1. Leaf Vein Density
The leaf vein density traits of S. lycopersicum, S. peruvianum and S. chilense are summarized in Figure 1. The ANOVA showed significant differences in total vein density (TotalVD; p = 0.025). S. lycopersicum was the species with the highest TotalVD (6.81 mm mm−2) followed by S. peruvianum (5.92 mm mm−2) and S. chilense (5.53 mm mm−2), although only S. lycopersicum and S. chilense differed significantly according to the Fisher test (Figure 1A). Major vein density (MVD) was significantly different between the species (p = 0.014), although only the extremes (S. chilense and S. lycopersicum) differed significantly (Figure 1B). Contrary to TotalVD, S. chilense showed the highest MVD (0.33 mm mm−2), followed by S. peruvianum (0.28 mm mm−2) and S. lycopersicum (0.26 mm mm−2). Minor vein density (mVD) was significantly different among the three species (p < 0.001). S. lycopersicum showed the highest mVD (5.71 mm mm−2), followed by S. peruvianum (4.94 mm mm−2) and S. chilense (3.01 mm mm−2) (Figure 1D). Finally, the K–W test showed significant differences in the ratio of major vein density to minor vein density (MVD/mVD; p = 0.001), with S. chilense (0.11) showing significantly higher median than S. lycopersicum and S. peruvianum (0.045 and 0.06, respectively; Figure 1C).
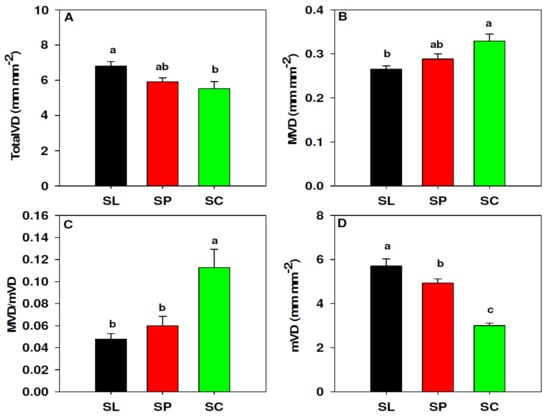
Figure 1.
Differences in leaf venation between S. lycopersicum (black, SL), S. peruvianum (red, SP) and S. chilense (green, SC). Total vein density, TotalVD (A), major vein density, MVD (B), ratio of MVD to mVD, MVD/mVD (C), minor vein density, mVD (D). Values in (A,B,D) represent the mean and the error bars represent standard error. Values in (C) represent the median analyzed by Kruskal–Wallis analysis and error bars represent the 75% percentile. Different letters indicate significant differences (p < 0.05) according to Fisher (A,B,D) and Dunn’s (C) tests.
3.2. Leaf Hydraulic Conductance
Leaf hydraulic traits are summarized in Figure 2. We found significant differences in water flux (E; p = 0.005), with S. chilense showing significantly higher median (4.58 mmol m−2 s−1) than S. peruvianum (0.70 mmol m−2 s−1, respectively) (Figure 2A). S. lycopersicum (2.22 mmol m−2 s−1) was not statistically different from the other two species. The three species differed significantly in leaf water potential (ΨLeaf; p < 0.001). S. chilense showed significantly less negative ΨLeaf (−0.24 MPa) than S. lycopersicum and S. peruvianum (−0.47 and −0.59 MPa, respectively) (Figure 2B). Area-adjusted leaf hydraulic conductance (KLeaf) showed significant differences between species (p < 0.001). S. chilense had a higher median KLeaf (18.42 mmol m−2 s−1 MPa−1) than S. peruvianum (1.24 mmol m−2 s−1 MPa−1), whereas S. lycopersicum (4.43 mmol m−2 s−1 MPa−1) was not statistically different from both species (Figure 2C). Total leaf hydraulic conductance (K) also showed significant differences between species (p = 0.002). Similar to KLeaf, S. chilense had a higher median for K (0.046 mmol s−1 MPa−1) than S. peruvianum (0.00655 mmol s−1 Mpa−1), while S. lycopersicum (0.0183 mmol s−1 Mpa−1) was not statistically different from both species (Figure 2D).
