Abstract
Oil palm (Elaeis guineensis Jacquin) tree yields may be negatively affected by biotic factors such as Black weevil (Rhynchophorus palmarum L.; Coleoptera: Curculionidae) and Spear rot (Fusarium spp.). This study aimed to identify, model the temporal progress, and correlate Black weevil (BW) and Spear rot (SR) in the highly susceptible varieties INIAP-Tenera and IRHO, under field conditions in Ecuador between 2016 and 2020. Morphological analysis of BW and morphomolecular study of the causal agent of SR allowed us to identify Rhynchophorus palmarum and Fusarium solani, respectively, as biotic factors that affect oil palm trees in Ecuador. The number of adults increased in both genotypes over the years, but much more in INIAP-Tenera (in 2017 and 2019). The logistic model, having a smaller mean square of the residue, was the non-linear model that best explained the SR epidemic in both susceptible genotypes. The incidence of dead palms was higher in INIAP-Tenera trees compared to those of IRHO in the year 2020. Dead plants and the number of insects, and these with the fresh fruit bunches, were correlated significantly. To our knowledge, this is the first report on the positive correlation between dead plants (%) by SR and BW, and the study of both biotic factors in Ecuador.
1. Introduction
Oil palm (Elaeis guineensis Jacquin) is an economically important perennial crop due to its high oil content and is currently becoming one of the major oil crops of the world. Its importance lies in the fact that palm oil produces about 3 times more oil than coconut oil, 7 times more oil than rapeseed, and almost 10 times more oil than soybean [1]. In Ecuador, this crop is one of the most important. According to The Ecuadorian National Institute of Statistics and Censuses (INEC, for its acronym in Spanish) [2] the harvested area and annual oil palm production in 2019 were 200908 hectares and 2.3 million tons, respectively, with Santo Domingo and Esmeraldas being the provinces that concentrate 31% and 41% of the total oil palm crop. However, according to the same institution, the annual production of oil palm has registered a harvested area and oil production reduction of 10% and 18%, respectively, compared to the previous year. This decrease seems to be associated with the attack of pests and diseases. Oil palm crops are under pressure from numerous insect pests and diseases worldwide and in Latin America [1,3].
Although the oil palm pests may be classified as leaf defoliators (Psychidae and Limacodidae), bagworms (Psychidae), nettle caterpillars (Limacodidae), crown attackers, rhinoceros beetle (Scarabaeoidea), and the bunch moth (Pyralidae) [1], the most destructive is the Black weevil (BW) (Rhynchophorus palmarum L.; Coleoptera: Curculionidae). This insect has a direct impact on the oil palm crop in Latin America [4]. Insect larval and pupal stages are generally found inside the trunk (formation of galleries in trees stipe) and can damage the meristem, quickly causing oil palm mortality or in a few months [5]. BW is characterized by traveling long distances in a short time, favoring its feeding and reproduction [6], accumulating average lifetime flight distances for males and females of ~268 km and ~220 km, respectively [7]. Additionally, this insect has an aggregated spatial distribution, forming localized foci from 175 to 710 m in radius [8]. Once established in the area, Rhynchophorus populations can be difficult to suppress, from which they can expand their range and threaten both commercial and native palms [9]. One of the main management methods is the individuals’ capture through pheromone traps with S-Rhynchophorol (C8H16O) addition as an attractant [10]. The increase in trap numbers can negatively affect the BW population through massive capture and interruption of insect mating [5]. On the other hand, monitoring insects in the crop can provide important data in each sector of the farm where the traps are placed, contributing to better planning and use of management strategies. As result, the use of synthetic insecticides may be considerably reduced within the crop. The use of traps can also help in the study of the fluctuation of BW population.
Spear rot (SR) is considered the most important oil palm disease in different countries, including Ecuador [11,12,13], because it considerably reduces the fruit bunches yield [5]. In fact, during the 1960s, this disease practically destroyed all the 2800 ha of E. guineensis trees that had been planted in the Turbo region of Northern Colombia [14]. The pathogen initially develops in the bud above the meristematic zone, producing necrotic lesions on the sides of the lance-shaped leaf (youngest, unexpanded leaf) [15]. Symptomatic trees show damage to the photochemical system that affects the processes by which it captures and transports energy, causing a physiological imbalance [3]. The spatial distribution of diseased palms initially is random, but a grouped dispersal of diseased trees may be observed as the number of cases increases [16,17]. Although the SR management strategy is to eliminate diseased trees, the disease may temporarily increase in healthy individuals [17].
SR is a very important phytosanitary problem, and until recently was an unknown pathology [11]. For the first time in the Esmeraldas province, Ecuador, this disease was associated with Fusarium solani and F. oxysporum in oil palm, from buds, infected samples, leaves, meristem tips, and roots, identified by morphological, molecular, and pathogenetic analysis by Ronquillo et al. [13]. SR was also reaffirmed with F. oxysporum by Rivas et al. [18] using only molecular techniques, and they even found an isolate of F. proliferatum associated with symptomatic oil palm trees. However, in countries like Colombia, Phytophthora palmivora has been found to be the causal agent of oil palm SR [15]. In Ecuador, this disease is characterized by young leaves yellowing, and flag leaf rot progressing to the meristematic tissues causing palm death [13]. There are suggestions that two different SR syndromes coexist, both affecting oil palm in the country. The first is a very fast collapse of the spear and decay of its meristematic zone, and the second is frequent shortening of newly emerged fronds and slower rot and death [12,17]. However, symptoms in oil palm plants could be different in Brazil, Colombia, and Ecuador [19]. For example, wet rot meristem and the recovery of diseased plants do not occur in Brazilian plants. The use of genetic material resistant or tolerant to bud rot is part of strategies to combat disease [20]. Although genetic resistance is one of the strategies that is likely to offer a solution in the medium and long term [14], in Ecuador it is still nonexistent.
