The Effect of Agrotechnical Factors on Fusarium Mycotoxins Level in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Field
2.2. Weather Conditions
2.3. Soil Conditions
2.4. Mycotoxins Analysis
2.5. Statistical Analysis
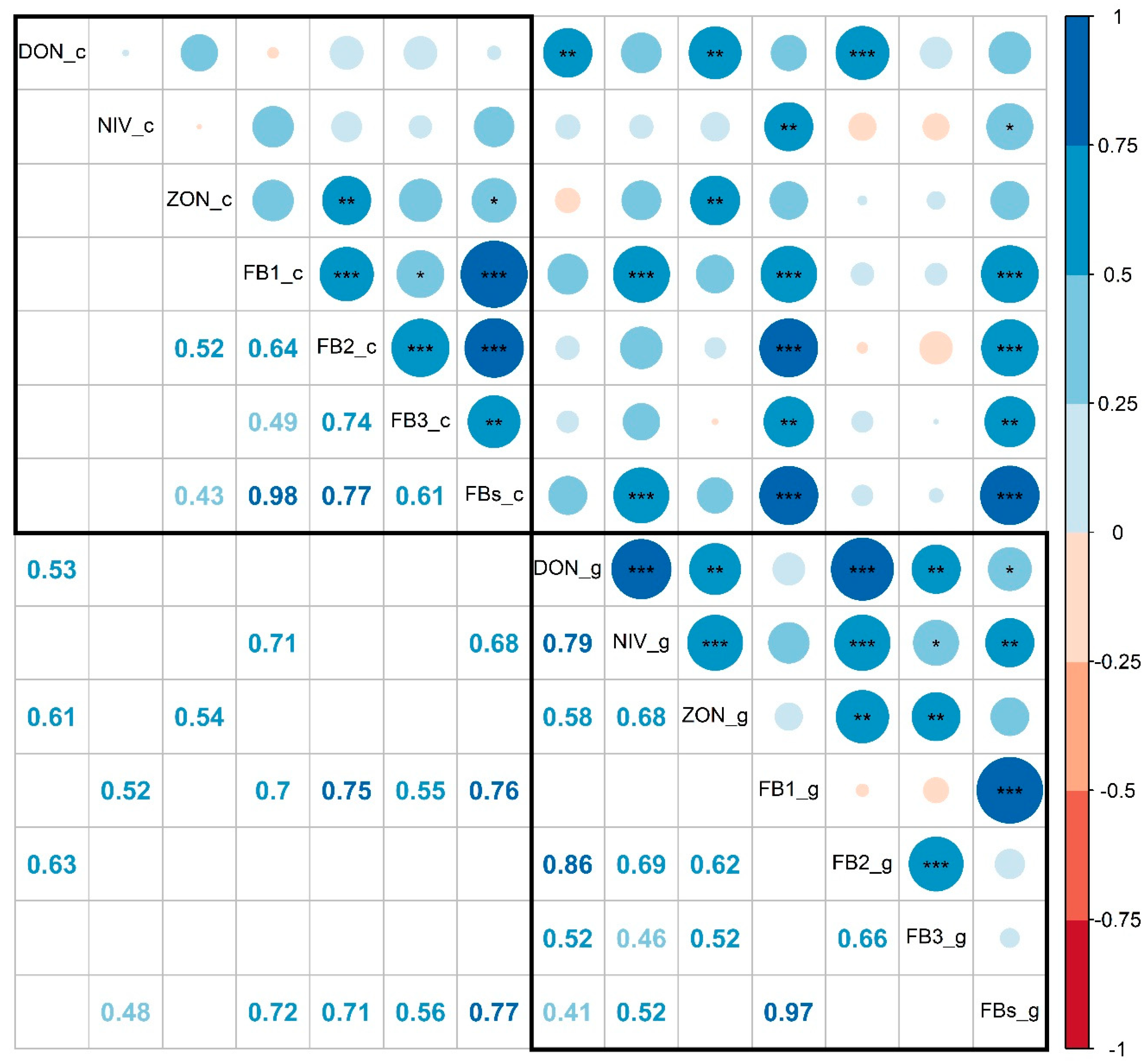
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Szulc, P.; Bocianowski, J.; Nowosad, K.; Michalski, T.; Waligóra, H.; Olejarski, P. Assessment of the Influence of Fertilization and Environmental Conditions on Maize Health. Plant Prot. Sci. 2018, 54, 174–182. [Google Scholar] [CrossRef]
- Szulc, P.; Bocianowski, J.; Kruczek, A.; Waśkiewicz, A.; Beszterda, M.; Goliński, P. Profile of Fusarium Mycotoxins in Various Maize (Zea mays L.) hybrids. Pol. J. Environ. Stud. 2015, 24, 707–715. [Google Scholar]
- Bocianowski, J.; Szulc, P.; Waśkiewicz, A.; Nowosad, K.; Kobus-Cisowska, J. Ergosterol and Fusarium Mycotoxins Content in Two Maize Cultivars under Different forms of Nitrogen Fertilizers. J. Phytopathol. 2019, 167, 516–526. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, R.; Suby, S.B.; Kaur, J.; Sekhar, J.C.; Soujanya, P.L. An overview of Crop Loss Assessment in Maize. Maize J. 2018, 7, 56–63. [Google Scholar]
- Blandino, M.; Reyneri, A.; Vanara, F.; Tamietti, G.; Pietri, A. Influence of Agricultural Practices on Fusarium Infection, Fumonisin and Deoxynivalenol Contamination of Maize Kernels. World Mycotoxin J. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Da Costa, R.V.; Simon, J.; Cota, L.V.; Da Silva, D.D.; De Almeida, R.E.M.; Lanza, F.E.; Lago, B.C.; Pereira, A.A.; Campos, L.J.M.; Figueiredo, J.E.F. Yield Losses in Off-Season Corn Crop Due to Stalk Rot Disease. Pesqui. Agropecu. Bras. 2019, 54, e00283. [Google Scholar] [CrossRef]
- Del Rio Garcia, J.C.; Moreno, R.C.; Pinton, P.; Mendoza, E.S.; Oswald, I.P. Evaluation of the Cytotoxicity of Aflatoxin and Fumonisin in Swine Intestinal Cells. Rev. Iberoam. de Micol. 2007, 24, 136–141. [Google Scholar] [CrossRef]
