Abstract
Grape pomace is a fibrous food with satisfactory quantities of residual sugars. It meets the desirable characteristics for conservation in the form of silage for later use in animal feed, mainly for ruminant herbivores. Fresh grape pomace was subdivided into three treatment groups: grape pomace as a control, grape pomace treated with an inoculum of lactic acid bacteria, and grape pomace treated with zeolite. The treatments were performed in micro-silos over 90 days. There was a significant change (p < 0.05) in the chemical characteristics, content of biologically active compounds, and fermentative characteristics during the silage of all treatments. After 30, 60 and 90 days of ensiling, silages treated with inoculum and zeolite had better fermentation quality indicated by significantly (p < 0.05) lower pH and ammonia-nitrogen contents compared with those of the control. Also, the additives have decreased the total polyphenols and tannins for 97% in average which confirmed that lactic acid bacteria and zeolite positively effect on the degradation of polyphenols and tannins in grape pomace silage. The Flieg score was calculated and the values were above 80% what refers to excellent silage. In conclusion, our results suggest that inoculant and zeolite supplementation improves the quality of grape pomace silage for later use in animal feed.
1. Introduction
The wine industry produces a large amount of unprocessed plant waste, known as grape pomace. Approximately 25% of the total mass of grapes used in wine production ends up as biowaste, which is, globally, around 9 million tons of waste every year [1]. In Croatia, the wine industry produces 15,000 tons of solid waste annually. A wide distribution of the leading grape variety in Croatia, called Graševina, is responsible for producing the largest share of wine waste [2]. Grape pomace consists of skins, seeds, and stems, and is considered a valuable by-product because grapes are rich in biologically active compounds. Improperly disposed grape pomace presents an environmental risk in terms of soil and water pollution. To reduce the amount of waste, a small portion of pomace is used for oil production or polyphenol extraction, or is processed into animal feed [3]. However, grapes are a seasonal fruit and large quantities of pomace are generated in a short period, making it difficult to store in a way that ensures the maintenance of desirable chemical characteristics. To preserve the stability of fresh plant material, the biomass can be dried; for example, vacuum drying is proven to be an effective method for conserving fresh biomass. However, vacuum drying requires additional equipment, which increases the final cost of storage, especially on a large industrial scale [2]. For the said reasons, ensilage is another method that can be applied in such cases [4]. Ensilage is a fermentative process used for the conservation of by-products. Lactic acid bacteria facilitate the fermentation of moist biomass under anaerobic conditions. As a result of converting water-soluble carbohydrates into organic acids, the pH value rapidly drops, which inhibits the growth of undesirable micro-organisms, such as yeast and mold. Therefore, the further decomposition and deterioration of the biomass are prevented and long-term storage under those conditions is provided [5]. When grape pomace is ensiled, it can produce high-quality feed for ruminant animals, such as cows or sheep. The fermentation process can produce a valuable by-product called biogas, which can be used as a renewable energy source. This can be economically beneficial for wineries that have a lot of disposed grape pomace. In addition to its potential as a feed source, the ensiling of grape pomace offers ecological advantages. Ensiling contributes to a reduction in agricultural waste by minimizing the amount of pomace left in the fields. To prevent greenhouse gas production during silage, it is advisable to monitor lactate degradation and butyrate formation by clostridia, as the production of methane can adversely affect the silage quality. The quantities of greenhouse gas emissions also depend on factors such as the forage type, dry matter concentration, and silage additives [6].
Grape pomace is especially desirable for ensiling because of its already low pH value and high content of polyphenols and lipids, which prevent protein degradation [7]. Polyphenolic compounds have already attracted a large market interest as antioxidants that can be used as additives for various applications and that exert numerous health-promoting effects on humans [8]. In contrast, polyphenol effects have been only recently studied in animal diets, and effects such as an enhanced oxidative stability of meat, the modulation of intestinal microbiota, and a reduction in synthetic antioxidant additions such as vitamin E have been reported [9]. Monitoring the total polyphenolic content during ensilage is essential for the quality control of the process. Polyphenols, being natural antioxidants, play a crucial role in enhancing the overall quality and nutritional value of silage. Variations in the polyphenolic content can serve as indicators of potential issues, such as spoilage during storage or excessive heating in the process, due to their degradation [10]. Moreover, polyphenols aid in optimizing the nutritional quality of the silage for specific animal diets [9]. Tannins are naturally occurring phenolic compounds that may exhibit positive effects on human health and ruminant performance. Nevertheless, concentrations higher than 50 g kg−1 dry matter (DM) generally decrease feed intake and reduce nutrient availability [11]. Since white grapes contain much higher concentrations of tannins, decreasing their concentration during silage is desirable. Tannins are capable of binding proteins, minerals, and carbohydrates, which can negatively affect the nutritional value of the ensiled biomass [12]. The main goal of ensilage is to improve the microbiological and nutritional quality of by-products [13].
