Abstract
Background: Due to its unknown etiology, the objective diagnosis and therapeutics of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) are still challenging. Generally, the patient-reported outcome (PRO) is the major strategy driving treatment response because the patient is the most important judge of whether changes are meaningful. Methods: In order to determine the overall characteristics of the main outcome measurement applied in clinical trials for CFS/ME, we systematically surveyed the literature using two electronic databases, PubMed and the Cochrane Library, throughout June 2020. We analyzed randomized controlled trials (RCTs) for CFS/ME focusing especially on main measurements. Results: Fifty-two RCTs out of a total 540 searched were selected according to eligibility criteria. Thirty-one RCTs (59.6%) used single primary outcome and others adapted ≥2 kinds of measurements. In total, 15 PRO-derived tools were adapted (50 RCTs; 96.2%) along with two behavioral measurements for adolescents (4 RCTs; 7.7%). The 36-item Short Form Health Survey (SF-36; 16 RCTs), Checklist Individual Strength (CIS; 14 RCTs), and Chalder Fatigue Questionnaire (CFQ; 11 RCTs) were most frequently used as the main outcomes. Since the first RCT in 1996, Clinical Global Impression (CGI) and SF-36 have been dominantly used each in the first and following decade (26.1% and 28.6%, respectively), while both CIS and Multidimensional Fatigue Inventory (MFI) have been the preferred instruments (21.4% each) in recent years (2016 to 2020). Conclusions: This review comprehensively provides the choice pattern of the assessment tools for interventions in RCTs for CFS/ME. Our data would be helpful practically in the design of clinical studies for CFS/ME-related therapeutic development.
1. Introduction
Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a debilitating disease characterized by medically unexplained chronic severe fatigue for at least 6 months along with key symptoms such as unrefreshing sleep, postexertion malaise (PEM), impairments in memory or concentration, and/or orthostatic intolerance [1]. The daily lives of patients are heavily impeded, which leads to unemployment for approximately half of patients and being home- or bed-bound for one quarter [2]. The prevalence of CFS/ME is suggested to be approximately 1–2% worldwide [3], and the annual economic cost for medical care is estimated to be up to USD 10,000 per patient in the US [4].
Although various etiologies of CFS/ME, such as autonomic and neurological dysfunction, abnormalities in mitochondrial function, and aberrant gut microbiota, have been hypothesized, they have not yet been clearly revealed [5]. Recently, this disease has become considered a multisystem neuroimmune disease [1]. To date, various randomized controlled trials (RCTs) for therapeutics have been conducted; however, no effective therapy for CFS/ME exists [6]. Recently, the PACE trial, a large-scale clinical study of cognitive behavior therapy (CBT) and graded-exercise therapy (GET), was reported to be effective for CFS/ME [7]. There is however a fair amount of controversy surrounding this PACE trial, likely due to the debates regarding its efficacy and criticisms by researchers and patients due to judgments of restoration as well as side effects [8].
On the other hand, the absence of objective biomarkers of CFS/ME raises a problem for the actual diagnosis of this illness. In addition, clinical evaluations of treatment responses are also dependent on self-reported assessments of symptom severity, leading to potential trouble during the investigation of new therapeutics [9]. Accordingly, methodologically well-designed tools to assess the valuable responses of treatments for CFS/ME are very important. To date, diverse patient-reported outcome (PRO) measurements have been developed and used to assess fatigue status in clinics, such as the Checklist Individual Strength (CIS) scale, Chalder Fatigue Questionnaire (CFQ), and Multidimensional Fatigue Inventory (MFI) [10,11,12]. Many clinical studies, however, have adopted various fatigue-nonspecific instruments, including the 36-item Short Form Health Survey (SF-36), Clinical Global Impression (CGI), and Sickness Impact Profile-8 (SIP-8) [13,14,15]. In fact, researchers need to carefully review the available measurements and choose the most optimized one for the purpose of their own clinical studies. However, it is not easy for researchers to choose the appropriate measurement instruments for CFS/ME-related studies due to the absence of well-established international guidelines.
To identify the assessment tools that help in the clinical study process for CFS/ME, we comprehensively reviewed the primary measurements used in RCTs and determined changes in the use of these measurements.
2. Methods
2.1. Data Sources and Search Terms
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [16], a systematic literature survey was performed using two electronic literature databases, PubMed and the Cochrane Library, throughout June 2020. The search terms were encephalomyelitis/chronic fatigue syndrome, ME/CFS, encephalomyelitis, ME, chronic fatigue syndrome, CFS, randomized controlled trial, RCT, and clinical trial. The trial type was limited to RCTs, and all languages were included.
2.2. Eligibility Criteria
Selected articles for this study were determined by the following inclusion criteria: (1) RCTs or randomized controlled crossover trials, (2) patients with CFS/ME as participants, (3) an evaluation of the efficacy of the intervention for CFS/ME treatment, and (4) fatigue-related measurement or outcome. The exclusion criteria were as follows: (1) articles with no full text and (2) studies without mention of the primary or main outcome. We did not have a criterion based on the number of participants in RCTs.