- Cota, L.V.; Da Costa, R.V.; Silva, D.D.; Casela, C.R.; Parreira, D.F. Quantification of Yield Losses Due to Anthracnose Stalk Rot on Corn in Brazilian Conditions. J. Phytopathol. 2012, 160, 680–684. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Waes, J.G.-V.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.A. Fumonisins Disrupt Sphingolipid Metabolism, Folate Transport, and Neural Tube Development in Embryo Culture and In Vivo: A Potential Risk Factor for Human Neural Tube Defects among Populations Consuming Fumonisin-Contaminated Maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef]
- Dilkin, P.; Direito, G.; Simas, M.; Mallmann, C.A.; Correa, B. Toxicokinetics and Toxicological Effects of Single Oral Dose of Fumonisin B1 Containing Fusarium Verticillioides Culture Material in Weaned Piglets. Chem. Interact. 2010, 185, 157–162. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium Species and Mycotoxins Associated with Head Blight in Small-Grain Cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Sopterean, L.M.; Puia, C. The Major Mycotoxins Produced by Fusarium Fungi and Their Effects. ProEnvironment 2012, 5, 55–59. [Google Scholar]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Bottalico, A. Fusarium Diseases of Cereals: Species Complex and Related Mycotoxin Profiles, in Europe. J. Plant Pathol. 1998, 80, 85–103. [Google Scholar]
- Szulc, P.; Bocianowski, J.; Waśkiewicz, A.; Beszterda, M. Impact of Applying Different Nitrogen Fertilizers on the Level of Fumonisins in Maize Hybrids Grain. Prog. Plant Prot. 2012, 52, 306–309. [Google Scholar]
- Tangendjaja, B.; Rachmawati, S.; Wina, E. Mycotoxin Contamination on Corn Used by Feed Mills in Indonesia. Indones. J. Agric. Sci. 2008, 9, 68–76. [Google Scholar] [CrossRef]
- Jagła, M.; Szulc, P.; Ambroży-Deręgowska, K.; Mejza, I.; Kobus-Cisowska, J. Yielding of Two Types of Maize Cultivars in Relation to Selected Agrotechnical Factors. Plant Soil Environ. 2019, 65, 416–423. [Google Scholar] [CrossRef]
- Stępień, Ł.; Waśkiewicz, A. Sequence Divergence of the Enniatin Synthase Gene in Relation to Production of Beauvericin and Enniatins in Fusarium Species. Toxins 2013, 5, 537–555. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Irzykowska, L.; Drzewiecka, K.; Bocianowski, J.; Dobosz, B.; Weber, Z.; Karolewski, Z.; Krzyminiewski, R.; Goliński, P. Plant-Pathogen Interactions During Infection Process of Asparagus with Fusarium spp. Open Life Sci. 2013, 8, 1065–1076. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Maresca, M. From the Gut to the Brain: Journey and Pathophysiological Effects of the Food-Associated Trichothecene Mycotoxin Deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.; Løes, A.-K.; Kristoffersen, A. Influence of Agronomic and Climatic Factors on Fusariumin Festation and Mycotoxin Contamination of Cereals in Norway. Food Addit. Contam. Part A 2012, 29, 1129–1140. [Google Scholar] [CrossRef]
- Steinkellner, S.; Langer, I. Impact of Tillage on the Incidence of Fusarium spp. in Soil. Plant Soil 2004, 267, 13–22. [Google Scholar] [CrossRef]
- Mahalakshmi, V.; Bidinger, F.R. Evaluation of Stay-Green Sorghum Germplasm Lines at ICRISAT. Crop. Sci. 2002, 42, 965–974. [Google Scholar] [CrossRef][Green Version]
- Michalski, T.; Perkowski, J.; Stachowiak, J. Yielding of Grain Maize and Presence of Fusarium Toxinis in Depence on Harvest Time. J. Plant Prot. Res. 2000, 40, 271–279. [Google Scholar]
- Piacentini, K.C.; Rocha, L.O.; Savi, G.D.; Carnielli-Queiroz, L.; Fontes, L.D.C.; Corrêa, B. Assessment of Toxigenic Fusarium Species and Their Mycotoxins in Brewing Barley Grains. Toxins 2019, 11, 31. [Google Scholar] [CrossRef]
- Blandino, M.; Scarpino, V.; Giordano, D.; Sulyok, M.; Krska, R.; Vanara, F.; Reyneri, A. Impact of Sowing Time, Hybrid and Environmental Conditions on the Contamination of Maize by Emerging Mycotoxins and Fungal Metabolites. Ital. J. Agron. 2017, 12, 215–224. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Torelli, E.; Firrao, G.; Bianchi, G.; Saccardo, F.; Locci, R. The Influence of Local Factors on the Prediction of Fumonisin Contamination in Maize. J. Sci. Food Agric. 2012, 92, 1808–1814. [Google Scholar] [CrossRef]
- Bocianowski, J.; Szulc, P.; Tratwal, A.; Nowosad, K.; Piesik, D. The Influence of Potassium to Mineral Fertilizers on The Maize Health. J. Integr. Agric. 2016, 15, 1286–1292. [Google Scholar] [CrossRef]
- Szulc, P. Organic and Mineral Fertilization of Maize Affecting Prevalence of Fusarium Diseases (Fusarium spp.) and European Corn Borer (Ostrinia nubilalis Hbn.). Prog. Plant Prot. 2013, 53, 498–502. [Google Scholar]