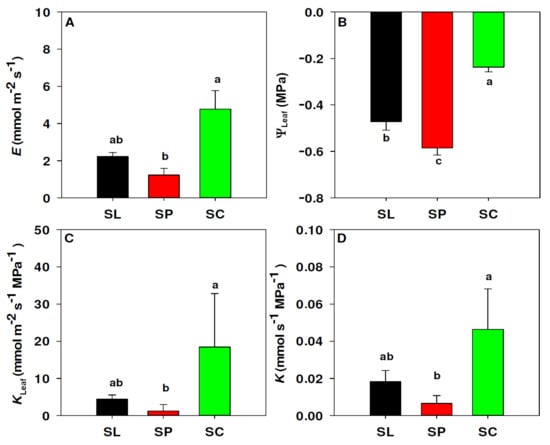
Figure 2.
Differences in leaf hydraulics between S. lycopersicum (black, SL), S. peruvianum (red, SP) and S. chilense (green, SC). Water flux E (A), water potential ΨLeaf (B), area-adjusted leaf hydraulic conductance KLeaf (C) and total leaf hydraulic conductance K (D). Values in (A,C,D) represent the median and 75% percentile. Values in (B) represent the mean and standard error. Different letters represent significant differences (p < 0.05) according to Fisher (B) and Dunn’s (A,C,D) tests.
3.3. Leaf Water Isotopic Enrichment
The isotopic enrichment of leaf water and related variables are summarised in Figure 3. The three species differed significantly in Δ18O (p < 0.001) and Δ2H (p = 0.003). S. lycopersicum showed significantly higher Δ18O and Δ2H (11.4‰ and 42.7‰, respectively; Figure 3A,B) than S. peruvianum (6.9‰; 30.6‰) and S. chilense (6.5‰; 25.8‰). S. peruvianum and S. chilense, however, were not significantly different. Effective path length (Lss), either calculated from 18O or 2H (Figure 3C,D), showed significant differences among species (p = 0.009 and p = 0.003, for Lss18O and Lss2H, respectively). S. peruvianum showed significantly higher Lss (301.7 mm, averaging 18O and 2H estimates) than S. lycopersicum (27.50 mm) and S. chilense (48.5 mm). The Lss of S. lycopersicum and S. chilense did not differ significantly.
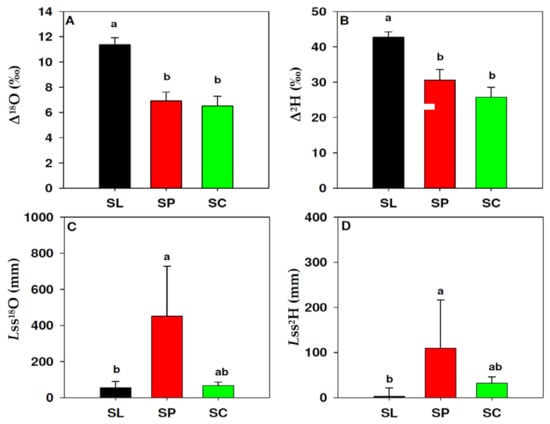
Figure 3.
Leaf water enrichment traits in S. lycopersicum (black, SL), S. peruvianum (red, SP) and S. chilense (green, SC). Δ18O and Δ2H, oxygen (A) and hydrogen (B) leaf water isotopic enrichment; Lss18O and Lss 2H, effective path length, calculated from Δ18O and Δ2H. Values in (A,B) represent the mean and standard error. Values in (C,D) represent the median and 75% percentile. Letters indicate significant differences (p < 0.05) according to Fisher (A,B) and Dunn’s (C,D) tests.
3.4. Leaf Conductance to Water Vapour and δ13C
Leaf conductance to water vapour (gw) showed significant differences between species (p = 0.005). The gw of S. chilense (247.90 mmol m2 s−1) was higher than S. peruvianum (32.01 mmol m2 s−1), whereas S. lycopersicum (131.54 mmol m2 s−1) was not significantly different from the other two species (Figure 4A). Carbon isotope composition (δ13C) also differed significantly between species (p < 0.001): S. lycopersicum (−28.34‰) was less negative than S. peruvianum and S. chilense (−29.69 and −30.13 ‰, respectively) (Figure 4B).
