Abstract
Agricultural production systems based on the application of synthetic chemical inputs are changing to more ecological management systems. In this context, rhizosphere microorganisms are considered fundamental to improving soil fertility and providing protection to the host plant. The objective of this study was to perform co-inoculation of Sechium edule (Jacq.) Sw. plants (chayote) with Rhizophagus intraradices and Azospirillum brasilense to reduce Phytophthora capsici damage. The chayote seeds were established in bags, and their inoculation was evaluated alone and in combination with R. intraradices and A. brasilense, in addition to inoculating the stem 14 days after planting with P. capsici. Eight treatments were distributed completely at random, with four repetitions. Morphological and physiological yield variables were recorded at 28, 56, and 84. It was found that S. edule treatment with R. intraradices and A. brasilense increased dry matter allocation in the morphological and physiological performance components. The biomass of plants inoculated with P. capsici and biofertilized with R. intraradices and A. brasilense decreased by 27%, which is relevant, since, under field conditions, plants infected with P. capsici die. Petiole biomass and leaf area decreased during the three evaluation periods with the presence of P. capsici. The other components had a differential response.
1. Introduction
Agricultural systems use large amounts of external inputs, such as agrochemicals, to achieve their production goals. High energy consumption, especially of a fossil nature, is used in this process [1]. This agricultural production model tends to be unsustainable, and consequently, alternative inputs are required even more so when considering that the demand for food produced without agrochemicals has increased [2,3], which requires the use of natural components to control growth, yield, and diseases in plants. In this context, special interest has been focused on improving crop nutrition and protection through the inclusion of microorganisms capable of establishing symbiosis with the root system of the host plant and favoring its functioning, especially under stress conditions.
Beneficial microorganisms have been considered for use as biological fertilization agents or biofertilizers, which the Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food (SAGARPA) [4] has promoted in Mexico due to evidence that they increase annual crop yields and reduce carbon dioxide emissions. The biofertilization of various microorganisms favors the nutrition of the host plant through various mechanisms of action and, concomitantly, reduces the severity of the attack by radical pathogens, as reported with Phytophthora capsici in horticultural plants when endomycorrhizal fungi are applied [5,6].
In Mexico, the production of chayote (Sechium edule (Jacq.) Sw.) (Cucurbitaceae), a vegetable for export, ranges from 54 [7], to 130–136 t ha−1 [8], and generates more than 27,700 local jobs for every 35 ha yr−1, which increases its importance as a social crop [9]. In general, the planting density in technified orchards of S. edule in mesophilic forest conditions (1200–1400 m) ranges from 100 to 130 plants ha−1, and one plant produces up to 1.0 t ha−1 in a period of 6 to 8 months [9].
The planted area in Mexico is 2953 ha [7], and in all regions, P. capsici has been recorded as one of the fungi responsible for stem rot (vital node) and chayote root rot (S. edule (Jacq.) Sw.), thus showing, in addition, different degrees of aggressiveness [10,11]. With this background, the effectiveness of co-inoculation of Sechium edule plants (chayote) with Rhizophagus intraradices and Azospirillum brasilense to reduce P. capsici damage was evaluated. We considered the hypothesis that co-inoculation can increase the nutrition of S. edule plants and reduce the damage to or loss of plants caused by P. capsici, attributed to the effect of competition for infection sites, by acting as a biocontrol.
2. Materials and Methods
The present research was carried out in the greenhouse of the Rosario Izapa Experimental Field of the National Institute for Forestry, Agricultural, and Livestock Research (INIFAP) in Tuxtla Chico, Chiapas (14°58′28.89″ LN, 92°09′19.64″ LO and 435 m altitude). The climate is generally warm and humid, with the influence of summer rains and monsoon. The average annual rainfall is 4720 mm and the average temperature, considered isothermal, is 25.4 °C.
2.1. Plant Material
Seeds of Costa Rica-type chayote (S. edule var. virens levis), provided by the Interdisciplinary Research Group of Sechium edule in Mexico, from the area of conservation and genetic improvement, were used (19°08′48″ N, 97°57′00″ W, at 1340 m). The fruits were cut between 36 and 38 days after anthesis—that is, 18–20 days after horticultural maturity [12]—and transported to the experimental site one day later. Due to the recalcitrant and endocarpic nature of the seed, the fruit of S. edule containing the seed is normally planted. Sowing was carried out two days after cutting.
2.2. Experimental Setup, Substrate, and Microorganisms
One chayote seed (one fruit) was planted per bag. The substrate used was a mixture of andosol molic soil and washed river sand in a 1:1 (v/v) ratio. With this, 6.0 kg polyethylene bags, perforated at the bottom to promote drainage, were filled, and randomly distributed on metal tables at a height of 1.0 m from the ground. Physicochemically, the substrate had the following components and characteristics: sand, 82.76%; silt, 11%; clay, 6.24%; crumbly sand texture; organic matter, 2.85%; pH, 5.50; N, 0.13%; P, 7 mg kg−1; K, 13 mg kg−1; Na, 16 mg kg−1; Mg, 12 mg kg−1; Ca, 96 mg kg−1; cation exchange capacity (CEC), 0.68 mg 100 g−1; and electrical conductivity (EC), 0.055 dS m−1.
