Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study
Abstract
1. Introduction
- Would steam sterilization result in a significant change in dimensions of biocompatible resin materials’ 3D printed test bodies?
- Would the dimensional accuracy of test bodies be similar across all the groups, i.e., proprietary and third-party biocompatible resin materials?
2. Materials and Methods
2.1. Equipment and Materials
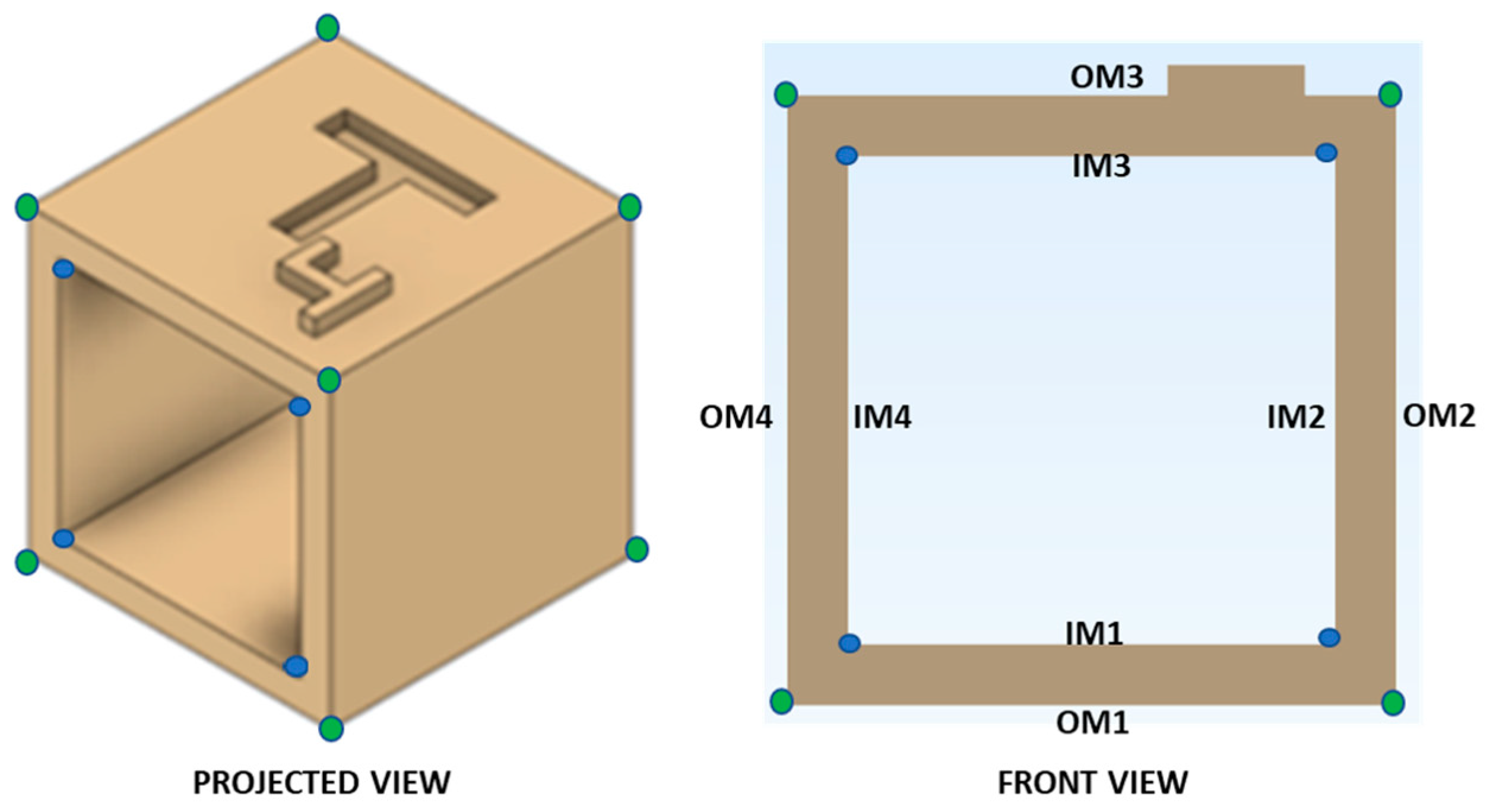
2.2. Computer-Aided Design (CAD) Modeling of Reference Test Body
2.3. 3D Printing of Test Bodies
2.4. Post-Processing and Post-Curing Processes of 3D Printed Test Bodies
2.5. Sterilization Method
2.6. Data Collection and Assessment of Dimensional Accuracy
2.7. Statistical Analysis
3. Results
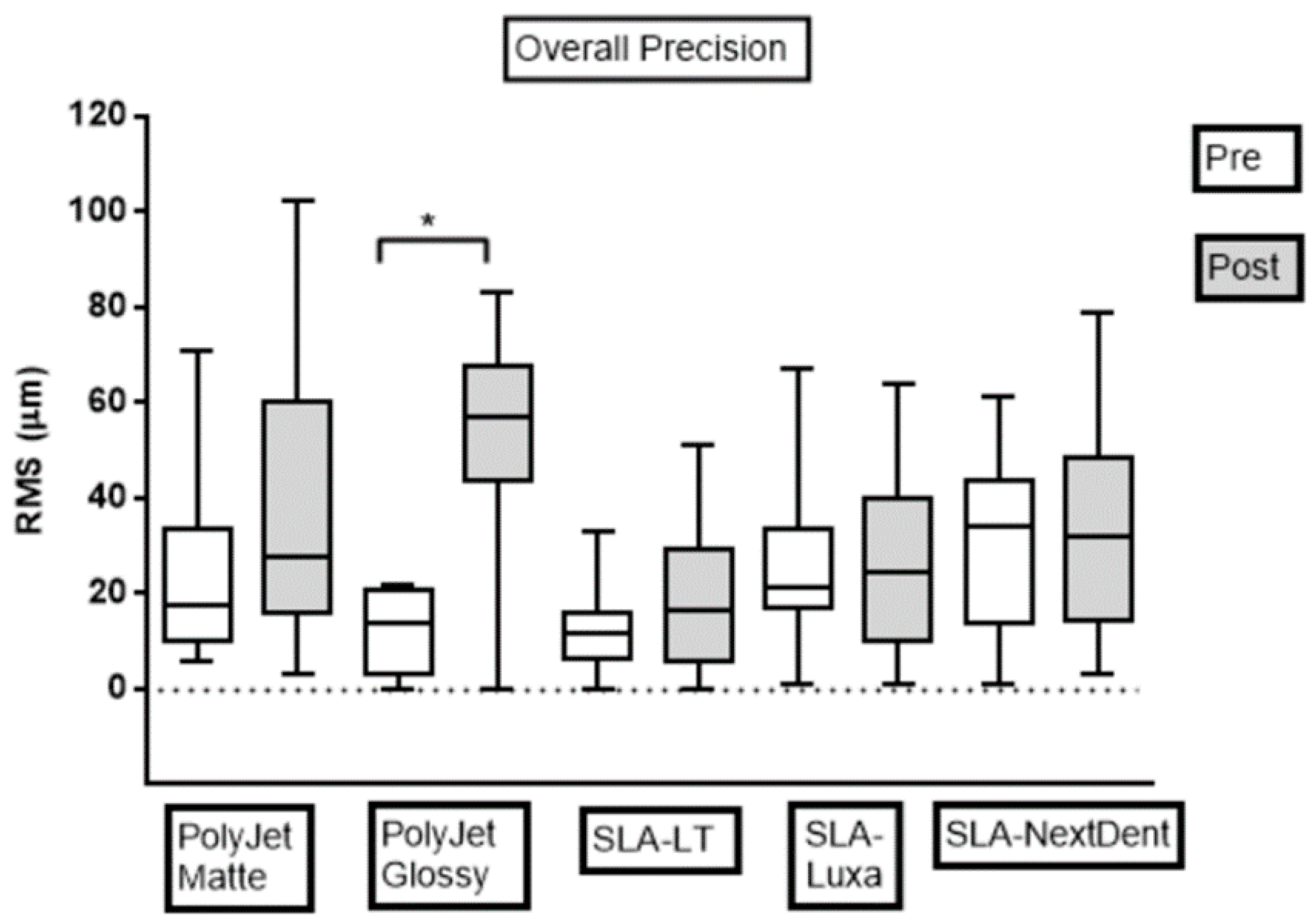
3.1. Precision Assessment
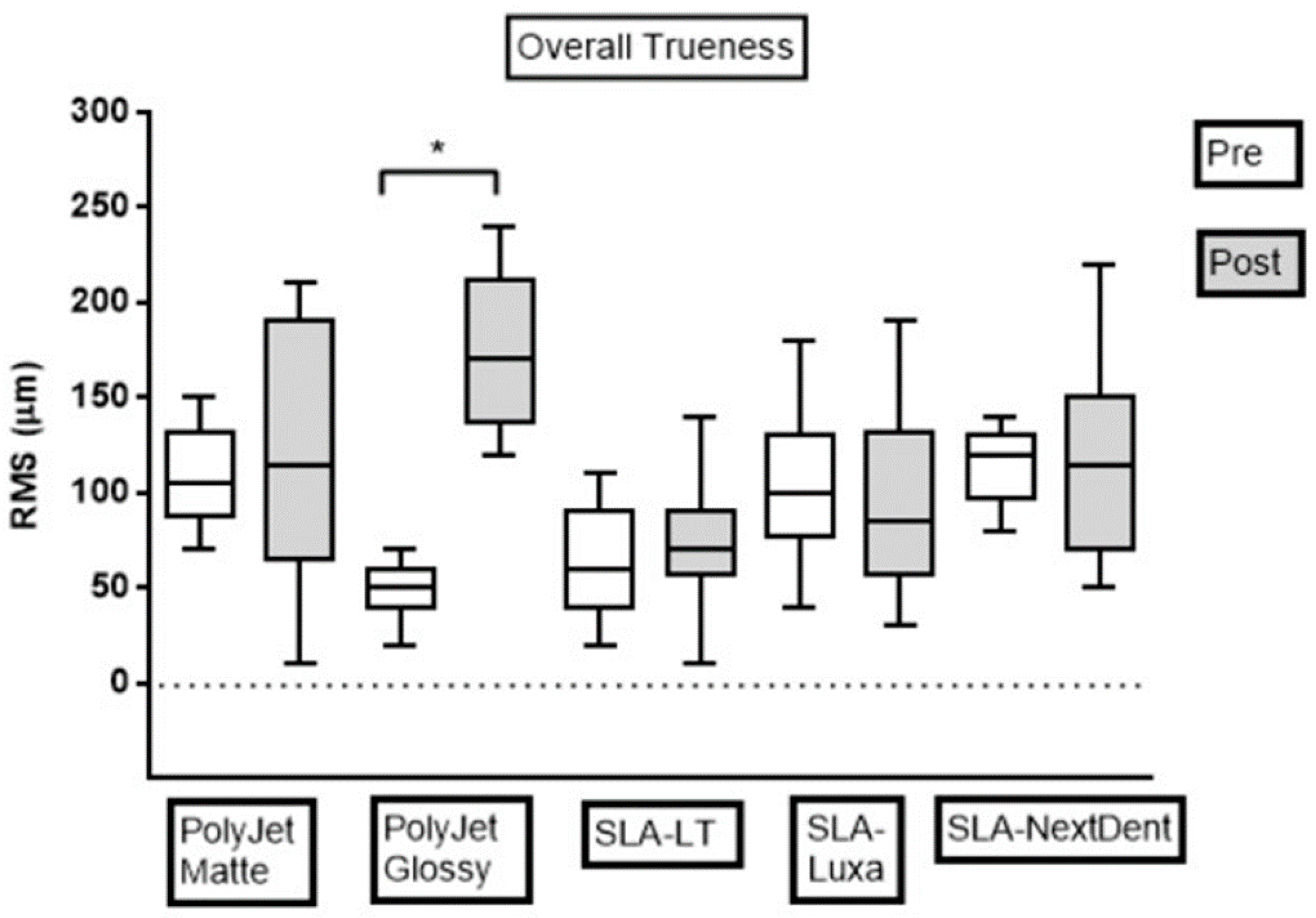
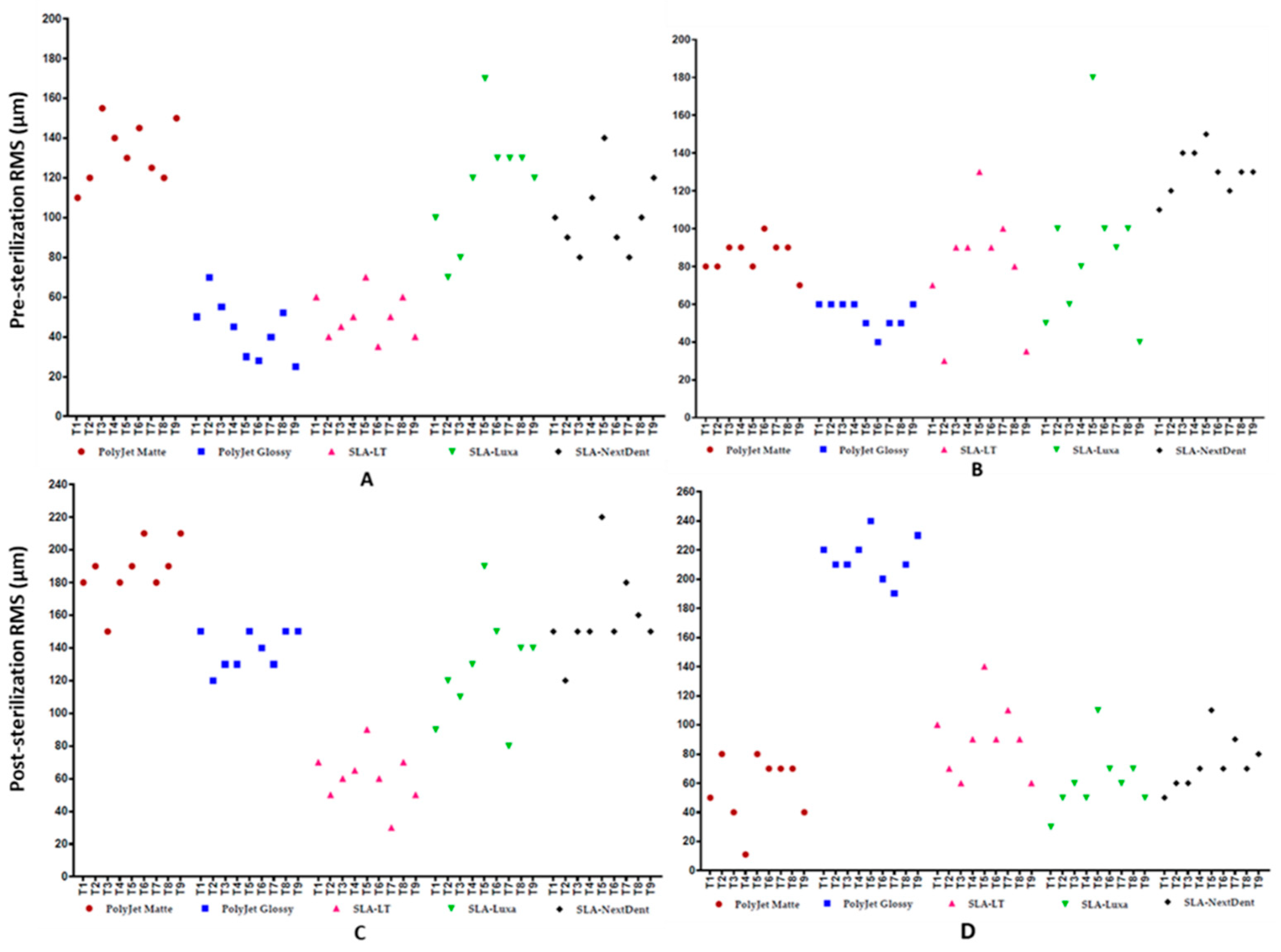
3.2. Trueness Assessment
3.3. Deviations Distribution Pattern of Test Bodies regarding Position on 3D Printer’s Build Platform
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| CAD | Computer-aided design |