2.3. Data Extraction and Analysis
We extracted data on general features of RCTs, such as the number of participants, age, intervention, and treatment period, along with the primary outcome measurement instrument (subscales, items, range of scores, versions, and application of cutoff scores for recruitment).
As a descriptive analysis, this study did not need to apply statistical analyses. Regarding the treatment period, the mean and standard deviation (SD) are presented.
3. Results
3.1. General Characteristics of RCTs
A total of 540 articles were initially identified from the PubMed and Cochran databases, and 52 articles met the inclusion criteria for this study (Figure 1). Forty-eight RCTs (92.3%) were performed with adult patients (n = 5872), while 4 RCTs (7.7%) were performed with adolescent subjects (n = 387). Twenty-six RCTs evaluated the efficacy of pharmacologic interventions, and 27 RCTs were conducted to evaluate nonpharmacologic interventions. The mean treatment period was 15.0 ± 9.3 weeks (Table 1).
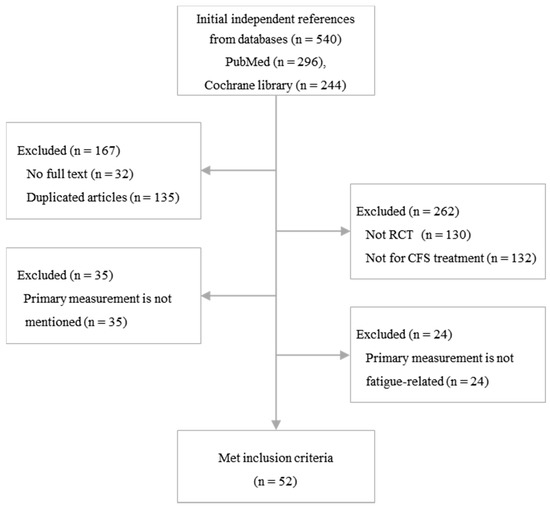
Figure 1.
Flow chart of the study.

Table 1.
Study characteristics.
In terms of the number of primary outcomes in RCTs, 31 RCTs (59.6%) used a single primary outcome (29 RCTs with adults and 2 RCTs with adolescents). Fifteen RCTs (28.8%) adopted two kinds of main measurements (with adult patients), while six RCTs (11.5%) used three kinds of measurements (four RCTs with adults and two with adolescents) as a primary outcome (Table 1).
3.2. Characteristics of Primary Measurements in RCTs
As shown in Figure 2, the 52 RCTs used 17 kinds of methodological instruments, which were classified into survey-based measurements (15 instruments in 50 RCTs) and behavioral measurements (two instruments in four RCTs). All RCTs with adults adopted survey-based measurements, while four RCTs with adolescent patients adopted behavioral (two RCTs) and/or survey-based (two RCTs) measurements (Table 1).
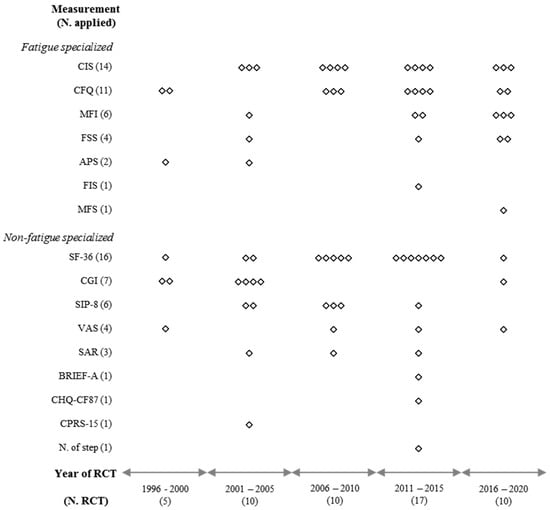
Figure 2.
Graphical display for quinquennial distribution of primary measurements.
Among the 17 kinds of instruments, the SF-36 was most frequently used (30.8%), followed by the CIS (26.9%), CFQ (21.2%), CGI (13.5%), MFI (11.5%), and SIP-8 (11.5%) (Table 2). Twenty-four RCTs adopted at least one subscale score from these measurement instruments, most commonly the fatigue severity score of CIS (12 RCTs) or physical function score of SF-36 (10 RCTs) (Table 2). Alternatively, these instrument-derived scores were applied as cutoff scores for participant inclusion, such as the fatigue severity score of the CIS (11 RCTs), total score of the SIP-8 (six RCTs), physical function score of the SF-36 (four RCTs), and total score of the CFQ (four RCTs) (Supplementary Table S1).

Table 2.
Measurement instruments in RCTs.
3.3. Quinquennial Distribution of Primary Measurements for RCTs
Since the first report of an RCT for CFS/ME using GET in 1997, the number of RCTs has increased, reaching a maximum from 2011 to 2015 (17 RCTs) (Figure 2). In the earliest decade (1996–2005), the CGI was dominantly used as a measurement instrument as the primary outcome in RCTs (6 out of 15 RCTs). During the following decade (2006–2015), the SF-36, CIS, and CFQ were preferred (19 out of 27 RCTs). In the last 5 years (2016–2020), the CIS and MFI have been frequently employed as primary outcome measurements (6 out of 10 RCTs) (Figure 2).