2.3. Microorganisms
Co-inoculation can enhance plant growth through mechanisms attributed to the positive effects of Azospirillum, which are mainly attributed to improved root development and mycorrhiza-improved nutrient status of the host plant, mainly with the transportation of P and the bioprotective effect of mycorrhization against soil-borne fungal pathogens. The co-inoculation of S. edule plants was justified by the expected advantages of improving plant nutrition under stress conditions due to P. capsici infection in addition to inducing competition for infection sites in the roots. In this regard, Ju et al. [13], mention the benefits of co-inoculation with rhizobacteria and rhizobium in alfalfa plants, observing a reduction in oxidative damage and an improvement in the antioxidation capacity of the plant in addition to observing less microbial diversity in the rhizosphere.
Rhizophagus intraradices (Schenck & Sm.) Walker & Schuessler (RI) was acquired at the INIFAP Campo Experimental Rosario Izapa, Chiapas, with 40 spores g−1 of soil plus mycelium and rootlets as sources of inoculum.
Azospirillum brasilense Tarrand, Krieg, & Döbereiner (AB) was obtained at the Benemérita Universidad Autónoma de Puebla (BUAP) with 9 × 106 bacteria g−1 of peat (information contained in the products). The P. capsici (PC) strain was provided by the Colegio de Postgraduados, Campus Montecillo, and cultured from a purified hyphal tip in V8 agar culture medium.
2.4. Application of Microorganisms
At the time of sowing, 20 g of the biofertilizer of each of the microorganisms was applied in the cavity of the corresponding treatment. The base of the fruit with the seed was in direct contact with the biofertilizers. Subsequently, two-thirds of the fruit was covered with the same substrate to avoid burning. The plants were irrigated with distilled water.
The application of P. capsici was inoculated at the base of the stem 14 days after planting (DAP). For this purpose, the fully sporulated strain with sporangium formation was separated from the medium with sterile distilled water and was gauged at 150 mL and refrigerated for 15 min to induce the release of zoospores. Subsequently, it was allowed to rest at room temperature for 2 min, and from this preparation, 2 mL plant−1 was applied at the base of the stem (vital node).
2.5. Treatments, Repetitions, and Experimental Design
The treatments included the inoculation of the microorganisms alone or combined, plus the control, with and without the application of P. capsici. A total of eight treatments were distributed completely at random with four repetitions. Irrigation was carried out every third day at the rate of one liter of water per plant (Table 1).

Table 1.
Description of the treatments and abbreviations used in the study.
2.6. Morphological and Physiological Variables
The morphological variables, including the number of leaves at 28, 56, and 84 DAP, the mean number of vines and the average length of four vines at 84 DAP, were recorded. The aerial and root parts of the plant were harvested at 84 days, and they were weighed on a semi-analytical balance (Ohaus Adventurer Pro, California USA) after drying in a forced air oven at 60–75 °C to a constant weight. The leaf area (cm2) was measured using a leaf area integrator (LI-COR, LI 3000, LI-COR Biosciences, Lincoln, NE, USA).
2.7. Histological Sections and Microscopy
Histological sections were made on a rotary microtome (Spencer, American Optical Company model 820, NY USA) and cut transversely to a thickness of 15 µm. The cut sections were placed in a flotation bath with hot water (43–45 °C) and gelatin. Finally, they were mounted on glass slides sequentially. They were deparaffinized by melting the paraffin and eliminating the excess contained in the tissues by means of three changes of xylol at intervals of 3 min each, and they were hydrated with two changes of ethyl alcohol, both absolute and at 96%, for 3 min in both cases. At the end of this procedure, the sections were placed in safranin (SIGMA-ALDRICH, St. Louis, MO, USA) for 24 h [14].
The sections remained for 24 h in safranin, after which they were rinsed three times with running water for 25 min in each rinse. Next, they were washed with picric acid-ethanol 96%, ammonia-ethanol 96% and absolute ethanol. Fast green was added for five seconds, followed by clove oil with a short exposure time. To remove excess dye (fast green), a mixture of clove oil–xylol–absolute alcohol was used; the sections were rinsed in xylol for 3 min and finally mounted in synthetic resin for observation [10]. Scanning electron microscopy was performed according to the protocol of Bozzola and Russell [15].
2.8. Statistical Analysis
The results were evaluated using the program SAS (Software as a Service) version 8.1 (SAS 1999-2000) [16] based on a completely randomized analysis. Differences between treatment means were compared using Tukey’s test (p ≤ 0.05).
3. Results
3.1. Morphological Components
The initial number of leaves (28 DAP) increased 35% more compared to the control when inoculating S. edule with R. intraradices or A. brasilense together with P. capsici. However, when co-inoculated with R. intraradices + A. brasilense and P. capsici, the number of leaves was similar to that of the control, without statistical difference (Figure 1). On the other hand, at 56 DAP, the co-inoculation of the two beneficial microorganisms in interaction with P. capsici induced an increase of 24% in the number of leaves compared to the control.