| AM | Additive manufacturing |
| SLA | Stereolithography |
| UV | Ultraviolet |
| LT | Dental LT Clear |
| Luxa | LuxaPrint Ortho Plus |
| NextDent | NextDent Ortho Clear |
| IPA | Isopropyl alcohol |
| RMS | Root mean square |
| SD | Standard deviation |
| STL | Standard tessellation language |
| OM | Outer measurement |
| IM | Inner measurement |
| PLA | Polylactic acid |
| PDMS | Polydimethylsiloxane |
| PTEG | Polyethylene Terephthalate Glycol |
| UV | Ultraviolet |
References
- Rustemeyer, J.; Busch, A.; Sari-Rieger, A. Application of computer-aided designed/computer-aided manufactured techniques in reconstructing maxillofacial bony structures. Oral Maxillofac. Surg. 2014, 18, 471–476. [Google Scholar] [CrossRef]
- Hirsch, D.L.; Garfein, E.S.; Christensen, A.M.; Weimer, K.A.; Saddeh, P.B.; Levine, J.P. Use of Computer-Aided Design and Computer-Aided Manufacturing to Produce Orthognathically Ideal Surgical Outcomes: A Paradigm Shift in Head and Neck Reconstruction. J. Oral Maxillofac. Surg. 2009, 67, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc Eng. 2019, 