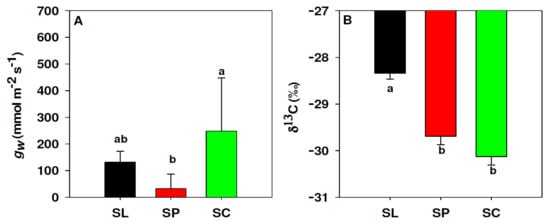
Figure 4.
Differences in leaf conductance to water vapour, gw (A) and leaf isotope composition of 13C, δ13C (B) between S. lycopersicum (black, SL), S. peruvianum (red, SP) and S. chilense (green, SC). Values in (A) represent the median and 75% percentile. Values in (B) represent the mean and standard error. Letters indicate significant differences (p < 0.05) according to Fisher (B) and Dunn’s (A) tests.
3.5. Correlation Analysis
The results of Pearson (rp, upper triangle) and Spearman (rs, lower triangle) correlation analysis among the most relevant variables are summarized in Table 1. Correlation analysis showed that leaf venation had a strong influence on leaf hydraulic variables. Interestingly, mVD was negatively correlated with KLeaf (rp = −0.67, p < 0.001) and positively correlated with Δ18O (rp = 0.73, p < 0.01), Δ2H (rp = 0.78, rs =0.78, p < 0.001) and δ13C (rp = 0.67, p < 0.01). Major vein density showed generally weaker correlations than mVD, and the opposed sign, i.e., positive with KLeaf (rp = 0.49, p < 0.05), and negative with Δ18O, Δ2H, Lss and δ13C (rs between −0.57 and −0.51, p < 0.05). The ratio between MVD and mVD, MVD/mVD, was also positively associated with KLeaf (rp = 0.77, p < 0.001). However, MVD/mVD was negatively correlated with Δ18O, Δ2H and δ13C, although these correlations showed a better adjustment with the Spearman rank correlation (rs between −0.71 and −0.76, p < 0.01). TotalVD was positively related to Δ18O (rp = 0.72, p < 0.001), Δ2H (rp = 0.74, p < 0.001) and δ13C (rp = 0.66, p < 0.01). On the other hand, Lss was negatively associated with KLeaf (rs = −0.61, p < 0.05) and Δ18O (rs = −0.53, p < 0.01) and positively correlated with MVD (rs = 0.51, p < 0.05).

Table 1.
Correlation analysis of leaf hydraulics, isotopes and venation traits in the three species here studied. Upper triangle corresponds to result of Pearson correlation; lower triangle corresponds to Spearman correlation. KLeaf, area-adjusted leaf hydraulic conductance (mmol m−2 s−1 MPa−1); Δ18O and Δ2H, oxygen and hydrogen isotopic enrichment of leaf water (‰); Lss, mean effective path length (mm); δ13C, carbon isotope composition (‰); TotalVD, MVD and mVD, total, major and minor vein density (mm mm−2).
3.6. Principal Component Analysis
To assess the association between variables, we performed a PCA with the most relevant variables (Figure 5A). The first two components of the PCA explained ca. 76% of the variability in leaf traits. The first axis (PC1) captured most of the variability associated with leaf hydraulic traits across the three species studied, explaining 56% of the total variation. The first axis (PC1) showed particularly strong loadings for mVD, MVD/mVD and KLeaf. The second axis (PC2) explained ca. 20% of the total variation, and only showed a strong loading for Lss. In general, a tight association between variables was observed (i.e., narrow angle between vectors), which was consistent with the correlation analysis (Table 1). Traits linked to higher water transport capacity were positively correlated with PC1 (e.g., MVD, MVD/mVD and KLeaf), whereas traits associated with lower water transport capacity (e.g., mVD) were negatively correlated. All the traits included in the PCA had a strong influence, and clearly differentiated the species in three groups, representative of contrasting hydraulic strategies (Figure 5B). S. lycopersicum and S. chilense fell on opposite sides of PC1. S. lycopersicum showed higher values of mVD, δ13C, TotalVD and Δ18O, which is consistent with a conservative hydraulic strategy. Conversely, S. chilense showed higher values of MVD/mVD, KLeaf, ΨLeaf and MVD, indicative of a water-spender hydraulic strategy. On the other hand, S. peruvianum had intermediate scores along PC1, but was clearly discriminated by PC2, being mainly associated with high values of Lss, and, to a lesser extent, low values of ΨLeaf, Δ18O, δ13C and KLeaf.
