Abstract
Red palm weevil (RPW) is a dangerous pest that infects the date palm tree and makes tunnels in the tree’s trunk. RPW infection is followed by secondary invaders of microorganisms that cause degradation of the trunk tissues leading to toppling the crown and death of the tree in a short time. This study showed that Fusarium oxysporum Schlecht. was the common fungal species isolated from the root and trunk tissues of the date trees infested with RPW, which recorded 100% of frequency. Pathogenicity of 4 isolates of F. oxysporum was confirmed on date palm seeds and seedlings. The results confirmed that all isolates involved in rot of the germinated seeds (40–100% incidence), root rot, and death of palm seedlings (20–100%) under artificial infection as well as degradation of date palm petioles. Application of 20 L/palm of systemic fungicide (Topsin) at 1% as foliar and soil drench of date palm for successive three times successfully reduced development of the deterioration and suppressed the growth of F. oxysporum. Interestingly this fungicide did not suppress the growth of Trichoderma viride Pers. So, our results recommend this fungicide to suppress the pathological and degradable activities of F. oxysporum during the integrated pest management of RPW on date palm trees.
1. Introduction
Date palm (Phoenix dactylifera L.) is an important fruit crop grown in tropical and subtropical countries [1,2]. Arab countries of the Middle East and North Africa have about 100 million date palms cultivated in one million hectares [3]. Dates are considered a good source of human food because they have a considerable quantity of simple sugars, in addition, they could provide some antioxidants such as phenol. Some dates were characterized by antibacterial, antifungal, and antitumor properties [4,5,6,7].
Four decades ago, cultivation of date palms in the Middle East became under threat because of the red palm weevil “RPW” (Rhynchophorus ferrugineus Olivier) that was reported in more than 50% of the cultivated area [8,9]. The pest infects relatively young trees (below 20-years-old) and the grubs bore into the plant tissue causing tunnels on the trunk and base of the frond petiole. Along with the infestation, oozing out of fluid from the tunnels and odor of fermentation is smelt. In case of and prolonged infestation, the trees die shortly [10].
The disease becomes more complicated when the RPW is associated with other microbial pathogens such as fungi that cause wilt and root rot of the trees. This may cause deterioration and death of palm trees in a short time [11]. Deterioration of date palm by facultative saprophytic fungi i.e., Botryodiplodia theobromae Pat., Thielaviopsis paradoxa (De Seynes) Höhn, Rhizoctonia solani Kühn, Fusarium solani (Mart.) Sacc., and F. oxysporum Schlecht. was reported [4,12,13,14]. Other saprophytic fungi including Aspergillus niger van Tieghem, Alternaria alternata Fries) Keissler, and Penicillium Link spp. were isolated from the collapsed tissues of date palm trees [15,16]. The infestation of trunk tissues with these fungal species could be facilitated by the tunnel made by the RPW grubs or could be transmitted by the pest itself during infestation [17]. During management practices of RPW, farmers may not pay an attention to the dangerous effect of the associated microflora that will cause a severe infection of root rot or wilt for the trees. Consequently, the trees may suffer from a complex of diseases that will eventually lead to weakness or even a complete death. There were some reports about the low efficacy of pesticides, pheromone traps or biocontrol agents in suppression of RPW and reduction of date palm loss because of such complicated diseases [8,18,19]. From the point of integrated pest management (IPM) view, our investigation considers an effective management strategy in which both pesticide and fungicide should be applied to prevent the RPW and their associated pathogenic fungi to prevent the prolonged loss in date palm trees.
2. Materials and Methods
2.1. Isolation of Fungi Associated with Rotten Tissues of Date Palm Infested with RPW
Thirty samples of rotten roots were collected from date palm trees (cv. Zaghlol) showing RPW symptoms in Kafr El-Abida village, El-Mahalla El-Kobra District, El-Gharbeia Governorate, Egypt. Pieces of root tissues were picked up, washed under tap water three times, then their surfaces were sterilized by sodium hypochlorite 1% for 2 min. Thereafter, the sterilized root pieces were washed three times with sterilized distilled water and dried between two layers of sterilized filter papers (Whatman No. 1). Sterilized pieces were plated on potato dextrose agar medium (PDA) supplemented with streptomycin sulfate (50 µg/mL) to suppress the bacterial growth. The plates were incubated at 27 °C for 3–5 days. The emerged fungi were isolated and purified using single spore and hyphal tip culture techniques PDA. The purified cultures were kept on PDA slants for further work. All species were identified based on their macro and microscopic characteristics with the aid of specific manuals [20,21,22,23].