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.; Perrault, D.; Stevanovic, M.; Ghiassi, A. Surgical applications of three-dimensional printing: A review of the current literature & how to get started. Ann. Transl. Med. 2016, 4, 456. [Google Scholar] [CrossRef] [PubMed]
- Turbush, S.K.; Turkyilmaz, I. Accuracy of three different types of stereolithographic surgical guide in implant placement: An in vitro study. J. Prosthet. Dent. 2012, 108, 181–188. [Google Scholar] [CrossRef]
- Diment, L.E.; Thompson, M.S.; Bergmann, J.H.M. Clinical efficacy and effectiveness of 3D printing: A systematic review. BMJ Open 2017, 7, e016891. [Google Scholar] [CrossRef]
- Abo Sharkh, H.; Makhoul, N. In-House Surgeon-Led Virtual Surgical Planning for Maxillofacial Reconstruction. J. Oral. Maxillofac. Surg. 2020, 78, 651–660. [Google Scholar] [CrossRef]
- Pajot, T.; Benichou, L.; Moreau, E.; Tallon, V.; Meningaud, J.P.; Khonsari, R.H.; Ketoff, S. Implementation of a digital chain for the design and manufacture of implant-based surgical guides in a hospital setting. J. Stomatol Oral. Maxillofac. Surg. 2019, in press. [Google Scholar] [CrossRef]