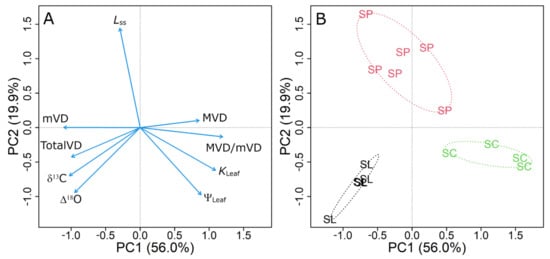
Figure 5.
Principal component analysis (PCA) for leaf hydraulics, isotopes and venation variables among the three species here studied. The variable loading plot (A) shows the variables as blue arrows: ΨLeaf, leaf water potential; KLeaf, leaf hydraulic conductance; MVD, mVD and MVD/mVD, major, minor vein density and major/minor vein ratio; Lss, mean effective path length; δ13C, leaf carbon isotope composition; Δ18O, isotopic enrichment of leaf water. The scores plot (B) shows the score of each replicate along the PCA axis, indicating the species grouping: S. lycopersicum (SL), S. peruvianum (SP) and S. chilense (SC) and their respective ellipsoid hulls.
4. Discussion
4.1. Contrasting Hydraulic Strategies in Tomato Wild Relatives
The three tomato species assessed showed contrasting water transport capacities, which were partly explained by differences in leaf venation, in agreement with previous studies [3,5,7,8]. To the best of our knowledge, wild tomato species have not been characterized under a hydraulic approach, but their TotalVD and KLeaf fell in the range of other herbaceous plants [7,8,20]. Nevertheless, KLeaf showed a wide range across species, from S. peruvianum, in the lowest range for Angiosperms, to the upper-intermediate values of S. lycopersicum and S. chilense [3,5,12,20]. Across the three species, KLeaf was best explained by the ratio between MVD and mVD, supporting the postulated functions of major and minor veins as main water suppliers and modulators of the extra-xylematic pathways, respectively [6,9]. Contrary to our first hypothesis, we found that S. chilense had the highest MVD, but the lowest mVD, which resulted in a high KLeaf. This reveals a water-spender strategy for this species, contrasting with the rather conservative responses of S. lycopersicum and S. peruvianum. In this regard, the microhabitat preferences of S. chilense, which is found in rocky soils and ravines, indicates that this species relies on a highly effective water uptake, taking advantage of deep or interstitial water reservoirs, as previously reported for other deep-rooted semiarid species [32,34]. Conversely, S. peruvianum showed intermediate vein densities, but the lowest KLeaf. Anatomical traits could explain the contrasting water transport capacity of S. lycopersicum and S. chilense but did not explain the lower KLeaf of S. peruvianum, pointing towards large extra-xylematic limitations to water flow in this species [9,20]. This is further supported by the high Lss values (in the upper range of herbaceous species [11,12,18]), indicative of a particularly tortuous water pathway from the xylem to the sites of evaporation, i.e., lower Kox [11,12,16,21]. This decline in KLeaf due to low Kox cannot be solely attributed to a smaller mVD (intermediate between S. lycopersicum and S. chilense), pointing towards functional differences in the mesophyll and/or bundle sheath [20]. Overall, the striking differences between S. peruvianum and S. chilense evidence that the two species have distinct ecological niches, despite a partially overlapping distribution. The strong hydraulic limitations of S. peruvianum suggest that this species is mainly adapted to dry environments, but with relatively low evaporative demand, such as the fog oases in coastal Peru [29,32]. Conversely, the adaptation of S. chilense to more continental habitats, with higher insolation and VPD, would require a higher water transport capacity to prevent leaf dehydration [3,26,27]. According to Tapia et al. [30], S. chilense also exhibits a stronger osmotic adjustment and proline accumulation than S. peruvianum, which would contribute to the maintenance of leaf turgor. In our study, the two species also differed in gw, with higher values in S. chilense than in S. peruvianum, in agreement with the proposed coordination between Kleaf