Abstract
Hemp cultivation faces challenges due to the adoption of dioecious cultivars, which suffer from biomass loss and fibre heterogeneity. In contrast, monoecious cultivars offer simultaneous fibre and seed production, albeit with lower fibre quality. Understanding the drying characteristics and storage requirements of hemp seeds is crucial for effective post-harvest management. This study explored the moisture sorption and desorption isotherms of two common Canadian hemp seed varieties, Altair (dioecious) and CanMa (monoecious), by using both saturated salt solution (SSS) and thin-layer drying methods. Their isotherms were also compared with the published isotherm of Finola—a common dioecious variety in Europe. The thin-layer drying method yielded higher EMC values than the SSS method due to incomplete equilibrium attainment. Larger EMC differences existed between different seed types (dioecious vs. monoecious), and this difference was small between the same seed types (dioecious vs. dioecious). The GAB equation provided the most accurate prediction of equilibrium moisture contents for both varieties.
1. Introduction
Hemp, a dicotyledonous plant, exhibits both monoecious (possessing male and female flowers on the same plant) and dioecious (having separate male and female plants) characteristics [1,2,3]. The varieties based on these characteristics exhibit considerable differences in height, maturity, seed size, and hull thickness [4,5,6,7,8]. Traditional hemp cultivation encountered difficulties in seed production due to the adoption of dioecious cultivars. These cultivars consisted of a significant proportion of male plants that were preferred for their superior quality in textile applications. However, a drawback was observed as the male plants entered the senescence phase shortly after flowering, resulting in a loss of biomass and an increase in fibre heterogeneity. In response to these challenges, modern hemp cultivation has shifted its focus towards the development of monoecious cultivars. These cultivars possess the advantage of simultaneously producing fibre and seed, although with a lower quality fibre output [9,10,11]. The height of a crop plays a vital role in determining its ultimate use. Shorter varieties are characterized by lower fibre content and are more suitable for grain seed production. On the other hand, taller varieties are considered “dual purpose” as they yield both high-quality grains and a substantial volume of desirable best fibre for fibre production. The physical properties (such as dimensions, sphericity, average surface area, bulk and true density, porosity, weight of 1000 kernels, filling and emptying angles of repose, and coefficients of friction on surfaces) of the seeds produced for different purposes have significant differences [12]. These differences might influence their drying characteristics such as the isotherm (the relationship among temperature, equilibrium moisture content, and equilibrium relative humidity).
In March of 1998, Health Canada began to license industrial hemp under the controlled drugs and substances act 1997. This provided legislative authority to allow the production of hemp in Canada and thus an extensive breeding program started across the country [13,14,15,16]. The licensing involved the breeding of new varieties, research, and cultivation of certified seeds with THC (delta—9—Tetrahydrocannabinol) below the 0.3% maximum limit. This led to the development of different certified varieties across Canada. As per Industrial Hemp regulations (IHR), 84 certified varieties were approved for the 2023 growing season in Canada.
Varieties grown for the grain production purpose are different from those grown for the dual purpose based on their higher oil content and chemical composition. Altair (dioecious) and CanMa (monoecious) are two such varieties that are grown for dual and grain production purposes, respectively. Based on the 2021 Industrial Hemp licensing statistics released by the Canadian government, Altair predominantly thrived in Alberta, a western prairie province, with a nationwide cultivation area of 130 hectares. In contrast, CanMa was most commonly grown in Quebec, an eastern province, with a total cultivation area of approximately 848 hectares throughout Canada. Finola (Dioecious) was widely planted in Canada.
Hemp seeds are harvested when they reach the stage of shattering, typically when around 70% of the seeds are ripe, containing approximately 22–30% moisture (MC, wet basis). The recommended moisture content for safe storage of Finola® hemp seeds is 8% [17]. Therefore, safe storage of hemp seed requires prompt drying after harvest to prevent spoilage. Guidelines for the natural air-drying of hemp seeds during storage have been established. However, a gap in knowledge arises when considering the widespread utilization of newer varieties because of their difference in isotherms.
For most crop grains, such as cereal grains and pulses, moisture sorption and desorption isotherms have been developed. These isotherms serve as pivotal tools for predicting the moisture content of stored grain seeds under known air conditions, including specified relative humidity (RH) and temperature. They play an integral role in guiding farmers and grain storage managers in making informed decisions regarding various post-harvest operations, such as drying, aeration, and storage. Jian et al. (2018) developed the moisture sorption and desorption isotherms of Finola hemp seeds and discussed the effects of different percentages of dockage on their physical characteristics [12]. Finola (dioecious; origin: Finland) was the first variety of hemp seed that was brought to Canada after it became popular in Europe. However, Finola hemp seeds are dioecious and have a distinct origin, hailing from Finland. While its isotherm characteristics are important for consideration, they may not entirely represent the broader spectrum of hemp seed varieties that have been bred and cultivated in Canada and beyond. The kernel size of Altair is larger than CanMa. Kernel size also influences the isotherm of desorption and sorption [18]. There is no study reporting the isotherm characteristics of any single purpose variety or any other dual purpose dioecious variety that was either bred in Canada or other than Finola. It is not known whether the equilibrium moisture content between different types of seed (such as monoecious vs. dioecious) and different kernel sizes has a larger difference than that between the same type of seed. This study aimed to bridge this critical gap by establishing moisture sorption and desorption isotherms of two distinct Canadian-bred hemp seed varieties. These experiments encompass different levels of dockage, allowing for a comprehensive exploration of the impact of this variable on moisture equilibrium. Furthermore, we endeavor to compare the outcomes of our experiments with those from the Finola variety, providing insights into potential differences between monoecious and dioecious varieties.
