Relationship between Soluble (Pro)Renin Receptor and Renin Activity in Patients with Severe Heart Failure
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.3. Method for Measurement of PRA, PRC and s(P)RR
2.4. Statistical Analysis
2.5. Ethical Approval
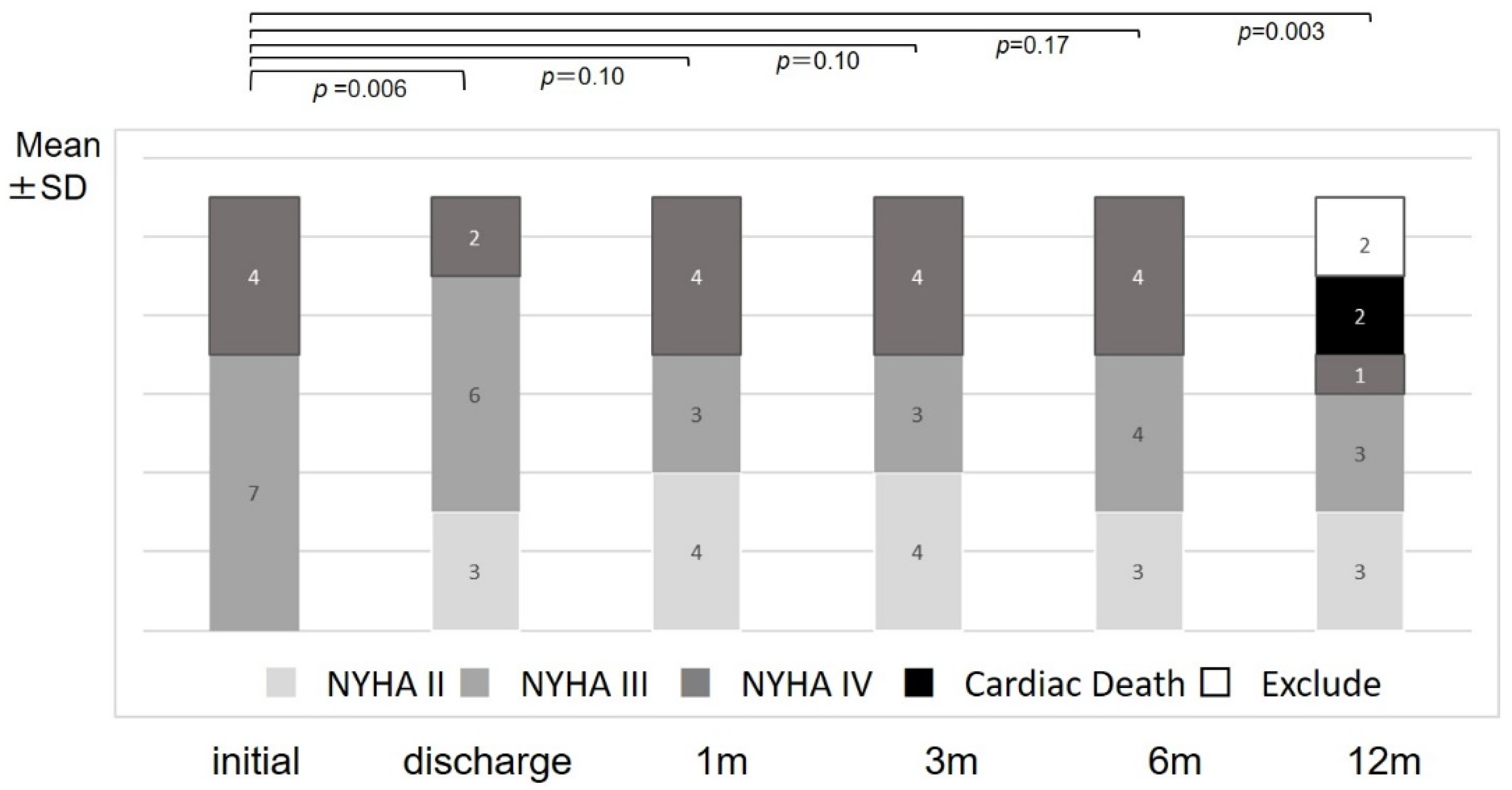
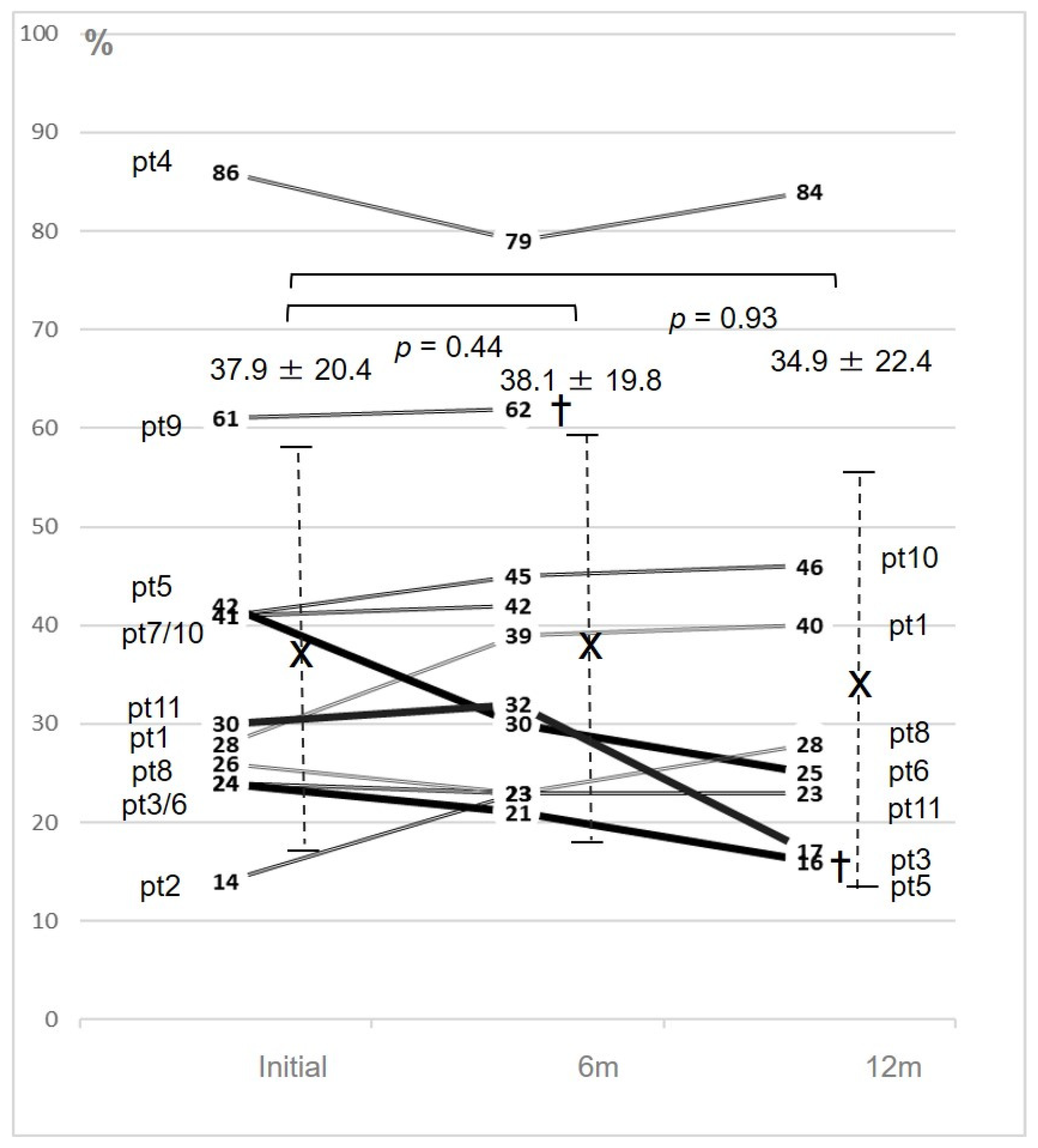
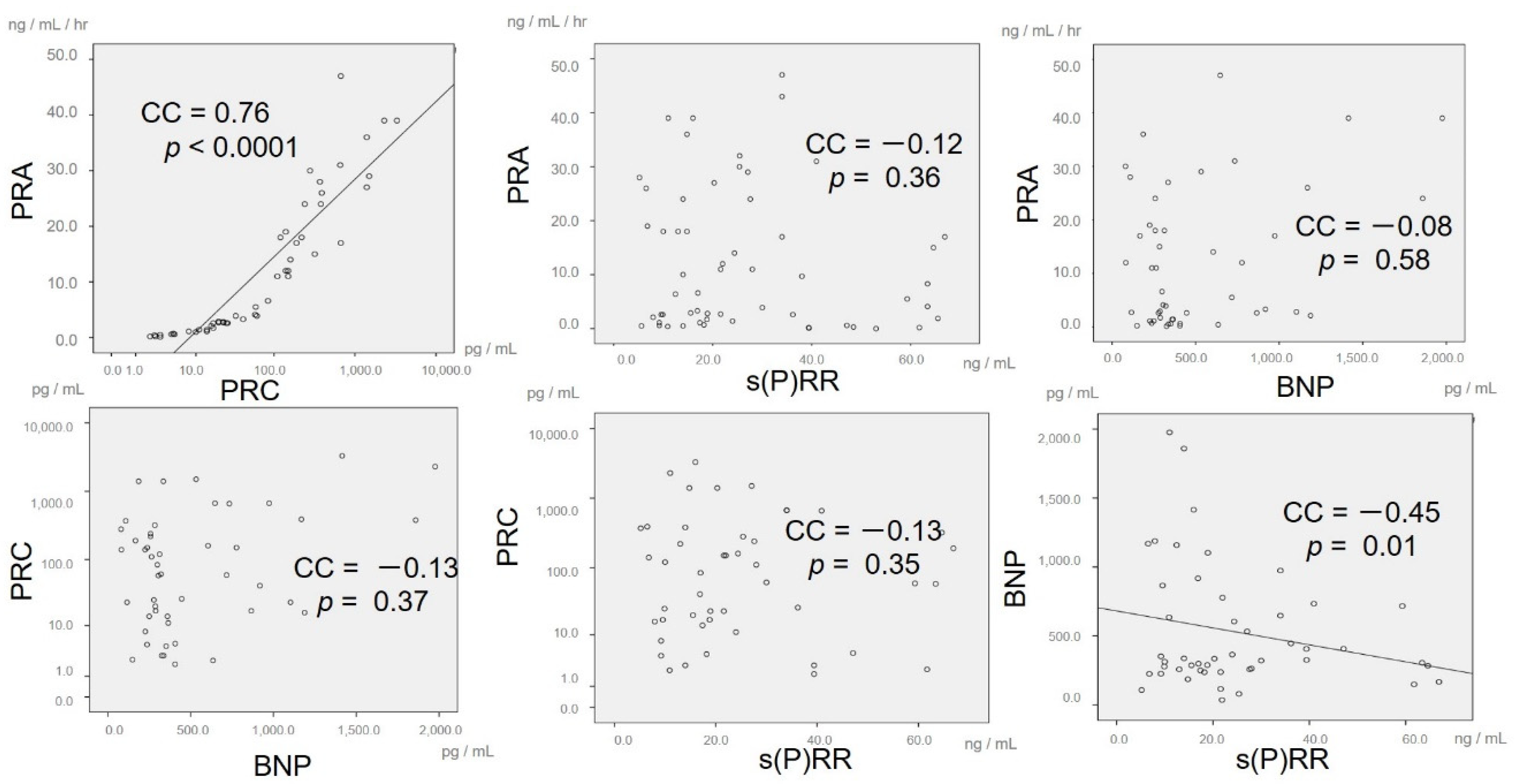
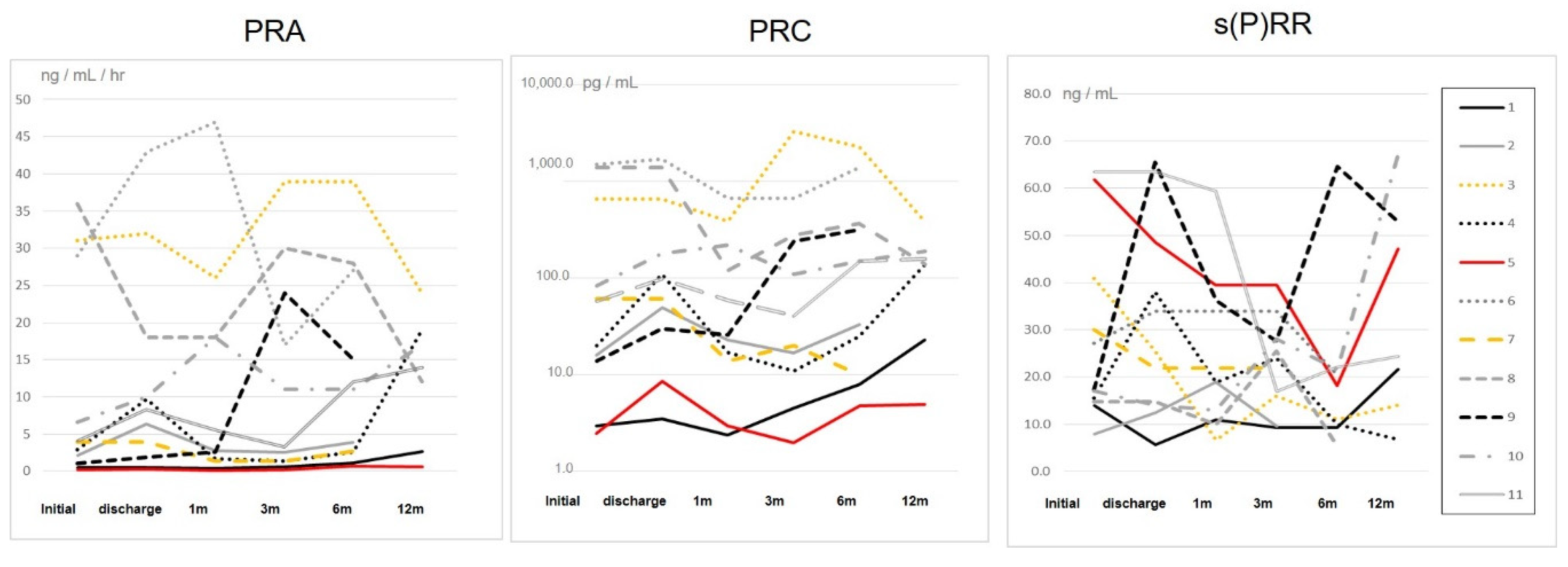
3. Results
4. Discussion
4.1. Major Findings
4.2. Regulation of RAS Activity and s(P)RR in Severe HF
4.3. Clinical Implications
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerd, H.; Douglas, L.M. Pathophysiology of Heart Failure. In Eugene Braunwald. A Textbook of Cardiovascular Medicine, 10th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 455–457. [Google Scholar]
- Douglas, L.M. Management of Patients with Heart Failure with Reduced Ejection Fraction. In Eugene Braunwald. A Textbook of Cardiovascular Medicine, 10th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 522–533. [Google Scholar]
- Tsutamoto, T.; Sakai, H.; Tanaka, T.; Fujii, M.; Yamamoto, T.; Wada, A.; Ohnishi, M.; Horie, M. PRA/PRC shows the internal renin activity of the heart failure patient, and it is reported that a convalescence prediction becomes. Circ. J. 2007, 71, 915–921. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Subodh, V.; Milan, G.; Daniel, T.H.; Hwee, T.; Sunayna, G.; Salim, Y.; Eva, M.L. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur. Heart J. 2011, 32, 2135–2142. [Google Scholar]