It is well-known that some oil palm diseases and insect pests have an interaction [4,8,17] and that SR could be transmitted by R. palmarum in oil palm crops [5]. Despite this fact, the mode of transmission is still unknown. On the other hand, for rational insect pest management practices to be developed, a sound knowledge of palm pathology and ecology of the associated insect pests is a prerequisite [21]. With the countless attempts to identify the SR causal agent, the disease worsening, and the BW in Ecuador over several decades, the use of a new research focus has become imperative, such as an epidemiological study of both biotic factors. In many cases, an analysis of this magnitude may be enough to implement or formulate a general management strategy to solve the problem [22].
The modeling of diseases and pests, as well as the damage they can cause to the crops, could help characterize their development and could lead to statements about future disease levels [16,23]. In the case of SR and BW, the reduction in the proportion of productive trees per unit area affects yield dramatically in oil palm trees [5,24]. Disease progress can be modeled using both linear and non-linear models [22]. For SR, the non-linear Gompertz and logistic models present the best predictions concerning the real data [25]. From a practical point of view, a given epidemic caused by diseases or pests in an agro-productive system may or may not lead to damage levels sufficient to cause crop losses [23]. From that perspective, there are more questions than answers regarding this disease and pest. On the other hand, despite many efforts made to identify the causal organism of bud or SR, the disease has advanced considerably in oil palm areas in Ecuador.
No studies exist evaluating SR progress and BW population fluctuation, as well as the synergism of these both biotic factors on oil palm trees in Ecuador. The hypothesis proposed for this work is that the temporal progress of insect and disease negatively affects palms [5]. An epidemiological study of the disease and population of R. palmarum under natural conditions in oil palm-susceptible trees can reveal valuable information about the possible interaction between both organisms and help in the selection of efficient crop management strategies. In the present study, we also evaluate and discuss the damage and losses caused by both biotic factors. Therefore, this study aimed to model the progress of Black weevil and Spear rot, as well as its damages in two highly susceptible oil palm trees, for the first time under field conditions in Ecuador.
2. Materials and Methods
2.1. Geographic Location and Agroclimatic Characteristics of the Study Area
The research was carried out between 2016 and 2020, at the San Sebastian farm located in Quinindé city, Esmeraldas’ province, Ecuador, at 00°5′5.3″ N 79°40′8.6″ W. The commercial oil palm crop is located at 208 meters above sea level, with an average annual air temperature of 24.7 °C, relative humidity of 87%, and accumulated rainfall of 2704.3 mm. The soil is a clay loam, classified as an Inceptisol. The farm climate has a humid mega-thermal tropical climate according to Köppen’s classification [26] characterized by having a rainy season and a very marked dry season. The climatological data (Table 1) used in the present study were obtained from the Ecuadorian National Institute of Meteorology and Hydrology (INAMHI).

Table 1.
Observed meteorological conditions between 2016 and 2020. San Sebastián Farm, Esmeraldas province, Ecuador.
2.2. Field Experiment Conditions
Field experiments were conducted in a completely randomized design over a commercial oil palm crop farm during five growing seasons (2016–2020). Palm trees of the INIAP-TENERA and IRHO varieties, planted in 1999 and 2005, respectively, and established at 9 × 9 m distance, were used in the research. The farm was distributed in eight plots, with a 7.8 (±2.6) ha each, totaling 962 plants.
Agronomic crop management was carried out during the five years of research. Oil palm trees were fertilized only at the first year of the crop establishment, with a mixture (2 kg 3 plant−1) of nitrogen (20%), phosphorus (5%), potassium (13%), magnesium oxide (3.4%), and sulfur (1.9%). The phytosanitary pruning was carried out every July by removing old leaves or those that lost more than 50% of the leaf tissue. The weeds were controlled using a brush cutter every two to three months. No irrigation system was installed on the farm, instead, crop water needs were cope with rainfall. The oil palm bunches were harvested every 15 days in each farm plot.
2.3. Quantification of Black Weevil
Bucket traps were made from plastic containers (20 L), with semi-open lateral openings at the top (8 × 12 cm), to allow insects entry (Figure S1A), and were placed next to the tree one meter high from the soil base (Figure S1B). A synthetic trap was placed around the container up to the edge of the openings. Sugar cane pieces (10 g) smeared with a 250 mL (2:1) water and molasses solution were used as bait, which was changed weekly. Subsequently, the Rhynchophorol aggregation pheromone was hung inside the container, and changed every 3 months (Figure S1C). One trap per hectare was placed and monitored for weevil presence weekly.
2.4. Capture and Morphological Characterization of Rhynchophorus palmarum
Sampling
Female adults of Rhynchophorus palmarum (10 specimens) were collected from three trees randomly chosen in the different plots, which presented the characteristic crater symptom. For this, the base of the tree stem was cut with a chainsaw, until reaching its meristem, because the larvae tunnel into the crown and trunk (Figure S1D,E).
2.5. Characterization of Adult Insects
The taxonomic identity of adult specimens associated with dead palm trees was corroborated with the keys of Chamorro [27] and Vásquez et al. [28]. The observations, photographs, and illustrations for the morphological descriptions were performed in the Laboratory of Entomology from the Ecuadorian National Plant Protection Organization (AGROCALIDAD), using the stereomicroscope Olympus SZX16 (Olympus, Center Valley, PA, USA) with double objectives for 3.5× to 100× zoom, associated with a reflection and transmission led lights, as well a high-resolution camera Olympus CellSens imaging software (version 1.12, Olympus, Tokyo, Japan).
2.6. Spear Rot Assessment
The incidence of dead trees (%) was evaluated in all individuals of both genotypes in each plot (1121 ± 373 trees on average) during the five years (Figure S2). Dead trees were considered to be all those that exhibited among 80.1% and 100% of the injured lance-shaped leaf (severity scale 5), lacked functional leaves or in the worst case did not have leaves, and were devoid of inflorescence and fruits (Figure S2G,H), showing even symptoms of the crater (Figure S1H) [29]. The percentage of dead palms concerning the total number of trees planted in each plot was calculated.