2. Materials and Methods
2.1. Sample Preparation
The CanMa variety was directly harvested from a field situated in Southern Manitoba and transferred to the laboratory. The Altair variety was obtained from Uniseeds Inc., located in Cobden, ON, Canada. Upon arrival at the laboratory, the moisture content (MC) of CanMa and Altair was measured to be 7.7 ± 0.3% and 10.0 ± 0.1% (standard error was reported in this article), respectively. The determination of moisture content was conducted using the oven drying method at a temperature of 103 °C for 5 h, following the protocols established by the Canadian Grain Commission (2016) [19] and Jian et al. (2018) [20].
The samples underwent a cleaning process utilizing a three-dimensional shaker (Sweco® Vibro-Energy® Separators, Sweco, Florence, NC, USA) and four sieves with varying opening sizes (5.56, 2.03, 1.65, and 0.51 mm) to effectively separate the unwanted dockage from the hemp seeds. The dockage mainly included fines, chaff, and broken seeds. Following the separation of dockage from the samples, the cleaned CanMa seeds were collected on the sieve with a 1.65 mm opening, while the cleaned Altair seeds were gathered on the sieve with a 2.03 mm opening. Any remaining particles collected on the other sieves were categorized as dockage.
The cleaned seeds prepared from the above-mentioned cleaning process were divided into two equal portions to facilitate desorption and sorption isotherm investigations. For the desorption study, the moisture content of the cleaned seeds was adjusted to 20% by incorporating a predetermined quantity of water, following the methodology outlined by Jian et al. (2018) [20]. These samples were designated as the “wet samples”. To achieve wet samples containing varying proportions of dockage (0%, 5%, 10%, and 15% by weight), an appropriate amount of dockage, derived from both the cleaning process and an elevator, was blended with the wet sample. The dockage fraction exhibited a size distribution of 45.4% for sizes ≤ 2.00 mm, 48.4% for sizes ranging from 2.00 to 3.35 mm, and 6.2% for sizes ≥ 3.35 mm, consistent with the characterization performed by Jian et al. (2018) [20] to enable data comparison. The wet sample and dockage were homogeneously mixed for 5 min utilizing a grain mixer (Big Cat, Type B, Red Lion, Inc., Winnipeg, MB, Canada).
The remaining half of the cleaned seeds underwent drying until reaching a moisture content (MC) of approximately 5%. This was achieved by spreading the hemp seeds, approximately 5 mm thick, on a table under ambient conditions (temperature: 22 to 28 °C, relative humidity: 30 to 45%) for 2 wk. Subsequently, these dried seeds were combined with dockage in desired weight proportions to obtain samples with dockage levels of 0%, 5%, 10%, and 15%. These samples were denoted as “dry samples with 0%, 5%, 10%, and 15% dockage”. The dry samples, each containing a different percentage of dockage, were utilized for the sorption study. Prior to testing, all prepared samples were stored in double-layer plastic bags at a controlled temperature of 5 ± 1 °C for 2 wk to ensure uniform moisture distribution in each sample.
2.2. Sorption and Desorption Study
2.2.1. Saturated Salt Solution (SSS) Method
The recommended methodology outlined by the European Project COST 90’s, as described by Spiess and Wolf, 1983, was adopted for the study [21]. Saturated solutions of MgCl2, Mg(NO3)2, NaNO2, NaCl, and KNO3 were prepared at about 40 °C. Pails containing the saturated solutions, following the methods established by several researchers [22,23,24], were utilized to house both dry and wet samples of the two varieties, each with varying percentages of dockage. Desorption and sorption isotherms of the samples, with different dockage levels, were measured at temperatures of 10, 20, 30, and 35 °C. The reason for choosing these temperatures and relative humidities was that these temperatures and relative humidities cover the range of temperatures and relative humidities that the seed can encounter after harvest or during storage. The samples (each sample was about 250 g) were packed in mesh clothes and tied on the top to form a bag, which were kept inside the pails containing a particular salt solution at the bottom. The pails were sealed (except the sampling time) using a lid so that each pail can maintain the desired temperature and RH condition. This experimental setup facilitated the complete exposure of the entire sample to the specified testing conditions. To monitor the moisture content (MC) changes in the samples over time, weekly sampling (10 g from each replicate) was conducted for the samples maintained at temperatures above 20 °C, while sampling was conducted once every two weeks for samples at 20 °C and below. During the entire testing period, heavy mold multiplication on hemp seeds was not observed. The experiment was terminated once three consecutive readings displayed a difference of less than 0.1 percentage points of the moisture contents among them. The MC determined at that time was considered as the equilibrium moisture content (EMC). Each tested condition was performed in triplicate.
2.2.2. Thin-Layer Drying Method
The wet samples, prepared in triplicate, were subjected to drying using a thin-layer dryer, as developed by Tabatabaee et al. (2004) [25]. The drying process took place at 30, 35, and 40 °C, along with relative humidity (RH) of 30%, 50%, and 70% at each temperature condition. The tested temperatures and RH are the conditions used for natural air-drying. The principle of the dryer is based on psychometric chart, and the desired RH was achieved with less than 3% errors. A constant air velocity of 0.2 m/s was maintained throughout the drying process. To ensure system stability, the dryer was operated for a minimum of 24 h before initiating the tests.
Each drying test involved approximately 150 g of the wet sample, and its mass was recorded at 15 min intervals to track the changes in mass, following the methodology outlined by Jian et al. (2018) [20]. The moisture content of the samples was determined at the beginning and end of the drying tests. The MC measured at the end of the drying process was designated as the equilibrium moisture content (EMC) achieved through the thin-layer drying method. The drying rate was determined by the equation described by Jian et al. (2018) [20] as follows:
in which (dMC/dt)n = drying rate (kg water per kg dry matter per hour) at time n; tn, tn−1, tn+1 are the drying time (h) at n, n − 1 and n + 1, respectively. MCn, MCn−1, and MCn+1 are the moisture contents at time n, n − 1, and n + 1, respectively.