Various types of additives can be applied early in the process to ensure that fermentation occurs appropriately and to improve the silage quality. These additives can be chemical or microbiological (also known as starter cultures or inoculants), with microbiological additives being more commonly used [14]. Chemical additives such as acids and salts improve the aerobic stability of the process. Urea can also be beneficial by increasing the protein content and reducing the fiber in silage, making it more nutritious [13]. The inoculant is a product composed of strains of one or more species of micro-organisms, and it must be viable at the time of use. The main positive effects of the use of these micro-organisms in silages are a reduction in dry matter losses, an increase in the production of microbial metabolites of interest, the inhibition of undesirable micro-organisms, and an improvement in the microbiological and nutritional quality [14]. The benefits of inoculated silage include the inhibition of pathogenic micro-organisms, lower concentrations of toxins and undesirable compounds, interactions with ruminal micro-organisms, and changes in rumen fermentation (e.g., a reduction in methane production) [15]. Recently, much attention has been directed towards zeolites—additives from the group of mycotoxin adsorbents that considerably reduce the toxic effects of the metabolic products of mildew, which are present in many feeds. The results of previous research indicated that zeolite had a positive effect on the quality of corn, alfalfa, red clover, and ryegrass silages as well as the silages of sugar beet pulp, in the way that they bound moisture and improved the activity of bacteria in the silages [16]. Irrespective of the ensilage approach applied, the main goal of ensilage is to improve the microbiological and nutritional quality of by-products [13].
The objective of this study was to evaluate the impact of different additives on the quality of grape pomace silage. Briefly, Graševina grape pomace was ensiled over 90 days in three different experiments: grape pomace as a control, grape pomace with an inoculum of lactic acid bacteria, and grape pomace with zeolite. After 30, 60, and 90 days of ensiling, chemical analyses of the grape pomace were performed. The following parameters were determined: the dry matter (DM), water-soluble carbohydrate (WSC), crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), total polyphenol (TP), and total tannin (TT) content. Furthermore, the fermentative characteristics of the grape pomace were determined, such as the pH, silage acids (lactic, acetic, and butyric acids), the ammonia–nitrogen (NH3-N) content, and the Flieg score, which describes the silage quality.
2. Materials and Methods
2.1. Materials
Graševina grape pomace (Vitis vinifera) was obtained from the Croatian native grape cultivar Kutjevo d.d. in September 2021. All solvents were purchased from Kemika d.d. (Zagreb, Croatia) and were of HPLC grade.
2.2. Silage of Grape Pomace
After harvest and wine production, the grape pomace was used for ensilage. Three different treatments were carried out: grape pomace as a control, grape pomace with an inoculum of lactic acid bacteria (0.0002% w/w) (SIL-ALL 4 × 4+ FVA, Nutritech, Auckland, New Zealand), and grape pomace with zeolites (0.2% w/w) (zeolite clinoptilolite, Heiltropfen, London, UK). The commercial inoculum comprised 4 bacterial strains (Lactobacillus plantarum, Pediococcus acidilactici, Pedioccus pentosaceus, and Propionibacterium acidipropionici), 4 enzymes (α-amylase, cellulase, β-glucanase, and xylanase), sucrose, and silica. The additives were mixed homogenously with the grape pomace.
In detail, each treatment was carried out in three replicates of 0.5 kg each, and they were packed into air-tight polyethylene bags. A total of 27 bags (3 treatments × 3 ensiling days × 3 replicates) were stored at a temperature of 18–20 °C. At 30, 60, and 90 days of ensiling, 9 bags (3 treatments × 3 replicates) were opened and the chemical composition and fermentative characteristics were analyzed. Initial fresh grape pomace samples were taken before ensiling and the same analysis was carried out.
2.3. Extract Preparation and Sample Analysis
The amount of total dry matter and the residual moisture content of Graševina grape pomace were determined using a conventional dryer (Instrumentaria d.d., Sesvete, Croatia) at 103 ± 2 °C with a pressure ≤100 mmHg (13.3 kPa) [2]. The WSCs were extracted in distilled water in a ratio of 1:10. The extraction was performed for 2 h in a water bath at 70 °C, and after the extraction, the mixture was filtered through a filter paper. The WSC content in the supernatant was determined using the phenol–sulfuric method described by Nielsen [17]. The total nitrogen (TN) was determined using an elemental analyzer with a spectrophotometer (LaboMed UV-VIS, Los Angeles, CA, USA), and the crude protein content was calculated via multiplying the TN by 6.25. The NDF and ADF were determined according to Van Soest et al. [18]. The extraction of phenolic compounds was carried out using an 80% (w/w) methanol solution according to the method described by Palma and Barroso [19], with some modifications. After the extraction, the mixture was filtered through a filter paper and the total phenolic (TP) content was determined using the Folin–Ciocalteu method [20]. The absorbance was measured at 760 nm (Specord 50 PLUS UV/VIS spectrophotometer, Analytik, Jena, Germany) and the results were expressed as the grams of gallic acid equivalent per kilogram of dry matter (g GAE kg−1 DM). The extraction of tannins was carried out according to the method described by Hagerman et al. [21] using 70% (w/w) acetone. After the extraction, the mixture was filtered through a filter paper and the total tannin (TT) content was determined using the Bate-Smith method as described by Ribéreau-Gayon et al. [22]. The absorbance was measured at 550 nm (Specord 50 PLUS UV/VIS spectrophotometer, Analytik, Jena, Germany) and the results were expressed as the tannin mass over the mass of dry matter (g kg−1). For the determination of pH, the extract was prepared as described by Ni at el. [23] and the pH was measured using a digital pH meter (Mettler Toledo, Greifensee, Switzerland). The organic acid concentrations were determined using high-performance liquid chromatography (HPLC) [2]. The NH3-N content was analyzed using Nessler’s method as described by Merck [24]. All the analyses were performed in triplicate. The parameter of silage quality, the Flieg score, was calculated using the following equation [25]:
Flieg score = [220 + (DM − 15)] − 40∙pH
2.4. Statistical Analysis
The statistical analyses were performed using Statistica 13.0 (Tibco Software Inc., Palo Alto, Santa Clara, CA, USA). Differences between the means of the chemical characteristics and the fermentative characteristics during the grape pomace silage were tested using a two-way analysis of variance (two-way ANOVA) at the significance level of p < 0.05, followed by Tukey’s honestly significant difference (HSD) test. The SS (sum of squares) in total, the SS among subjects, the SS between subjects A and B, the MS (mean square) for subjects A and B, the df (degrees of freedom) for subjects A and B, and the F-ratio for subjects 1 and 2 were the parameters used for the two-way ANOVA. A two-way ANOVA is a statistical method that tests the group means of two or more factors. This type of analysis examines groups that have been divided into multiple categories based on the values of both independent variables. The formulas for two-way ANOVA are given in Table 1:

Table 1.