and gas exchange [5,19]. Our values are in range with those previously reported for S. peruvianum [4] (40–70 mmol m−2 s−1) and other tomato cultivars [27,30] (60–100 mmol m−2 s−1), whereas for S. chilense we found higher gw than in Tapia et al. [30] (60–80 mmol m−2 s−1). It should be noted that our approach to estimating gw might be biased due to the assumption of steady-state water flow, which may not be the case if partial dehydration occurs (i.e., transpiration exceeding water inflow). However, this would cause an underestimation of gw, rather than an overestimation, and mainly in species showing a significant drop in leaf water potential, such as S. peruvianum. Most likely, the generally higher gw in our study would be due to the more limiting light conditions in previous works (150–300 µmol m−2 s−1 [27,30]; 1000 µmol m−2 s−1 in our case), particularly for S. chilense, the species most adapted to high radiation. Interestingly, S. lycopersicum showed an intermediate water use pattern, being closer to the conservative strategy of S. peruvianum. Although the results from a single cultivar (var. ‘Moneymaker’) cannot be extrapolated to the wide diversity of domesticated tomatoes, our values of vein density, Kleaf and gw are similar to those reported by Du et al. [27] for the cultivars ‘Jinpeng’ and ‘Zhongza’, and also agree with the gw reported by Tapia et al. [30] for cherry tomatoes (var. ‘cerasiforme’), considered the ancestral form of domesticated tomato [32]. Besides the role of artificial selection, the similarities between S. lycopersicum and S. peruvianum may reflect the comparable habitat preferences of tomato ancestors [29,32].
4.2. Implications of Hydraulic Constraints for Water Use Efficiency
The PCA analysis further evidenced that the hydraulic strategy of S. peruvianum was more conservative than the other two species, but without a significant gain in WUEi, as estimated from δ13C. Indeed, despite their contrasting water use patterns, S. peruvianum and S. chilense displayed similar values for δ13C, and both were more negative than S. lycopersicum (i.e., less WUEi). Comparing S. peruvianum with S. lycopersicum, the lower δ13C values were not linked to a higher gw (scenario 2 in [22]) but would be explained by a lower photosynthetic capacity. In this regard, it has been reported in a wide range of tomato wild relatives that variation in mesophyll conductance to CO2 (gm) strongly affects photosynthesis, with mesophyll limitations exceeding stomatal limitations [31]. Conversely, the low δ13C of S. chilense, associated with high KLeaf and gw, would be consistent with a productive, water-spender strategy [scenario 1 in [22]). At this point, it has been argued that the leaf diffusion of CO2 and H2O share some common pathways, and hence KLeaf and gm are correlated, but this is still a matter of debate [11,12,20,21,38]. Although the KLeaf of S. peruvianum was not statistically lower than S. lycopersicum, it was more limited hydraulically, according to the higher Lss, and slightly lower mVD. Besides a direct relationship between KLeaf and gm, Ferrio et al. [11] also reported a negative association between Lss and gm in grapevine, further suggesting that mesophyll limitations may be responsible for the low δ13C observed in S. peruvianum. Notably, MVD and mVD were inversely correlated across species, evidencing a tradeoff between water transport (high MVD and MVD/mVD), and reducing mesophyll limitations to gas exchange (high mVD), contradicting the general trends described by Brodribb et al. [5]. Again, this suggests that, for this particular set of species, water transport capacity and WUEi responded independently to selective pressures. Most likely, this involves different combinations of mesophyll and stomatal limitations [31], which encourages further research on the links between gm and KLeaf across tomato wild relatives.