- Vergaro, G.; Emdin, M.; Iervasi, A.; Zyw, L.; Gabutti, A.; Poletti, R.; Mammini, C.; Giannoni, A.; Fontana, M.; Passino, C. Prognostic Value of Plasma Renin Activity in Heart Failure. Am. J. Cardiol. 2011, 108, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.; Delarue, F.; Burcklé, C.; Bouzhir, L.; Giller, T.; Sraer, J.D. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Investig. 2002, 109, 1417–1427. [Google Scholar] [CrossRef]
- Batenburg, W.W.; de Bruin, R.J.; van Gool, J.M.; Müller, D.N.; Bader, M.; Nguyen, G.; Danser, A.H. Aliskiren-binding increases the half life of renin and prorenin in rat aortic vascular smooth muscles cells. Arter. Thromb. Vasc. Biol. 2008, 28, 1151–1157. [Google Scholar] [CrossRef]
- Ichihara, A.; Hayashi, M.; Kaneshiro, Y.; Suzuki, F.; Nakagawa, T.; Tada, Y.; Koura, Y.; Nishiyama, A.; Okada, H.; Uddin, M.; et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J. Clin. Investig. 2004, 114, 1128–1135. [Google Scholar] [CrossRef]
- Yoshikawa, A.; Aizaki, Y.; Kusano, K.; Kishi, F.; Susumu, T.; Iida, S.; Ishiura, S.; Nishimura, S.; Shichiri, M.; Senbonmatsu, T. The (Pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertention Res. 2011, 34, 599–605. [Google Scholar] [CrossRef]
- Cousin, C.; Bracquart, D.; Contrepas, A.; Corvol, P.; Muller, L.; Nguyen, G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 2009, 53, 1077–1082. [Google Scholar] [CrossRef]
- Gonzalez, A.A.; Lara, L.S.; Luffman, C.; Seth, D.M.; Prieto, M.C. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 2011, 57, 859–864. [Google Scholar] [CrossRef]