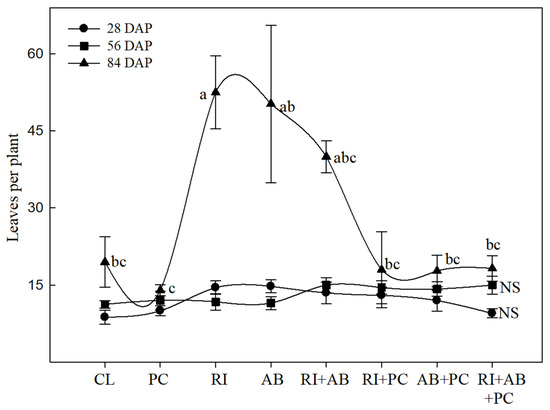
Figure 1.
Number of leaves per plant of S. edule var. virens levis Costa Rica Type due to the effect of biofertilization with R. intraradices and/or A. brasilense in interaction with P. capsici. The vertical line indicates ± standard error. CV = 25% for 28 DAP, 21.9% for 56 DAP and 48.5% for 84 DAP. Different letters represent significant differences (p < 0.05).
At 84 DAP, the most notable increase in the number of leaves was presented in the biofertilized treatments with R. intraradices, counting 52 leaves; in A. brasilense, counting 50 leaves; and in the double biofertilization (R. intraradices + A. brasilense), counting 40 leaves, and they were statistically different (p ≤ 0.05) to the other treatments. The lowest number of leaves occurred in the control and in the treatments where P. capsici was inoculated (Figure 1).
The length of the vines was shorter in the controls with and without P. capsici, at an average of 17 cm. In the case of the treatments biofertilized with R. intraradices and/or A. brasilense alone plus the inoculation of P. capsici, a reduction in the length of the vines of up to 70% was also recorded, compared to the same treatments without P. capsici. In the case of double biofertilization, the average length of the vines was 56 cm and when P. capsici was inoculated, it was 15 cm (Figure 2). It should be noted that introducing P. capsici in the treatments colonized by R. intraradices led to the development of one more vine compared to treatment with A. brasilense and the double symbiosis.
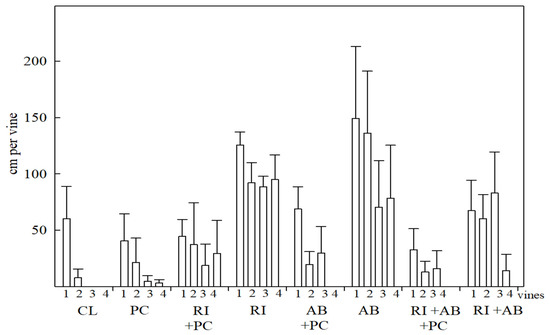
Figure 2.
Length of vines in plant of S. edule var. virens levis Costa Rica Type due to the effect of biofertilization with R. intraradices and/or A. brasilense in interaction with P. capsici. Values at 84 DAP. The vertical line indicates ± the standard error.
3.2. Physiological Components
The allocation of dry matter to different components of the plants showed changes over time between treatments. The application of P. capsici on plants biofertilized with the microorganisms, R. intraradices and A. brasilense, decreased the biomass of S. edule by 8% compared to the same treatments without the application of the pathogen. This may be the effect of the increase observed in the necrotic root system of plants inoculated with P. capsici. However, compared to the average biomass of the controls with and without P. capsici, the biomass of biofertilized plants inoculated with P. capsici was 230% higher (Table 2).

Table 2.
Comparisons of means of dry weight of root, stem, leaf blade, petiole, and leaf area of S. edule var. virens levis Costa Rica Type, biofertilized with A. brasilense and R. intraradices and inoculated with P. capsici in an andosol-molic soil from Soconusco, Chiapas.
The initial growth of S. edule assigned an average of 25, 32, 37, and 6% photosynthates to the root, stem plus vines, leaf blade, and petiole, respectively. In the second sampling (56 DAP), there was an increase in the allocation of biomass in the physiological components of yield, expressed in the leaf blade, representing about 50% of the total plant, which decreased the biomass in the root system. This growth in S. edule was also observed at 84 DAP, observing that the yield components with the greatest changes were the leaf blade and the root system. When P. capsici was applied, root growth decreased in all treatments during the three samplings and was even more restricted in the controls with P. capsici. In this treatment, the number of roots with external necrosis increased. The total number of plants killed by necrosis was three for the CL treatment and one that was biofertilized with AB + PC (Figure 3 and Figure 4).

Figure 3.
Sequence of damage in the basal vine (vital node) of S. edule var. virens levis Costa Rica Type. (A–C) 22 days after inoculation with P. capsici. (D)Transversal histological section of the stem, with coenocytic mycelial hyphae of P. capsici in the vascular bundle 30 days after inoculation. (E) Total necrosis of the adult plant basal guide taken in the field.

Figure 4.
Phytophthora capsici on seed root tissue of S. edule var. virens levis Costa Rica Type. (A,B) sporangia and zoospores. (C) in germinated seed root tissue. Scanning electron microscope micrographs. 500×, 3000×, 2500×, respectively.