2.2. Count of Fungi of Date Palm Trunk Infected with RPW
The total count of fungal species isolated from date palm trunk (30 samples) infested with RPW was counted in 10 g of the date palm trunk tissues (cv. Zaghlol). Each sample (10 g of tree trunk) was added to 90 mL of sterilized distilled water in Erlenmeyer Flask 250 mL and shaken for 30 min. The dilution adjusted at 1 × 10−4, and then one ml of each sample was spread on Petri dish (9 cm diameter) containing 20 mL of Martin, agar medium supplemented with streptomycin sulfate (50 µg/mL). Three dishes were used as replicates from each sample. The inoculated dishes were incubated at 27 °C for 3–6 days, during which the emerged fungal colonies were counted. Fungal species were purified and identified as mentioned above.
2.3. Pathogenicity of F. oxysporum Isolates on the Germinated Seed of Date Palm
Inoculum of four isolates of F. oxysporum was prepared from 10-days-old cultures grown on PDA at 27 °C in sterilized distilled water (106 conidia/mL). Date palm seeds of cv. Zaghlol were surface sterilized with 2% of sodium hypochlorite for 3 min before their germination between two layers of wetted sterilized filter papers in Petri dishes (18-cm in diameter) for 15 days in dark incubation condition at 35 ± 2 °C. Four batches of 30 germinated seeds were artificially infected by soaking in the inoculum suspension of one of each of the fungal isolates for 30 min. In the control, 30 germinated seeds were soaked in sterilized water. The inoculated germinated seeds were transferred into new Petri dishes (18-cm in diameter) containing wetted sterilized filter papers to follow up the disease progress. Three dishes were used as replicates for each Fusarium isolate (10 seeds/dish).
The dishes were incubated for 15 days at 27 °C. The percentage of the rot of date palm germinated seeds was recorded according to Ziedan, Farrag and Sahab [12].
2.4. Pathogenicity Potential of F. oxysporum Isolates on Date Palm Seedlings
A six-month-old seedling of date palm (cv. Zaghlol) was cultivated in a glass bottle of 500 mL containing peat moss infected with 10 mL of inoculum of each isolate of F. oxysporum (106 conidia/mL). Fifteen bottles were used for each isolate. In the control, peat moss was mixed with 100 mL sterilized water (without fungal infection). The bottles were incubated at 35 ± 2 °C and 85% relative humidity for 30 days. Plants were watered regularly, with avoidance of wetting the foliage at 5-days intervals. Percentage of root rot of date palm seedlings and disease severity on the shoot and root system was determined according to Ziedan, et al. [24]. Disease severity on the shoot system was determined using a linear scale from 0 to 4; where 0 = no wilted leaf, 1 = one leaf wilted, 2 = two leaves wilted, 3 = three leaves wilted, and 4 = whole plant wilted. The disease severity on root system was estimated as follows: 0 = no discoloration on root, 1 = 1–25% brown discoloration of root 2 = 26–50% brown discoloration, 3 = 51–75% brown discoloration, and 4 = 76–100% brown discoloration using the following formula:
where: n = number of plants in each numerical disease grade; r = Number of the disease grade and N = Total number of plants multiplied by the maximum numerical disease grade.
Disease severity = Σ (n × r) × 100/N
2.5. Degradation Potential of Date Palm Petioles Tissue by F. oxysporum Isolates
The degradation potential of F. oxysporum fungal isolates was tested on fresh pieces of date palm petioles (about 50 g of tissues per isolate) and were cut in small pieces (5 g/piece). The pieces were sterilized by dipping in sodium hypochlorite 1% for 3 min, and then they were dried between two sterilized layers of filter papers. Fifteen pieces were used as replicates for each fungal isolate (5 g/piece). The infection was done by spraying 10 mL of conidial suspension (106/mL) of each isolate on the petiole tissues’ pieces, while pieces sprayed with sterilized distilled water were served a control (15 pieces). Each sterilized piece was incubated in a plastic cage at 28 ± 2 °C for 30 days. Loss of pieces weight was determined according to Al-Hamdany, et al. [25]. The experiment was repeated twice.