- Danser, A.H. The increase in renin during renin inhibition: Does it result in harmful effects by the (pro)renin receptor? Hypertens. Res. 2010, 33, 4–10. [Google Scholar] [CrossRef]
- Faris, R.; Flather, M.D.; Purcell, H.; Poole-Wilson, P.A.; Coats, A.J. Diuretics for heart failure. Cochrane Database Syst. Rev. 2012, 15, CD003838. [Google Scholar] [CrossRef]
- Domanski, M.; Tian, X.; Haigney, M.; Pitt, B. Diuretic use, progressive heart failure, and death in patients in the DIG study. J. Card Fail. 2006, 12, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.Z.; Danny, S.S. Tolvaptan: A novel diuretic in heart failure management. J. Tehran Heart Cent. 2016, 13, 1–5. [Google Scholar]
- Kida, K. New insights into tolvaptan treatment and the renin-angiotensin-aldosterone system. Int. Heart J. 2017, 31, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Kanda, A.; Hirose, I.; Noda, K.; Ishida, S. Systemic factors related to soluble (pro)renin receptor in plasma of patients with proliferative diabetic retinopathy. PLoS ONE 2017, 12, e0189696. [Google Scholar] [CrossRef]
- Hamada, K.; Taniguchi, Y.; Shimamura, Y.; Inoue, K.; Ogata, K.; Ishihara, M.; Horino, T.; Fujimoto, S.; Ohguro, T.; Yoshimoto, Y.; et al. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 848–856. [Google Scholar] [CrossRef]
- Morimoto, S.; Ando, T.; Niiyama, M.; Seki, Y.; Yoshida, N.; Watanabe, D.; Kawakami-Mori, F.; Kobori, H.; Nishiyama, A.; Ichihara, A. Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens. Res. 2014, 37, 642–648. [Google Scholar] [CrossRef]