4. Discussion
Dead plants were recorded in the CL treatment, as well as one dead plant in the AB + PC treatment, even though it was expected that in PC, all the plants would die; nevertheless, lower values were observed in the length of the vines (Figure 2). The AB, RI, and RI + AB treatments stood out, demonstrating their possible effect on plant nutrition. However, the RI + PC, AB + PC and RI + AB + PC plants showed survival and medium to low values of vine length, without registering plant loss.
Similar behavior was observed for the number of leaves (Figure 1), where the plants inoculated with RI, AB, and RI + AB, were outstanding compared to those inoculated with PC.
The foregoing is relevant, as successful cultivation of S. edule is identified by the formation of a canopy of vines that protects the youngest and most productive vines, since it is grown in a horizontal structure. S. edule produces one to two fruits per node longitudinally; therefore, the longer the vines are, the greater the number of fruits will be [8]. In terms of plant survival in the field, RI + PC treatment, as well as AB + PC, is relevant for its possible protective effect.
In other tropical perennial crops, such as Theobroma cacao L. and Coffea arabica L., without application of P. capsici, increases in the number of leaves are cited when endomycorrhizal fungi or nitrogen-fixing bacteria such as A. brasilense are applied [17]. In relation to the number of vines per plant, an increase was recorded in the treatments without the application of P. capsici, but the effect was outstanding with the individual biofertilization of R. intraradices and A. brasilense compared to when the two microorganisms were applied together.
The aforementioned response is attributed to the improvement in the sanitary condition of plants biofertilized with endomycorrhizal fungi by reducing the root tissue necrotized by P. capsici [18]. Endomycorrhizal fungi, when interacting with radical pathogens, trigger a host plant defense response by stimulating phytoalexins, chitinases, arginine, isoflavonoids, and glucanolytic enzymes [19].
In the case of root dry weight, at 28 DAP, an increase of 42% was observed in the R. intraradices + P. capsici treatment; however, the most contrasting increase was recorded with the individual or combined biofertilization with microbial biofertilizers at 56 and 84 DAP.
Stem dry weight presented the most contrasting changes in biomass at 84 DAP; in this case, all treatments with biofertilizers, alone or in combination, and with P. capsici presented the highest values and were statistically different from the controls (p ≤ 0.05). In other tropical plants such as T. cacao and C. arabica, increased stem growth was observed when biofertilizing with R. intraradices [20].
The effect of increased growth of biofertilized plants is attributed, on the one hand, to the ability of A. brasilense to induce the growth of the root system, or to the external growth of the mycelium due to R. intraradices, which serves as an extension of the root system, thus contributing to the absorption of nutrients, such as phosphorus [21]. On the other hand, it is attributed to the bioprotection and fortification of the cell wall by the increased production of polysaccharides for lignification [22], which hinders the penetration of the pathogen [23]. A precursor of lignification in vegetables is β-glucosidase, which was identified in S. edule [24], and is involved in several functions in plants, forming part of the cell wall [25,26].
The above effects may be related to the benefits that occur in plant–microorganism symbiosis. In the case of Azospirillum, the root system of plants is increased through the production of growth-regulating substances [27], and the increase in root biomass favors the ability to explore and transport nutrients to the host plant [28]. Authors such as Akhtar and Siddiqui [29], observed a delay in the development of Phytophthora with mycorrhizal colonization and added that the pathogen did not penetrate the arbuscle. This structure is responsible for the release of nutrients into the cell [30], being unaltered, the flow of nutrients is maintained, which results in an increase in the biomass of biofertilized plants.
In Ocimum basilicum, Copetta et al. [31], cited an increase in phenols in the tissue when colonized by endomycorrhizal fungi, as well as an increase in the production of root hairs. In Carica papaya L. cv. Sunrise inoculated with Glomus mosseae, a decrease was observed in the effect of Fusarium, Pythium, Phytophthora, Rhizoctonia, Verticillium, and root-knot nematodes (Meloidogyne spp.) or lesion nematodes (Pratylenchus sp.) [5], and the severity of infection in tomato with Glomus macrocarpus or Glomus fasciculatum after 20 days of being infected by Fusarium oxysporum f. sp. lycopersici decreased between 75% and 78% [32].
Reyes et al. [33], warned about the differential effects of Phytophthora spp. by endomycorrhizal fungi isolates on Capsicum annuum L. In general, the defense mechanisms responsible for disease inhibition in mycorrhizal plants could also be explained by improved nutrition of the host plant due to the transportation of phosphorus, other nutrients, and water as well as competition with the pathogen for root colonization space [32].
In general, the biomass increased with the individual and combined application of R. intraradices and A. brasilense, compared to the controls with and without P. capsici (Table 2).
Similar results have been reported in other crops when biofertilized with endomycorrhizal fungi and/or nitrogen-fixing bacteria, such as in C. arabica [34], and forest species such as Tabebuia donnell-smithii [35], and Cedrela odorata [36].