Formulas for two-way ANOVA.
3. Results and Discussion
3.1. Effect of Additives and Storage Time on the Chemical Characteristics of Grape Pomace Silage
The main characteristics of fresh grape pomace and the effect of additives and storage time on the chemical composition of grape pomace silage are presented in Table 2. In general, there was a significant change in the chemical characteristics after 30 days of the silage of grape pomace in all the treatments in comparison to fresh grape pomace; with a longer silage period, the chemical characteristics did not change considerably, and the values were consistent until the end of the process.

Table 2.
Chemical composition of fresh and ensiled grape pomace. Results are presented as average value ± standard errors.
One of the factors that affected the ensiling process was the DM content of the plant. According to the literature, to obtain good-quality silage, the interval needs to be between 280 and 400 g kg−1 to avoid high nutrient losses [4]. As these authors have reported, dry matter values within this range contributed to a reduction in nutrient losses, which can be leached together with excess moisture. The grape pomace used in our experiment for ensiling showed an adequate dry matter content, and at the end of all treatments, the values were higher than 400 g kg−1, which indicated no significant nutrient loss. Also, an increase in the dry matter content was noticed in the treatment with zeolite, and similar results have been reported by Herremans et al. [16]. In this previous study, they found that an application of zeolite (1%) increased the content of dry matter in ryegrass and red clover silage compared to a control. Good fermentation probably results in total dry matter losses of less than 10 to 12%, and poor fermentation coupled with poor storage conditions results in total dry matter losses of greater than 20%. Losses of dry matter may also come from runoff, oxidation, and the loss of volatile organic compounds [26]. In this work, the control and grape pomace with an inoculum had dry matter losses of 8.75% and 12.00%, respectively, which indicates good fermentation. On the other hand, the experiment with zeolite showed an increase in dry matter of 6.58%, which was expected and is in line with the literature.
The concentration of WSCs during the silage was influenced by factors such as the temperature, moisture content, fertilizers, additives, and time of ensiling. In the ensiling process, carbohydrates are the key source of fermentable substrates. In an anaerobic environment, water-soluble carbohydrates are converted into organic acids with the help of lactic acid bacteria [27]. The initial value of WSCs was 54.83 g kg−1 DM, which corresponds to the values reported in the literature [28]. As expected, the WSC concentrations decreased rapidly in all the grape pomace silages over the first 30 days of ensiling (Table 2), especially in the experiment with lactic acid bacteria. The higher WSC content in the control silage (48.13 g kg−1 DM) and lower content in the inoculant and zeolite treatments 30 days after the start of the silage could be explained by an inhibition of microbial growth by the tannins in the grape pomace silage. After 90 days of ensiling, the final WSC contents were 13.40, 11.78, and 7.88 g kg−1 DM for the control, inoculum, and zeolite treatments, respectively. The decrease in the WSC content was mainly due to oxygen consumption by plant cells in the silages in the early stage of ensiling, followed by the fermentation of water-soluble carbohydrates by micro-organisms into lactic acid; as a consequence, the pH decreased [29]. Furthermore, the CP content in fresh grape pomace before the silage process was 96.25 g kg−1 DM, and the approximate values have been reported by some authors [10,28]. There were no significant changes in the CP values after 90 days of ensilage. Similar results were obtained by Massaro Junior et al. [4] and Fitri et al. [30]. Herremans et al. [16] found that an application of zeolite (1%) increased the content of crude protein in red clover silage compared to the control.
The nutritional value of grape pomace silage is significantly lower than that of other forages, resulting from a high proportion of structural carbohydrates. Generally, there is great variation in the ADF and NDF values of grape pomace in the literature. For the ADF, the values are between 312.00 and 593.10 g kg−1 DM, and for the NDF, the values are between 439.70 and 631.00 g kg−1 DM [4,28], which correspond to the values obtained in this experiment. A detergent fiber analysis showed that the content of ADF and NDF in grape pomace silages increased after 90 days of the silage process compared to the content before the ensilage. Alipour et al. [31] and Spanghero et al. [32] also found an increase in the ADF and NDF content in grape pomace 30 and 45 days after the start of ensilage. Certain inoculants contain bacteria that could secrete specific enzymes, mainly cellulases and xylanases, that may contribute to degrading these structures and increasing fiber digestion [33].