2.7. Morphological and Molecular Characterization of Microorganism
Sampling
Tissue samples affected by SR were collected in November 2020 from three trees randomly chosen in some of the farm plots, showing a severity scale [29] of 2 (among 20.1% and 40% of the injured lance-shaped leaf, Figure S2A–C), 3 (among 40.1% and 60% of the injured lance-shaped leaf), and 4 (among 60.1% and 80% of the injured lance-shaped leaf, Figure S2D–F). The trees were cut at the base of the stem using a chainsaw and their leaves were carefully separated. Then, the bud above the meristematic zone (stipe) of the lance-shaped leaf was collected, and immediately stored in previously identified Ziploc plastic bags, to be transferred in a cooler box to the laboratory.
2.8. Isolation of Microorganisms
Infected lance-shaped leaf was cut into 4 to 5 mm pieces and surface disinfected by immersion and shaking in sodium hypochlorite (0.5%) for 30 s and rinsed with sterile distilled water twice. Dried samples were seeded in Petri dishes containing semi-selective culture media potato dextrose agar (PDA) with antibiotics and pimaricin, ampicillin, rifampicin, and pentachloronitrobenzene (PARP) to isolate fungi and oomycetes, respectively. The Petri dishes were kept at room temperature (24 ± 3 °C) and for 12 h under fluorescent light for four days. Pure fungal cultures were obtained from the mycelial colonies.
2.9. Morphological Characterization
Vegetative (phialides) and reproductive (macroconidia and microconidia) structures of 10-day colony were characterized microscopically. For this, microscopic preparations were mounted in a drop of sterile distilled water on glass slides. The width and length were measured on 50 randomly selected structures, using a camera coupled to a microscope (100×) and software for measuring and calibration.
2.10. Molecular Characterization
Fungal genomic DNA was extracted from mycelium grown from the edge of the fungal colony of an 8-day culture, using a protocol with minor modifications according to Gupta et al. [30]. A genomic region of the extracted material was amplified, using the ITS region of ribosomal DNA using universal primers ITS, RPB2, and EF1a [31,32]. The products obtained from the amplification were purified and sequenced on a 3730 XL DNA Analyzer (Applied Biosystems, Macrogen, Seoul, Korea).
Bioinformatic analysis was performed by concatenating the three sequences obtained from (ITS + EF + RPB2) by trimming the corresponding sequences for each region for the six different Fusarium spp. queried in the GenBank containing the three sequences (ITS, EF, rpb2). Fusarium isolates include the F. solani isolate ColPat-359, Neocosmospora sp. FSSC 28, strain CPC 28194, F. fujikuroi strain CBS 257.52, F. fujikuroi isolate HJGF1-2, F. dimerum strain CBS 108944, and F. cf. dimerum ATS65. Alignment was performed using MUSCLE in MEGAX [33] and the best model was used (Kimura 2-Paramete, assuming that a certain fraction of sites is evolutionarily invariable). A bootstrap test was performed with 1000 replications.
2.11. Fruit Bunches Yield
Fruit bunches were harvested in trees of both varieties, using a Malay-type sickle every 15 days. The annual yield (tons) was obtained from the total production of fruits during the respective year. To obtain financial income per hectare, the value of each ton of fruit bunches was multiplied by the mean FAOSTAT historical data value for Ecuador (195 USD).
2.12. Statistical Analyses
Data obtained over time from the quantification of the incidence of dead plants (once a year) and the number of adult insects per trap (once a week) in the entire growing area (eight plots) were used for the analyses.
For plant disease progress analysis, the disease progress rate (r) was calculated using the logistic model equation of Berger [34] (Equation (1)) as follows:
where = disease proportion (0 < <1), = logit (), = rate, and = time; and the Gompertz model equation [35,36] (Equation (2)) is as follows:
where is a position parameter, = rate, and = time, and the exponential model equation (Equation (3)) as follows:
where is the initial value, is the rate, and t is the time.
Mean values of epidemiological parameters, as well as dead plants, number of insects, and fruit bunches yield evaluated at the last year of evaluation were compared using unpaired t-tests (p < 0.05). Correlation tests were performed to evaluate the association between dead plants and insects using the Pearson correlation test. For this, the Shapiro–Wilk normality test was used for both variables. All calculations were performed using the statistical packages Generalized Nonlinear Models (GNM) described by Turner and Firth [37], Nonlinear Regression for Agricultural Applications (NLRAA) Archontoulis and Miguez [38], and the epifitter available in Rstudio CRAN library [39].
3. Results
3.1. Brief Description of The Adult Species
The adult specimen considered here was assigned to the curculionid subfamily Dryophthorinae, based on the following characteristics: tarsal formula 4-4-4; mandible with two broad lobes; ventral space between antennal scrobes rugosus with several long, slender setae; middle and hind tibiae without distal spines; pygidium convex dorsally; setae beneath third tarsal segment covering one-half the entire area; subrostrum oval; antenna thick; scutellum produced posteriorly; body completely black; and large eyes, that elongate in an oval, narrowly or widely separated above (Figure S3).
3.2. Characterization of Plant Pathogen
In the morphological characterization of biological agents, the fungal strain FIAG-Fs1 presented mycelial growth with a yellowish-brown color, both at the top and at the bottom on Petri dish containing the potato dextrose agar culture media, producing microconidia, macroconidia, and long phialides (Figure S4). Mycelium, microconidia (6.4 ± 4.5 ×1.4 ± 1.0), macroconidia (24.6 ± 4.0 × 5.2 ± 1.4), and long phialides (45.4 ± 4.4) hyaline were observed. On the other hand, phylogenetic analysis of the concatenated sequences ITS, RPB2, and EF1a showed 100%, 99.3%, and 97.8% similarity to Fusarium solani, respectively (Figure S5).