The petiole was the plant structure that did not show contrasting changes in its dry weight at 28 DAP (without statistical differences); however, differences were observed at 56 and 84 DAP. The biofertilized treatment with AB + PC increased petiole biomass at 56 DAP (p ≤ 0.05), and at 84 DAP, the highest values in petiole biomass were in the double symbiosis treatment without P. capsici, but all treatments with the biofertilizers were clustered in the first statistical group and surpassed the controls (p ≤ 0.05). This plant structure was the most sensitive in the allocation of dry matter to biomass.
The initial leaf blade biomass was lower with the application of P. capsici (p ≤ 0.05). At 84 DAP, the highest dry matter allocation to the leaf blade of chayote was observed with the biofertilizer microorganisms and without P. capsici, and the lowest was observed with the controls. The leaf area was strongly affected by the inoculation with P. capsici from the beginning of the research. The biofertilized plants increased the leaf area and were statistically different (p ≤ 0.05). The effect on these increases in plants inoculated with RI, and/or AB has also been observed in C. arabica [17] and Coffea canephora [37].
In O. basilicum biofertilized with endomycorrhizal fungi [31], reported an increase in the number of glandular trichomes, which, in addition to being resistance barriers, are sites of accumulation of secondary metabolites [38], that block the action of pathogens and herbivores, while also regulating the biological activity of cytokinins [39,40]. The inoculation of S. edule plants with the microorganisms was beneficial, since even when inoculated with PC, the biomass reduction was only 27%, which is relevant, since, under field conditions, infected plants die (Figure 3E), as demonstrated by Siddiqui and Akhtar in their tomato research [41].
Alarcón et al. [42], mentioned that when inoculating Solanum lycopersicum L. with Glomus mosseae, it favored growth indicators and reduced infestation by the nematode Meloidogyne spp. Other authors such as Perez-Moncada [43], reported that inoculation with three types of Glomus macrocarpum and one of Rhizoglomus intraradices decreased the absorption of cadmium in Theobroma cacao L. plants, registering the above as benefits to reduce stress in the plant. Evaluations carried out [44], with Rhizophagus aggregatus and a consortium of Glomus claroides, Rhizophagus diaphanous, and Paraglomus albidum on seedlings of varieties of Coffea arabica L. showed that inoculation with the consortium registered the highest values in seedling growth, phosphorus assimilation, and health.
Studies carried out with R. intraradices [45], showed that the phosphorus concentration, water potential in leaves, photosynthetic rate, transpiration, stomatal conductance, and efficiency in the use of water increased significantly in barley plants. The results of combined inoculation with R. intraradices and Paenibacillus mucilaginosus showed that the inoculated Poncirus trifoliata seedlings registered higher levels of antioxidant enzymes than the non-inoculated ones did [46]. Tiwari et al. [47], working with O. basilicum plants that were invaded by the nematode M. incognita, applied bioinoculants based on Bacillus megatarium and B. subtilis, showing improvements in plant growth and oil production and an outstanding reduction in infestation of the nematode. The foregoing is relevant since it presents a friendly alternative for the environment and human health to avoid the use of synthetic nematicides. This shows the advantages of the symbiotic association between plants and microorganisms, as shown in the present case with S. edule and P. capsici.
In relation to the possible greenhouse effects, the plants grew in conditions with a permanent temperature of 26–28 °C. The above based on [48], who reported that S. edule registers an ambient temperature of 26 °C for the exchange of gases and water relations, for the best rate of assimilation of CO2 and 28 °C for the beginning of stomatal closure. Relative humidity was controlled at 85% to maintain adequate turgor that allows water potential without permanent wilting, which impacts stomatal conductance.
5. Conclusions
Plants of S. edule var. virens levis of the Costa Rica type inoculated with P. capsici and biofertilized with R. intraradices and A. brasilense showed a decrease of only 27% in their biomass. The petiole biomass and leaf area were the plant components with the lowest growth during the three evaluation periods in the presence of P. capsici. As with root biomass, other components responded differently with time, and at the end of the evaluation, the components in the stem indicated more decreases. The use of co-inoculation with symbiotic microorganisms is advantageous to improve the nutrition and bioprotection of S. edule plants in the presence of P. capsici.
Author Contributions
Conceptualization, J.C.-I.; Data curation, M.I.A.-L.; Formal analysis, J.F.A.-M.; Investigation, G.O.-H.; Software, J.F.A.-C.; Writing—original draft, J.C.-I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
(Non-governmental organization) for the genetic material.
Acknowledgments
To the Interdisciplinary Research Group at Sechium edule in Mexico.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cepal. Foro de los Países de América Latina y el Caribe Sobre el Desarrollo Sostenible. Informe de la Segunda Reunión del Foro de los Países de América Latina y el Caribe sobre el Desarrollo Sostenible. Santiago, Chile, 2018. Available online: https://repositorio.cepal.org/bitstream/handle/11362/43843/S1800706_es.pdf?sequence=1&isAllowed=y (accessed on 17 April 2021).
- de Hodson, J.E. Bioeconomía: El futuro sostenible. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2018, 42, 188–201. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; De Vos, O.J.; Korthals, G.W.; Bruggen, A.H.C. Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. App. Soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- SAGARPA. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Nota de Prensa Núm. 270. Trabaja SAGARPA Para Mitigar Efectos del Cambio Climático en México. 2016. Available online: https://www.gob.mx/sagarpa/prensa/trabaja-sagarpa-para-mitigar-efectos-del-cambio-climatico-en-mexico (accessed on 14 June 2016).