4.3. Potential and Limitations of Water Isotopes as Indicators of Hydraulic Constraints
Although the observed trends in KLeaf and Lss partly agree with our hypothesis (e.g., in the case of S. peruvianum), our results confirm that this association is not as straightforward as originally proposed. Other studies did not find a clear association between Lss and KLeaf [39], or even discuss the existence of a causal association between them, attributing it to methodological limitations and modeling assumptions [17,40]. One of these limitations is that evaporative gradients within the leaf may not be restricted to the mesophyll [41]. To solve this issue, the Farquhar–Gan model [41] considered not only a Péclet effect in the mesophyll, but also radial and longitudinal Péclet effects within the xylem network, which cause a progressive enrichment of leaf water from the midvein to the leaf margin. According to this model, major veins act as a sink of enriched water from minor veins, and these in turn act as a sink of enriched water from the mesophyll. Under the assumption that the Péclet effect is different for each leaf tissue (major and minor veins, mesophyll), Lss could be related to anatomical traits such as vein density, just because leaf vein fraction influences the Péclet effect and thus the overall isotopic enrichment in leaf water [21,40]. However, it should be noted that in our study Lss showed only a weak negative correlation with MVD, contrary to what would be expected if Lss variations were driven by the fraction of water in leaf veins, as described in [40]. In particular, the high Lss values in S. peruvianum could not be explained by the (intermediate) values in vein density. Furthermore, within this species, Lss was negatively correlated with KLeaf (rs = −0.94, p = 0.017), whereas vein traits were not significantly correlated with Lss and KLeaf (p = 0.136–1.000). Hence, despite the above-mentioned limitations, our study suggests that Lss may still reflect differences in extra-xylematic water pathways, beyond anatomical effects. In this regard, this association deserves further exploration in a broad range of species and environments, e.g., taking advantage of recent advances in isotope laser spectroscopy, which allow non-destructive, continuous measurements of isotope fractionations within the leaves [21].
5. Conclusions
We confirmed our first hypothesis (more conservative water use in wild relatives) only for S. peruvianum, which shows a clear water-saving strategy, whereas S. chilense performs as a typical water spender. Although both species can grow in dry habitats, these strategies reflect adaptations to different levels of atmospheric water demand. Interestingly, vein density cannot explain the low KLeaf of S. peruvianum, which is mostly attributable to high resistances in mesophyll water pathways, as suggested by the high Lss. Our second hypothesis (higher WUEi in wild relatives) was rejected, and indeed the domesticated tomato showed the highest WUEi. In the case of S. chilense, the low WUEi is consistent with high stomatal conductances and a water-spender strategy. Conversely, the low WUEi in the water-saving S. peruvianum points towards the mesophyll limitations of photosynthesis, indirectly evidenced through the unexpectedly low δ13C and the large effective pathlength Lss. From a methodological point of view, we support the use of Lss to assess changes in the mesophyll components of KLeaf, independent from vein density, as was the case for S. peruvianum.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13030525/s1, Supplementary Table S1.
Author Contributions
Conceptualization, D.B.-A. and J.P.F.; methodology, D.B.-A. and J.P.F.; formal analysis, D.B.-A. and J.P.F.; resources, G.T. and J.P.F.; writing—original draft preparation, D.B.-A.; writing—review and editing, J.P.F. and G.T.; funding acquisition, J.P.F. All authors have read and agreed to the published version of the manuscript.
Funding
J.P.F. was supported by VRID-216.111.062-1 (Universidad de Concepción, Chile) and Grupo de Referencia H09_20R (Gobierno de Aragón, Spain).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
We thank Felipe Aburto and Alejandro Atenas (Laboratorio de Investigación en Suelos, Aguas y Bosques, Univ. Concepción) and Pilar Sopeña (Laboratorio de Silvicultura, Universitat de Lleida) for their assistance with isotope analysis. We thank Marcela Rodriguez and Roberto A. Rodríguez Ríos (Univ. Concepción) for the use of binocular microscopes and for providing materials for staining, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Passport information from wild tomato accession.
Table A1.
Passport information from wild tomato accession.
