Abstract
Herpes zoster (HZ) caused by varicella zoster virus (VZV) reactivation is characterized as a vesicular rash of unilateral distribution that can also cause multiple complications; such as post-herpetic neuralgia; ophthalmic zoster; and other neurological issues. VZV can also increase incident hemorrhagic or ischemic complications by causing inflammatory vasculopathy. Thus; emerging epidemiological and clinical data recognizes an association between HZ and subsequent acute strokes or myocardial infarction (MI). This study reviewed published articles to elucidate the association between HZ and cerebrovascular and cardiac events. Individuals exposed to HZ or herpes zoster ophthalmicus had 1.3 to 4-fold increased risks of cerebrovascular events. Higher risks were noted among younger patients (age < 40 years) within one year after an HZ episode. The elevated risk of CV events diminished gradually according to age and length of time after an HZ episode. The putative mechanisms of VZV vasculopathy were also discussed. Several studies showed that the development of herpes zoster and herpes zoster ophthalmicus increased the risks of stroke; transient ischemic attack; and acute cardiac events. The association between VZV infection and cardiovascular events requires further studies to establish the optimal antiviral treatment and zoster vaccination to reduce zoster-associated vascular risk
1. Introduction
Herpes zoster (HZ) is an infectious disease induced by the reactivation of the varicella zoster virus (VZV) from its latent state in the sensory ganglia. VZV is a double-stranded DNA alpha herpesvirus that can be transmitted by direct airborne transmission. Primary infection with VZV in childhood is known as chickenpox, and then the latent VZV may develop HZ (shingles) in adults. HZ usually starts with a prodromal phase with severe, sharp radicular pain and paresthesia spreading in the affected dermatomes. It then proceeds via vesicular eruption on an erythematous base in the dermatomes within 48–72 h. During the acute phase, the erythematous maculopapular rash evolves into vesicles, pustules, and finally scabs. The lesions exfoliate for approximately 10 days, and the skin usually returns to its intact state after 2–4 weeks. The lesion is mostly in the thoracic region (>50%), but is also possible in the ophthalmic, cervical, and lumbosacral regions. Herpes zoster ophthalmicus (HZO) involves the ophthalmic branch of the trigeminal nerve and can occur in approximately 10% to 20% of HZ cases [1]. Reactivated VZV can introduce complications of post-herpetic neuralgia, myelitis, meningoencephalitis, and VZV vasculopathy. The association between vascular diseases and VZV reactivation has been as described herein. However, the vascular risk among patient age or post-herpes zoster duration remains poorly elucidated. The aim of this review is to determine the correlation between HZ or HZO and the occurrence of cerebrovascular and cardiac events on age stratification and various post-herpes zoster time periods.
2. Studies Investigating the Association Between HZ and CVD or Cerebrovascular Disease
Comprehensive searches of electronic databases (PubMed, Scopus, Google Scholar, and the Cochrane Library) were performed (date from database inception to February 28, 2019) to identify relevant studies written in English. Text headings and medical subject heading (MeSH) terms used in the searches included HZ, shingles, HZO, stroke, TIA, cerebrovascular disease, and MI. Fifteen studies examined the associations between unspecified HZ types and the following: Non-specified stroke [2,3,4,5,6,7,8,9], ischemic stroke [7,10,11], hemorrhagic stroke [7], transient ischemic attack (TIA), a composite of stroke and TIA [12,13], myocardial infarction (MI) [2,5,9,10,11], acute coronary syndromes (MI and unstable angina) [14], and incident coronary artery disease, including angina and MI (Table 1, Table 2) [15]. There were also eight studies regarding HZO and cardiovascular disease (CVD), five studies of non-specified stroke [2,3,6,7,16], and studies with ischemic stroke [10], hemorrhagic stroke [7], and MI [10] as endpoints (Table 3). The follow-up time periods ranged from 1 week to 24 years. Six meta-analysis studies were also enrolled to investigate the correlation between HZ and cerebrovascular risk [17,18,19,20,21,22].