- Jaizme-Vega, M.C.; Rodríguez-Romero, A.S. Integración de microorganismos benéficos (hongos micorrícicos y bacterias rizosféricas) en agrosistemas de las islas Canarias. Agroecología 2008, 3, 33–39. Available online: http://revistas.um.es/agroecologia/article/view/95491/91801 (accessed on 17 April 2021).
- Gómez-Dorantes, N.; Carreón-Abud, Y.; Fernández-Pavía, P.S. Reducción de la susceptibilidad a Phytophthora capsici Leonian causante de la pudrición de raíz en jitomate (Solanum lycopersicum L.). Biológicas 2008, 10, 100–108. Available online: https://www.biologicas.umich.mx/index.php?journal=biologicas&page=article&op=view&path%5B%5D=44 (accessed on 14 April 2021).
- SIAP. Servicio de Información Agroalimentaria y Pesquera Información Datos Abiertos. 2019. Available online: http://infosiap.siap.gob.mx/gobmx/datosAbiertos.php (accessed on 14 April 2021).
- Cadena-Iñiguez, J.; Arévalo-Galarza, M.L.; Avendaño-Arrazate, C.H.; Soto-Hernández, R.M.; Ruiz-Posadas, L.; Santiago-Osorio, E.; Acosta-Ramos, M.; Cisneros-Solano, V.M.; Aguirre-Medina, J.F.; Ochoa-Martínez, D. Production, genetics, postharvest management, and pharmacological characteristics of Sechium edule (Jacq.) Sw. Fresh Prod. 2007, 1, 41–53. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/0706/FP_1(1)/FP_1(1)41-53o.pdf (accessed on 14 April 2021).
- Cadena-Iñiguez, J.; Becerril-Román, E. Generación y Reporte de Casos de Éxito en el Sector Rural. AgroProductividad 2016, 9, x–xviii, Suplemento Noviembre. Available online: https://revista-agroproductividad.org/index.php/agroproductividad/article/view/868 (accessed on 17 April 2021).
- Olguín-Hernández, G.; Valdovinos-Ponce, G.; Cadena-Iñiguez, J.; Arévalo-Galarza, L. Etiología de la Marchitez de Plantas de Chayote (Sechium edule) en el Estado de Veracruz. Rev. Mex. Fitopatol. 2013, 31, 161–169. Available online: http://www.scielo.org.mx/pdf/rmfi/v31n2/v31n2a7.pdf (accessed on 11 January 2021).
- Andrade-Luna, M.I.; Espinosa-Victoria, D.; Gómez-Ro-dríguez, O.; Cadena Iñiguez, J.; Arévalo-Galarza, M.L.; Trejo Téllez, L.I.; Delgadillo-Martínez, J. Severity of a Phytophthora capsici strain in chayote Sechium edule plants at growth chamber level. Rev. Mex. Fitopatol. 2016, 35, 40–57. [Google Scholar] [CrossRef]
- Romero-Velázquez, S.D.; Tlapal-Bolaños, B.; Cadena-Iñiguez, J.; Nieto-Ángel, D.; Arévalo-Galarza, M.L. Hongos causantes de enfermedades postcosecha en chayote (Sechium edule (Jacq.) Sw.) y su control in vitro. Agron. Costarric. 2015, 39, 19–32. Available online: http://www.mag.go.cr/rev_agr/v39n02_019.pdf (accessed on 17 April 2021). [CrossRef]
- Ju, W.; Liu, L.; Jin, X.; Duan, C.; Cui, Y.; Wang, J.; Ma, D.; Zhao, W.; Wang, Y.; Fang, L. Co-inoculation effect of plant-growth-promoting rhizobacteria and rhizobium on EDDS assisted phytoremediation of Cu contaminated soils. Chemosphere 2020, 254. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0045653520309176 (accessed on 17 April 2021). [CrossRef] [PubMed]
- Leyva, M.S.G.; Lora Trejo, L.; Cárdenas Soriano, E.; Valdovinos Ponce, G. Patogénesis de la roya blanca Puccinia horiana henn. en una variedad susceptible de crisantemo [Chrysanthemum morifolium (Ramat.) Hemsl.]. Rev. Mex. Fitopatol. 2001, 19, 191–196. Available online: https://www.researchgate.net/publication/238754809_Pathogenic_and_molecular_variability_of_Puccinia_horiana_Henn_isolates (accessed on 17 April 2021).
- Bozzola, J.J.; Russell, D.L. Electron Microscopy. In Principles and Techniques for Biologists; Jones and Bartlett Publishers: London, UK, 1992; pp. 16–63, 332–356. [Google Scholar]
- SAS Institute Inc. SAS/ETS 9.2. User Guide; SAS Institute Inc.: Cary, NC, USA, 2008; Available online: http://morgan.dartmouth.edu/Docs/sas92/support.sas.com/documentation/cdl/en/biig/60946/PDF/default/biig.pdf (accessed on 24 September 2020).