2.6. Management of Deterioration on Date Palm Tree Infested with RPW and Associated F. oxysporum
Systemic fungicide Topsin M-70 (Thiophanate-methyl) was prepared at 1% by dissolving 200 g in 20 L of water and applied one week later after application of the insecticide Malacun (1%) (Malathion) manufactured by National Chemical Company for Chemicals Production, Alexandria, Egypt following the manufacturing procedures by dissolving 400 g in 20 L of water and it was used as a spray. The application of fungicide and insecticide was carried out as foliar spray on the tree trunk and soil drench around trunk base of date palm trees (Cv. Zaghlol), three times during spring (March) summer (July), and autumn (October). In this experiment, 20 date palm trees were involved. One year after the first treatment, suppression of deterioration was observed and only Trichoderma viride was isolated from the newly growing lateral root. The fungus was identified according to Kumar et al. [26].
2.7. Statistical Analysis
The obtained data were tested for homogeneity and analyzed using the One-Way ANOVA test. The mean was compared for the significant difference using Duncan’s multiple range test or t-test of two independent samples (in case of 2 treatments only) at p < 0.5 according to [27].
3. Results
3.1. Root Rot and Fungi Associated with RPW Infestation
Seven fungal species were isolated from the roots of date palm trees infested with RPW, Fusarium solani was the most frequent fungus that recorded 54.2%. Rhizoctonia solani appeared in 5.9% of samples, however, Botryodiplodia sp. was observed in 2.9% of the examined root samples. Aspergillus was represented by three species with moderate to low frequencies, however, Trichoderma was seen in the frequency of 2.9% (Table 1). In most all cases the roots, from which microorganisms were isolated, showed root rot symptoms with a black discoloration, maceration of root tissue, dryness, and lysis of cortex tissue (Figure 1).

Table 1.
Frequency % of fungi associated with RPW during an infestation of date palm roots.

Figure 1.
Root rot symptoms of date palm infested with the RPW. Healthy normal roots (A) and infested roots showing a black discoloration and maceration (B).
3.2. Fungal Counts Associated with Date Palm Tree Trunk Infested with RPW
To follow the succession of the fungal species in date palm trees infested RPW, we examined two types of infestation: recently infested tissues and degraded tissue (old infested). Results showed that F. oxysporum was the only species isolated from 100% of the recently infested tissues (Table 2). However, this fungus was completely absent from the degraded tissues it was substituted with two saprophytes: Aspergillus niger and Penicillium sp. which were isolated in high count (16.0 × 104/g). Figure 2 confirms the presence of fungal mycelia in both recently infested degraded tissues, however, the degraded tissues were intensively occupied by the fungal mycelia.

Table 2.
Fungal count (CFU) and frequency % on date palm infected with RPW.

Figure 2.
The appearance of fungal mycelia in the date palm trunk infested with RPW. Recently infested trunk (left), degraded tissue (right).
3.3. Pathogenicity of F. oxysporum on Germinated Seeds of Date Palm
The results confirmed the pathogenicity of the four isolates of F. oxysporum on date palm germinated seeds and caused a clear rot on the radicals of the germinated seeds. Isolate No. 1 and No. 3 involved 100% of disease incidence, however the other 2 isolated cause 40–50% incidence (Table 3). Fusarium oxysporum isolates typically produced the symptoms of root rot including brown discoloration, maceration, and even the appearance of white mycelia on the germinated seeds (Figure 3).

Table 3.
Pathogenicity test of F. oxysporum isolates on date palm germinated seeds.

Figure 3.
Symptoms of root rot disease caused by four isolates of F. oxysporum on germinated seeds of date palm. Healthy (uninfected) germinated seeds (0), necrotic radicals infected with F. oxysporum (1–4) showing different degrees of rot and brown discoloration.