3.2. Effect of Additives and Storage Time of Grape Pomace Silage on Biologically Active Compounds
Polyphenols and tannins are biologically active compounds that may provide positive impacts on human health due to their anti-tumor, anti-inflammatory, anti-viral, anti-fungal, anti-bacterial, anti-parasitic, and cardio-protective effects [8]. In past decades, the polyphenol valorization of agro-food industry by-products emerged in order to provide added value to unexploited by-products. In addition, polyphenol’s global market is ever-growing, since these compounds may be used as additives to improve a product’s potential health benefits across a wide range of industries, such as the cosmetic, pharmaceutical, and food industries [7,32]. On the other hand, an elevated polyphenol content in silage may have a negative impact on animal production. Polyphenols may inhibit the activity of certain digestive enzymes and modulate the gut microbiota, thus negatively affecting digestion. Nevertheless, polyphenols have also been reported to improve animal gut health due to their antioxidant and anti-inflammatory properties [34]. Due to all the aforesaid results, their preservation during ensiling is crucial. Therefore, during grape pomace ensiling, the TP and TT contents were closely monitored.
The TP content in fresh Graševina grape pomace was 58.80 g kg−1 DM (Figure 1a). Antonić et al. [3] reported that the TP content in white grape pomace varies from 2.80 to 87.00 g kg−1 DM. The content of polyphenols decreased rapidly in all the grape pomace silages over the first 30 days of ensiling. On average, 82.24% of the polyphenols were degraded during the first 30 days. At the end of the experiment, 97.67% of the polyphenols had been degraded, on average, in all the monitored treatments. Winkler et al. [35], on the other hand, noted that 50% of the TPs in grape pomace ensiled for 56 days was degraded. Of the three silage treatments, after 90 days, the lowest TP degradation was seen in the treatment with zeolite (96.84%), while the control and inoculum treatments exhibited lower TP retention (98.21 and 97.96% of TPs degraded, respectively). The degradation of polyphenols has also been reported in studies by Alipour and Rouzbehan [31] and Winkler et al. [35]. This degradation generally occurs due to polyphenol polymerization and oxidation [35].
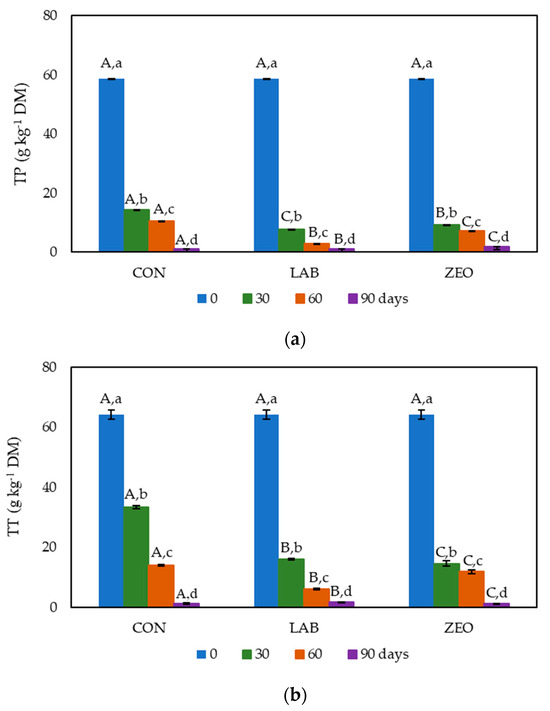
Figure 1.
TP (a) and TT (b) content during 90 days of ensiling. Results are expressed as average values ± standard errors (CON-control; LAB-inoculum of lactic acid bacteria; ZEO-zeolite); n = 3. A–C The same superscript capital letters within a row denote no significant differences (p > 0.05) between the values obtained for the different treatments regarding the control sample according to Tukey’s ANOVA. a–d The same superscript lowercase letters within a column denote no significant differences (p > 0.05) between values obtained for different days of storage according to Tukey’s ANOVA.
The TT content was monitored over the 90 days of grape pomace ensiling (Figure 1b). In fresh grape pomace, the TT content was 64.16 g kg−1 DM. A similar TT content in white grape pomace has already been reported in the literature [36]. As in the case of TPs, the most considerable number of tannins were degraded during the first 30 days of ensiling (66.71%, on average). This was slightly less evident in the control, where it was 47.92%. Nevertheless, after 90 days, the control treatment, together with the zeolite treatment, had the lowest TT content (1.24 and 1.19 mg g−1 DM, respectively). At the end of ensiling, only 2.15% of the tannins, on average, were preserved. Condensed tannin degradation during grape pomace ensiling has also been reported by Alipour and Rouzbehan [31], whereby after 30 days of ensiling, only 27.56% of the tannins remained in the grape pomace silage. The presented results (Figure 1) indicate that ensiling is not a suitable method for polyphenol and tannin preservation. Therefore, other methods for biologically active compound conservation should be applied.