- Aguirre-Medina, J.F.; Aguirre-Cadena, J.F.; Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H. Biofertilización en Plantas de la Selva Húmeda Tropical, 1st ed.; Editorial Colegio de Postgraduados: Montecillo, Edo. De México, México, 2012; 99p. [Google Scholar]
- Jaizme-Vega, M.C. La vida en el suelo. In Papel de los Microorganismos en la Agroecología; Afonso-Carrillo, J., Ed.; Instituto de Estudios Hispánicos de Canarias. Puerto de la Cruz: Tenerife, Agricultura en Canarias, Spain, 2002; pp. 145–172. [Google Scholar]
- Slezack, S.; Negrel, J.; Bestel-Corre, G.; Dumas-Gaudot, E.; Gianinazzi, S. Purification and partial amino acid sequencing of a mycorrhiza-related chitinase isoform from Glomus mosseae-inoculated roots of Pisum sativum L. Planta 2001, 213, 781–787. [Google Scholar] [CrossRef]
- Aguirre-Medina, J.F.; Mendoza-López, A.; Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H. La Biofertilización del cacao (Theobroma cacao L.) en vivero con Azospirillum brasilense Tarrand, Krieg et Döbereiner y Glomus intraradices Schenk et Smith. Interciencia 2007, 32, 1–6. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-18442007000800010 (accessed on 17 April 2021).
- Smith, S.; Anderson, I.; Smith, F. Mycorrhizal associations and phosphorus acquisition: From cells to ecosystems. Annu. Rev. Plant Biol. 2015, 48, 409–440. [Google Scholar] [CrossRef]
- Jalali, B.L.; Jalali, I. Mycorrhiza in plant disease control. In Handbook of Applied Mycology; Arora, K., Rai, B., Mujerki, K.G., Knudsen, G.R., Eds.; Dekker: New York, NY, USA, 1991; pp. 131–154. [Google Scholar]
- Fusconi, A.; Gnavi, E.; Trotta, A.; Berta, G. Apical meristems of tomato roots and their modifications induced by arbuscular mycorrhizal and soilborne pathogenic fungi. New Phytol. 1999, 142, 505–516. [Google Scholar] [CrossRef]
- Espíndola-Mateos, S.; Matías Cervantes, C.A.; Zenteno, E.; Slomianny, M.C.; Alpuche, J.; Hernández-Cruz, P.; Martínez-Cruz, R.; del Pina Canseco, M.S.; Pérez-Campos, E.; Sánchez Rubio, M.; et al. Purification and Partial Characterization of ß-Glucosidase in Chayote (Sechium edule). Molecules 2015, 20, 19372–19392. [Google Scholar] [CrossRef]
- Escamilla-Treviño, L.L.; Chen, W.; Card, M.L.; Shih, M.-C.H.; Cheng, C.-L.; Poulton, J.E. Arabidopsis thaliana ß-glucosidases BGLU45 and BGLU46 hydrolyse monolignol glucosides. Phytochemistry 2006, 67, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; De-Bashan, L. Chapter Two-How the Plant Growth-Promoting Bacterium Azospirillum Promotes Plant Growth-A Critical Assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar] [CrossRef]
- Ngwene, B.; Gabriel, E.; George, E. Influence of different mineral nitrogen sources (NO3−-N vs. NH4+-N) on arbuscular mycorrhiza development and N transfer in a Glomus intraradices–cowpea symbiosis. Mycorrhiza 2013, 23, 107–117. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Siddiqui, Z.A. Arbuscular mycorrhizal fungi as potential bioprotectants against plant pathogens. In Mycorrhizae: Sustainable Agriculture and Forestry; Siddiqui, Z.A., Akhtar, M.S., Futai, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 61–97. [Google Scholar]
- Fitter, A.H. What is the link between carbon and phosphorus fluxes in arbuscular mycorrhizas? A null hypothesis for symbiotic function. New Phytol. Lett. 2006, 172, 3–6. [Google Scholar] [CrossRef]
- Copetta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular hairs and essential oil production in Ocimum basilicum. Mycorrhiza 2006, 16, 485–494. [Google Scholar] [CrossRef]
- Kapoor, R. Induced Resistance in Mycorrhizal Tomato is correlated to Concentration of Jasmonic Acid Induced Resistance in Mycorrhizal Tomato is correlated to Concentration of Jasmonic. Acid. Online. J. Biol. Sci. 2008, 8, 49–56. Available online: https://thescipub.com/ojbs/issue/361 (accessed on 17 April 2021). [CrossRef]
- Reyes, T.A.; Quiñones, A.E.E.; Rincón, E.G.; López, P.L. Micorrización en Capsicum annuum L. para promoción de crecimiento y bioprotección contra Phytophthora capsici L. Rev. Mex. Cienc. Agric. 2016, 7, 857–870. Available online: http://www.scielo.org.mx/scielo.php?pid=S2007-9342016000400857&script=sci_abstract (accessed on 17 April 2021). [CrossRef]
- Aguirre-Medina, J.F.; Moroyoqui-Ovilla, D.M.; Mendoza-López, A.; Cadena-Iñiguez, J.; Avendaño-Arrazate, C.H.; Aguirre-Cadena, J.F. Aplicación de A. brasilense y G. intraradices a Coffea arabica en vivero. Agron. Mesoam. 2011, 22, 1–10. Available online: https://www.scielo.sa.cr/pdf/am/v22n1/a09v22n1.pdf (accessed on 17 April 2021).