3.4. Pathogenicity of F. oxysporum on Seedlings of Date Palm
The four isolates of F. oxysporum caused a high percentage of seedling mortality (80%) except No. 3 that showed 20% of mortality of date palm seedlings after 30 days from infection. All isolates recorded high root rot percentage and disease severity on the shoot and root systems of date palm seedlings (Table 4). They recorded 80–100% of the disease incidence. The disease severity was 1.2–3.4 on the shoot system and 3.0–3.6 on the root system (Figure 4).

Table 4.
Root rot incidence and severity of F. oxysporum isolates on date palm seedlings.
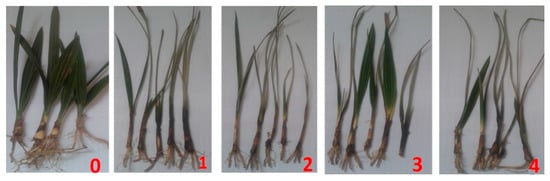
Figure 4.
Root rot severity F. oxysporum of on seedlings of date palm. Heathy uninfected control (0), and infected seedling with different isolates (1–4).
3.5. Degradation Potential of F. oxysporum Isolates on Date Palm
The degradable potential of F. oxysporum isolates was tested in vitro on artificially infected date palm petioles and the loss of petioles weight was consider as an indicator of the degree of degradation. Data in Table 5 showed that the greatest loss in date palm petioles weight was attributed to F. oxysporum isolate No. 4 which reduced the weight by 17.66% followed by isolates 3 which involved in 10.99% reduction in the petioles’ wright. Both other isolated involved in the reduction of the petioles weigh by 8.12% and 7.41%.

Table 5.
Degradation of F. oxysporum isolates on date palm tree.
3.6. Management of Deterioration of Date Palm Infected by (RPW)
Systemic fungicide (Topsin M-70) 10 g/L was applied on date palm plants (cv. Zaghlol) by the rate of 20 L/tree as a foliar spray on the trunks and as soil drench 3 times during (March, July, and October). The results confirmed the efficacy of the fungicide to suppress fungal growth and reduced the degradation process. Recovery signs were observed as the date tree trunk showing good formation of aerial roots inside the tunnels as well as the formation of external lateral roots outside the trunk square (Figure 5). Furthermore, when we reisolated the fungi from the aerial roots inside the tunnels, we did not get any pathogenic species, but Trichoderma viride was only isolated from the treated date palm trunks.
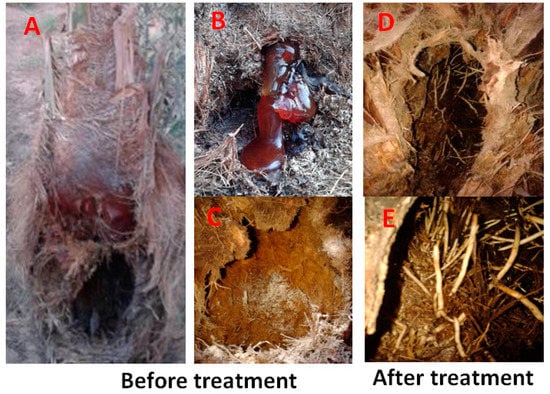
Figure 5.
The figure shows the symptoms of RPW infestation before and after treatment with the fungicide. Before treatment, the tunnels made by the pest is full of degraded tissues (A), the ooze of dark brown fluid is seen (B) and/or occupied by the fungal (C). Whereas after treatment the tunnels are free of mycelia with inhiation of the aerial roots (D), then the aerial roots are well developed, and degradation of the tissues was obscured (E).