2.3. Empirical Models of Isotherms
Jian and Jayas (2022) [18], present a comprehensive exposition of non-linear equations, including the Modified Henderson equation, Modified Chung–Pfost equation, Modified Halsey equation, Modified Oswin equation, and Guggenheim–Anderson–deBoer (GAB) equations, which are commonly employed for modelling sorption and desorption isotherms [18]. These equations were utilized in a regression analysis of the equilibrium moisture content (EMC) data obtained through the saturated salt solution method. The primary objective was to identify the equation that demonstrated the lowest mean squared error (MSE) between the predicted and measured EMC values, while also achieving the highest coefficient of determination (R2). This enabled the selection of the equation that provided the best fit to the data among the available options.
The experimental data obtained from the thin-layer drying experiments were analyzed using various empirical models, namely Lewis (Newton), Henderson and Pabis, Modified Henderson and Pabis, Page, Modified Page, Modified Page II, two-term exponential, two-term logarithmic, Verma, and Hii models [18]. These models were selected based on their relevance and detailed descriptions provided by Jian and Jayas (2022) [18], which justify their applicability in representing the drying behavior of the samples [18].
2.4. Data Analysis
There were three replicates for each tested condition. Tukey test was conducted to compare the MC of the hemp seeds at different percentages of dockage under the same temperature and RH. For both hemp seed varieties, the data with different percentages of dockage and at the same RH and temperature were pooled because there was no significant difference under this condition (Tukey test, all F > 0.14, p > 0.93, N = 20). Paired t-tests were conducted to determine any difference between the EMC measured and predicted at different RH conditions at constant temperature conditions. The predicted EMC was the calculated EMC by using the developed best empirical models of isotherms.
Jian et al. (2018) [20] conducted the isotherm study of Finola under the same conditions (10, 20, 30 °C temperatures at 34, 54, 64, 75, and 94% RH each) and same procedure as tested in our study (for both sorption and desorption) [20]. Both Altair and Finola are dioecious, while CanMa is monoecious. Therefore, their isotherms were also compared. Difference in the EMCs for each of the comparable group, i.e., Finola-CanMa (dioecious-monoecious), Finola-Altair (dioecious-dioecious), and CanMa-Altair (monoecious- dioecious) were calculated for each tested condition. Paired-t tests were conducted to compare between the groups.
3. Results
3.1. Isotherms under Saturated Salt Solution Condition
The lowest EMC value of the Altair variety under desorption conditions was 5.8 ± 0.0% at 35 °C and 34% RH (Figure 1). This condition represented the driest state tested. The highest EMC value under desorption conditions was 13.7 ± 0.0% at 10 °C and 94% RH. This condition represented the wettest state tested. The highest EMC value under sorption conditions was 14 ± 0.0% at 10 °C and 94% RH, while the lowest EMC under sorption conditions was 5.6 ± 0.0% at 35 °C and 34% RH. These differences of the highest and lowest EMC between sorption and desorption indicated the hysteresis of the Altair variety.
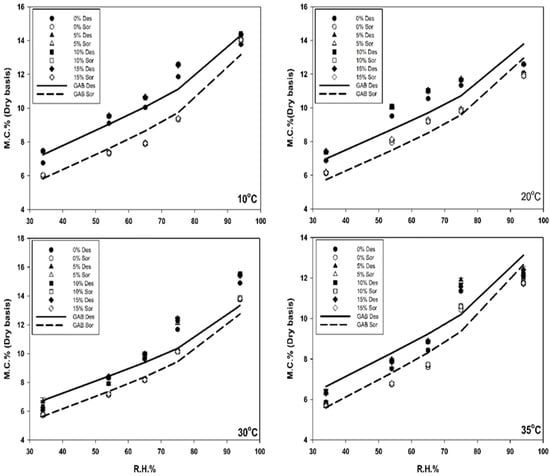
Figure 1.
Isotherm of Altair with different dockage percentages (0, 5, 10, and 15%) under desorption and adsorption conditions and at different environmental conditions (temperature: 10, 20, 30, 35 °C; and RH from 34 to 94%) generated by salt solutions. In the graph, the line shows the value predicted by the developed GAB equation and different symbols show the measured values with different dockage percentages. In the legend, Des = desorption, and Sor = sorption.
Similar trends were observed for the CanMa variety (Figure 2). The lowest EMC for desorption was 6.1 ± 0.0% at 35 °C and 34% RH, while the highest EMC value reached 14.0 ± 0.0% at 10 °C and 94% RH. The lowest and highest EMC values were 5.9 ± 0.0% and 14.1 ± 0.0%, respectively, under the corresponding sorption conditions. These results indicated that the CanMa variety also had hysteresis and consistently exhibited higher EMC values (about 0.3 to 0.5 percentage points) than Altair under both sorption and desorption conditions.
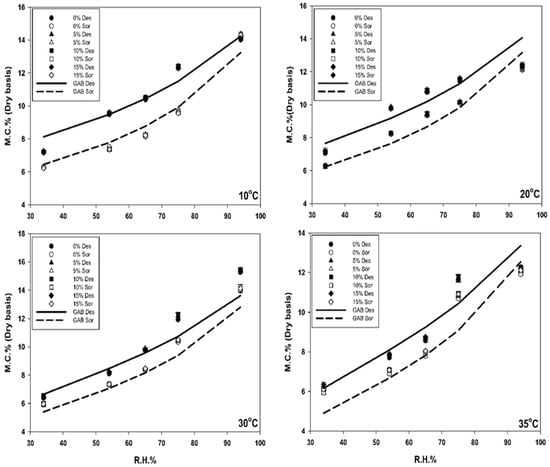
Figure 2.