3.3. Temporal Analysis of Black Weevil and Spear Rot
The number of adult insects increased over the years (Figure 1), reaching a median of 17 insects on average. The INIAP-Tenera trees (17 insects on average) obtained the highest number of insects in the years 2017 and 2019 (Figure 1A), compared to the IRHO ones (15 insects on average). Among August and October, an increase in insects was observed (Figure 1B).
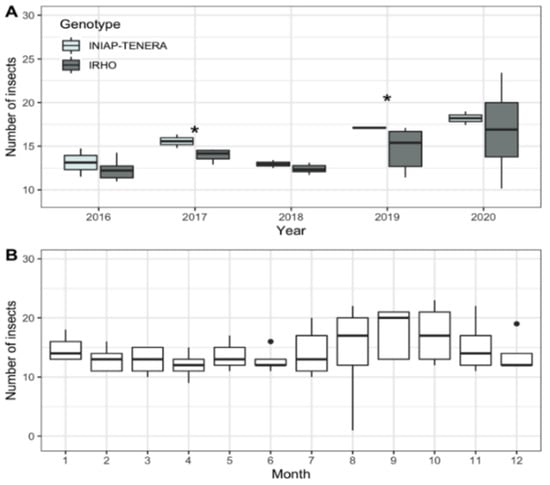
Figure 1.
Box plots of the total number of insects for different years (2016–2020) (A) and monthly mean (B) on INIAP-TENERA and IRHO oil palm trees. San Sebastián Farm, Esmeraldas province, Ecuador. Black dots represent outliers. * indicate the statistical difference.
In general, the temporal SR progress (a disease caused by a monocyclic pathogen) generated a polyetic epidemic (continuous epidemic over the years) in the INIAP-TENERA (Figure 2A) and IRHO (Figure 2B) genotypes, increasing the incidence of dead trees over time. Exponential (Figure 2 A) and logistic (Figure 2B) models better represented the epidemic, observing its curves closest to the SR evaluation points. The residuals of each epidemiological model evaluated higher dispersion points in the INIAP-Tenera genotype only in 2019 and 2020, using the three epidemiological models (Figure 3). In contrast, the points in genotype IRHO were closer to the line only in the logistic model (Figure 3).
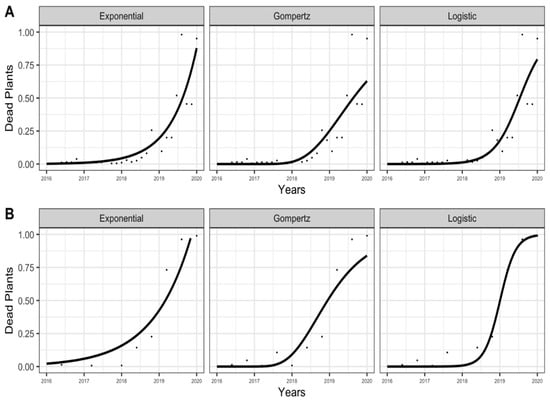
Figure 2.
Adjusted progress curves of Spear rot for the dead plant proportion on INIAP-TENERA (A) and IRHO (B) oil palm trees, using exponential, Gompertz, and logistic epidemiological models evaluated between 2016 and 2020. San Sebastián Farm, Esmeraldas province, Ecuador.
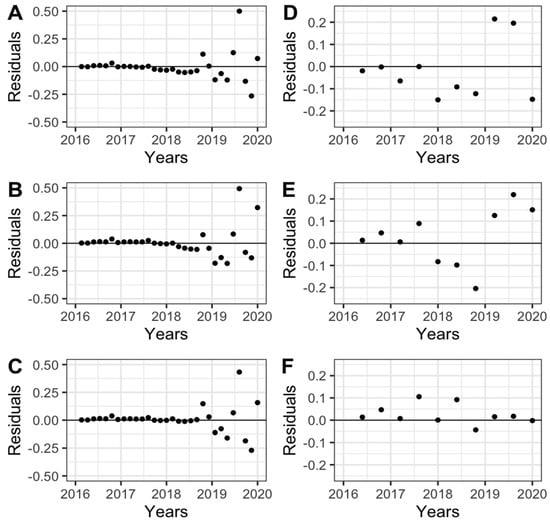
Figure 3.
Residuals of the adjusted progress curves of Spear rot for the dead plant proportion on INIAP-Tenera (A–C) and IRHO (D–F) oil palm trees, using exponential (A,D), Gompertz (B,E), and logistic (C,F) epidemiological models evaluated between 2016 and 2020. San Sebastián Farm, Esmeraldas province, Ecuador.
Concerning the epidemiological parameter obtained from dead plants (Table 2), using the exponential model, both the initial disease () and the disease progress rate () were on average six times higher in the var. INIAP TENERA, compared to the other genotype. On the other hand, using the logistic model, the r was five-fold higher in trees from INIAP TENERA, compared to those of the other variety. The three non-linear models generated high regression coefficients (between 0.89765 and 0.98962) and were significant (between 0.00083 and 0.00772) for the var. INIAP TENERA (Table 2). However, for the IRHO genotype, all non-linear models presented regression coefficients smaller than 0.80622, being significant (less than 0.00001) only in the exponential and the logistic models (Table 2). The mean square of the remainder was lower in the INIAP TENERA (0.02541) and IRHO (0.42430) genotypes using the logistic model compared to others (Table 2).

Table 2.
Epidemiological parameters using the logistic, exponential and Gompertz models, obtained from dead plants incidence by Spear rot in trees of varieties INIAP TENERA and IRHO. San Sebastián Farm, Esmeraldas province, Ecuador.