| Accession Number | Taxon | Latitude | Longitude | Elevation (m.a.s.l) | Location-Country |
|---|---|---|---|---|---|
| Q958 | S. peruvianum | 18°14′31.9″ | 70°9′8.7″ | 355 | Tarapaca, Chile |
| Q966 | S. chilense | 18°16′52.7″ | 69°18′50.4″ | 3290 | Tarapaca, Chile |
References
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Skelton, R.P.; West, A.G.; Dawson, T.E. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc. Nat. Acad. Sci. USA 2015, 112, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Chatelet, D.S.; Pasquet-kok, J.; Rawls, M.; Donoghue, M.J.; Edwards, E.J.; Sack, L. Hydraulic basis for the evolution of photosynthetic productivity. Nat. Plants 2016, 2, 16072. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Field, T.S.; Jordan, G.J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef]
- McKown, A.D.; Cochard, H.; Sack, L. Decoding leaf hydraulics with a spatially explicit model: Principles of venation architecture and implications for its evolution. Am. Nat. 2010, 175, 447–460. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of leaf hydraulic conductance with dehydration: Relationship to leaf size and venation architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef]
- Cochard, H.; Nardini, A.; Coll, L. Hydraulic architecture of leaf blades: Where is the main resistance? Plant Cell Environ. 2004, 27, 1257–1267. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Cuntz, M.; Offermann, C.; Siegwolf, R.; Saurer, M.; Gessler, A. Effect of water availability on leaf water isotopic enrichment in beech seedlings shows limitations of current fractionation models. Plant Cell Environ. 2009, 32, 1285–1296. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Pou, A.; Florez-Sarasa, I.; Gessler, A.; Kodama, N.; Flexas, J.; Ribas-Carbó, M. The Péclet effect on leaf water enrichment correlates with leaf hydraulic conductance and mesophyll conductance for CO2. Plant Cell Environ. 2012, 35, 611–625. [Google Scholar] [CrossRef]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriquí, M.; Díaz-Espejo, A.; Douthe, C.; Dreyer, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Lloyd, J. Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In Stable Isotopes and Plant Carbon-Water Relations; Ehleringer, J.R., Hall, A.E., Farquhar, G.D., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1993; pp. 47–70. [Google Scholar] [CrossRef]
- Barbour, M.M.; Farquhar, G.D. Do pathways of water movement and leaf anatomical dimensions allow development of gradients in H218O between veins and the sites of evaporation within leaves? Plant Cell Environ. 2004, 27, 107–121. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Barbeta, A.; Bush, R.T.; Eichstaedt, R.; Ferrio, J.P.; Flanagan, L.B.; Gessler, A.; Martín-Gómez, P.; Hirl, R.T.; Kahmen, A.; et al. Do 2H and 18O in leaf water reflect environmental drivers differently? New Phytol. 2022, 235, 41–51. [Google Scholar] [CrossRef]
- Cuntz, M.; Ogée, J.; Farquhar, G.D.; Peylin, P.; Cernusak, L.A. Modelling advection and diffusion of water isotopologues in leaves. Plant Cell Environ. 2007, 30, 892–909. [Google Scholar] [CrossRef]
- Loucos, K.E.; Simonin, K.A.; Song, X.; Barbour, M.M. Observed relationships between leaf H218O Péclet effective length and leaf hydraulic conductance reflect assumptions in Craig-Gordon model calculations. Tree Physiol. 2015, 35, 16–26. [Google Scholar] [CrossRef]
- Hommel, R.; Siegwolf, R.; Saurer, M.; Farquhar, G.D.; Kayler, Z.; Ferrio, J.P.; Gessler, A. Drought response of mesophyll conductance in forest understory species–impacts on water-use efficiency and interactions with leaf water movement. Physiol. Plant. 2014, 152, 98–114. [Google Scholar] [CrossRef]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef]
- Barbour, M.M. Understanding regulation of leaf internal carbon and water transport using online stable isotope techniques. New Phytol. 2017, 213, 83–88. [Google Scholar] [CrossRef]
- Fardusi, M.J.; Ferrio, J.P.; Comas, C.; Voltas, J.; Resco de Dios, V.; Serrano, L. Intra-specific association between carbon isotope composition and productivity in woody plants: A meta-analysis. Plant Sci. 2016, 251, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Gyenge, J.; Fernández, M.E.; Sarasola, M.; Schlichter, T. Testing a hypothesis of the relationship between productivity and water use efficiency in Patagonian forests with native and exotic species. For. Ecol. Manag. 2008, 255, 3281–3287. [Google Scholar] [CrossRef]