Isotherm of CanMa with different dockage percentages (0, 5, 10, and 15%) under desorption and adsorption conditions and at different environmental conditions (temperature: 10, 20, 30, 35 °C; and RH from 34 to 94%) generated by salt solutions. In the graph, the line shows the value predicted by the developed GAB equation and different symbols show the measured values with different dockage percentages. In the legend, Des = desorption, and Sor = sorption.
3.2. Desorption Isotherm under Thin-Layer Drying Condition
The EMC obtained for both varieties using the thin-layer method was higher than that determined by the SSS method at the same temperature and RH (Figure 1, Figure 2 and Figure 3). It varied by 0.5% on average. Both varieties had the same EMC at 40 °C and 30% RH (4.5 ± 0.0%). The EMC of Altair and CanMa was 8.2 ± 0.1% and 8.1 ± 0.1%, respectively, at 30 °C and 70% RH, which is not significantly different. Therefore, CanMa had a similar EMC as Altair (minimum 0.1% difference) under the same drying condition when the temperature was lower than 35 °C.
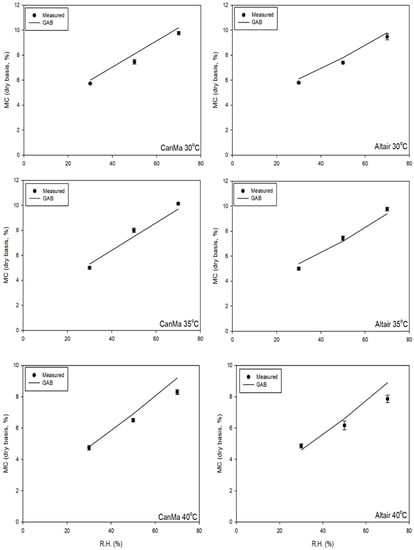
Figure 3.
Isotherm of CanMa and Altair samples under thin layer drying conditions (temperature: 30, 35 and 40 °C, RH: 30, 50, 70%). In the graph, the line shows the values predicted by the developed GAB equation and different symbols show the measured equilibrium moisture contents.
During drying, only the falling rate period was observed for both varieties (Figure 4). Both varieties showed the first and second falling rates of drying. For both varieties, the maximum drying rate was around 0.2 to 0.3 kg water per kg dry matter per hr at 40 °C at 30% RH, and the drying rate was lower than 0.1 kg kg−1 h−1 for the second falling rate when the seeds had a lower than 13% MC (Figure 4). When the temperature was lower than 35 °C, both varieties had a drying rate lower than 0.2 kg kg−1 h−1. The maximum drying time was over 10 h (Figure 4).
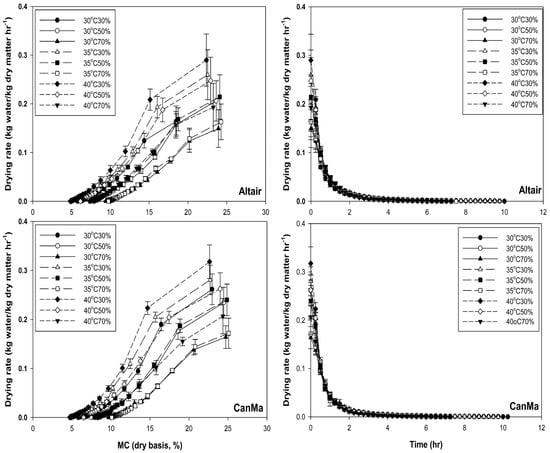
Figure 4.
Drying rate of CanMa and Altair hemp seeds under different drying temperatures, RHs, and drying times using the thin-layer drying method.
3.3. Empirical Models
For both varieties, all the tested empirical models exhibited higher than 0.9 R2 values (Table 1). Regarding the salt solution study, the GAB equation and Modified Henderson equation were the best equations for predicting both desorption and sorption EMC for both varieties because they had the lowest MSE. These two equations yielded similar predicted values for most conditions due to their comparable R2 and MSE values. There were no significant differences observed between the predicted and measured moisture contents under both desorption and sorption conditions (Paired t-test and the highest t and p values were 0.046 and 0.96, respectively, Figure 1 and Figure 2). The largest discrepancy between the measured and predicted EMC was observed at ≥30 °C when RH was above 90%. This finding aligned with previous studies [20,26]. These researchers also recommended the application range of the GAB model up to 90 to 95% RH. The parameter values of the developed models fell within the range described in the literature and were consistent with previous studies on different oilseeds [27,28,29,30,31,32]. Other regressed equations, such as the Modified Oswin equation, exhibited lower than 0.8 R2 values, indicating limited applicability in predicting EMC for oilseed samples.

Table 1.
Parameter values of the best fitted equations to predict isotherms of the two varieties of hemp seeds measured by saturated salt solution and thin-layer drying methods.
Under thin-layer drying conditions, the GAB equation was identified as the best equation, as it displayed the lowest mean square error compared to the Modified Halsey equation, despite both equations having a similar R2 value of 0.93. The predicted values of these two equations were almost identical for most conditions.
For both varieties, the Page model and modified Page model exhibited the highest R2 values (~0.98) and lowest MSE across all conditions, indicating a better fit compared to other tested models (Table 2). Some models (such as Modified Henderson and Pabis, Modified Page II, two-term exponential, Verma, and Hii models) yielded k (regressed drying constant) values with a standard error more than 100 times the mean value, or even infinite values. These models were deemed unsuitable and were rejected. No negative k values were obtained in this study, in contrast to the findings reported by Jian et al. (2018) [20] for Finola.

Table 2.
Drying constant (k, mean ± SE) and R2 of best fitted empirical models of the hemp seed varieties under thin-layer drying conditions.