- Watanabe, N.; Bokuda, K.; Fujiwara, T.; Suzuki, T.; Mito, A.; Morimoto, S.; Seung, C.J.; Egawa, M.; Yoshie, A.; Suzuki, F.; et al. Soluble (Pro)Renin Receptor and Blood Pressure During Pregnancy A Prospective Cohort Study. Hypertension 2012, 60, 1250–1256. [Google Scholar]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; John, G.F.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonza’lez-Juanatey, J.R.; Harjola, V.-P.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar]
- Wegman-Ostrosky, T.; Soto-Reyes, E.; Vidal-Millán, S.; Sánchez-Corona, J. The renin-angiotensin system meets the hallmarks of cancer. J. Renin. Angiotensin Aldosterone Syst. 2015, 16, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Atucha, A.; Izag, A. Sex differences in the aging pattern of renin–angiotensin system serum peptidases. Biol. Sex Differ. 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Linghui, G.D.; Shenglin, Z.; Linrui, L.; Xin, G.; Dongxia, W.; Dachang, W.; Ke, W.; Yan, L. Soluble (pro)renin receptor in elderly chronic heart failure patients. Peptides 2019, 111, 152–157. [Google Scholar]
- Obradovic, O.; Loncar, G.; Radenovic, S.; Tahirovic, E.; Heidecke, H.; Schulze-Forster, K.; Muller, D.; Busjahn, A.; Buttner, P.; Veskovic, J.; et al. Soluble (pro)renin receptor in elderly chronic heart failure patients. Front. Biosci. 2020, 25, 1836–1850. [Google Scholar] [CrossRef]
- Fukushima, A.; Kinugawa, S.; Homma, T.; Makita, Y.; Furihata, T.; Abe, T.; Suga, T.; Takada, S.; Kadoguchi, T.; Okita, K.; et al. Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int. J. Cardiol. 2013, 168, 4313–4314. [Google Scholar] [CrossRef]