4. Discussion
Rhynchophorus ferrugineus is an epidemic pest of date palm causing considerable damage to palm trees and reducing productivity [8]. During the life cycle, the pest is feeding on soft tissues inside the palm tree causing tunnels inside the tree trunk. Also, thick oozing yellow to brown fluid accomplished fermentation odor was noticed inside and around opening tunnels [28]. So, many saprophytic and pathogenic microorganisms could be associated with the pest infestation either carried by the pest body or as secondary invaders after pest entrance inside the tree trunk. The assumption was confirmed by the findings of many researchers who isolated many fungi and bacteria after RPW infestation [17,29]. Botryodiplodia theobromae, Thielaviopsis paradoxa, Rhizoctonia solani, and Fusarium spp. were among the dangerous fungal species that were isolated from the date palm infested with RPW in previous works [4,12,13,14]. Our study revealed that Aspergillus, Botryodiplodia, Fusarium, Rhizoctonia, and Trichoderma were the common genera isolated from the date palm roots infested with RPW. Isolation of these microorganisms from date palm tree as endophytic or seed-borne fungi were confirmed previously [30,31,32].
Fusarium oxysporum was the most frequent species associated with date palm trees infested with RPW. When F. oxysporum was tested for pathological potential, it caused root rot on the germinated seeds and seedlings. It involved in mortality of the seedlings and the wilt of shoot system of date palm seedlings. However, F. oxysporum is known as a main causal agent of root rot and wilt disease on many crops [33], to the best of our knowledge it is the first to report its deleterious effect on date palm seedlings in Egypt. It was noticed that the deterioration of date palm trunk infested with red weevil increased due to the synergistic effect of the secondary infection of fungi which could produce secondary metabolites and volatile toxic materials such as ethanol, acetic acid, and acyl acetate which move down to roots and apical meristem causing quick deterioration of date palm tissue [34]. These products were reported as toxic material to the date palm tissues [35] and negatively affected the growth of plant roots and seedlings [36]. In this study, F. oxysporum was reported as the main pathogen for date palm seedlings, and was recovered the date palm tissue showing a high degree of degradation.
Application of systemic fungicide Topsin M-70 (1%) three times per year successfully suppressed the deterioration of the infested date palm with the RPW and enhanced aerial rooting formation in the tree trunk, to indicate the applicability of such fungicide as a part in IPM of the RPW of the date palm to prevent the successive occupation of the infested trunk with the secondary invaders and accelerate the curing of the trees in a short time. In similar strategies fungicides: Carbendazim, Score, and Benlate (1 g/L) were successfully applied to prevent the fungal infection in tissue culture of different date palm cultivars [15,16]. Interestingly, Topsin did not suppress Trichoderma viride which was the only fungal species isolated from the treated date palm trunks. This observation confirms the selectivity of the fungicide against pathogenic fungi. It was reported that T. viride was highly compatible with many fungicides such as Mancozeb, Carboxin, and Thiram for management of Fusarium wilt of pigeon pea caused by F. oxysporum f.sp. udum [37]. Furthermore, occupation of the aerial roots by T. viride could promote the formation of aerial roots inside the trunks until the cavity will be closed. The results agreed with reports that approved T. viride colonization in the soil and roots as a biocontrol agent against soil-borne pathogenic fungi with high compatibility with fungicides in seed and soil treatments under greenhouse and field conditions [38]. So, the study suggests the application of a mix of pesticide and fungicide as a new approach to suppress the activity of fungi associated with RPW infestation and their synergistic deterioration.
5. Conclusions
The study explored the deleterious effect of fungi associated with RPW infestation and reported F. oxysporum as the main pathogen infecting the germinating seeds and seedling of date palm. It recommends the application of a systemic fungicide as an important ingredient during the application of IPM of RPW on date palm trees to achieve the best result and recovering the tree in a short time.