Table 1.
Studies that investigated herpes zoster and the risk of stroke or transient ischemic attack.

Table 2.
Studies that investigated herpes zoster and the risk of myocardial infarction.

Table 3.
Studies that investigated herpes zoster ophthalmicus and the risk of cardiovascular or cerebrovascular disease.
3. Herpes Zoster and Risk of Stroke or Myocardial Infarction
Recent epidemiological studies (n = 15) from Asia (Taiwan [3,14,15,16] and Korea [9,11,12]), Iran [8], Europe (the United Kingdom [2,6], Germany [7], Demark [13], and Sweden [4]), and the United States [5,10] showed an increased incidence of stroke or MI in patients with a recent history of zoster. The characteristics of the 15 studies are displayed in Table 1, Table 2 and Table 3. Different databases were used in these studies, including the Taiwanese National Health Insurance Research Database (NHIRD) [3,14,15,16], the United Kingdom’s Clinical Practice Research Datalink (CPRD) and the Health Improvement Network (THIN) general practice databases [2,6], the Danish registry [13], the Swedish registry [4], the German Pharmacoepidemiological Research Database (GePaRD) [7], the US Medicare Database [10] and Olmsted County residents [5], and the Korean Health Insurance Database [9,11,12]. The study designs included a case-control study [8], retrospective cohort studies [2,3,4,5,11,12,13,14,15,16], a propensity score-matching approach [9], and self-controlled case series analyses [6,7,10] with adjustment for age, sex, and CVD risk factors (for example, hypertension diabetes, congestive heart failure, dyslipidemia, ischemic heart disease, atrial fibrillation, intermittent arterial claudication, carotid stenosis, and valvular heart disease). Among these studies, Sundström et al. [4] used only age and sex for adjustment, and Breuer et al. [2] and Langan et al. [6] further controlled for smoking and obesity. Schink et al. [7] and Minassian et al. [10] used a self-controlled case series design that made within-person comparisons to control for confounders (Table 1, Table 2 and Table 3).
The earliest studies used the Taiwan NHIRD to examine the association between HZ and cerebrovascular events. Kang et al. [3] reported a 30% increase in CVD risk (hazard ratio (HR), 1.31; 95% confidence interval (CI), 1.06–1.60) after HZ episode and a four folds increased risk (HR, 4.28; 95% CI, 2.01–9.03) after an HZO episode within one year. Lin et al. [16] found that HZ was a significant risk factor for ischemic stroke (HR, 4.52; 95% CI, 2.45–8.33), but not for hemorrhagic stroke. Sreenivasan et al. [13] also reported an increased risk of stroke after HZ episodes in a Denmark population-based cohort study between 1995 and 2008. A total of 4876 subjects had a stroke during the follow-up period among 117,926 HZ patients. An increased risk of stroke after treatment for herpes zoster was observed for the following one year (incident rate ratio (IRR), 2.27; 95% CI, 1.83–2.82). Using the 2002–2010 THIN Database, Breuer et al. [2] evaluated the risk of stroke, TIA, and MI in 106,601 HZ patients with one episode of herpes zoster with 213,202 controls matched for age, sex, and general practice. Increased risks of MI (HR, 1.10; 95% CI, 1.05–1.16) and TIA (HR, 1.15; 95% CI, 1.09–1.21) were found. The risk was highest in patients under 40 years of age as risk of stroke, TIA, and MI (HR, 1.74, 2.42, and 1.49). The risk disappeared in the analysis of all age populations (HR, 1.02; 95% CI, 0.98–1.07). Sundström et al. [4] reported a 1.34-fold (IRR, 1.34; 95% CI, 1.12–1.62) increased risk of stroke within 1 year after HZ episodes in all age groups in a Swedish register-based cohort controlling for age and sex. Minassian et al. [10] used the Medicare Database to study subjects more than 65 years old with an HZ diagnosis