Sokač et al. [2] reported a high stability for polyphenols and tannins during grape pomace drying. The authors noted that grape pomace drying at 70 °C ensured the highest level of secondary metabolite retention. Therefore, drying could be an acceptable alternative to ensure secondary metabolite stability in grape pomace. Despite the above-mentioned polyphenol and tannin degradation in grape pomace silage, ensiling has been reported to increase the digestibility of grape pomace, and thus, improve its feed value [9]. Grape pomace is generally considered to be a low-energy source when compared to the common forage used in animal diets. Nevertheless, ensiling may improve its digestibility, ensuring a year-round feed source [37]. The application of grape pomace as feed does not bring an extremely high added value to the whole wine-making process; however, it decreases the amount of wasted by-product and ensures its complete usage.
3.3. Effect of Additives and Storage Time on the Fermentative Characteristics of Grape Pomace Silage
Table 2 illustrates the dynamics of the fermentation quality of grape pomace silage during ensiling. Generally, the fermentation quality of silages improved by a low pH, a high lactic acid content, and a low level of ammonia-N can be achieved through the treatment of silage with different additives, including inoculants, chemical matter, and enzymes [12,21,26]. The pH of fresh grape pomace was 5.88, and after 90 days of the ensiling process, the value decreased. At the end of the silage, the pH value in the control treatment and the treatment with LAB was 4.06, and in the treatment with zeolite, it was 4.27. The decrease in the pH was expected due to the fermentation of water-soluble carbohydrates by lactic acid bacteria (LAB), and as a result, organic acids (mainly lactic acid) were produced [29]. The pH value of silage is an important index for evaluating the success of silage; well-fermented silage should have a pH of 3.80~4.20 [38]. The amount of acid required to decrease the original pH of 6 to a stable pH depends on the contents of the silage dry matter, the water-soluble carbohydrate content, and the crude protein content. Furthermore, forages with a high dry matter content are fermented at a slower rate than forages with low dry matter because of low water activity [39]. There are different opinions on the suitability of pH values for silage: some authors define a pH value of 4.2 as the upper threshold for a positive assessment of silage [40], while other researchers state that the final pH is not important; what matters is the decreasing rate, as this parameter is more important for inhibiting a secondary fermentation occurrence [4].
Lactic acid should be the primary acid in good silages. This acid is stronger than the other acids present in silage (acetic, propionic, and butyric acids), and as mentioned before, it is usually responsible for the decrease in the pH value. Lactic acid should represent at least 65 to 70% of the total silage acids in a good silage [26]. In this study, the lactic acid content before the silage of grape pomace was 28.35 g kg−1 DM, and after 30 days, the values in all the treatments decreased. Moreover, after 60 days of silage, an increase in lactic acid was noted and, finally, it decreased until the end of the process (Table 3). The content of lactic acid was higher in the treatment with zeolite than in the control and inoculum treatments. Also, Đorđević et al. [41] found in their research that the application of zeolite increased the content of lactic acid in corn, alfalfa, and perennial ryegrass silage compared to a control.

Table 3.
Fermentative characteristics of the grape pomace silage. Results are presented as average value ± standard errors.
When lactic acid decreases, acetic acid increases (Table 3), which can be explained by the conversion of lactic acid to acetic acid with a prolonged ensilage time, in accordance with Ni et al. [23]. Der Bedrosian et al. [42] reported that some strains of lactic acid bacteria can utilize lactic acid anaerobically when sugar is limiting, which reduces the lactic acid concentration and increases the acetic acid concentration. Acetic acid is the acid with the second highest concentration in silage, usually ranging from 1 to 3%. The acetic acid content in silage is also affected by the dry matter content [38], while the acetic acid content decreases as the dry matter content increases, as also confirmed by the results of Juráček et al. [43]. Acetic acid is a promoter of aerobic stability during the ensiling process [44] and an effective inhibitor of fungi [45].
The butyric acid content was not determined in any of the grape pomace silage treatments. Furthermore, butyric acid is undesirable in silage due to its inhibitory effect on lactic acid bacteria and yeast growth. However, the presence of butyric acid in a range between 0.1% and 0.6% would not affect the silage quality [46]. Belém et al. [28] reported that the low values observed for butyric acid may be related to the low ammoniacal nitrogen in the silage, indicating low Clostridium spp. activity and a high silage quality. Besides the presence of butyric acid and lower-than-normal concentrations of lactic acid, clostridial silages are often characterized by a higher-than-normal pH and higher-than-normal concentrations of acetic acid and NH3-N [38].
Furthermore, the NH3-N concentration is a reliable indicator of protein degradation [25,44]. During the ensiling process, NH3-N accumulation can be explained by the activity of plant enzymes and fermentation by Clostridium and Enterobacter [47]. Usually, silage with high concentrations of NH3-N coupled with butyric acid may also have significant concentrations of other undesirable end products, such as amines, that may reduce animal performance [38]. Compared to the control, a significant reduction in ammonia was observed in the silages with the inoculant or zeolite opened after 30 and 60 days (Table 3). This may have been the result of the lower pH values, the activity of homofermentative lactic acid bacteria, or lactic acid production. Some authors [5,46] have also found that an inoculation with lactic acid bacteria or the use of zeolites significantly decreased the NH3-N levels compared with the controls. At the end of the silage, the lowest values of NH3-N were found for the treatment with zeolite in comparison with the control and the treatment with an inoculum (Table 3), which agrees with the literature [14,39].