3.4. Dead Plants by Spear Rot, Number of Black Weevil Adults, and Fruit Bunches Yield
Dead plant incidence caused by SR was 40% higher in the INIAP TENERA genotype when compared to the IRHO variety (Figure 4A). Meanwhile, the number of adult insects (Figure 4B) and the fruit bunches yield (Figure 4C) were similar in trees of both varieties.
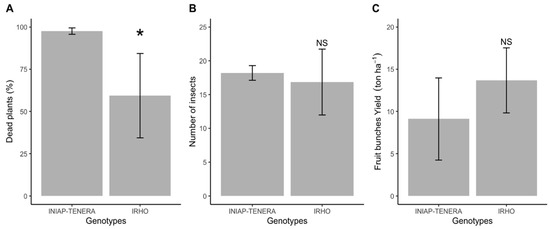
Figure 4.
Dead plants (%) by Spear rot (A), number of Black weevil adults (B) and fruit bunches yield (ton ha−1, C), on INIAP-TENERA (A) and IRHO (B) oil palm trees, evaluated in 2020. San Sebastián Farm, Esmeraldas province, Ecuador. * indicate the statistical difference between genotypes. NS indicate that there is no statistical difference between genotypes.
3.5. Relationship and Correlation among Variables
In our work, no correlation was observed between the total number of insects and the meteorological variables (Table 3).

Table 3.
Correlation coefficients (r) and probability values (p-values) between the total number of insects and meteorological variables. San Sebastián Farm, Esmeraldas province, Ecuador.
We noted a strong relationship between fresh fruit bunches (FFB) yield (Figure 5A), net income (NI) (Figure 5B), and number of insects (R2: 0.61; p-value: 7.967 × 10−7) and dead plants (R2: 0.78; p ≤: 5.617 × 10−13). The FFB was well determined by insects’ occurrence and death plants, with an R2 value of approximately 0.62, whether net income (NI) presented a value of 0.78, as may be observed in Table 4. The results of the multiple linear regression model showed that NI decreases 343.32 USD per year, and FFB yield also decreases 0.18 Ton ha−1 per year for each insect per trap. Nevertheless, for FFB and NI, the relations are more complicated, and it is necessary to individually analyze each component to improve our understanding. The number of insects and dead plants explains, in the mean, 70% of the FFB and NI variability during the five evaluated seasons (R2 between 0.61 and 0.78, shown in Table 4). The surfaces shown in Figure 5A,B indicate that the decrease in FFB and NI is associated with the increase of insects and death plants and that this relationship occurs in a hyperbolic pattern in most seasons (data not shown). The number of insects and dead plants explains, in the mean, 70% of the FFB and NI variability during the five evaluated years. The correlation between dead plants and the number of insects was significant (R2: 0.73; p ≤: 6.8× 10−8 Figure 6).
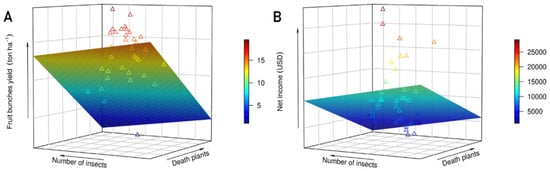
Figure 5.
Relation of the yield of the fruit bunches (A) and the net income (B) with the number of insects and dead plants in terms of the annual mean for the 2016–2020 season. San Sebastián Farm, Esmeraldas province, Ecuador.

Table 4.
Linear and angular coefficients of the multivariate linear models to estimate fruit bunches yield (ton ha−1) and net income (USD ha−1), considering the variables plant mortality and number of insects.
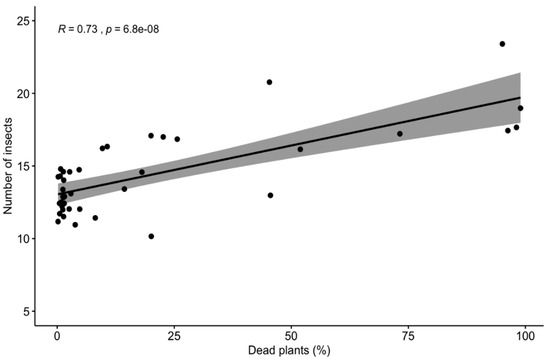
Figure 6.
Correlation between dead plants (%) and the number of insects quantified in INIAP-TENERA and IRHO oil palm trees.
4. Discussion
Despite the importance of BW and SR in oil palms in Ecuador, less research has been done to study both biotic factors. In our study, for the first time in the country, we describe the fluctuation of the BW population, model the temporal progress of SR, and evaluate the damage and losses of oil palms caused by both organisms. Additionally, we characterize both the insect pest and the fungus, something that is rarely done in field research.
Although it also used a selective medium for oomycetes, Fusarium solani (M98 strain) was only found in one of the three analyzed samples obtained from diseased trees. It is known that Phytophthora palmivora [12,15] and different Fusarium species, including F. solani [11,12,40,41,42] have been associated with oil palm SR trees in different places worldwide. In Ecuador, F. solani, F. oxysporum, and F. proliferatum have been associated with the disease [13,18]. Thus, our report of the association of F. solani with SR disease in Ecuador coincides with that of several authors from Ecuador, Colombia, and the Asian continent. It should be mentioned that this report is very important because, both in Ecuador and in several countries, these pathogens may rarely be isolated from infected palms and no positive results have ever been obtained by inoculating these fungi on healthy plants [17].
Differences in the number of insects of R. palmarum between genotypes were only in the years 2017 and 2019 over time. Perhaps, the age of the var. INIAP-Tenera could have influenced this behavior. Additionally, the number of adults increased in both genotypes over the years. The number of adults beetles can enhance from one year to the next, especially in diseased palm trees, rather than in healthy ones [5]. On the other hand, an increase in insects was observed in oil palm trees, between August and October, generally considered to be the dry season. These results are congruent with those of others experiments carried out both in Mexico and Brazil, where the number of captured insects may be affected by rainfall variation, increasing in the dry months, coinciding with the synchrony between the biological cycle of this beetle [4,8]. Although precipitation seems to negatively influence the number of beetles, the correlation between both parameters was not significant (p > 0.0605).