4. Discussion
In terms of CFS/ME, a symptom-based approach is a key strategy for not only therapy but also diagnosis because of its unknown etiology [1]. The Centers for Disease Control and Prevention (CDC) recommended symptomatic treatment based on the case definition of the Institute of Medicine (IOM) for providing alternative care for patients [68]. The subjective complaints and comprehension of the PROs are crucial in the diagnostic process as well as in evaluating therapeutic responses in clinical practice for CFS/ME. To provide practical guidance in choosing a suitable measurement in clinical studies for CFS/ME, we analyzed the primary outcome measurements in RCTs conducted to date.
Unlike common guidelines recommending single primary outcome measurement in RCTs [69], 21 (40.4%) of the 52 RCTs employed multiple primary measurements (Table 1). This might be due to the absence of a well-established measurement tool specialized for CFS/ME. Among the 17 tools used in the 52 RCTs, only two behavioral measurements (school attendance rate and the number of steps per day) were adopted in four RCTs that enrolled only adolescent participants (Table 1). It is generally well known that adolescent patients show a poorer school attendance rate than healthy controls [70]. The remaining RCTs (50 RCTs with 15 different tools) employed survey-based PRO measurements, likely for many subjective symptoms or disorders, including migraine, major depressive disorder, or anxiety [71,72,73]. We classified the measurements into two groups: nine nonfatigue specialized tools employed mainly in an earlier decade (1996 to 2005) and eight fatigue-specialized measurements which have been dominant since 2016 (Figure 2).
The SF-36, not specialized for fatigue, is the most frequently used measurement based on our data (16 RCTs) (Table 2). It has been broadly applied for measuring patients’ general health status in reference to health-related quality of life (HRQOL). It is well recognized that the HRQOL of CFS/ME sufferers is notoriously poor and has been linked to a 7-fold higher risk of suicide than healthy controls [74,75]. Therefore, the SF-36, especially the physical functioning subscale, was steadily employed as a primary measurement until 2015, often supportively combined with other fatigue-specialized measurements (10 RCTs), such as the CIS or CFQ. Likewise, the SIP-8 score assessing dysfunction of daily behaviors has been used as part of the primary outcome coupled with fatigue-specialized tools (Supplementary Table S1).
In regard to fatigue-specialized instruments, the fatigue severity subscale of the CIS and the total score of the CFQ (11-item version) were dominantly employed (Table 2). Both have been commonly endorsed for the evaluation of psychometric fatigue status in RCTs for CFS/ME and other disorders, including rheumatoid arthritis and fibromyalgia [76]. Both instruments assess not only physical but also mental fatigue status, such as concentration and motivation, and they are known to show a very high correlation in assessing fatigue severity [77]. In particular, the CFQ was employed mostly in trials conducted in the UK (9/11 adoptions), while the CIS was preferred in the Netherlands (12/14 adoptions). On the other hand, the MFI, markedly preferred in recent studies along with the CIS, was originally developed for assessing multifarious fatigue status in patients with cancer [12]. The MFI was one of the measures in the Wichita clinical study assessing over 30 kinds of measurements or parameters for CFS/ME in 2005, and the MFI was proven as a valid measurement [78]. Recently, the MFI was applied in a large-scale study to explore the cytokine signature that showed a positive correlation between serum levels of TGF-β and the severity of CFS/ME [79]. Both the MFI and CIS were created by Dutch researchers and contain 20 nearly identical questionnaire items. However, they have some differences in measurement method strategies: a maximum of 140 points with 7-point scales on the CIS versus a maximum of 100 points with 5-point scales on the MFI (Supplementary Table S2). Unlike the CFQ-11, the MFI and CIS adopt both positive and negative questions and measure PEM-related symptoms such as “I am tired very quickly or easily”, which is focused on as one of the recently established hallmark symptoms of CFS/ME [1].
In fact, numerous studies certified the validity and reliability of these commonly used instruments for CFS/ME, such as the CFQ-11, CIS, and MFI [10,78,80], while some researchers have pointed out the ceiling effects of these measurements, especially in clinical trials for treatments [81,82]. They are concerned with the possibility that sufferers of CFS/ME tend to report scores close to maximum, thereby hindering the accurate reflection of treatment response and the baseline condition. Most measurement tools (including CFS/ME-specific instruments) have non-CFS/ME-specific questionnaires, such as “I feel tired” or “I feel weak”, which are frequently complained of among general populations. Accordingly, many trials (most RCTs adopted CIS-based primary outcome) used cutoff scores in the process of participant inclusion (Supplementary Table S1). On the other hand, responders to the CFQ-11 will obtain high scores due to comparisons with “usual” or “last well-state”. Because most CFS patients have experienced many years of the disease with fluctuating symptoms, assessment methods involving comparisons to “usual” can hardly reflect not only deterioration in status but also treatment response [2]. Thus, some studies have adopted a modified CFQ-11 as a 10-point Likert scale (from 0 points for healthy conditions to 9 points for the worst status) in RCTs for drug development related to CFS/ME [41].