- Ballard, D.H.; Mills, P.; Duszak, R.; Weisman, J.A.; Rybicki, F.J.; Woodard, P.K. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Acad. Radiol. 2019, in press. [Google Scholar] [CrossRef]
- Chen, L.; Lin, W.-S.; Polido, W.D.; Eckert, G.J.; Morton, D. Accuracy, reproducibility, and dimensional stability of additively manufactured surgical templates. J. Prosthet. Dent. 2019, 122, 309–314. [Google Scholar] [CrossRef]
- Marei, H.F.; Alshaia, A.; Alarifi, S.; Almasoud, N.; Abdelhady, A. Effect of Steam Heat Sterilization on the Accuracy of 3D Printed Surgical Guides. Implant. Dent. 2019, 28, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/steam.html (accessed on 18 March 2020).
- Christensen, A.; Rybicki, F.J. Maintaining safety and efficacy for 3D printing in medicine. 3D Print. Med. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- George, E.; Liacouras, P.; Rybicki, F.J.; Mitsouras, D. Measuring and Establishing the Accuracy and Reproducibility of 3D Printed Medical Models. Radiographics 2017, 37, 1424–1450. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; McGee, K.; Morris, J.; Alexander, A.; Kuhlmann, J.; Vrieze, T.; McCollough, C.H.; Matsumoto, J. Anatomic modeling using 3D printing: Quality assurance and optimization. 3D Print. Med. 2017, 3, 6. [Google Scholar] [CrossRef]
- Chepelev, L.; Wake, N.; Ryan, J.; Althobaity, W.; Gupta, A.; Arribas, E.; Santiago, L.; Ballard, D.H.; Wang, K.C.; Weadock, W.; et al. RSNA Special Interest Group for 3D Printing. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): Guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print. Med. 2018, 4, 11. [Google Scholar] [CrossRef]
- Infection control recommendations for the dental office and the dental laboratory. ADA council on scientific affairs and ADA council on dental practice. J. Am. Dent. Assoc. 1996, 127, 672–680. [Google Scholar]
- Shqaidef, A.; Ayoub, A.F.; Khambay, B.S. How accurate are rapid prototyped (RP) final orthognathic surgical wafers? A pilot study. Br. J. Oral. Maxillofac. Surg. 2014, 52, 609–614. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- The 3D Printing Dental Market is Booming. Available online: https://www.3dnatives.com/en/3d-printing-dental-market-170120184/ (accessed on 4 April 2020).