Finally, the silage quality can be expressed by the Fleig score, which is the relationship between the dry matter content and the pH value of the silage [48]. The Flieg point was determined for all the treatments, and the value for the fresh grape pomace was 61.85. After the first 30 days of ensiling, the Flieg score increased significantly and the maximum values were achieved; these values were 141.74 for the control, 133.33 for the treatment with the inoculum of lactic acid bacteria, and 144.08 for the treatment with zeolite. At the end of the ensiling, the values decreased by 7.90%, on average, in comparison to the values determined 30 days after the process. The Flieg score had a value >80 for excellent silage, 61–80 for good, 41–60 for medium, 21–40 for weak, and 0–20 for poor silage [48,49]. Considering the mentioned scale and the calculated values for the Flieg score, in all the treatments, the silage was of a great quality. Similar results to those obtained in this work were reported by Zehra Saricicek et al. [25] for corn silage.
4. Conclusions
The present study illustrates the chemical and fermentative characteristics during grape pomace silage carried out in different treatments: control, with an inoculum of lactic acid bacteria, and with zeolite. After 30 days, the silages treated with additives had a better fermentation quality, as indicated by a significantly (p < 0.05) lower pH and NH3-N content and a higher lactic acid content compared with those of the control. Thus, our results suggest that inoculant and zeolite supplementation can improve the quality of grape pomace silage for its use in animal feed, mainly for ruminant herbivores.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13122264/s1, Table S1. p-values of two-way ANOVA of chemical composition of the fresh and the ensiled grape pomace; Table S2. p-values of two-way ANOVA of fermentative characteristics of the grape pomace silage.
Author Contributions
Conceptualization, I.R.R. and V.G.; methodology, I.R.R., D.U. and V.G.; software, V.G., A.J.T. and T.S.C.; validation, I.R.R., D.U., A.J.T. and T.J.; formal analysis, V.G., A.D., T.S.C. and A.P.; investigation, V.G., A.D., T.S.C. and A.P.; resources, I.R.R.; data curation, V.G., A.D., T.S.C. and A.P.; writing—original draft preparation, V.G., T.S.C., A.D. and A.P.; writing—review and editing, V.G., T.S.C., A.D., A.P., A.J.T., T.J., I.R.R. and D.U.; visualization, V.G., T.S.C., A.D. and A.P.; supervision, I.R.R. and T.J.; project administration, I.R.R.; funding acquisition, I.R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Union through the European Regional Development Fund, Competitiveness and Cohesion 2014–2020 (KK.01.1.1.07.0007).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and Supplementary Materials.
Acknowledgments
The authors warmly acknowledge Kutjevo d.d. (Kutjevo, Croatia) for providing the raw Graševina grape pomace biomass.
Conflicts of Interest
The co-author Pušek worked at the faculty at the time of performing the research. However, there are no potential commercial interests because this firm has nothing with the silage or any other similar work. The authors declare no conflict of interest.
References
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green Processing and Biotechnological Potential of Grape Pomace: Current Trends and Opportunities for Sustainable Biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Sokač, T.; Gunjević, V.; Pušek, A.; Tušek, A.J.; Dujmić, F.; Brnčić, M.; Ganić, K.K.; Jakovljević, T.; Uher, D.; Mitrić, G.; et al. Comparison of Drying Methods and Their Effect on the Stability of Graševina Grape Pomace Biologically Active Compounds. Foods 2022, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Massaro Junior, F.L.; Bumbieris Junior, V.H.; Zanin, E.; da Silva, L.d.D.F.; Galbeiro, S.; Pereira, E.S.; Neumann, M.; Mizubuti, I.Y. Effect of Storage Time and Use of Additives on the Quality of Grape Pomace Silages. J. Food Process. Preserv. 2020, 44, e14373. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Zhao, S.; Wang, Y. Lactobacillus Plantarum Inoculants Delay Spoilage of High Moisture Alfalfa Silages by Regulating Bacterial Community Composition. Front. Microbiol. 2020, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Schmithausen, A.J.; Deeken, H.F.; Gerlach, K.; Trimborn, M.; Weiß, K.; Büscher, W.; Maack, G.C. Greenhouse Gas Formation during the Ensiling Process of Grass and Lucerne Silage. J. Environ. Manag. 2022, 304, 114142. [Google Scholar] [CrossRef]
- Ke, W.C.; Yang, F.Y.; Undersander, D.J.; Guo, X.S. Fermentation Characteristics, Aerobic Stability, Proteolysis and Lipid Composition of Alfalfa Silage Ensiled with Apple or Grape Pomace. Anim. Feed Sci. Technol. 2015, 202, 12–19. [Google Scholar] [CrossRef]