- Szulc, P. The Effect of The Sum of Absolute Values of Nutrient Status Indexes in Plants of Two Hybrid Types of Maize (Zea mays L.) on Dynamics of Dry Matter Accumulation in Initial Vegetation Period at varied Soil Nitrogen and Magnesium Resources. Fresenius Environ. Bull. 2013, 22, 2616–2624. [Google Scholar]
- Szulc, P.; Bocianowski, J.; Nowosad, K.; Rybus-Zając, M.; Waligóra, H.; Michalski, T. The Dynamics of a Dry Matter Accumulation in the Initial Period of Growth of Four Varieties of the “Stay-Green” Type of Maize (Zea mays L.). Pak. J. Bot. 2017, 49, 1017–1022. [Google Scholar]

| Years | Values of Coefficient (K) | |||||||
|---|---|---|---|---|---|---|---|---|
| IV | V | VI | VII | VIII | IX | X | Mean | |
| 2013 | 0.39 | 1.97 | 2.01 | 0.77 | 0.51 | 1.91 | 0.45 | 1.14 |
| 2014 | 1.48 | 1.78 | 0.83 | 0.71 | 0.96 | 0.81 | 0.83 | 1.06 |
| Specifications | Years | |
|---|---|---|
| 2013 | 2014 | |
| P [mg P kg−1 dm soil] | 38.0 | 127.0 |
| K [mg K kg−1 dm soil] | 111.0 | 261.0 |
| Mg [mg Mg kg−1 dm soil] | 23.0 | 36.0 |
| pH [w 1 mol dm−3 KCl] | 4.80 | 4.70 |
| Compound | Detection | λ [nm] | Chromatographic Column | LOD [ng g−1] |
|---|---|---|---|---|
| Zearalenone—ZON | PDA and FLD | λex = 274 λem = 440 | Nova Pak C-18 (150 × 3.9 mm) | 1.0 |
| Deoxynivalenol—DON | PDA | 224 | Nova Pak C-18 (300 × 3.9 mm) | 5.0 |
| Nivalenol—NIV | PDA | 224 | Nova Pak C-18 (300 × 3.9 mm) | 5.0 |
| Fumonisins—FBs | FLD | λex = 335 λem = 440 | XBridge C-18 (3.0 × 100 mm) | 2.0 |
| Source of Variation | d.f. | DON | NIV | ZON | FB1 | FB2 | FB3 | FBs |
|---|---|---|---|---|---|---|---|---|
| Blocks | 2 | 20.16 | 12.09 | 1.855 | 109.6 | 17.07 | 1.141 | 72.6 |
| A | 1 | 397.96 | 5.02 | 292.004 * | 31,617.7 ** | 1093.38 * | 6.214 * | 45,527.9 ** |
| Residual 1 | 2 | 28.52 | 34.79 | 3.366 | 125.6 | 20.49 | 0.24 | 256.1 |
| B | 1 | 4918.12 ** | 2.65 | 15.402 | 3717.6 | 85.95 ** | 3.939 | 2882.2 |
| A × B | 1 | 19.42 | 62.22 | 1015.588 *** | 5297.3 | 128.48 *** | 3.614 | 6759.5 |
| Residual 2 | 4 | 141.8 | 12.66 | 4.572 | 1014.5 | 1.46 | 1.741 | 909.9 |
| C | 1 | 282.42 | 733.91 ** | 19.931 | 73,172.7 *** | 1160.83 *** | 20.869 ** | 95,569.8 *** |
| A × C | 1 | 35.76 | 4.17 | 168.263 *** | 2860.6 ** | 45.14 | 5.012 | 3899 ** |
| B × C | 1 | 33.48 | 114.42 | 196.871 *** | 522.3 | 476.97*** | 0.345 | 2050.3 * |