- John, J.V.M.; Milton, P.; Akshay, S.D.; Jianjian, G.; Martin, P.L.; Adel, R.R.; Jean, L.R.; Victor, C.S.; Scott, D.S.; Karl, S.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar]
- Kinouchi, K.; Ichihara, A.; Itoh, H. Functional characterization of (pro)renin receptor in association with V-ATPase. Front. Biosci. 2011, 16, 3216–3223. [Google Scholar]
- Takahashi, K.; Hiraishi, K.; Hirose, T.; Kato, I.; Yamamoto, H.; Shoji, I.; Shibasaki, A.; Kaneko, K.; Satoh, F.; Totsune, K. Expression of (pro)renin receptor in the human brain and pituitary, and co-localisation with arginine vasopressin and oxytocin in the hypothalamus. J. Neuroendocrinol. 2010, 22, 453–459. [Google Scholar] [CrossRef]
- Watanabe, K.; Dohi, K.; Sugimoto, T.; Yamada, T.; Sato, Y.; Ichikawa, K.; Sugiura, E.; Kumagai, N.; Nakamori, S.; Nakajima, H.; et al. Short-term effects of low-dose tolvaptan on hemodynamic parameters in patients with chronic heart failure. J. Cardiol. 2012, 60, 462–469. [Google Scholar] [CrossRef]
- Jujo, K.; Saito, K.; Ishida, I.; Furuki, Y.; Kim, A.; Suzuki, Y.; Sekiguchi, H.; Yamaguchi, J.; Ogawa, H.; Hagiwara, N. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail. 2016, 3, 177–188. [Google Scholar] [CrossRef]
- Kinugawa, K.; Sato, N.; Inomata, T. Effects of Tolvaptan on Volume Overload in Patients with Heart Failure Meta-Analysis of Randomized Controlled Trials. Int. Heart J. 2018, 59, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