- Van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- International Organization for Standardization. Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 1: General Principles and Definitions—Technical Corrigendum 1; ISO 5725–1:1994/Cor 1:1998; International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- Ender, A.; Mehl, A. Accuracy of complete-arch dental impressions: A new method of measuring trueness and precision. J. Prosthet. Dent. 2013, 109, 121–128. [Google Scholar] [CrossRef]
- Legocki, A.T.; Duffy-Peter, A.; Scott, A.R. Benefits and Limitations of Entry-Level 3-Dimensional Printing of Maxillofacial Skeletal Models. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Hatz, C.R.; Msallem, B.; Aghlmandi, S.; Brantner, P.; Thieringer, F.M. Can an entry-level 3D printer create high-quality anatomical models? Accuracy assessment of mandibular models printed by a desktop 3D printer and a professional device. Int. J. Oral. Maxillofac. Surg. 2020, 49, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Juneja, M.; Thakur, N.; Kumar, D.; Gupta, A.; Bajwa, B.; Jindal, P. Accuracy in dental surgical guide fabrication using different 3-D printing techniques. Addit. Manuf. 2018, 22, 243–255. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Cho, J.-W.; Chang, N.-Y.; Chae, J.-M.; Kang, K.-H.; Kim, S.-C.; Cho, J.-H. Accuracy of three-dimensional printing for manufacturing replica teeth. Korean J. Orthod. 2015, 45, 217–225. [Google Scholar] [CrossRef]
- Silva, D.N.; Gerhardt de Oliveira, M.; Meurer, E.; Meurer, M.I.; Lopes da Silva, J.V.; Santa-Bárbara, A. Dimensional error in selective laser sintering and 3D-printing of models for craniomaxillary anatomy reconstruction. J. Craniomaxillofac. Surg. 2008, 36, 443–449. [Google Scholar] [CrossRef]
- Barker, T.M.; Earwaker, W.J.S.; Lisle, D.A. Accuracy of stereolithographic models of human anatomy. Australas. Radiol. 1994, 38, 106–111. [Google Scholar] [CrossRef]
- Ibrahim, D.; Broilo, T.L.; Heitz, C.; de Oliveira, M.G.; de Oliveira, H.W.; Nobre, S.M.W.; dos Santos Filho, J.H.G.; Silva, D.N. Dimensional error of selective laser sintering, three-dimensional printing and PolyJet™ models in the reproduction of mandibular anatomy. J. Craniomaxillofac. Surg. 2009, 37, 167–173. [Google Scholar] [CrossRef]
- Choi, J.Y.; Choi, J.H.; Kim, N.K.; Kim, Y.; Lee, J.K.; Kim, M.K.; Lee, J.H.; Kim, M.J. Analysis of errors in medical rapid prototyping models. Int J. Oral. Maxillofac. Surg. 2002, 31, 23–32. [Google Scholar] [CrossRef]
- Hazeveld, A.; Huddleston Slater, J.J.R.; Ren, Y. Accuracy and reproducibility of dental replica models reconstructed by different rapid prototyping techniques. Am. J. Orthod. Dentofacial. Orthop. 2014, 145, 108–115. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.; Kim, G.B.; Hong, D.; Kwon, J.; Park, J.W.; Kim, N. Accuracy of a simplified 3D-printed implant surgical guide. J. Prosthet. Dent. 2019, in press. [Google Scholar] [CrossRef]