The three nonlinear models showed high regression coefficients and were significant for the var. INIAP-Tenera. Meanwhile, all nonlinear models presented regression coefficients less than 0.80622 for the IRHO genotype, being significant only in the exponential and the logistic models. Interestingly, both the Gompertz and logistic models can present the most concordant values between the predictions made by the models and the real data for Spear rot [25]. Either way, the lower mean square of the remaining data was obtained in both susceptible genotypes using the logistic model, followed by the exponential model. Thus, it seems plausible to suppose that there may be some difference between the genotypes evaluated by us and those of other investigations. Spear rot progress curves may be adjusted to the Gompertz model, especially susceptible progenies of oil palm. However, the disease progression in more resistant progenies tends to follow the monomolecular model [41]. On the other hand, the adjustment of traditional epidemiological models to the data, despite the high correlation coefficients, does not correctly explain the behavior of the disease, having to be demonstrated with the mean square of the residue (MSR) for each curve or residuals. In this regard, Bergamin et al. [22] in Brazil also obtained similar results but rejected this interpretation due to the poor result of the residues. They chose to explain the data with a more-than-exponential model, a model that is oblivious to the growth of diseases caused by biotic pathogens. Taking this information as a reference, it could be that the logistics model, having a lower MSR, would be the nonlinear model that would better explain the epidemic in both susceptible genotypes.
Dead palms incidence by SR was higher in INIAP-Tenera trees when compared to those of IRHO in the year 2020. Two hypotheses may be puts forward to explain this result: (1) the age of trees and (2) the pathogen inoculum source. Oil palm INIAP-Tenera trees were established in the field with a difference of six years compared to var. IRHO. Thus, both disease plants and dead trees’ trunks of INIAP-Tenera can represent direct sources of infection [5,43].
In oil palm, whether cultivated in large areas or small farms, pests and diseases avert normal healthy growth and can cause large yield reductions. If a great outbreak is not controlled on time, severe defoliation will happen and that would outcome in crop yield losses [44]. Thus, the crop loss is a direct result of oil palm being killed by the grub’s development in the palm trunk.
The percentage of dead plants as well as the presence of several insects were positively correlated. Generally, the number of pests may be significantly higher in the holes made at the base of diseased oil palm trees with SR, compared to healthy individuals analyzed on the same plots [17,25]. The hypothesis of spread of SR by mechanical transmission through harvesting and pruning has no empirical support, mainly due to the random occurrence of diseased trees, and it is plausible to assume that the causal agent of SR could be vector-borne like R. palmarum [16]. Perhaps, the contact of the insect with the pathogen in root crown tissues of diseased trees would spread F. solani structures to healthy trees more quickly. The damage caused by the larvae is only visible long after infestation, and by the time the first symptoms of the attack appear, they are so serious that they generally result in the death of the tree. Thus, the association between R. palmarum and SR disease may be a cause of death and a great loss to the oil palm plantations over time [5]. At present, the latter factor can reduce further expansion of palm oil crops and the survival of existing ones, making it likely that SR levels increase not only in Ecuador, but also worldwide [45].
As final considerations, we corroborate our hypothesis formulated at the beginning of the work related to synergism between R. palmarum and SR disease. This and the rest of the findings presented in this study are of great importance to both Ecuadorian and world palm cultivation, especially to researchers dedicated to the management of both biotic factors. For example, from a practical point of view, the epidemiological analysis of SR may be enough for the implementation or formulation of a general management strategy to manage the disease [22]. Something similar may be emphasized around the population fluctuation of BW, being able to take more efficient management measures, especially during the months of highest incidence. Even detection efficiency could be increased by using other types of traps such as Picusan, which are more effective than bucket traps [46]. Finally, the significant interaction found between SR and R. palmarum puts everyone on alert, especially government control agencies such as Agrocalidad, because both biotic factors should be managed together and not separately.
5. Conclusions
In conclusion, the BW morphological analysis and the morphomolecular study of the causal agent of SR allowed us to identify Rhynchophorus palmarum and Fusarium solani, respectively, as the biotic factors that affect oil palm trees in this part of Ecuador. The number of adults increased in both genotypes over the years, but much more in INIAP-Tenera oil palm trees (years 2017 and 2019), especially between August and October, months that are generally considered to be the dry season. Regarding temporal analysis, SR progress curves are adjusted to the non-linear logistic model. The incidence of dead palms was higher in INIAP-Tenera trees compared to those of IRHO in the year 2020. To our knowledge, this is the first report on the positive correlation among dead plants (%) by SR and BW, and the study of both biotic factors in Ecuador.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agriculture12020257/s1, Figure S1: Capture of Black weevil in traps and on IRHO oil palm trees. Figure S2: Spear rot in IRHO and INIAP-TENERA oil palm trees. Figure S3: Male adult, habitus Rhynchophorus palmarum L. Figure S4: The monosporic Fusarium solani strain FIAG-Fs1 isolated from INIAP-TENERA-symptomatic plants with Spear rot. Figure S5: Phylogenetic analysis of the concatenated sequences ITS + EF + rpb2 of the M98 isolate with the other six different accessions queried in GenBank.
Author Contributions
L.A.G.-Q., A.L.S.-B. and F.R.G.-F. conceived and designed the research. L.A.G.-Q. carried out experiments in the field for 5 years. L.A.G.-Q., D.P. and F.R.G.-F. conducted experiments, analyzed the data, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JOYAPALMA CIA. LTDA and Oil Extractor LA JOYA EXTRAJOYA CIA. LTDA. company.
Institutional Review Board Statement
Not applicable.