Although no confirmative pathophysiology of CFS/ME has been identified, some new findings have been highlighted, such as aberrant composition of the gut microbiome and altered serotonergic metabolism within the brain [83,84]. In addition, several studies investigating objective parameters for diagnostic and severity assessments, including elevated levels of TGF-β and nanoelectronic assays, have been conducted [79,85]. One group also found a reduction of red blood cell deformability in patients with CFS/ME [86]. Along with these advances in knowledge, it is necessary that a CFS/ME-specialized measurement instrument be developed to reflect the clinical severity and treatment response and objective biomarkers be discovered to ensure CFS/ME.
5. Conclusions
This systematic review provides a comprehensive overview of the choice of primary measurements in RCTs for CFS/ME to date. Approximately 40% of RCTs applied multiple primary measurements. Of the 17 kinds of measurement tools, the SF-36 (nonfatigue specific measurement) had been most frequently applied through 2015, while two fatigue-specific measurements, the CIS and MFI, have been frequently employed in recent trials. Our data will be helpful in the practical design of clinical studies for CFS/ME-related therapeutic development.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/11/3463/s1, Table S1: Summary of RCTs included in this review, Table S2: General characteristics of self-reported survey measurements used in RCTs.
Author Contributions
D.-Y.K. searched the literature, then extracted and analyzed the data. J.-S.L. participated in discussion. D.-Y.K. and C.-G.S. wrote the manuscript. C.-G.S. supervised the whole process of this study with initial design. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (NRF-2018R1A6A1A03025221).
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Institute of Medicine of the National Academes. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; Committee on the diagnostic criteria for ME/CFS; Institute of Medicine: Washington, DC, USA, 2015. [Google Scholar]
- Castro-Marrero, J.; Faro, M.; Zaragozá, M.C.; Aliste, L.; De Sevilla, T.F.; Alegre, J. Unemployment and work disability in individuals with chronic fatigue syndrome/myalgic encephalomyelitis: A community-based cross-sectional study from Spain. BMC Public Health 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Benton, M.C.; Valentine, L.M.; Johnson, A.; Torres-Harding, S.R. The Economic impact of ME/CFS: Individual and societal costs. Dyn. Med. 2008, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, D.; Annesley, S.J.; Fisher, P.R. Pathological Mechanisms Underlying Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Diagnostics 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Lee, J.-S.; Park, S.-Y.; Kim, S.-J.; Son, C.-G. Systematic review of randomized controlled trials for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 7–12. [Google Scholar] [CrossRef]
- White, P.D.; Goldsmith, K.A.; Johnson, A.L.; Potts, L.; Walwyn, R.; De Cesare, J.C.; Baber, H.L.; Burgess, M.; Clark, L.V.; Cox, D.L.; et al. Comparison of adaptive pacing therapy, cognitive behavior therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet 2011, 377, 823–836. [Google Scholar] [CrossRef]
- Shepherd, C.B. PACE trial claims for recovery in myalgic encephalomyelitis/chronic fatigue syndrome–true or false? It’s time for an independent review of the methodology and results. J. Health Psychol. 2017, 22, 1187–1191. [Google Scholar] [CrossRef]
- Cleare, A.J.; Reid, S.; Chalder, T.; Hotopf, M.; Wessely, S. Chronic fatigue syndrome. BMJ Clin. Evid. 2015, 2015, 1101. [Google Scholar]
- Vercoulen, J.H.; Swanink, C.M.; Fennis, J.F.; Galama, J.M.; Van Der Meer, J.W.; Bleijenberg, G. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994, 38, 383–392. [Google Scholar] [CrossRef]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Smets, E.; Garssen, B.; Bonke, B.; De Haes, J. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Stewart, A.L.; Hays, R.D.; Ware, J.E. The MOS Short-form General Health Survey. Med. Care 1988, 26, 724–735. [Google Scholar] [CrossRef]
- Guy, W. ECDEU Assessment Manual for Psychopharmacology; U.S. Department of Health, Education, and Welfare: Washington, DC, USA, 1976.