- Aguirre Medina, J.F.; Culebro Cifuentes, F.; Cadena-Iñiguez, J.; Aguirre-Cadena, J.F. Crecimiento de Tabebuia Donnell-Smithii (Rose) Inoculada con Hongos Micorrizicos y Azospirillum brasilense. Agrociencia 2014, 48, 331–345. Available online: http://www.scielo.org.mx/pdf/agro/v48n3/v48n3a8.pdf (accessed on 17 April 2021).
- Aguirre-Medina, J.F.; Mina-Briones, F.O.; Cadena-Iñiguez, J.; Dardón-Zunun, J.D.; Hernández-Sedas, D.A. Crecimiento de Cedrela odorata L. Biofertilizada con Rhizophagus intraradices y Azospirillum brasilense en vivero. Rev. Chapingo Ser. Cienc. For. y del Ambiente 2014, 177–186. [Google Scholar] [CrossRef]
- Ibarra-Puón, J.C.; Aguirre-Medina, J.F.; Ley-De Coss, A.; Cadena-Iñiguez, J.; Zavala-Mata, A. Inoculación de Coffea canephora (Pierre) ex Froehner con Rhizophagus intraradices (Schenck et Sm.) Walker et Schuessler y Azospirillum brasilense Tarrand, Krieg et Döbereiner en vivero. Rev. Chapingo Ser. Hortic. 2014, 20, 201–213. [Google Scholar] [CrossRef]
- Traw, M.B.; Dawson, T.E. Differential induction of trichomes by three herbivores of black mustard. Oecologia 2002, 131, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Morant, A.V.; Jørgenesen, K.; Jørgenesen, C.; Paquette, S.M.; Sánchez-Pérez, R.; Møller, B.L.; Bak, S. ß-glucosidase as detonators of plant chemical defense. Phytochemistry 2008, 69, 1795–1813. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Bak, S.; Møller, B.L. Cyanogenesis in plants and arthropods. Phytochemistry 2008, 69, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.A.; Akhtar, M.S. Synergistic effects of antagonistic fungi and a plant growth promoting rhizobacterium, an arbuscular mycorrhizal fungus, or composted cow manure on populations of Meloidogyne incognita and growth of tomato. Biocontrol. Sci. Techn. 2008, 18, 279–290. [Google Scholar] [CrossRef]
- Alarcón, A.; Boicet, T.; Godefoy, M.; Bacilio-Jiménez, M.; Ceiro, W.; Bazán, Y. Effect of arbuscular mycorrhizas and Meloidogyne spp. on tomato (Solanum lycopersicum L.). Rev. Protección Veg. 2013, 28, 219–223. Available online: http://scielo.sld.cu/scielo.php?pid=S1010-7522013000300010&script=sci_arttext&tlng=pt (accessed on 17 April 2021).
- Pérez Moncada, U.A.; Ramírez Gómez, M.; Serralde Ordoñez, D.P.; Peñaranda Rolón, A.M.; Wilches Ortiz, W.A.; Ramírez, L.; Rengifo Estrada, G.A. Hongos formadores de micorrizas arbusculares (HFMA) como estrategia para reducir la absorción de cadmio en plantas de cacao (Theobroma cacao). Terra Latinoam. 2019, 37, 121–130. [Google Scholar] [CrossRef]
- Hernández-Acosta, E.; Trejo-Aguilar, D.; Rivera-Fernández, A.; Ferrera Cerrato, R. La micorriza arbuscular como biofertilizante en cultivo de café. Terra Latinoam. 2020, 38, 613–628. [Google Scholar] [CrossRef]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef]
- Wang, P.; Wu, S.H.; Wen, M.-X.; Wang, Y.; Wu, Q.-S. Effects of combined inoculation with Rhizophagus intraradices and Paenibacillus mucilaginosus on plant growth, root morphology, and physiological status of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under different levels of phosphorus. Sci. Hort. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- Tiwari, S.; Pandey, R.; Gross, A. Identification of Rhizospheric Microorganisms That Manages Root Knot Nematode and Improve Oil Yield in Sweet Basil (Ocimum basilicum L.). Agronomy 2021, 11, 570. [Google Scholar] [CrossRef]
- Cadena-Iñiguez, J.; Ruiz-Posadas, L.M.; Trejo-López, C.; Sánchez-García, P.; Aguirre-Medina, J.F. Regulación del intercambio de gases y relaciones hídricas en chayote (Sechium edule (Jacq.) Swartz). Rev. Chapingo Ser. Hortic. 2001, 7, 21–35. Available online: https://www.researchgate.net/profile/Jorge-Cadena-Iniguez/publication/315630210_ (accessed on 17 April 2021). [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).