Acknowledgments
Authors are grateful to Hector Cedeño Pinargote and Sebastián Alzamora Donoso, Oil Extraction Company ‘La Joya Extrajoya’ CIA. LTDA, for having provided the facilities to carry out the present research. Likewise, we thank Bergamin Filho and Naga Raju Maddela for the revision of the manuscript, and José Velásquez, Efrén Santos, and Alejandro Mieles for the aid in identifying the biotic agents under study.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Nair, P.K. Tree Crops. Harvesting Cash from the World’s Important Cash Crops, 1st ed.; Springer Nature: Cham, Switzerland, 2021; pp. 249–285. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Censos (INEC). Boletín Técnico. Encuesta de Superficie y Producción Agropecuaria Continua, 1st ed.; INEC: Quito, Ecuador, 2020; pp. 1–13. Available online: https://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_agropecuarias/espac/espac-2019/Boletin%20Tecnico%20ESPAC_2019.pdf (accessed on 14 June 2021).
- Aucique-Pérez, C.E.; Daza, E.S.; Ávila-Diazgranados, R.A.; Romero, H.M. Chlorophyll a fluorescence and leaf temperature are early indicators of oil palm diseases. Sci. Agric. 2020, 77, 1–6. [Google Scholar] [CrossRef]
- Landero-Torres, I.; Presa-Parra, E.; Galindo-Tovar, M.E.; Leyva-Ovalle, O.R.; Murguía-González, J.; Valenzuela-González, J.E.; García-Martínez, M.Á. Variación temporal y espacial de la abundancia del Picudo negro (Rynchophorus palmarum L., Coleoptera: Curculionidae) en cultivos de palmas ornamentales del centro de Veracruz, México. Southwest. Entomol. 2015, 40, 179–188. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; Fernandes, F.L.; De Sousa Ramalho, F.; Zanuncio, J.C.; Serrão, J.E. Interactions between the bud rot disease of oil palm and Rhynchophorus palmarum (Coleoptera: Curculionidae). J. Econ. Entomol. 2016, 109, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Murguía-González, J.; Landero-Torres, I.; Leyva-Ovalle, O.R.; Galindo-Tovar, M.E.; Llarena-Hernández, R.C.; Presa-Parra, E.; García-Martínez, M.A. Efficacy and cost of trap–bait combinations for capturing Rhynchophorus palmarum L. (Coleoptera: Curculionidae) in ornamental palm polycultures. Neotrop. Entomol. 2018, 47, 302–310. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Hoddle, C.D.; Milosavljević, I. Quantification of the life time flight capabilities of the south American palm weevil, Rhynchophorus palmarum (L.) (Coleoptera: Curculionidae). Insects 2021, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.; Farias, P.R.; Rodrigues, K.C.; Tinôco, R.; Santos, A.V.; Marssena, R.T. Distribuição espacial de Rhynchophorus palmarum em palma de óleo no Estado do Pará, Amazônia. Cienc. Agrar. 2016, 59, 22–31. [Google Scholar] [CrossRef]
- Milosavljević, I.; El-Shafie, H.A.F.; Faleiro, J.R.; Hoddle, C.D.; Lewys, M.; Hoddle, M.S. Palmageddon: The wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest. Sci. 2019, 92, 143–156. [Google Scholar] [CrossRef]
- Saïd, I.; Kaabi, B.; Rochat, D. Evaluation and modeling of synergy to pheromone and plant kairomone in American palm weevil. Chem. Cent. J. 2011, 5, 14. [Google Scholar] [CrossRef]
- Suwandi–Akino, S.; Kondo, N. Common spear rot of oil palm in Indonesia. Plant Dis. 2012, 96, 537–543. [Google Scholar] [CrossRef][Green Version]
- de Assis Costa, O.Y.; Tupinambá, D.D.; Bergmann, J.C.; Barreto, C.C.; Quirino, B.F. Fungal diversity in oil palm leaves showing symptoms of fatal yellowing disease. PLoS ONE 2018, 13, e0191884. [Google Scholar] [CrossRef]
- Ronquillo-Narváez, M.; de Jensen, C.E.; Bernal, G. Fusarium spp. asociados a la pudrición del cogollo de la palma aceitera (Elaeis guineensis Jacq.) en Ecuador. J. Agrie. Univ. P. R. 2013, 97, 135–148. [Google Scholar]
- De Franqueville, H. Oil palm bud rot in Latin America. Exp. Agric. 2003, 39, 225–240. [Google Scholar] [CrossRef]
- Torres, G.A.; Sarria, G.A.; Varon, F.; Coffey, M.D.; Elliott, M.L.; Martinez, G. First report of Bud rot caused by Phytophthora palmivora on African oil palm in Colombia. Plant Dis. 2010, 94, 1163. [Google Scholar] [CrossRef] [PubMed]
- Van De Lande, H.L.; Zadoks, J.C. Spatial patterns of spear rot in oil palm plantations in Surinam. Plant Pathol. 1999, 48, 189–201. [Google Scholar] [CrossRef]
- Perthuis, B. Association of the soil insect Scaptocoris minor Berg (Heteroptera, Cydnidae) with foci of bud-rot diseases of oil palm in eastern Ecuador. Int. J. Trop. Insect. Sci. 2020, 41, 1883–1887. [Google Scholar] [CrossRef]
- Rivas-Figueroa, F.; Herrera-Isla, L.; Borrás-Hidalgo, O. Molecular diagnosis of Fusarium spp. isolates associated to bud rot of Oil palm in Ecuador. Biotecnol. Apl. 2015, 32, 2221–2223. [Google Scholar]
- Swinburne, T. Fatal yellows, bud rot and spear rot of African oil palm—A comparison of the symptoms of these diseases in Brazil, Ecuador and Colombia. Planter 1993, 69, 15–23. [Google Scholar]
- Avila-Diazgranados, R.A.; Daza, E.S.; Navia, E.; Romero, H.M. Response of various oil palm materials (Elaeis guineensis and Elaeis oleifera × Elaeis guineensis interspecific hybrids) to bud rot disease in the southwestern oil palm-growing area of Colombia. Agron. Colomb. 2016, 34, 74–81. [Google Scholar] [CrossRef]
- Gitau, C.W.; Gurr, G.M.; Dewhurst, C.F.; Fletcher, M.J.; Mitchell, A. Insect pests and insect-vectored diseases of palms. Aust. J. Entomol. 2009, 48, 328–342. [Google Scholar] [CrossRef]
- Bergamin Filho, A.; Amorim, L.; Laranjeira, F.F.; Berger, R.D.; Hau, B. Análise temporal do amarelecimento fatal do dendezeiro como ferramenta para elucidar sua etiologia. Fitopatol. Bras. 1998, 23, 391–396. [Google Scholar]
- Savary, S.; Nelson, A.D.; Djurle, A.; Esker, P.D.; Sparks, A.; Amorim, L.; Bergamin Filho, A.; Caffi, T.; Castilla, N.; Garrett, K.; et al. Concepts, approaches, and avenues for modelling crop health and crop losses. Eur. J. Agron. 2018, 100, 4–18. [Google Scholar] [CrossRef]
- Van De Lande, H.L. Spatio-temporal analysis of spear rot and ‘marchitez sorpresiva’ in African oil palm in Surinam. Neth. J. Plant Pathol. 1993, 99, 129–138. [Google Scholar] [CrossRef]
- Benítez-Sastoque, E.R. Epidemiología de la Pudrición del Cogollo de la Palma de Aceite. Ph.D. Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2011. Available online: https://repositorio.unal.edu.co/handle/unal/52477 (accessed on 14 June 2021).