- Bergner, M.; Bobbitt, R.A.; Carter, W.B.; Gilson, B.S. The Sickness Impact Profile: Development and Final Revision of a Health Status Measure. Med. Care 1981, 19, 787–805. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Vos-Vromans, D.C.W.M.; Smeets, R.J.E.M.; Huijnen, I.P.J.; Köke, A.J.; Hitters, W.M.G.C.; Rijnders, L.J.M.; Pont, M.; Winkens, B.; Knottnerus, J.A. Multidisciplinary rehabilitation treatment versus cognitive behavioural therapy for patients with chronic fatigue syndrome: A randomized controlled trial. J. Intern. Med. 2015, 279, 268–282. [Google Scholar] [CrossRef]
- Núñez, M.; Fernández-Solà, J.; Nuñez, E.; Fernández-Huerta, J.-M.; Godás-Sieso, T.; Gómez-Gil, E. Health-related quality of life in patients with chronic fatigue syndrome: Group cognitive behavioural therapy and graded exercise versus usual treatment. A randomised controlled trial with 1 year of follow-up. Clin. Rheumatol. 2011, 30, 381–389. [Google Scholar] [CrossRef]
- O’Dowd, H.; Gladwell, P.; Rogers, C.A.; Hollinghurst, S.; Gregory, A. Cognitive behavioural therapy in chronic fatigue syndrome: A randomised controlled trial of an outpatient group programme. Health Technol. Assess. 2006, 10, 1–121. [Google Scholar] [CrossRef]
- Peterson, P.K.; Pheley, A.; Schroeppel, J.; Schenck, C.; Marshall, P.; Kind, A.; Haugland, J.M.; Lambrecht, L.J.; Swan, S.; Goldsmith, S. A preliminary placebo-controlled crossover trial of fludrocortisone for chronic fatigue syndrome. Arch. Intern. Med. 1998, 158, 908–914. [Google Scholar] [CrossRef]
- Clark, L.V.; Pesola, F.; Thomas, J.M.; Vergara-Williamson, M.; Beynon, M.; White, P.D. Guided graded exercise self-help plus specialist medical care versus specialist medical care alone for chronic fatigue syndrome (GETSET): A pragmatic randomised controlled trial. Lancet 2017, 390, 363–373. [Google Scholar] [CrossRef]
- Pinxsterhuis, I.; Sandvik, L.; Strand, E.B.; Bautz-Holter, E.; Sveen, U. Effectiveness of a group-based self-management program for people with chronic fatigue syndrome: A randomized controlled trial. Clin. Rehabil. 2016, 31, 93–103. [Google Scholar] [CrossRef]
- Wiborg, J.F.; Van Bussel, J.; Van Dijk, A.; Bleijenberg, G.; Knoop, H. Randomised Controlled Trial of Cognitive Behaviour Therapy Delivered in Groups of Patients with Chronic Fatigue Syndrome. Psychother. Psychosom. 2015, 84, 368–376. [Google Scholar] [CrossRef]
- Burgess, M.; Andiappan, M.; Chalder, T. Cognitive Behaviour Therapy for Chronic Fatigue Syndrome in Adults: Face to Face versus Telephone Treatment—A Randomized Controlled Trial. Behav. Cogn. Psychother. 2011, 40, 175–191. [Google Scholar] [CrossRef]
- Tummers, M.; Knoop, H.; Bleijenberg, G. Effectiveness of stepped care for chronic fatigue syndrome: A randomized noninferiority trial. J. Consult. Clin. Psychol. 2010, 78, 724–731. [Google Scholar] [CrossRef]
- Wearden, A.; Dowrick, C.; Chew-Graham, C.; Bentall, R.P.; Morriss, R.K.; Peters, S.; Riste, L.; Richardson, G.; Lovell, K.; Dunn, G.; et al. Nurse led, home based self help treatment for patients in primary care with chronic fatigue syndrome: Randomised controlled trial. BMJ 2010, 340, c1777. [Google Scholar] [CrossRef]
- Hobday, R.A.; Thomas, S.; O’Donovan, A.; Murphy, M.; Pinching, A.J. Dietary intervention in chronic fatigue syndrome. J. Hum. Nutr. Diet. 2008, 21, 141–149. [Google Scholar] [CrossRef]
- Stulemeijer, M.; Jong, L.W.A.M.D.; Fiselier, T.J.W.; Hoogveld, S.W.B.; Bleijenberg, G. Cognitive behaviour therapy for adolescents with chronic fatigue syndrome: Randomised controlled trial. BMJ 2004, 330, 14. [Google Scholar] [CrossRef]
- Powell, P.; Bentall, R.P.; Nye, F.J.; Edwards, R.H.T. Randomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. BMJ 2001, 322, 387. [Google Scholar] [CrossRef]
- Tummers, M.; Knoop, H.; Van Dam, A.; Bleijenberg, G. Implementing a minimal intervention for chronic fatigue syndrome in a mental health centre: A randomized controlled trial. Psychol. Med. 2012, 42, 2205–2215. [Google Scholar] [CrossRef]
- Walach, H.; Bösch, H.; Lewith, G.; Naumann, J.; Schwarzer, B.; Falk, S.; Kohls, N.; Haraldsson, E.; Wiesendanger, H.; Nordmann, A.; et al. Effectiveness of Distant Healing for Patients with Chronic Fatigue Syndrome: A Randomised Controlled Partially Blinded Trial (EUHEALS). Psychother. Psychosom. 2008, 77, 158–166. [Google Scholar] [CrossRef]
- Montoya, J.G.; Anderson, J.N.; Adolphs, D.L.; Bateman, L.; Klimas, N.; Levine, S.M.; Garvert, D.W.; Kaiser, J.D. KPAX002 as a treatment for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A prospective, randomized trial. Int. J. Clin. Exp. Med. 2018, 11, 2890–2900. [Google Scholar]