Author Contributions
Conceptualization, methodology, investigation, E.-S.H.E.Z. and M.H.; validation, formal analysis, data curation, E.-S.H.E.Z., Y.S.M., M.H. and S.A.; writing—original draft preparation, writing—review and editing, E.-S.H.E.Z., S.A. and Y.S.M.; visualization, E.-S.H.E.Z. and M.H.; supervision, S.A.; project administration, M.H.; funding acquisition, Y.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFP-KKU-2020/2.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number IFP-KKU-2020/2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Dosary, N.M.; Al-Dobai, S.; Faleiro, J.R. Review on the management of red palm weevil Rhynchophorus ferrugineus Olivier in date palm Phoenix dactylifera L. Emirates J. Food Agri. 2016, 28, 34–44. [Google Scholar] [CrossRef]
- Chao, C.T.; Krueger, R.R. The date palm (Phoenix dactylifera L.): Overview of biology, uses, and cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- El Hadrami, A.; Al-Khayri, J.M. Socioeconomic and traditional importance of date palm. Emirates J. Food Agri. 2012, 24, 371. [Google Scholar]
- Zaid, A.; De Wet, P. Chapter II Origin, Geographical Distribution and Nutritional Values of Date palm. FAO Plant Produc. Protect. Pap. 1999, 29. [Google Scholar]
- Vayalil, P.K. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). J. Agric. Food Chem. 2002, 50, 610–617. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Baloch, M.K.; Saleem, S.A.; Baloch, A.K.; Baloch, W.A. Impact of controlled atmosphere on the stability of Dhakki dates. LWT-Food Sci. Technol. 2006, 39, 671–676. [Google Scholar] [CrossRef]
- Faleiro, J. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006, 26, 135–154. [Google Scholar]
- Abraham, V.; Faleiro, J.; Al Shuaibi, M.; Abdan, S.A. Status of pheromone trap captured female red palm weevils from date gardens in Saudi Arabia. J. Tropical Agri. 2006, 39, 197–199. [Google Scholar]
- Abraham, V.; Shuaibi, M.A.; Faleiro, J.; Abozuhairah, R.; Vidyasagar, P.S. An integrated management approach for red palm weevil Rhynchophorus ferrugineus Oliv. a key pest of date palm in the Middle East. J. Agric. Mar. Sci. [JAMS] 1998, 3, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.; Briscoe, B. The red palm weevil as an alien invasive: Biology and the prospects for biological control as a component of IPM. Biocontrol News Inf. 1999, 20, 35N–46N. [Google Scholar]
- Ziedan, E.; Farrag, E.; Sahab, A. Effect of Trichoderma harzianum against Thielaviopsis paradoxa and their pathological potential on date palm seedlings. Int. J. Agri. Technol. 2015, 11, 913–923. [Google Scholar]
- Ahmed, D.; Attya, M.F. Management of date palm root rot diseases by using some biological control agents under organic farming system. Novel Res. Microbiol. J. 2018, 2, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Alwahshi, K.J.; Saeed, E.E.; Sham, A.; Alblooshi, A.A.; Alblooshi, M.M.; El-Tarabily, K.A.; AbuQamar, S.F. Molecular identification and disease management of date palm sudden decline syndrome in the United Arab Emirates. Int. J. Mol. Sci. 2019, 20, 923. [Google Scholar] [CrossRef] [Green Version]
- Abass, M. Microbial contaminants of date palm (Phoenix dactylifera L.) in Iraqi tissue culture laboratories. Emirates J. Food Agri. 2013, 25, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Al-Mayahi, A.; Ahmed, A.; Al-Khalifa, A. Isolation and identification of associated fungi with the micropropagation of five different date palm cultivars and the effect of Benlate fungicides in their control. Basra J. Date Palm Res. 2010, 9, 79–97. [Google Scholar]
- Azmi, W.A. Identification and characterization of fungi associated with red palm weevil, Rhynchophorus ferrugineus: A microscopy study. Malays. J. Microsc. 2013, 9, 127–132. [Google Scholar]
- Salama, H.; Foda, M.; El-Bendary, M.; Abdel-Razek, A. Infection of red palm weevil, Rhynchophorus ferrugineus, by spore-forming bacilli indigenous to its natural habitat in Egypt. J. Pest Sci. 2004, 77, 27–31. [Google Scholar] [CrossRef]