3.4. Difference among Altair, CanMa, and Finola
For the Altair variety, the desorption EMC determined through the thin layer drying method differed by a minimum of 0.2 to a maximum of 0.9 percentage points compared to the SSS method. The difference ranges across different temperature and RH levels for CanMa from 0.1 to 0.6 percentage points, and 0.3 to 1 percentage points for Finola. Therefore, these three varieties had slightly different hysteresis.
Table 3 presents the difference in equilibrium moisture contents (EMCs) among the three varieties under the same tested condition. Finola exhibited higher sorption EMC values than CanMa and Altair, except at 94% relative humidity (RH). However, CanMa had higher desorption EMC than Finola and higher desorption and sorption than Altair at all tested conditions (Table 3). There were significant differences between Finola and CanMa (dioecious–monoecious) and between CanMa and Altair (monoecious vs. dioecious), while there was no significant difference between Finola and Altair (dioecious–dioecious) (Table 4). Therefore, a larger EMC difference might exist between different seed types, and this difference might be small between the same seed types.

Table 3.
Percentage point differences in the EMCs for the hemp seed varieties at the tested temperatures and RH conditions.

Table 4.
Statistical comparison (paired t-test) of the EMC difference between varieties.
4. Discussion
4.1. Differences Due to the Method of Isotherm Determination
The thin-layer drying method is a dynamic and time-efficient approach for determining sorption isotherms under any ventilation conditions (e.g., aeration, natural air-drying, and high-temperature drying). However, it does not represent the EMC exhibited by grain bulk during storage in grain bins. The EMC determined through thin-layer drying is generally higher than that obtained using the saturated salt solution method, which can take weeks to months to complete [33]. This discrepancy reflects the slow moisture changes or migration within the grain bulk during storage, unlike the rapid changes imposed in dynamic determinations [34]. Therefore, both dynamic and SSS methods should be used to determine the EMC, so the developed isotherms can be used for both drying and storage conditions.
Under both thin-layer drying and SSS conditions, moisture migration might be mainly caused by diffusion because the tested temperature was lower than 40 °C. Diffusion is a slow process under low temperature and it needs time [20]. Under thin-layer drying conditions, the water molecules inside grain kernels might not have sufficient time to migrate to the surface for evaporation, leading to an incomplete representation of the actual EMC [35,36,37]. Conversely, the SSS method allows for the gradual migration of moisture out of the grain kernel over an extended period under constant temperature and RH conditions, bringing the moisture content closer to the true EMC level. This study also confirms these findings.
4.2. Differences Due to Seeds Fit for Purpose and Their End Use
Breeding and genotype differences between hemp seed varieties arise from specific utilization and the requirements of the target population of female and male parts. The presence of both male and female parts within the same plant or in different plants of the same type results in variations in the end use. These differences are also evident in the seed’s physical and chemical properties of both types of plants. The composition and morphological characteristics of the seeds vary due to specific traits, leading to variations in moisture accumulation and migration under different conditions because differences in composition affect the affinity of water molecules to the seed, which is particularly evident in oilseeds with varying oil content. Higher oil content can be compensated by higher carbohydrate or other macronutrient levels, altering the internal bonding of water molecules, and influencing drying patterns. This explained the difference in EMC and hysteresis of different varieties under the same environmental conditions. The CanMa variety consistently demonstrated approximately 0.2% higher moisture content than Altair under the same temperature and RH conditions. This observation suggests inherent differences in the moisture accumulation and release characteristics between the two hemp seed varieties. Yang and Cenkowski (1993) also reported the difference in the EMC of the Canola variety Westar from the varieties of Gulle and Hecktor [38]. Similar differences in EMC had also been reported for different oilseeds [39,40,41]. Therefore, drying and storage of different varieties should use their corresponding isotherms because misusing might result in wet or over-dried grain during drying and/or storage, which is the main reasons of the grain spoilage [23].
The isotherm of seeds is influenced by the combination of its components, as the effects of individual components cannot be isolated. Matilla et al. (2018) and House et al. (2010) reported significant differences in lignin content, carbohydrate content in the hull, as well as the presence of acid and neutral detergent fibres in different hemp seed varieties [42,43,44]. Certain differences can be observed when varieties from different geographical areas are also compared. Gallaso et al. (2016) studied 20 genotypes prevalent in different parts of Europe (13 dioecious and 7 monoecious) and significant variability (p < 0.01) was observed among the analyzed genotypes for all biochemical traits [45]. This aligns with the results of Krese et al., (2004); Chen et al., (2010); and Vonapartis et al., (2015) [46,47,48]. Xu et al. (2021) reported the differences in physicochemical and other properties based on the differences in the genotypes of Hempseeds [49]. Those and our studies clearly indicated that not just the type of oilseed, but a difference in variety can also be a reason for the requirement of in-depth knowledge of varieties with different traits and purpose of use, especially for grain drying and storage.
The present study indicates that the two Canadian-bred varieties, irrespective of the type (dioecious and monoecious), had differences in EMC from Finola (dioecious), which is a known Finnish variety. The difference between the similar type of seeds, i.e., Finola and Altair (both dioecious), although bred from different geographical areas, was non-significant, whereas the difference was significant between the two monoecious–dioecious groups, i.e., Finola and CanMa, and CanMa and Altair. The difference in between the two Canadian bred varieties was higher than the difference found between Finola and CanMa. This depicts that the type of seeds has more impact on the moisture sorption characteristics of the seeds than the geographical traits for the same crop. These results can be important when selecting the location and conditions for the storage of the hemp seeds. This also places emphasis on the need for trait knowledge and information on the composition of the variety.
Jian et al. (2018) recommended that the safe storage moisture content of Finola was 8% [17]. Our study revealed that Finola had a higher EMC than CanMa and Altair under sorption conditions, while it had a lower EMC under desorption. Therefore, the safe storage EMC of CanMa and Altair should be close to 8% because the moisture content of seeds during storage might be between the sorption and desorption EMC. To reach this safe storage EMC, however, CanMa and Altair should be dried under higher temperatures or lower RH than Finola.