| A × B × C | 1 | 274.9 | 144.11 | 5.194 | 1565.8 * | 507.46 *** | 24.069 ** | 4489.4 ** |
| Residual 3 | 8 | 86.19 | 46.83 | 5.176 | 216.3 | 17.09 | 1.124 | 246.4 |
| Y | 1 | 52,134.15 *** | 982.2 *** | 4752.319 *** | 537,707.4 *** | 2195.24 *** | 42.056 *** | 598,540 *** |
| Y × A | 1 | 78.36 | 6.8 | 50 * | 10,616.9 *** | 68.9 * | 7.262 * | 11,803.6 *** |
| Y × B | 1 | 701.89 ** | 220.46 * | 49.187 * | 17,532.7 *** | 0.05 | 20.869 *** | 18,701.3 *** |
| Y × C | 1 | 191.8 | 304.47 ** | 171.499 *** | 643.1 | 7.25 | 14.796 ** | 354.2 |
| Y × A × B | 1 | 174 | 9.23 | 1887.395 *** | 0.6 | 524.9 *** | 24.41 *** | 731.7 |
| Y × A × C | 1 | 68.9 | 109.66 | 71.224 * | 1699.9 | 681.39 *** | 4.148 | 4263.7 * |
| Y × B × C | 1 | 456.4 ** | 413.66 *** | 17.533 | 2410.2 | 95.46 * | 0.231 | 1508.8 |
| Y × A × B × C | 1 | 280.29 * | 12.78 | 2.079 | 4681.7 * | 131.57 ** | 63.918 *** | 2396.9 |
| Residual 4 | 16 | 46.26 | 25.49 | 8.252 | 589.9 | 15.13 | 1.072 | 616.2 |
| Source of Variation | d.f. | DON | NIV | ZON | FB1 | FB2 | FB3 | FBs |
|---|---|---|---|---|---|---|---|---|
| Blocks | 2 | 280.2 | 105.91 | 47 | 173.2 | 15.563 | 3.244 | 337 |
| A | 1 | 17,415.1 *** | 1491.76 * | 1194.51 ** | 3126.5 | 509.929 | 5.985 | 6551.8 * |
| Residual 1 | 2 | 10.4 | 26.44 | 4.03 | 241.2 | 33.761 | 0.969 | 146 |
| B | 1 | 85,587.5 *** | 3549.56 *** | 1759.95 *** | 20,303.8 *** | 376.712 *** | 6.818 | 27,064.1 *** |
| A × B | 1 | 25,716.4 *** | 519.36 * | 70.4 | 342.7 | 204.311 ** | 0.006 | 1071 |
| Residual 2 | 4 | 275.7 | 30.02 | 14.53 | 179.5 | 3.247 | 1.077 | 173.4 |
| C | 1 | 44,890.8 *** | 8680.9 *** | 69 * | 13,939.1 *** | 33.617 | 0.263 | 15,469 *** |
| A × C | 1 | 82.1 | 426.8 * | 1.1 | 103 | 6.728 | 0.983 | 188.7 |
| B × C | 1 | 21,765.6 *** | 3101.99 *** | 0.16 | 229.3 | 1.364 | 9.747 * | 117.8 |
| A × B × C | 1 | 1928 * | 709.4 ** | 510.32 *** | 11 | 109.656 ** | 0.064 | 183.3 |
| Residual 3 | 8 | 200.8 | 37.41 | 7.9 | 405.1 | 7.37 | 1.491 | 368.4 |
| Y | 1 | 289,978 *** | 49,823.08 *** | 640.28 *** | 169,158.2 *** | 2106.618 *** | 10.277 * | 211,960.9 *** |
| Y × A | 1 | 22,097.4 *** | 3094.28 *** | 96.19 * | 3040.2 ** | 401.074 *** | 0.088 | 5694.4 *** |
| Y × B | 1 | 47,004.5 *** | 1046.92 *** | 0.13 | 23,647 *** | 83.029 * | 6.969 | 25,679.4 *** |