- Blockmans, D.; Persoons, P.; Van Houdenhove, B.; Bobbaers, H. Does Methylphenidate Reduce the Symptoms of Chronic Fatigue Syndrome? Am. J. Med. 2006, 119, 167.e23–167.e30. [Google Scholar] [CrossRef] [PubMed]
- Janse, A.; Worm-Smeitink, M.; Bleijenberg, G.; Donders, R.; Knoop, H. Efficacy of web-based cognitive–behavioural therapy for chronic fatigue syndrome: Randomised controlled trial. Br. J. Psychiatry 2018, 212, 112–118. [Google Scholar] [CrossRef]
- Roerink, M.E.; Bredie, S.J.; Heijnen, M.; Dinarello, C.A.; Knoop, H.; Van Der Meer, J.W. Cytokine Inhibition in Patients With Chronic Fatigue Syndrome. Ann. Intern. Med. 2017, 166, 557. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, S.L.; Bleijenberg, G.; Uiterwaal, C.S.P.M.; Kimpen, J.L.L.; Van De Putte, E.M. Effectiveness of internet-based cognitive behavioural treatment for adolescents with chronic fatigue syndrome (FITNET): A randomised controlled trial. Lancet 2012, 379, 1412–1418. [Google Scholar] [CrossRef]
- The, G.K.H.; Bleijenberg, G.; Buitelaar, J.K.; Van Der Meer, J.W.M. The Effect of Ondansetron, a 5-HT 3 Receptor Antagonist, in Chronic Fatigue Syndrome. J. Clin. Psychiatry 2010, 71, 528–533. [Google Scholar] [CrossRef] [PubMed]
- The, G.K.H.; Bleijenberg, G.; Van Der Meer, J.W.M. The Effect of Acclydine in Chronic Fatigue Syndrome: A Randomized Controlled Trial. PLoS Clin. Trials 2007, 2, e19. [Google Scholar] [CrossRef]
- Brouwers, F.; Van Der Werf, S.; Bleijenberg, G.; Van Der Zee, L.; Van Der Meer, J. The effect of a polynutrient supplement on fatigue and physical activity of patients with chronic fatigue syndrome: A double-blind randomized controlled trial. QJM Int. J. Med. 2002, 95, 677–683. [Google Scholar] [CrossRef]
- Prins, J.B.; Bleijenberg, G.; Bazelmans, E.; Elving, L.D.; De Boo, T.M.; Severens, J.L.; Krabbenborg, L.; Spinhoven, P.; Van Der Meer, J.W. Cognitive behaviour therapy for chronic fatigue syndrome: A multicentre randomised controlled trial. Lancet 2001, 357, 841–847. [Google Scholar] [CrossRef]
- Joung, J.-Y.; Lee, J.-S.; Cho, J.-H.; Lee, D.-S.; Ahn, Y.-C.; Son, C.-G. The Efficacy and Safety of Myelophil, an Ethanol Extract Mixture of Astragali Radix and Salviae Radix, for Chronic Fatigue Syndrome: A Randomized Clinical Trial. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Ng, S.-M.; Yiu, Y.-M. Acupuncture for chronic fatigue syndrome: A randomized, sham-controlled trial with single-blinded design. Altern. Ther. Health Med. 2013, 19, 21–26. [Google Scholar]
- Rimes, K.A.; Wingrove, J. Mindfulness-Based Cognitive Therapy for People with Chronic Fatigue Syndrome Still Experiencing Excessive Fatigue after Cognitive Behaviour Therapy: A Pilot Randomized Study. Clin. Psychol. Psychother. 2011, 20, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cleare, A.J.; Heap, E.; Malhi, G.S.; Wessely, S.; O’Keane, V.; Miell, J. Low-dose hydrocortisone in chronic fatigue syndrome: A randomised crossover trial. Lancet 1999, 353, 455–458. [Google Scholar] [CrossRef]
- Wearden, A.J.; Morriss, R.K.; Mullis, R.; Strickland, P.L.; Pearson, D.J.; Appleby, L.; Campbell, I.T.; Morris, J.A. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. Br. J. Psychiatry 1998, 172, 485–490. [Google Scholar] [CrossRef] [PubMed]
- McDermott, C.; Richards, S.; Thomas, P.W.; Montgomery, J.; Lewith, G. A placebo-controlled, double-blind, randomized controlled trial of a natural killer cell stimulant (BioBran MGN-3) in chronic fatigue syndrome. QJM Int. J. Med. 2006, 99, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.K.L.; Zachrisson, O.; Gottfries, C.-G.; Matousek, M.; Peilot, B.; Forsmark, S.; Schuit, R.C.; Carlsson, M.L.; Kloberg, A.; Carlsson, A. A randomised controlled trial of the monoaminergic stabiliser (−)-OSU6162 in treatment of myalgic encephalomyelitis/chronic fatigue syndrome. Acta Neuropsychiatr. 2017, 30, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Moss-Morris, R.; Sharon, C.; Tobin, R.; Baldi, J.C. A Randomized Controlled Graded Exercise Trial for Chronic Fatigue Syndrome: Outcomes and Mechanisms of Change. J. Health Psychol. 2005, 10, 245–259. [Google Scholar] [CrossRef]
- Blacker, C.V.R.; Greenwood, D.T.; Wesnes, K.A.; Wilson, R.; Woodward, C.; Howe, I.; Ali, T. Effect of Galantamine Hydrobromide in Chronic Fatigue Syndrome. JAMA 2004, 292, 1195–1204. [Google Scholar] [CrossRef]
- Zachrisson, O.C.; Regland, B.; Jahreskog, M.; Jonsson, M.; Kron, M.; Gottfries, C.-G. Treatment with staphylococcus toxoid in fibromyalgia/chronic fatigue syndrome--a randomised controlled trial. Eur. J. Pain 2002, 6, 455–466. [Google Scholar] [CrossRef]