4.3. Effect of Dockage
In the present study, the results indicated that the dockage did not influence the EMC levels and drying characteristics of the hemp seeds at any of the tested conditions. The sample consisted of a chaff as well as fines mixed at different levels. But the influence on the isotherm was negligible. This aligns with the results indicated by Bian et al. (2015) where the influence of chaff was observed in the bulk properties of wheat. This can be because of the low moisture content of the dockage [50]. The dry state of the dockage particles had moisture content much lower than the wet hemp seed samples, conditioned to around 14% M.C. Jian et al. (2019) [17] studied the safe storage times for Finola with dockage. It was concluded that a higher level of dockage (15%) had a slight effect on the germination of stored hemp seeds, but the major influence was due to the storage temperature and relative humidity. The results from the present study also indicated that different levels of dockage might not have any effect on the desorption and sorption of the hemp seeds irrespective of the varieties. However, this conclusion might not apply to the storage in bins because the dockage might have a higher moisture content (e.g., higher than 90%) than that tested under lab conditions.
4.4. Isotherm Models
GAB is a multi-molecular, localized, and homogeneous adsorption model. This model postulates the state of sorbate molecules in the second layer is identical to the one in superior layers, but different from those in the liquid state. The parameter a is the monolayer moisture content (%); b is a dimensionless GAB parameter called the correction factor; ΔHd and ΔHb are functions describing the heat of adsorption and condensation of the water vapor (J/mol), respectively. This study found GAB was the best model to predict the isotherm of both sorption and desorption. Jian et al. (2018) [20] also found GAB was the best model for Finola. These results indicated that the water sorption and desorption by hemp seeds had a surface absorption and desorption phenomena and associated kinetic.
The established isotherms exhibit a type II profile, characterized by a sigmoidal shape, a pattern commonly observed in various stored biomaterials. This shape suggests the formation of multiple layers extending infinitely once the monolayer phase has formed.
5. Conclusions
This study sheds light on the moisture sorption and desorption characteristics of two Canadian-bred hemp seed varieties, Altair and CanMa, in comparison to the established Finnish variety, Finola. Hemp’s dichotomous nature—dioecious and monoecious—gives rise to variations in seed properties that affect drying behavior. Hemp cultivation has transitioned from dioecious to monoecious varieties due to challenges in seed production, and this shift impacts seed properties and drying requirements. The study delved into moisture sorption behaviors through both the saturated salt solution (SSS) method and thin-layer drying, uncovering differences between the two methodologies.
Notably, CanMa consistently exhibited slightly higher equilibrium moisture content (EMC) values compared to Altair under both sorption and desorption conditions, highlighting the impact of monoecious breeding. Three varieties displayed hysteresis, indicating that sorption and desorption characteristics differ under the same environmental conditions. Importantly, the moisture sorption characteristics between the two Canadian-bred varieties exhibited more pronounced differences than those between Altair and the Finnish variety, Finola. Therefore, a more significant EMC difference existed between different seed types (dioecious vs. monoecious), while a smaller difference existed between identical seed types (dioecious vs. dioecious).
Furthermore, this study highlighted the limited impact of dockage, including chaff and fines, on hemp seed moisture behavior. The dockage showed negligible influence on EMC levels and drying characteristics. This finding is crucial for post-harvest handling and storage practices.
Empirical models, the Guggenheim–Anderson–deBoer (GAB) equation and Modified Henderson equation were the best in predicting sorption and desorption behaviors. These models offer valuable predictive capabilities for estimating moisture contents during various drying and storage scenarios.
Author Contributions
A.T.: Methodology, investigation, data collecting and analyzing, writing (original draft). F.J.: Conceptualization, funding acquisition, project administration, supervision, writing (review and editing). All authors have read and agreed to the published version of the manuscript.
Funding
The present work was supported by the Canadian Hemp Trade Alliance.
Data Availability Statement
Data are available upon request.
Acknowledgments
The authors would like to express their gratitude to the technicians Dale Bourns and Marcel Lehmann for their technical support, and Sristi Mundhada and T. Anukiruthika for their assistance during the study. We would also like to thank the Prairie Agricultural Machinery Institute (PAMI) for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deguchi, M.; Kane, S.; Potlakayala, S.; George, H.; Proano, R.; Sheri, V.; Curtis, W.R.; Rudrabhatla, S. Metabolic Engineering Strategies of Industrial Hemp (Cannabis sativa L.): A Brief Review of the Advances and Challenges. Front. Plant Sci. 2020, 11, 580621. [Google Scholar] [CrossRef]