| Y × C | 1 | 50,382.6 *** | 4526.22 *** | 296.76 *** | 2015.4 * | 5.488 | 1.821 | 2105.6 * |
| Y × A × B | 1 | 28,530 *** | 29.59 | 85.15 * | 1795.2 * | 124.711 ** | 5.103 | 2629.5 * |
| Y × A × C | 1 | 32.6 | 1197.3 *** | 69.72 * | 1486 * | 0.743 | 22.236 ** | 1087.1 |
| Y × B × C | 1 | 16,079.5 *** | 2834.69 *** | 156.49 ** | 231 | 19.14 | 12.99 * | 52.1 |
| Y × A × B × C | 1 | 3069 *** | 630.24 *** | 19.14 | 552.2 | 112.394 ** | 7.092 | 988.4 |
| Residual 4 | 16 | 160.2 | 33.76 | 13.64 | 267.6 | 9.944 | 2.175 | 307.2 |
| Factor | Factor Level | DON | NIV | ZON | FB1 | FB2 | FB3 | FBs |
|---|---|---|---|---|---|---|---|---|
| Year, Y | 2013 | 18.33 b ± 8.23 | 1.908 b ± 4.066 | 8.53 b ± 3.944 | 384.3 a ± 74.42 | 37.86 a ± 11.26 | 3.362 b ± 1.909 | 425.6 a ± 81.42 |
| 2014 | 84.24 a ± 19.17 | 10.96 a ± 10.815 | 28.43 a ± 12.865 | 172.7 b ± 44.07 | 24.34 b ± 10.53 | 5.235 a ± 2.569 | 202.2 b ± 53 | |
| HSD0.05 | 4.16 | 3.09 | 1.76 | 14.86 | 2.38 | 0.63 | 15.19 | |
| Methods of maize sowing, A | A1 | 48.4 a ± 35.43 | 6.108 a ± 10.473 | 16.01 b ± 13.22 | 252.8 b ± 104.2 | 26.33 b ± 11.39 | 3.939 b ± 1.786 | 283.1 b ± 110.8 |
| A2 | 54.16 a ± 37.8 | 6.755 a ± 8.143 | 20.95 a ± 14.15 | 304.2 a ± 136.4 | 35.87 a ± 12.48 | 4.658 a ± 2.934 | 344.7 a ± 145.6 | |
| HSD0.05 | 6.63 | 7.33 | 2.28 | 13.92 | 5.62 | 0.61 | 19.88 | |
| Types of hybrids, B | B1 | 61.41 a ± 38.75 | 6.197 a ± 7.083 | 19.05 a ± 13.06 | 287.3 a ± 137.9 | 29.76 b ± 13.89 | 4.585 a ± 1.62 | 321.6 a ± 148.2 |
| B2 | 41.16 b ± 31.41 | 6.667 a ± 11.221 | 17.91 a ± 14.71 | 269.7 a ± 108 | 32.44 a ± 11.69 | 4.012 a ± 3.045 | 306.1 a ± 115.6 | |
| HSD0.05 | 9.54 | 2.85 | 1.71 | 25.53 | 0.97 | 1.06 | 24.18 | |
| Methods of supplying NP fertilizer, C | C1 | 53.71 a ± 38.01 | 10.342 a ± 10.781 | 19.12 a ± 15.73 | 317.5 a ± 123.2 | 36.02 a ± 13.11 | 4.958 a ± 2.889 | 358.5 a ± 131.4 |
| C2 | 48.86 a ± 35.28 | 2.522 b ± 5.297 | 17.83 b ± 11.8 | 239.5 b ± 111.8 | 26.18 b ± 10.52 | 3.639 b ± 1.679 | 269.3 b ± 118.5 | |
| HSD0.05 | 6.18 | 4.56 | 1.52 | 9.79 | 2.75 | 0.71 | 10.45 |
| Factor | Factor Level | DON | NIV | ZON | FB1 | FB2 | FB3 | FBs |
|---|---|---|---|---|---|---|---|---|
| Year, Y | 2013 | 75.8 b ± 13.66 | 9.6 b ± 7.77 | 23.12 b ± 10.45 | 180.4 a ± 57.43 | 20.23 a ± 9.861 | 2.363 a ± 1.809 | 203 a ± 64.04 |