 : measurement of RAS activity; Admit: hospital admission; IC: informed consent; NYHA: New York Heart Association classification; UCG: ultrasonic cardiography; Dose: dose of tolvaptan and other diuretics; m: month(s).
: measurement of RAS activity; Admit: hospital admission; IC: informed consent; NYHA: New York Heart Association classification; UCG: ultrasonic cardiography; Dose: dose of tolvaptan and other diuretics; m: month(s).
 : measurement of RAS activity; Admit: hospital admission; IC: informed consent; NYHA: New York Heart Association classification; UCG: ultrasonic cardiography; Dose: dose of tolvaptan and other diuretics; m: month(s).
: measurement of RAS activity; Admit: hospital admission; IC: informed consent; NYHA: New York Heart Association classification; UCG: ultrasonic cardiography; Dose: dose of tolvaptan and other diuretics; m: month(s).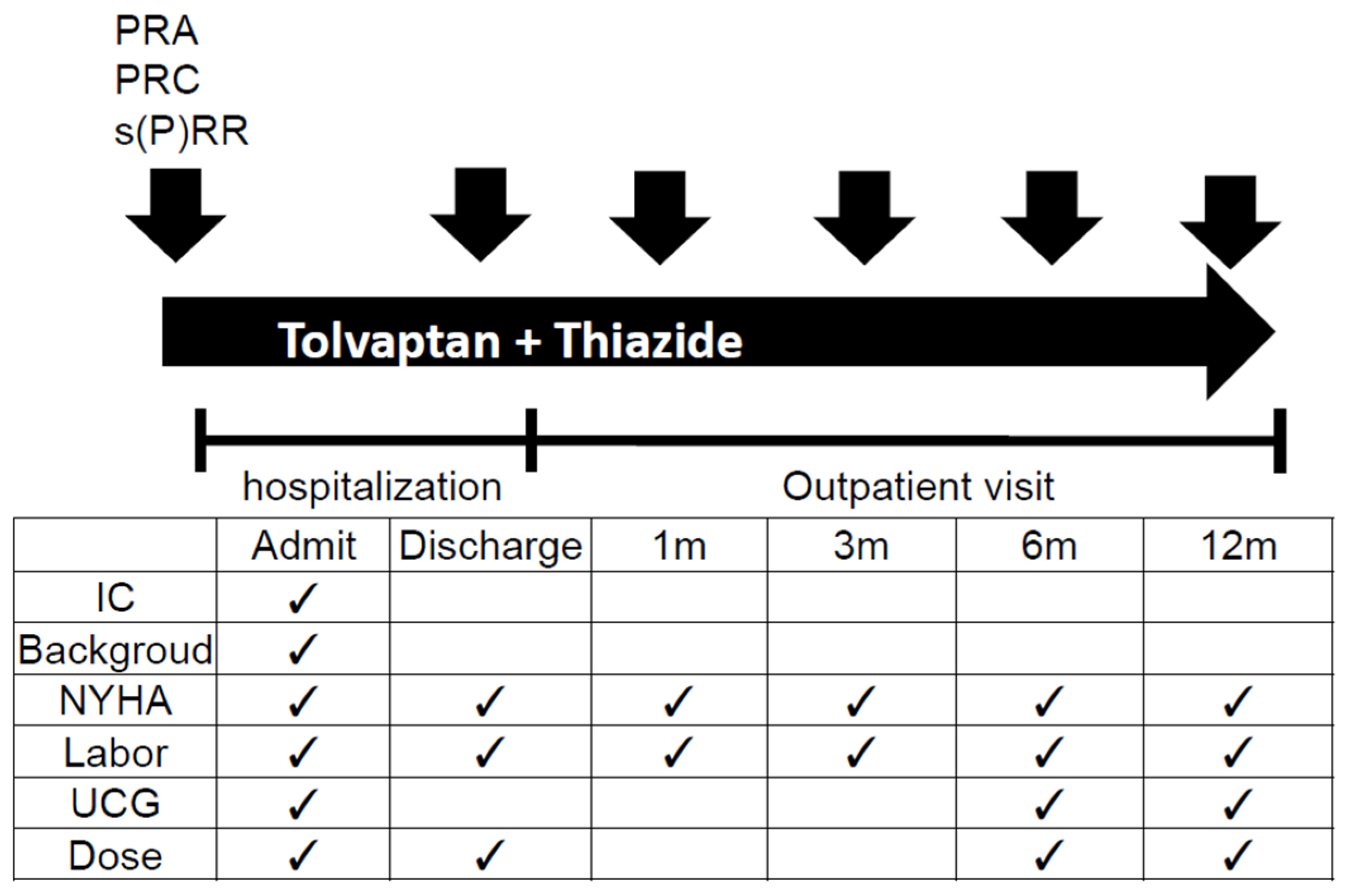

| Age | Sex | Etiology | Initial NYHA | Last NYHA | Initial EF (%) | Final EF (%) | Initial Cr/eGFR | BNP | PRA/ PRC | s(P)RR | Final Cr/eGFR | Frosemide Dose (mg) | Tolvaptan Dose (mg) | Final Tolvaptan | Furosemide Restart | Re- Admission | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 89 | m | DCM | III | II | 28 | 40 | 0.9/63.9 | 337 | 0.5/3.0 | 15 | 1.6/30.2 | 60 | 15 | 15 | yes | no | Study Fin | |
| 2 | 87 | m | DCM | III | NA | 14 | NA | 1.5/35.9 | 1188 | 2.1/16.0 | 8 | NA | 40 | 7.5 | NA | NA | NA | Excluded | |
| 3 | 67 | f | dHCM | IV | IV | 24 | 16 | 1.2/35.8 | 734 | 31.0/660 | 40.9 | 1.5/25.4 | 40 | 7.5 | 15 | yes | yes | Study Fin | |