- Rahemi, A.; Dhakal, R.; Temu, V.W.; Rutto, L.; Kering, M.K. Performance of Different-Use Type Industrial Hemp Cultivars under Mid-Atlantic Region Conditions. Agronomy 2021, 11, 2321. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Petit, J.; Trindade, L.M. The Complex Interactions Between Flowering Behavior and Fiber Quality in Hemp. Front. Plant Sci. 2019, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Sankari, H.S. Comparison of Bast Fibre Yield and Mechanical Fibre Properties of Hemp (Cannabis sativa L.) Cultivars. Ind. Crops Prod. 2000, 11, 73–84. [Google Scholar] [CrossRef]
- Eržen, M.; Čeh, B.; Kolenc, Z.; Bosancic, B.; Čerenak, A. Evaluation of Different Hemp (Cannabis sativa L.) Progenies Resulting from Crosses with Focus on Oil Content and Seed Yield. Ind. Crops Prod. 2023, 201, 116893. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Amaducci, S. Comparing Hemp (Cannabis sativa L.) Cultivars for Dual-Purpose Production under Contrasting Environments. Ind. Crops Prod. 2016, 87, 33–44. [Google Scholar] [CrossRef]
- Schumann, E.; Peil, A.; Weber, W.E. Preliminary Results of a German Field Trial with Different Hemp (Cannabis sativa L.) Accessions. Genet. Resour. Crop Evol. 1999, 46, 399–407. [Google Scholar] [CrossRef]
- Alaru, M.; Kukk, L.; Olt, J.; Menind, A.; Lauk, R.; Vollmer, E.; Astover, A. Lignin Content and Briquette Quality of Different Fibre Hemp Plant Types and Energy Sunflower. Field Crops Res. 2011, 124, 332–339. [Google Scholar] [CrossRef]
- Faux, A.-M.; Draye, X.; Lambert, R.; d’Andrimont, R.; Raulier, P.; Bertin, P. The Relationship of Stem and Seed Yields to Flowering Phenology and Sex Expression in Monoecious Hemp (Cannabis sativa L.). Eur. J. Agron. 2013, 47, 11–22. [Google Scholar] [CrossRef]
- Mihoc, M.; Pop, G.; Alexa, E.; Radulov, I. Nutritive Quality of Romanian Hemp Varieties (Cannabis sativa L.) with Special Focus on Oil and Metal Contents of Seeds. Chem. Cent. J. 2012, 6, 122. [Google Scholar] [CrossRef]
- Baldini, M.; Ferfuia, C.; Piani, B.; Sepulcri, A.; Dorigo, G.; Zuliani, F.; Danuso, F.; Cattivello, C. The Performance and Potentiality of Monoecious Hemp (Cannabis sativa L.). Cultivars as a Multipurpose Crop. Agronomy 2018, 8, 162. [Google Scholar] [CrossRef]
- Jian, F.; Yavari, S.; Narendran, R.B.; Jayas, D.S. Physical Properties of Finola® Hemp Seeds: Clean and Containing Dockages. Appl. Eng. Agric. 2018, 34, 1017–1026. [Google Scholar] [CrossRef]
- Fortenbery, T.R.; Bennett, M. Opportunities for Commercial Hemp Production. Rev. Agric. Econ. 2004, 26, 97–117. [Google Scholar] [CrossRef]
- Cherney, J.; Small, E. Industrial Hemp in North America: Production, Politics and Potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef]
- Small, E.; Marcus, D.; Janick, J.; Whipkey, A. Hemp: A New Crop with New Uses for North America. Trends New Crops New Uses 2002, 24, 284–326. [Google Scholar]
- Leson, G. Hemp Foods in North America. J. Ind. Hemp 2006, 11, 87–93. [Google Scholar] [CrossRef]
- Jian, F.; Al Mamun, M.A.; White, N.D.G.; Jayas, D.S.; Fields, P.G.; McCombe, J. Safe Storage Times of FINOLA® Hemp (Cannabis sativa) Seeds with Dockage. J. Stored Prod. Res. 2019, 83, 34–43. [Google Scholar] [CrossRef]
- Jian, F.; Jayas, D.S. Grains: Engineering Fundamentals of Drying and Storage; CRC Press: Boca Raton, FL, USA, 2022; ISBN 1000475255. [Google Scholar]
- Canadian Grain Commission. Canola and Rapeseed—Chapter 10. In Official Grain Grading Guide; Canadian Grain Commission: Winnipeg, MB, Canada, 2016. Available online: https://www.grainscanada.gc.ca/en/grain-quality/official-grain-grading-guide/pdf/10-canola-e.pdf (accessed on 20 June 2023).
- Jian, F.; Divagar, D.; Mhaiki, J.; Jayas, D.S.; Fields, P.G.; White, N.D.G. Static and Dynamic Methods to Determine Adsorption Isotherms of Hemp Seed (Cannabis sativa L.) with Different Percentages of Dockage. Food Sci. Nutr. 2018, 6, 1629–1640. [Google Scholar] [CrossRef]
- Spiess, W.E.L.; Wolf, W.R. Results of the COST 90 Project on Water Activity. In Physical Properties of Foods; Peleg, M., Bagley, E.B., Eds.; AVI Publishing Co. Inc.: Westport, CT, USA, 1983. [Google Scholar]
- Uribe, E.; Miranda, M.; Vega-Gálvez, A.; Quispe, I.; Clavería, R.; Di Scala, K. Mass Transfer Modelling During Osmotic Dehydration of Jumbo Squid (Dosidicus Gigas): Influence of Temperature on Diffusion Coefficients and Kinetic Parameters. Food Bioproc Technol. 2011, 4, 320–326. [Google Scholar] [CrossRef]
- López-Vidaña, E.C.; Castillo Téllez, M.; Pilatowsky Figueroa, I.; Santis Espinosa, L.F.; Castillo-Téllez, B. Moisture Sorption Isotherms, Isosteric Heat, and Gibbs Free Energy of Stevia Leaves. J. Food Process Preserv. 2021, 45, e15016. [Google Scholar] [CrossRef]
- Wexler, A.; Hasegawa, S. Relative Humidity-Temperature Relationships of Some Saturated Salt Solutions in the Temperature Range 0 Degree to 50 Degrees C. J. Res. Natl. Bur. Stand. (1934) 1954, 53, 19–26. [Google Scholar] [CrossRef]