| 2014 | 231.3 a ± 126.17 | 74.03 a ± 37.11 | 30.43 a ± 9.91 | 61.7 b ± 13.22 | 6.98 b ± 1.888 | 1.437 b ± 1.673 | 70.1 b ± 13.32 | |
| HSD0.05 | 7.74 | 3.56 | 2.26 | 10.01 | 1.93 | 0.9 | 10.73 | |
| Methods of maize sowing, A | A1 | 134.5 b ± 79.9 | 36.24 b ± 31.44 | 21.78 b ± 10.602 | 113 a ± 62.03 | 10.34 a ± 5.315 | 1.547 a ± 1.603 | 124.8 b ± 66.45 |
| A2 | 172.6 a ± 146.9 | 47.39 a ± 50.5 | 31.76 a ± 8.422 | 129.1 a ± 82.73 | 16.86 a ± 11.916 | 2.253 a ± 1.921 | 148.2 a ± 93.77 | |
| HSD0.05 | 4 | 6.39 | 2.49 | 19.29 | 7.22 | 1.22 | 15.01 | |
| >Types of hybrids, B | B1 | 195.8 a ± 149 | 50.41 a ± 49.41 | 32.83 a ± 8.26 | 141.6 a ± 89.51 | 16.4 a ± 11.459 | 2.277 a ± 1.91 | 160.3 a ± 99.09 |
| B2 | 111.3 b ± 52.9 | 33.21 b ± 31.75 | 20.72 b ± 9.513 | 100.5 b ± 43.97 | 10.8 b ± 6.687 | 1.523 a ± 1.604 | 112.8 b ± 49.98 | |
| HSD0.05 | 13.31 | 4.39 | 3.06 | 10.74 | 1.44 | 0.83 | 10.55 | |
| Methods of supplying NP fertilizer, C | C1 | 184.1 a ± 149.4 | 55.26 a ± 50.8 | 27.97 a ± 11.05 | 138.1 a ± 79.38 | 14.44 a ± 10.459 | 1.974 a ± 1.732 | 154.5 a ± 87.87 |
| C2 | 123 b ± 66.5 | 28.37 b ± 25.42 | 25.57 b ± 10.5 | 104 b ± 62.62 | 12.76 a ± 9.031 | 1.826 a ± 1.873 | 118.6 b ± 71.42 | |
| HSD0.05 | 9.43 | 4.07 | 1.87 | 13.4 | 1.81 | 0.81 | 12.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocianowski, J.; Szulc, P.; Waśkiewicz, A.; Cyplik, A. The Effect of Agrotechnical Factors on Fusarium Mycotoxins Level in Maize. Agriculture 2020, 10, 528. https://doi.org/10.3390/agriculture10110528
Bocianowski J, Szulc P, Waśkiewicz A, Cyplik A. The Effect of Agrotechnical Factors on Fusarium Mycotoxins Level in Maize. Agriculture. 2020; 10(11):528. https://doi.org/10.3390/agriculture10110528
Chicago/Turabian StyleBocianowski, Jan, Piotr Szulc, Agnieszka Waśkiewicz, and Adrian Cyplik. 2020. "The Effect of Agrotechnical Factors on Fusarium Mycotoxins Level in Maize" Agriculture 10, no. 11: 528. https://doi.org/10.3390/agriculture10110528
APA StyleBocianowski, J., Szulc, P., Waśkiewicz, A., & Cyplik, A. (2020). The Effect of Agrotechnical Factors on Fusarium Mycotoxins Level in Maize. Agriculture, 10(11), 528. https://doi.org/10.3390/agriculture10110528