- Yuan, X.; Xuan, M.; Tian, W.; Long, J. Application of digital surgical guides in mandibular resection and reconstruction with fibula flaps. Int J. Oral. Maxillofac. Surg. 2016, 45, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Török, G.; Gombocz, P.; Bognár, E.; Nagy, P.; Dinya, E.; Kispélyi, B.; Hermann, P. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy—Pilot study. BMC Oral. Health 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.S.; Gateno, J.; Bell, R.B.; Hirsch, D.L.; Markiewicz, M.R.; Teichgraeber, J.F.; Zhou, X.; Xia, J.J. Accuracy of a computer-aided surgical simulation protocol for orthognathic surgery: A prospective multicenter study. J. Oral. Maxillofac. Surg. 2013, 71, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Schouman, T.; Rouch, P.; Imholz, B.; Fasel, J.; Courvoisier, D.; Scolozzi, P. Accuracy evaluation of CAD/CAM generated splints in orthognathic surgery: A cadaveric study. Head Face Med. 2015, 25, 24. [Google Scholar] [CrossRef]
- Vercruyssen, M.; Laleman, I.; Jacobs, R.; Quirynen, M. Computer-supported implant planning and guided surgery: A narrative review. Clin. Oral Implant. Res. 2015, 26, 69–76. [Google Scholar] [CrossRef]
- Oth, O.; Dauchot, C.; Orellana, M.Á.; Glineur, R. How to Sterilize 3D Printed Objects for Surgical Use? An Evaluation of the Volumetric Deformation of 3D-Printed Genioplasty Guide in PLA and PETG after Sterilization by Low-Temperature Hydrogen Peroxide Gas Plasma. Open Dent. J. 2019, 13, 410–417. [Google Scholar] [CrossRef]
- Boursier, J.F.; Fournet, A.; Bassanino, J.; Manassero, M.; Bedu, A.S.; Leperlier, D. Reproducibility, accuracy and effect of autoclave sterilization on a thermoplastic three-dimensional model printed by a desktop fused deposition modelling three-dimensional printer. Vet. Comp. Orthop. Traumatol. 2018, 31, 422–430. [Google Scholar] [CrossRef]
- Shaheen, E.; Alhelwani, A.; Van De Casteele, E.; Politis, C.; Jacobs, R. Evaluation of Dimensional Changes of 3D Printed Models After Sterilization: A Pilot Study. Open Dent. J. 2018, 12, 72–79. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Marrelli, M.; Maletta, C.; Inchingolo, F.; Alfano, M.; Tatullo, M. Three-Point Bending Tests of Zirconia Core/Veneer Ceramics for Dental Restorations. Int. J. Dent. 2013, 2013, 831976. [Google Scholar] [CrossRef]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.-F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Shin, Y.-S.; Jung, H.-D.; Hwang, C.-J.; Baik, H.-S.; Cha, J.-Y. Precision and trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Unkovskiy, A.; Bui, P.H.-B.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef] [PubMed]
- Odeh, M.; Levin, D.; Inziello, J.; Lobo Fenoglietto, F.; Mathur, M.; Hermsen, J.; Stubbs, J.; Ripley, B. Methods for verification of 3D printed anatomic model accuracy using cardiac models as an example. 3D Print. Med. 2019, 29, 6. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, J.; Lee, J.; Cho, D.W. Three-Dimensional Printing of Tissue/Organ Analogues Containing Living Cells. Ann. Biomed. Eng. 2017, 45, 180–194. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential Use of Human Periapical Cyst-Mesenchymal Stem Cells (hPCy-MSCs) as a Novel Stem Cell Source for Regenerative Medicine Applications. Front. Cell Dev. Biol. 2017, 5, 103. [Google Scholar] [CrossRef]
- Barry, M.; Pearce, H.; Cross, L.; Tatullo, M.; Gaharwar, A.K. Advances in Nanotechnology for the Treatment of Osteoporosis. Curr. Osteoporos. Rep. 2016, 14, 87–94. [Google Scholar] [CrossRef]
| Group | Resin Code Name | Printing Profile Setting |
|---|---|---|
| 1 | PolyJet Matte | Matte profile |
| 2 | PolyJet Glossy | Glossy profile |
| 3 | SLA-LT | Dental LT resin profile |
| 4 | SLA-Luxa | Clear V2 resin-experimental profile |
| 5 | SLA-NextDent | Dental SG resin-experimental profile |