- Köppen, W. Classification of climates according to temperature, precipitation and seasonal cycle. Petermanns. Geogr. Mitt. 1918, 64, 193–203. [Google Scholar]
- Chamorro, M.L. An illustrated synoptic key and comparative morphology of the larvae of Dryophthorinae (Coleoptera, Curculionidae) genera with emphasis on the mouthparts. Diversity 2019, 11, 4. [Google Scholar] [CrossRef]
- Vásquez-Ordóñez, A.A.; Löhr, B.L.; Marvaldi, A.E. Comparative morphology of the larvae of the palm weevils Dynamis borassi (Fabricius) and Rhynchophorus palmarum (Linnaeus) (Curculionidae: Dryophthorinae): Two major pests of peach palms in the Neotropics. Pap. Avulsos. Zool. 2020, 60, e202060.27. [Google Scholar] [CrossRef]
- Martínez-López, G.; Torres, G.A. Presencia de la pudrición del cogollo de palma de aceite (PC) en plantas de vivero. Palmas 2007, 28, 13–20. [Google Scholar]
- Gupta, V.K.; Tuohy, M.G.; Gaur, R. Methods for high-quality DNA extraction from fungi. In Laboratory Protocols in Fungal Biology, 1st ed.; Gupta, V., Tuohy, M., Ayyachamy, M., Turner, K., O’Donovan, A., Eds.; Springer: New York, NY, USA, 2013; pp. 403–406. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Geiser, D.M.; del Mar Jiménez-Gasco, M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’donnell, K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant. Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.D. Comparison of the Gompertz and Logistic equations to describe plant disease progress. Phytopathology 1981, 71, 716–719. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Tjørve, K.M.; Tjørve, E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE 2017, 12, e0178691. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.; Firth, D. Generalized Nonlinear Models in R: An Overview of the Gnm Package Generalized Linear Models Preamble, 1st ed.; ESRC National Centre for Research Methods: Coventry, UK, 2020; pp. 1–52. Available online: https://eprints.ncrm.ac.uk/id/eprint/472/1/0607_overview_of_the_gnm_package.pdf (accessed on 14 June 2021).
- Archontoulis, S.V.; Miguez, F.E. Nonlinear Regression models and applications in agricultural research. J. Agron. 2015, 107, 786–798. [Google Scholar] [CrossRef]
- R Studio Team. RStudio. Available online: http://www.rstudio.com/ (accessed on 14 June 2021).
- Wattanapongsiri, A. A Revision to the Genera Rhynchophorus and Dynamis (Coleoptera: Curculionidae). Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1966. Available online: https://hdl.handle.net/1957/10673 (accessed on 14 June 2021).
- Monge, J.E.; Chinchilla, C.M.; Wang, A. Studies on the etiology of the crown disease / spear rot syndrome in oil palm. ASD. Oil Palm Papers 1993, 7, 1–16. [Google Scholar]
- Hafizi, R.; Salleh, B.; Latiffah, Z. Morphological and molecular characterization of Fusarium solani and F. oxysporum associated with crown disease of oil palm. Braz. J. Microbiol. 2013, 44, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Flood, J.; Keenan, L.; Wayne, S.; Hasan, Y. Studies on oil palm trunks as sources of infection in the field. Mycopathologia 2005, 159, 101–107. [Google Scholar] [CrossRef]
- Chung, G.F. Effect of pests and diseases on oil palm yield. In Palm Oil: Production, Processing, Characterization, and Uses, 1st ed.; Oi-Ming, L., Chin-Ping, T., Casimir, C.A., Eds.; Academic Press and AOCS Press: Beltsville, MD, USA, 2012; pp. 163–210. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Future scenarios for oil palm mortality and infection by Phytophthora palmivora in Colombia, Ecuador and Brazil, extrapolated to Malaysia and Indonesia. Phytoparasitica 2020, 48, 513–523. [Google Scholar] [CrossRef]
- Milosavljević, I.; Hoddle, C.D.; Mafra-Neto, A.; Gómez-Marco, F.; Hoddle, M.S. Use of digital video cameras to determine the efficacy of two trap types for capturing Rhynchophorus palmarum (Coleoptera: Curculionidae). J. Econ. Entomol. 2020, 113, 3028–3031. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).