- Araus, J.L.; Villegas, D.; Aparicio, N.; Del Moral, L.; El Hani, S.G.; Rharrabti, Y.; Ferrio, J.P.; Royo, C. Environmental factors determining carbon isotope discrimination and yield in durum wheat under Mediterranean conditions. Crop Sci. 2003, 43, 170–180. [Google Scholar] [CrossRef]
- Voltas, J.; Chambel, M.R.; Prada, M.A.; Ferrio, J.P. Climate-related variability in carbon and oxygen stable isotopes among populations of Aleppo pine grown in common-garden tests. Trees 2008, 22, 759–769. [Google Scholar] [CrossRef]
- Sack, L.; Tyree, M.T.; Holbrook, N.M. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol. 2005, 167, 403–413. [Google Scholar] [CrossRef]
- Du, Q.; Jiao, X.; Song, X.; Zhang, J.; Bai, P.; Ding, J.; Li, J. The response of water dynamics to long-term high vapor pressure deficit is mediated by anatomical adaptations in plants. Front. Plant Sci. 2020, 11, 758. [Google Scholar] [CrossRef]
- Moyle, L.C.; Muir, C.D. Reciprocal insights into adaptation from agricultural and evolutionary studies in tomato. Evol. Appl. 2010, 3, 409–421. [Google Scholar] [CrossRef]
- Bauchet, G.; Causse, M. Genetic diversity in Tomato (Solanum lycopersicum) and its wild relatives. In Genetic Diversity in Plants; InTech: London, UK, 2012; pp. 161–162. [Google Scholar] [CrossRef]
- Tapia, G.; Méndez, J.; Inostroza, L. Different combinations of morpho-physiological traits are responsible for tolerance to drought in wild tomatoes Solanum chilense and Solanum peruvianum. Plant Biol. 2016, 18, 406–416. [Google Scholar] [CrossRef]
- Muir, C.D.; Hangarter, R.P.; Moyle, L.C.; Davis, P.A. Morphological and anatomical determinants of mesophyll conductance in wild relatives of tomato (Solanum sect. Lycopersicon, sect. Lycopersicoides; Solanaceae). Plant Cell Environ. 2014, 37, 1415–1426. [Google Scholar] [CrossRef]
- Peralta, I.E.; Spooner, D.M. Classification of wild tomatoes: A review. Kurtziana 2000, 28, 45–54. [Google Scholar]
- Chetelat, R.; Pertuze, R.; Faundez, L.; Graham, E.; Jones, C. Distribution, ecology and reproductive biology of wild tomatoes and related nightshades from the Atacama Desert region of northern Chile. Euphytica 2009, 167, 77–93. [Google Scholar] [CrossRef]
- Palacio, S.; Azorín, J.; Montserrat-Martí, G.; Ferrio, J.P. The crystallization water of gypsum rocks is a relevant water source for plants. Nat. Commun. 2014, 5, 4660. [Google Scholar] [CrossRef]
- Martín-Gómez, P.; Barbeta, A.; Voltas, J.; Peñuelas, J.; Dennis, K.; Palacio, S.; Dawson, T.E.; Ferrio, J.P. Isotope-ratio infrared spectroscopy: A reliable tool for the investigation of plant-water sources? New Phytol. 2015, 207, 914–927. [Google Scholar] [CrossRef]
- Dongmann, G.; Nurnberg, H.W.; Forstel, H.; Wagener, K. On the enrichment of H218O in the leaves of transpiring plants. Radiat. Environ. Biophys. 1974, 11, 41–52. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic Composition of Plant Carbon Correlates With Water-Use Efficiency of Wheat Genotypes. Funct. Plant Biol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Loucos, K.E.; Simonin, K.A.; Barbour, M.M. Leaf hydraulic conductance and mesophyll conductance are not closely related within a single species. Plant Cell Environ. 2017, 40, 203–215. [Google Scholar] [CrossRef]
- Kahmen, A.; Simonin, K.; Tu, K.; Goldsmith, G.R.; Dawson, T.E. The influence of species and growing conditions on the 18-O enrichment of leaf water and its impact on ‘effective path length’. New Phytol. 2009, 184, 619–630. [Google Scholar] [CrossRef]
- Holloway-Phillips, M.; Cernusak, L.A.; Barbour, M.; Song, X.; Cheesman, A.; Munksgaard, N.; Stuart-Williams, H.; Farquhar, G.D. Leaf vein fraction influences the Péclet effect and 18O enrichment in leaf water. Plant Cell Environ. 2016, 39, 2414–2427. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Gan, K.S. On the progressive enrichment of the oxygen isotopic composition of water along leaves. Plant Cell Environ. 2003, 26, 801–819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).