- Tabatabaee, R.; Jayas, D.S.; White, N.D.G. Thin-Layer Drying and Rewetting Characteristics of Buckwheat. Can. Biosyst. Eng. 2004, 46, 3.19–3.24. [Google Scholar]
- Yanniotis, S.; Blahovec, J. Model Analysis of Sorption Isotherms. LWT-Food Sci. Technol. 2009, 42, 1688–1695. [Google Scholar] [CrossRef]
- Lazouk, M.-A.; Savoire, R.; Kaddour, A.; Castello, J.; Lanoisellé, J.-L.; Van Hecke, E.; Thomasset, B. Oilseeds Sorption Isoterms, Mechanical Properties and Pressing: Global View of Water Impact. J. Food Eng. 2015, 153, 73–80. [Google Scholar]
- Zomorodian, A.; Kavoosi, Z.; Momenzadeh, L. Determination of EMC Isotherms and Appropriate Mathematical Models for Canola. Food Biopro Proc. 2011, 89, 407–413. [Google Scholar] [CrossRef]
- Cassells, J.A.; Caddick, L.P.; Green, J.R.; Reuss, R. Isotherms for Australian Canola Varieties. In Proceedings of the Australian Postharvest Technical Conference, Canberra, NSW, Australia, 25–27 June 2003; pp. 59–63. [Google Scholar]
- Sun, D.-W. Comparison and Selection of EMC/ERH Isotherm Equations for Rice. J. Stored Prod. Res. 1999, 35, 249–264. [Google Scholar] [CrossRef]
- Sun, D.-W.; Byrne, C. Selection of EMC/ERH Isotherm Equations for Rapeseed. J. Agric. Eng. Res. 1998, 69, 307–315. [Google Scholar] [CrossRef]
- Chirife, J.; Timmermann, E.O.; Iglesias, H.A.; Boquet, R. Some Features of the Parameter k of the GAB Equation as Applied to Sorption Isotherms of Selected Food Materials. J. Food Eng. 1992, 15, 75–82. [Google Scholar] [CrossRef]
- Carter, B.P.; Campbell, G.S. Fundamentals of Moisture Sorption Isotherms; Decagon Devices, Inc.: Pullman, WA, USA, 2008. [Google Scholar]
- Penner, E.A.; Schmidt, S.J. Comparison between Moisture Sorption Isotherms Obtained Using the New Vapor Sorption Analyzer and Those Obtained Using the Standard Saturated Salt Slurry Method. J. Food Meas. Charac 2013, 7, 185–193. [Google Scholar] [CrossRef]
- Arlabosse, P.; Rodier, E.; Ferrasse, J.H.; Chavez, S.; Lecomte, D. Comparison Between Static and Dynamic Methods for Sorption Isotherm Measurements. Dry. Technol. 2003, 21, 479–497. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; McMinn, W.A.M.; Magee, T.R.A. Moisture Sorption Isotherm Characteristics of Food Products: A Review. Food Bioprod. Proc. 2002, 80, 118–128. [Google Scholar] [CrossRef]
- Schiffmann, R.F. Handbook of Industrial Drying; Taylor & Francis: Abingdon, UK, 2006; Volume 12, p. 315. [Google Scholar]
- Yang, W.H.; Cenkowski, S. Latent Heat of Vaporization for Canola as Affected by Cultivar and Multiple Drying-Rewetting Cycles. Cana Agric. Eng. 1993, 35, 195–198. [Google Scholar]
- Yang, W.H.; Cenkowski, S. Canola Isotherms. Cana Agric. Eng. 1995, 37, 169. [Google Scholar]
- Bianco, A.M.; Boente, G.; Pollio, M.L.; Resnik, S.L. Influence of Oil Content on Sorption Isotherms of Four Varieties of Peanut at 25 °C. J. Food Eng. 2001, 47, 327–331. [Google Scholar] [CrossRef]
- Pollio, M.L.; Resnik, S.L.; Chirife, J. Water Sorption Isotherms of Soybean Varieties Grown in Argentina. Int. J. Food Sci. Technol. 1987, 22, 335–338. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Pinela, J.; Ćirić, A.; Calhelha, R.C.; Soković, M.; Ferreira, I.C.F.R.; Barros, L.; Torija-Isasa, E.; de Cortes Sánchez-Mata, M. Chemical Composition and Biological Activities of Whole and Dehulled Hemp (Cannabis sativa L.). Seeds. Food Chem. 2022, 374, 131754. [Google Scholar] [CrossRef]
- Mattila, P.; Mäkinen, S.; Eurola, M.; Jalava, T.; Pihlava, J.-M.; Hellström, J.; Pihlanto, A. Nutritional Value of Commercial Protein-Rich Plant Products. Plant Foods Human. Nutri 2018, 73, 108–115. [Google Scholar]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the Quality of Protein from Hemp Seed (Cannabis sativa L.) Products through the Use of the Protein Digestibility-Corrected Amino Acid Score Method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Galasso, I.; Russo, R.; Mapelli, S.; Ponzoni, E.; Brambilla, I.M.; Battelli, G.; Reggiani, R. Variability in Seed Traits in a Collection of Cannabis sativa L. Genotypes. Front. Plant Sci. 2016, 7, 688. [Google Scholar] [CrossRef]
- Vonapartis, E.; Aubin, M.-P.; Seguin, P.; Mustafa, A.F.; Charron, J.-B. Seed Composition of Ten Industrial Hemp Cultivars Approved for Production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar]
- Chen, T.; He, J.; Zhang, J.; Zhang, H.; Qian, P.; Hao, J.; Li, L. Analytical Characterization of Hempseed (Seed of Cannabis sativa L.) Oil from Eight Regions in China. J. Diet. Suppl. 2010, 7, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus. Oil Content, Tocopherol Composition and Fatty Acid Patterns of the Seeds of 51 Cannabis sativa L. Genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Zhao, J.; Wang, W.; Griffin, J.; Li, Y.; Bean, S.; Tilley, M.; Wang, D. Hempseed as a Nutritious and Healthy Human Food or Animal Feed Source: A Review. Int. J. Food Sci. Technol. 2021, 56, 530–543. [Google Scholar] [CrossRef]
- Bian, Q.; Ambrose, R.P.K.; Subramanyam, B. Effect of Chaff on Bulk Flow Properties of Wheat. J. Stored Prod. Res. 2015, 64, 21–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).