- Cravotto, G.; Mariatti, F.; Gunjevic, V.; Secondo, M.; Villa, M.; Parolin, J.; Cavaglià, G. Pilot Scale Cavitational Reactors and Other Enabling Technologies to Design the Industrial Recovery of Polyphenols from Agro-Food by-Products, a Technical and Economical Overview. Foods 2018, 7, 130. [Google Scholar] [CrossRef]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling Grape Pomace With and Without Addition of a Lactiplantibacillus Plantarum Strain: Effect on Polyphenols and Microbiological Characteristics, In Vitro Nutrient Apparent Digestibility, and Gas Emission. Front. Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef]
- Alba-Mejía, J.E.; Dohnal, V.; Domínguez-Rodríguez, G.; Středa, T.; Klíma, M.; Mlejnková, V.; Skládanka, J. Ergosterol and polyphenol contents as rapid indicators of orchardgrass silage safety. Heliyon 2020, 9, e14940. [Google Scholar] [CrossRef]
- Peng, K.; Jin, L.; Niu, Y.D.; Huang, Q.; McAllister, T.A.; Yang, H.E.; Denise, H.; Xu, Z.; Acharya, S.; Wang, S.; et al. Condensed Tannins Affect Bacterial and Fungal Microbiomes and Mycotoxin Production during Ensiling and upon Aerobic Exposure. Appl. Environ. Microbiol. 2018, 84, e02274-17. [Google Scholar] [CrossRef]
- Fitri, A.; Obitsu, T.; Sugino, T. Effect of Ensiling Persimmon Peel and Grape Pomace as Tannin-Rich Byproduct Feeds on Their Chemical Composition and In Vitro Rumen Fermentation. Anim. Sci. J. 2021, 92, e13524. [Google Scholar] [CrossRef]
- Carvalho, B.F.; Sales, G.F.C.; Schwan, R.F.; Ávila, C.L.S. Criteria for Lactic Acid Bacteria Screening to Enhance Silage Quality. J. Appl. Microbiol. 2021, 130, 341–355. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage Review: Recent Advances and Future Uses of Silage Additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Han, H.; Wang, C.; Huang, Z.; Zhang, Y.; Sun, L.; Xue, Y.; Guo, X. Effects of Lactic Acid Bacteria-Inoculated Corn Silage on Bacterial Communities and Metabolites of Digestive Tract of Sheep. Fermentation 2022, 8, 320. [Google Scholar] [CrossRef]
- Herremans, S.; Decruyenaere, V.; Beckers, Y.; Froidmont, E. Silage Additives to Reduce Protein Degradation during Ensiling and Evaluation of In Vitro Ruminal Nitrogen Degradability. Grass Forage Sci. 2019, 74, 86–96. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual, 1st ed.; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Palma, M.; Barroso, C.G. Ultrasound-Assisted Extraction and Determination of Tartaric and Malic Acids from Grapes and Winemaking by-Products. Anal. Chim. Acta 2002, 458, 119–130. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Hagerman, A.E. Extraction of Tannin from Fresh and Preserved Leaves. J. Chem. Ecol. 1988, 14, 453–461. [Google Scholar] [CrossRef]
- Gayon, P.R.; Stonestreet, E. Dosage Des Tanins Du Vin Rouge et Determination de Leur Structure. Chim. Anal. 1966, 48, 188–196. [Google Scholar]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of Lactic Acid Bacteria and Molasses Additives on the Microbial Community and Fermentation Quality of Soybean Silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Merck, E. The Testing of Water: A Selection of Chemical Methods for Practical Use, 10th ed.; Merck Darmstadt: Darmstadt, Germany, 1964; pp. 7–13. [Google Scholar]
- Zehra Saricicek, B.; Yildirim, B.; Kocabas, Z.; Ozgumus Demir, E. Effect of Storage Time on Nutrient Composition and Quality Parameters of Corn Silage Mısır Silajının Besin Madde Kompozisyonu ve Silaj Kalite Parametreleri Üzerine Depolama Süresinin Etkileri. Turkish J. Agric. Sci. Technol. Türk Tarım-Gıda Bilim ve Teknol. Derg. 2016, 4, 934–939. [Google Scholar]
- Kung, L., Jr. Understanding the Biology of Silage Preservation to Maximize Quality and Protect the Environment. In Proceedings of the California Alfa & Forage Symposium and Corn/Cereal Silage Conferenxe, Visalia, CA, USA, 1–2 December 2010; pp. 1–14. [Google Scholar]
- Ali, M.F.; Tahir, M. An Overview on the Factors Affecting Water-Soluble Carbohydrates Concentration during Ensiling of Silage. J. Plant Environ. 2021, 3, 63–80. [Google Scholar] [CrossRef]
- Belém, C.d.S.; de Souza, A.M.; de Lima, P.R.; de Carvalho, F.A.L.; Queiroz, M.A.Á.; da Costa, M.M. Digestibility, Fermentation and Microbiological Characteristics of Calotropis Procera Silage with Different Quantities of Grape Pomace. Ciênc. Agrotecnologia 2016, 40, 698–705. [Google Scholar] [CrossRef]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage Processing and Strategies to Prevent Persistence of Undesirable Microorganisms. Anim. Feed Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Fitri, A.; Obitsu, T.; Sugino, T.; Jayanegara, A. Ensiling of Total Mixed Ration Containing Persimmon Peel: Evaluation of Chemical Composition and In Vitro Rumen Fermentation Profiles. Anim. Sci. J. 2020, 91, e13403. [Google Scholar] [CrossRef]