| PolyJet Matte | PolyJet Glossy | SLA-LT | SLA-Luxa | SLA-NextDent | ||
|---|---|---|---|---|---|---|
| Measurement | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Precision | OM | 31.0 ± 20.1 a | 9.25 ± 8.4 b | 8.5 ± 4.1 b | 26.7 ± 21.3 a,b | 23.5 ± 17.7 a,b |
| IM | 14.5 ± 11.3 a | 16.88 ± 4.4 a | 20.5 ± 11.2 a | 23.4 ± 10.8 a,b | 38.4 ± 15.2 b | |
| Overall | 24.4 ± 18.7 a,b | 12.3 ± 7.9 a | 13.3 ± 9.6 a | 25.4 ± 17.5 a,c | 29.5 ± 17.9 b,c | |
| Trueness | OM | 132.2 ± 13.9 a | 41.1 ± 16.2 b | 48.9 ± 12.7 b | 116.7 ± 30.0 a,c | 101.1 ± 19.0 c |
| IM | 85.6 ± 8.8 a,b | 54.4 ± 7.3 a,c | 74.4 ± 32.8 b,c | 88.9 ± 41.1 b | 128.9 ± 10.5 d | |
| Overall | 108.9 ± 26.5 a | 47.8 ± 13.9 b | 61.7 ± 27.5 b | 102.8 ± 37.7 a | 115.0 ± 20.6 a |
| PRE | PolyJet Matte | PolyJet Glossy | SLA-LT | SLA-Luxa | SLA-NextDent |
|---|---|---|---|---|---|
| PolyJet Matte | |||||
| PolyJet Glossy | 0.08 | ||||
| SLA-LT | 0.14 | 0.99 | |||
| SLA-Luxa | 0.99 | 0.06 | 0.09 | ||
| SLA-NextDent | 0.82 | <0.05 * | <0.05 * | 0.91 | |
| POST | PolyJet Matte | PolyJet Glossy | SLA-LT | SLA-Luxa | SLA-NextDent |
| PolyJet Matte | |||||
| PolyJet Glossy | 0.32 | ||||
| SLA-LT | 0.06 | <0.05 * | |||
| SLA-Luxa | 0.43 | <0.05 * | 0.85 | ||
| SLA-NextDent | 0.99 | 0.15 | 0.15 | 0.67 | |
| PolyJet Matte | PolyJet Glossy | SLA-LT | SLA-Luxa | SLA-NextDent | ||
|---|---|---|---|---|---|---|
| Measurement | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Precision | OM | 50.8 ± 29.7 a | 38.0 ± 24.7 a,b | 16.8 ± 12.0 b | 35.3 ± 15.8 a,b | 46.0 ± 19.4 a |
| IM | 17.5 ± 11.1 a | 70.5 ± 8.0 | 20.5 ± 17.6 a | 10.3 ± 6.4 a | 17.8 ± 14.5 a | |
| Overall | 37.5 ± 28.9 a,b | 51.0 ± 25.4 b | 18.3 ± 14.2 a | 25.3 ± 17.8 a | 34.7 ± 22.3 a,b | |
| Trueness | OM | 186.7 ± 18.0 a | 138.9 ± 11.7 b | 72.2 ± 19.9 | 127.8 ± 33.1 b | 158.9 ± 27.6 a,b |
| IM | 81.1 ± 19.0 a,c | 214.4 ± 15.1 | 90.0 ± 25.5 b,c | 61.1 ± 22.1 a | 76.7 ± 20.0 a,b | |
| Overall | 133.9 ± 57.2 a | 176.7 ± 41.0 | 81.1 ± 23.9 b | 94.4 ± 43.8 a,b | 117.8 ± 48.3 a,b |
| PRE | PolyJet Matte | PolyJet Glossy | SLA-LT | SLA-Luxa | SLA-NextDent |
|---|---|---|---|---|---|
| PolyJet Matte | |||||
| PolyJet Glossy | <0.05 * | ||||
| SLA-LT | <0.05 * | 0.52 | |||
| SLA-Luxa | 0.95 | <0.05 * | <0.05 * | ||
| SLA-NextDent | 0.96 | <0.05 * | <0.05 * | 0.64 | |
| POST | PolyJet Matte | PolyJet Glossy | SLA-LT | SLA-Luxa | SLA-NextDent |
| PolyJet Matte | |||||
| PolyJet Glossy | <0.05 * | ||||
| SLA-LT | <0.05 * | <0.05 * | |||
| SLA-Luxa | 0.61 | <0.05 * | 0.89 | ||
| SLA-NextDent | 0.8 | <0.05 * | 0.15 | 0.52 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. https://doi.org/10.3390/jcm9051506
Sharma N, Cao S, Msallem B, Kunz C, Brantner P, Honigmann P, Thieringer FM. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. Journal of Clinical Medicine. 2020; 9(5):1506. https://doi.org/10.3390/jcm9051506
Chicago/Turabian StyleSharma, Neha, Shuaishuai Cao, Bilal Msallem, Christoph Kunz, Philipp Brantner, Philipp Honigmann, and Florian M. Thieringer. 2020. "Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study" Journal of Clinical Medicine 9, no. 5: 1506. https://doi.org/10.3390/jcm9051506
APA StyleSharma, N., Cao, S., Msallem, B., Kunz, C., Brantner, P., Honigmann, P., & Thieringer, F. M. (2020). Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. Journal of Clinical Medicine, 9(5), 1506. https://doi.org/10.3390/jcm9051506