| 4 | 86 | m | Aged heart | III | III | 86 | 84 | 1.2/44.3 | 287 | 2.9/20.0 | 15.5 | 1.5/34.3 | 60 | 15 | 15 | yes | no | Study Fin | |
| 5 | 81 | f | VD | III | II | 42 | 25 | 1.2/34.5 | 149 | 1.2/34.5 | 61.7 | 0.9/43.7 | 40 | 7.5 | 15 | no | no | Study Fin | |
| 6 | 58 | m | DCM | IV | †(IV) | 24 | 23 | 0.9/65.1 | 533 | 0.9/65.1 | 27.1 | 1.1/53.0 | 60 | 15 | 15 | no | yes | LVAS imp | |
| 7 | 72 | f | VD | IV | NA | 41 | NA | 1.4/30.0 | 203 | 1.4/30.0 | 30 | NA | 60 | 15 | NA | no | NA | Excluded | |
| 8 | 71 | m | CAD | IV | III | 26 | 28 | 0.9/68.0 | 186 | 0.9/68.0 | 14.8 | 0.75/77.9 | 40 | 7.5 | 15 | no | yes | Study Fin | |
| 9 | 88 | m | VD | III | †(IV) | 61 | NA | 1.6/31.7 | 250 | 1.6/31.7 | 17.4 | 2.7/17.5 | 40 | 7.5 | 7.5 | yes | yes | Death by HF | |
| 10 | 63 | m | CAD | III | II | 41 | 17 | 0.9/64.7 | 299 | 0.9/64.7 | 17.9 | 1.0/56.0 | 80 | 15 | 15 | no | no | Study Fin | |
| Av | 76.4 ± 10.8 | 3.5 ± 0.5 | 2.8 ± 0.8 | 37.9 ± 20.4 | 34.9 ± 22.4 | 1.2 ± 0.3/ | 426.8 ± 318 | 1.2 ± 0.31/ | 28.2 ± 20.1 | 1.3 ± 0.3/ | 51.0 ± 13.8 | 10.9 ± 3.9 | 13.3 ± 3.3 | ||||||
| 46.1 ± 15.8 | 46.1 ± 15.0 | 42.0 ± 18.1 | |||||||||||||||||
 |  | ||||||||||||||||||
| * | ** | ||||||||||||||||||
| case | ACEI/ARB | Dose (mg) | BB | Dose (mg) | MRA | Dose (mg) | |
|---|---|---|---|---|---|---|---|
| 1 | Olmesartan | 10 to 20 | Carvedilol | 5 | NA | ||
| 2 | NA | Carvedilol | 5 | NA | |||
| 3 | Enalapril | 2.5 | Carvedilol | 0.625 to 1.25 | Spironolactone | 50 | |
| 4 | Losartan | 25 | Carvedilol | 5 | Eplerenone | 25 | |