- Llácer, E.; Dembilio, O.; Jacas, J. Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a microencapsulated formulation against Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J. Econ. Entomol. 2010, 103, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.; Tousson, T.; Marasas, W. Fusarium Species an Illustrated Manual of Identification; The Pennsylvania State University Press: University Park: London, UK, 1983; p. 193. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; American Phytopathological Society (APS Press): Saint Paul, MN, USA, 1998. [Google Scholar]
- Dugan, F.M.; Dugan, F.M. The Identification of Fungi: An Illustrated Introduction with Keys, Glossary, and Guide to Literature; The American Phytopathological Society: St. Paul, MN, USA, 2006. [Google Scholar]
- Moubasher, A. Soil Fungi in Qatar and Other Arab Countries; The Centre for Scientific and Applied Research, University of Qatar: Doha, Qatar, 1993. [Google Scholar]
- Ziedan, E.; Khattab, A.; Alamri, S.A.; Mohamed, H. Molecular characterization of Alcaligenes faecalis and Pseudomonas aeruginosa causing root rot of date palm. Int. J. Agric. Biol. 2020, 23, 183–189. [Google Scholar]
- Al-Hamdany, M.; Jaber, H.; Kadhem, A.; Sabar, J. Role of Chalara radicicola in date palm trees decline. Arab J. Plant Prot. 2011, 29, 118–121. [Google Scholar]
- Kumar, V.; Verma, D.K.; Pandey, A.K.; Srivastava, S. Trichoderma spp.: Identification and Characterization for Pathogenic Control and its Potential Application. In Microbiology for Sustainable Agriculture, Soil Health, and Environmental Protection; Apple Academic Press: New York, NY, USA, 2019; p. 223. [Google Scholar]
- Snedecor, G.; Cochran, W. Statistical Methods; Iowa State University: Iowa, IA, USA, 1980. [Google Scholar]
- Kaakeh, W. Toxicity of imidacloprid to developmental stages of Rhynchophorus ferrugineus (Curculionidae: Coleoptera): Laboratory and field tests. Crop Protect. 2006, 25, 432–439. [Google Scholar] [CrossRef]
- Khiyami, M.; Alyamani, E. Aerobic and facultative anaerobic bacteria from gut of red palm weevil (Rhynchophorus ferrugineus). Afr. J. Biotechnol. 2008, 7, 10. [Google Scholar]
- Chobba, I.B.; Elleuch, A.; Ayadi, I.; Khannous, L.; Namsi, A.; Cerqueira, F.; Drira, N.; Gharsallah, N.; Vallaeys, T. Fungal diversity in adult date palm (Phoenix dactylifera L.) revealed by culture-dependent and culture-independent approaches. J. Zhejiang Univ. Sci. B 2013, 14, 1084–1099. [Google Scholar] [CrossRef] [Green Version]
- Ammar, M.; El-Naggar, M. Date palm (Phoenix Dactylifera L.) fungal diseases in Najran, Saudi Arabia. Int. J. Plant Pathol. 2011, 2, 126–135. [Google Scholar] [CrossRef]
- Bokhary, H. Seed-borne fungi of date-palm, Phoenix dactylifera L. from Saudi Arabia. Saudi J. Biol. Sci. 2010, 17, 327–329. [Google Scholar] [CrossRef] [Green Version]
- de Lamo, F.J.; Takken, F.L. Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front. Plant Sci. 2020, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Monzer, A.-E.; Abd El-Rahman, R. Effect on Heterorhabditis indica of substances occurring in decomposing palm tissues infested by Rhynchophorus Ferrugineus. Nematology 2003, 5, 647–652. [Google Scholar] [CrossRef]
- El-Sohaimy, S.; Hafez, E. Biochemical and nutritional characterizations of date palm fruits (Phoenix dactylifera L.). J. Appl. Sci. Res. 2010, 6, 1060–1067. [Google Scholar]
- Gussin, E.; Lynch, J. Effect of local concentrations of acetic acid around barley roots on seedling growth. New Phytol. 1982, 92, 345–348. [Google Scholar] [CrossRef]
- Meena, R.; Arsia, S.; Jain, Y.; Dongre, M. Compatibility of fungicides with Trichoderma viride against fusarium wilt caused by Fusarium udum. Int. J. Agric. Sci. 2018, 10, 5268–5271. [Google Scholar]
- Ashwani, T.; Rajesh, K.; Nandini, G.; Shailesh, P. Compatibility of Trichoderma viride for selected fungicides and botanicals. Int. J. Plant Pathol. 2012, 3, 89–94. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).