- Alipour, D.; Rouzbehan, Y. Effects of Ensiling Grape Pomace and Addition of Polyethylene Glycol on In Vitro Gas Production and Microbial Biomass Yield. Anim. Feed Sci. Technol. 2007, 137, 138–149. [Google Scholar] [CrossRef]
- Spanghero, M.; Salem, A.Z.M.; Robinson, P.H. Chemical Composition, Including Secondary Metabolites, and Rumen Fermentability of Seeds and Pulp of Californian (USA) and Italian Grape Pomaces. Anim. Feed Sci. Technol. 2009, 152, 243–255. [Google Scholar] [CrossRef]
- Adesogan, A.T.; Arriola, K.G.; Jiang, Y.; Oyebade, A.; Paula, E.M.; Pech-Cervantes, A.A.; Romero, J.J.; Ferraretto, L.F.; Vyas, D. Symposium Review: Technologies for Improving Fiber Utilization. J. Dairy Sci. 2019, 102, 5726–5755. [Google Scholar] [CrossRef]
- Mariatti, F.; Gunjević, V.; Boffa, L.; Cravotto, G. Process Intensification Technologies for the Recovery of Valuable Compounds from Cocoa By-Products. Innov. Food Sci. Emerg. Technol. 2021, 68, 102601. [Google Scholar] [CrossRef]
- Winkler, A.; Weber, F.; Ringseis, R.; Eder, K.; Dusel, G. Determination of Polyphenol and Crude Nutrient Content and Nutrient Digestibility of Dried and Ensiled White and Red Grape Pomace Cultivars. Arch. Anim. Nutr. 2015, 69, 187–200. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Characterization of Polyphenols and Antioxidant Potential of White Grape Pomace Byproducts (Vitis vinifera L.). J. Agric. Food Chem. 2013, 61, 11579–11587. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable Wineries through Waste Valorisation: A Review of Grape Marc Utilisation for Value-Added Products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage Review: Interpretation of Chemical, Microbial, and Organoleptic Components of Silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Rizk, C.; Mustafa, A.F.; Phillip, L.E. Effects of Inoculation of High Dry Matter Alfalfa Silage on Ensiling Characteristics, Ruminal Nutrient Degradability and Dairy Cow Performance. J. Sci. Food Agric. 2005, 85, 743–750. [Google Scholar] [CrossRef]
- Fu, Z.; Sun, L.; Hou, M.; Hao, J.; Lu, Q.; Liu, T.; Ren, X.; Jia, Y.; Wang, Z.J.; Ge, G. Effects of Different Harvest Frequencies on Microbial Community and Metabolomic Properties of Annual Ryegrass Silage. Front. Microbiol. 2022, 13, 971449. [Google Scholar] [CrossRef]
- Đorđević, N.; Grubić, G.; Adamović, M.; Stojanović, B. The Influence of Inoculant and Zeolite Supplementation on Quality of Silages Prepared from Whole Maize Plant, Lucerne and Perennial Ryegrass. In Proceedings of the International Congres: Food Technology, Quality and Safety, Novi Sad, Serbia, 13–15 November 2007; pp. 51–56. [Google Scholar]
- Der Bedrosian, M.C.; Nestor, K.E.; Kung, L. The Effects of Hybrid, Maturity, and Length of Storage on the Composition and Nutritive Value of Corn Silage. J. Dairy Sci. 2012, 95, 5115–5126. [Google Scholar] [CrossRef]
- Juráček, M.; Bíro, D.; Šimko, M.; Gálik, B.; Rolinec, M.; Hanušovský, O.; Vašeková, P.; Kolláthová, R.; Barantal, S. Fermentation Quality and Dry Matter Losses of Grape Pomace Silages with Urea Addition. J. Int. Sci. Publ. Agric. Food 2019, 7, 173–178. [Google Scholar]
- Schmidt, R.J.; Kung, L. The Effects of Lactobacillus Buchneri with or without a Homolactic Bacterium on the Fermentation and Aerobic Stability of Corn Silages Made at Different Locations. J. Dairy Sci. 2010, 93, 1616–1624. [Google Scholar] [CrossRef]
- Le Lay, C.; Coton, E.; Le Blay, G.; Chobert, J.M.; Haertlé, T.; Choiset, Y.; Van Long, N.N.; Meslet-Cladière, L.; Mounier, J. Identification and Quantification of Antifungal Compounds Produced by Lactic Acid Bacteria and Propionibacteria. Int. J. Food Microbiol. 2016, 239, 79–85. [Google Scholar] [CrossRef]
- Öztürk, Y.E.; Gülümser, E.; Mut, H.; Başaran, U.; Çopur Doğrusöz, M. A Preliminary Study on Change of Mistletoe (Viscum album L.) Silage Quality According to Collection Time and Host Tree Species. Turkish J. Agric. For. 2022, 46, 104–112. [Google Scholar] [CrossRef]
- Bai, J.; Ding, Z.; Ke, W.; Xu, D.; Wang, M.; Huang, W.; Zhang, Y.; Liu, F.; Guo, X. Different Lactic Acid Bacteria and Their Combinations Regulated the Fermentation Process of Ensiled Alfalfa: Ensiling Characteristics, Dynamics of Bacterial Community and Their Functional Shifts. Microb. Biotechnol. 2021, 14, 1171–1182. [Google Scholar] [CrossRef]
- Eliş, S.; Özyazici, M.A. Determination of the Silage Quality Characteristics of Different Switchgrass (Panicum virgatum L.) Cultivars. Appl. Ecol. Environ. Res. 2019, 17, 15755–15773. [Google Scholar] [CrossRef]
- Wang, M.; Gao, R.; Franco, M.; Hannaway, D.B.; Ke, W.; Ding, Z.; Yu, Z.; Guo, X. Effect of Mixing Alfalfa with Whole-Plant Corn in Different Proportions on Fermentation Characteristics and Bacterial Community of Silage. Agriculture 2021, 11, 174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).