| 5 | Enalapril | 2.5 | Carvedilol | 1.25 to off | NA | ||
| 6 | Enalapril | 10 to 5 | Carvedilol | 7.5 to 5 | Spironolactone | 50 to 25 | |
| 7 | Enalapril | 5 | NA | Spironolactone | 50 | ||
| 8 | Enalapril | 5 to 2.5 | Bisoprolol | 1.25 | Spironolactone | 25 to off | |
| 9 | Valsartan | 40 | Carvedilol | 5 | NA | ||
| 10 | Enalapril | 2.5 | Carvedilol | 3.75 | NA | ||
| 11 | NA | Carvedilol | 5 | Spironolactone | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, Y.; Tsutsui, K.; Yamada, Y.; Kato, R.; Muramatsu, T.; Senbonmatsu, T. Relationship between Soluble (Pro)Renin Receptor and Renin Activity in Patients with Severe Heart Failure. J. Clin. Med. 2020, 9, 4110. https://doi.org/10.3390/jcm9124110
Ikeda Y, Tsutsui K, Yamada Y, Kato R, Muramatsu T, Senbonmatsu T. Relationship between Soluble (Pro)Renin Receptor and Renin Activity in Patients with Severe Heart Failure. Journal of Clinical Medicine. 2020; 9(12):4110. https://doi.org/10.3390/jcm9124110
Chicago/Turabian StyleIkeda, Yoshifumi, Kenta Tsutsui, Yoshihiro Yamada, Ritsushi Kato, Toshihiro Muramatsu, and Takaaki Senbonmatsu. 2020. "Relationship between Soluble (Pro)Renin Receptor and Renin Activity in Patients with Severe Heart Failure" Journal of Clinical Medicine 9, no. 12: 4110. https://doi.org/10.3390/jcm9124110
APA StyleIkeda, Y., Tsutsui, K., Yamada, Y., Kato, R., Muramatsu, T., & Senbonmatsu, T. (2020). Relationship between Soluble (Pro)Renin Receptor and Renin Activity in Patients with Severe Heart Failure. Journal of Clinical Medicine, 9(12), 4110. https://doi.org/10.3390/jcm9124110