- Rowe, P.C.; Calkins, H.; DeBusk, K.; McKenzie, R.; Anand, R.; Sharma, G.; Cuccherini, B.A.; Soto, N.; Hohman, P.; Snader, S.; et al. Fludrocortisone Acetate to Treat Neurally Mediated Hypotension in Chronic Fatigue Syndrome. JAMA 2001, 285, 52–59. [Google Scholar] [CrossRef]
- Fulcher, K.Y.; White, P.D. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. BMJ 1997, 314, 1647. [Google Scholar] [CrossRef]
- Park, S.B.; Kim, K.-N.; Sung, E.; Lee, S.Y.; Shin, H.C. Human Placental Extract as a Subcutaneous Injection Is Effective in Chronic Fatigue Syndrome: A Multi-Center, Double-Blind, Randomized, Placebo-Controlled Study. Biol. Pharm. Bull. 2016, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Windthorst, P.; Mazurak, N.; Kuske, M.; Hipp, A.; Giel, K.E.; Enck, P.; Nieß, A.; Zipfel, S.; Teufel, M. Heart rate variability biofeedback therapy and graded exercise training in management of chronic fatigue syndrome: An exploratory pilot study. J. Psychosom. Res. 2017, 93, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Kogelnik, A.M.; Bhangoo, M.; Lunn, M.R.; Flamand, L.; Merrihew, L.E.; Watt, T.; Kubo, J.; Paik, J.; Desai, M. Randomized clinical trial to evaluate the efficacy and safety of valganciclovir in a subset of patients with chronic fatigue syndrome. J. Med. Virol. 2013, 85, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Weatherley-Jones, E.; Nicholl, J.P.; Thomas, K.J.; Parry, G.J.; McKendrick, M.W.; Green, S.T.; Stanley, P.J.; Lynch, S.P. A randomised, controlled, triple-blind trial of the efficacy of homeopathic treatment for chronic fatigue syndrome. J. Psychosom. Res. 2004, 56, 189–197. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Stojanovic, M.; Drid, P.; Hoffman, J.R.; Sekulic, D.; Zenic, N. Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome. Nutrients 2016, 8, 72. [Google Scholar] [CrossRef]
- Arnold, L.M.; Blom, T.J.; Welge, J.A.; Mariutto, E.; Heller, A. A Randomized, Placebo-Controlled, Double-Blinded Trial of Duloxetine in the Treatment of General Fatigue in Patients with Chronic Fatigue Syndrome. Psychosomatics 2015, 56, 242–253. [Google Scholar] [CrossRef]
- Friedberg, F.; Adamowicz, J.L.; Caikauskaite, I.; Seva, V.; Napoli, A. Efficacy of two delivery modes of behavioral self-management in severe chronic fatigue syndrome. Fatigue Biomed. Health Behav. 2016, 4, 158–174. [Google Scholar] [CrossRef]
- Kim, J.-E.; Seo, B.-K.; Choi, J.-B.; Kim, H.-J.; Kim, T.H.; Lee, M.-H.; Kang, K.-W.; Kim, J.-H.; Shin, K.-M.; Lee, S.H.; et al. Acupuncture for chronic fatigue syndrome and idiopathic chronic fatigue: A multicenter, nonblinded, randomized controlled trial. Trials 2015, 16, 314. [Google Scholar] [CrossRef]
- Olson, L.G.; Ambrogetti, A.; Sutherland, D. A Pilot Randomized Controlled Trial of Dexamphetamine in Patients With Chronic Fatigue Syndrome. J. Psychosom. Res. 2003, 44, 38–43. [Google Scholar] [CrossRef]
- Fluge, Ø.; Bruland, O.; Risa, K.; Storstein, A.; Kristoffersen, E.K.; Sapkota, D.; Næss, H.; Dahl, O.; Nyland, H.; Mella, O. Benefit from B-Lymphocyte Depletion Using the Anti-CD20 Antibody Rituximab in Chronic Fatigue Syndrome. A Double-Blind and Placebo-Controlled Study. PLoS ONE 2011, 6, e26358. [Google Scholar] [CrossRef]
- Chalder, T.; Deary, V.; Husain, K.; Walwyn, R. Family-focused cognitive behaviour therapy versus psycho-education for chronic fatigue syndrome in 11- to 18-year-olds: A randomized controlled treatment trial. Psychol. Med. 2009, 40, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, L.M.; Preuss, H.G.; MacDowell, A.L.; Chiazze, L.; Birkmayer, G.D.; Bellanti, J.A. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann. Allergy Asthma Immunol. 1999, 82, 185–191. [Google Scholar] [CrossRef]
- Young, J.L. Use of lisdexamfetamine dimesylate in treatment of executive functioning deficits and chronic fatigue syndrome: A double blind, placebo-controlled study. Psychiatry Res. 2013, 207, 127–133. [Google Scholar]
- Castro-Marrero, J.; Cordero, M.D.; Segundo, M.J.; Sáez-Francàs, N.; Calvo, N.; Román-Malo, L.; Aliste, L.; De Sevilla, T.F.; Alegre, J. Does Oral Coenzyme Q10 Plus NADH Supplementation Improve Fatigue and Biochemical Parameters in Chronic Fatigue Syndrome? Antioxid. Redox Signal. 2015, 22, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Sulheim, D.; Fagermoen, E.; Winger, A.; Andersen, A.M.; Godang, K.; Müller, F.; Rowe, P.C.; Saul, J.P.; Skovlund, E.; Øie, M.G.; et al. Disease Mechanisms and Clonidine Treatment in Adolescent Chronic Fatigue Syndrome. JAMA Pediatr. 2014, 168, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Treatment of ME/CFS. Available online: https://www.cdc.gov/me-cfs/treatment/index.html (accessed on 20 July 2020).
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Politis, J.; Garnham, C.; Scheinberg, A.; Tollit, M.A. School Functioning in Adolescents with Chronic Fatigue Syndrome. Front. Pediatr. 2018, 6, 302. [Google Scholar] [CrossRef]
- Peng, K.-P.; Wang, S.-J. Migraine diagnosis: Screening items, instruments, and scales. Acta Anaesthesiol. Taiwanica 2012, 50, 69–73. [Google Scholar] [CrossRef]
- Lee, Y.; Rosenblat, J.D.; Lee, J.; Carmona, N.E.; Subramaniapillai, M.; Shekotikhina, M.; Mansur, R.B.; Brietzke, E.; Lee, J.-H.; Ho, R.C.; et al. Efficacy of antidepressants on measures of workplace functioning in major depressive disorder: A systematic review. J. Affect. Disord. 2018, 227, 406–415. [Google Scholar] [CrossRef]
- Lazor, T.; Tigelaar, L.; Pole, J.D.; De Souza, C.; Tomlinson, D.; Sung, L. Instruments to measure anxiety in children, adolescents, and young adults with cancer: A systematic review. Support. Care Cancer 2017, 25, 2921–2931. [Google Scholar] [CrossRef]
- Hvidberg, M.F.; Brinth, L.S.; Olesen, A.V.; Petersen, K.; Ehlers, L. The Health-Related Quality of Life for Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLoS ONE 2015, 10, e0132421. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.; Webb, R. Suicide risk in people with chronic fatigue syndrome. Lancet 2016, 387, 1596–1597. [Google Scholar] [CrossRef]
- Hewlett, S.; Dures, E.; Almeida, C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS). Arthritis Care Res. (Hoboken) 2011, 63, S263–S286. [Google Scholar] [CrossRef]
- De Vries, J.; Michielsen, H.J.; Van Heck, G.L. Assessment of fatigue among working people: A comparison of six questionnaires. Occup. Environ. Med. 2003, 60, 10–15. [Google Scholar] [CrossRef]
- Reeves, W.C.; Wagner, D.; Nisenbaum, R.; Jones, J.F.; Gurbaxani, B.M.; Solomon, L.; Papanicolaou, D.A.; Unger, E.R.; Vernon, S.D.; Heim, C. Chronic Fatigue Syndrome—A clinically empirical approach to its definition and study. BMC Med. 2005, 3, 19. [Google Scholar] [CrossRef]
- Montoya, J.G.; Holmes, T.H.; Anderson, J.N.; Maecker, H.T.; Rosenberg-Hasson, Y.; Valencia, I.J.; Chu, L.; Younger, J.W.; Tato, C.M.; Davis, M.M. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc. Natl. Acad. Sci. USA 2017, 114, E7150–E7158. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Chalder, T. Measuring fatigue in clinical and community settings. J. Psychosom. Res. 2010, 69, 17–22. [Google Scholar] [CrossRef]
- Murdock, K.W.; Wang, X.S.; Shi, Q.; Cleeland, C.S.; Fagundes, C.P.; Vernon, S.D. The utility of patient-reported outcome measures among patients with myalgic encephalomyelitis/chronic fatigue syndrome. Qual. Life Res. 2016, 26, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Sasusa. PACE: The Research That Sparked a Patient Rebellion and Challenged Medicine; Sense about Science USA: New York, NY, USA, 2016. [Google Scholar]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kashi, A.A.; Davis, R.W.; Phair, R. The IDO Metabolic Trap Hypothesis for the Etiology of ME/CFS. Diagnostics 2019, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Esfandyarpour, R.; Kashi, A.; Nemat-Gorgani, M.; Wilhelmy, J.; Davis, R.W. A nanoelectronics-blood-based diagnostic biomarker for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Proc. Natl. Acad. Sci. USA 2019, 116, 10250–10257. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.K.; Schmidt, B.R.; Wilhelmy, J.; Nguyen, V.; Abugherir, A.; Do, J.K.; Nemat-Gorgani, M.; Davis, R.W.; Ramasubramanian, A.K. Red blood cell deformability is diminished in patients with Chronic Fatigue Syndrome. Clin. Hemorheol. Microcirc. 2019, 71, 113–116. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).