between 2006 and 2011. Among them, 42,954 had incident ischemic stroke and 24,237 had incident MI. In a self-controlled case series, a 2.4-fold increased rate of ischemic stroke (IRR, 2.37; 95% CI, 2.17–2.59) and a 1.7-fold increased rate of MI (IRR, 1.68; 95% CI, 1.47–1.92) were reported during the first week after an HZ episode. However, the risk of both incidents gradually decreased during the following 6 months. Because of limited power, this study showed no evidence of MI or stroke prevention by vaccination against zoster [10]. Previous three studies using the Korean Health Insurance Database found increased risks of stroke, TIA, and MI in HZ patients. Kwon et al. [12] reported higher risk of stroke/TIA (IRR, 1.90; 95% CI, 1.85–1.95) and Kim et al. [9] further found that HZ was associated with a 35% increased risk of stroke (HR 1.35; 95% CI, 1.18–1.54) and a 59% increased risk of MI (HR 1.59; 95% CI, 1.27–2.01) in a propensity score-matched analysis. In addition, Seo et al. [11] showed that severe HZ requiring hospitalization increased the risk of MI (HR, 1.83; 95% CI, 1.35–2.48) and ischemic stroke (HR, 1.52; 95% CI, 1.21–1.92). In the USA, Yawn et al. [5] compared 4862 individuals older than 50 years versus 19,433 control matched individuals and found a recent episode of herpes zoster was associated with a higher risk of stroke or MI. They found a greater risk of stroke at 3 months’ post-HZ (odds ratio (OR), 1.53; 95% CI, 1.10–2.33), but the association between HZ and MI at 3 months was not robust in a sensitivity analysis. According to the meta-analysis results reported by Yang et al. [19], the combined relative risk (RR) was 1.36 (95% CI, 1.10–1.67), which demonstrated that HZ patients had a 36% greater risk of developing stroke (Table 4). The analysis also identified that HZ was associated with ischemic stroke (RR, 1.99; 95% CI, 1.04–3.81), but not hemorrhagic stroke (RR, 1.86; 95% CI, 0.76–4.54). Recently, Patterson et al. [23,24] estimated the risk of TIA, stroke, MI, and composite vascular events using the Market Scan Commercial and Medicare 2007 to 2014 dataset linked with Electronic Medical Records (EMR). A 1.56-fold increase risk of TIA (95% CI, 1.13–2.15) and a statistically insignificant 1.40-fold increase of stroke (95% CI, 0.93–2.11) in 23,339 HZ subjects compared to 46,378 propensity-matched (sociodemographic and clinical factors) controls [24]. The adjusted IRR disclosed a 1.3-fold increase in composite vascular events (95% CI, 1.03–1.65) in the HZ group [23]. In summary, the incidence rate of post herpes zoster associated cardiovascular events are 9.56 to 17.98 per 1000 person-years in stroke/TIA [11,12,15] and 3.68 to 5.47 per 1000 person-years in myocardial infarction [11,14]. There was a significant association between HZ and stroke or MI in these epidemiology studies. Regarding the stroke subtype, an increased risk was observed in ischemic stroke, but not in hemorrhagic stroke after HZ infection [18].

Table 4.
Summary of meta-analysis results of herpes zoster and herpes zoster ophthalmicus with stroke or myocardial infarction.
4. Herpes Zoster Ophthalmicus and Risk of Stroke
HZO is HZ reactivation in the ophthalmic branch of the trigeminal nerve, the nerve that provides sensation to the eyes and forehead. Kang et al. [3] and HR, 4.52; 95% CI, 2.45–8.33 in Lin et al. [16] Antiviral treatment for zoster had no effect on the incidence of subsequent cerebral vascular disease [16]. Langan et al. [6] reported a 3-fold increased risk of stroke from 5–12 weeks following HZO (IRR, 3.38; 95% CI, 2.18–5.24). However, Breuer et al. [2] found a non-significant risk of stroke post-HZO (HR, 1.03; 95% CI, 0.77–1.39) using UK databases (CPRD and THIN) to investigate the association between HZO and stroke risk. Using the GePaRD, Schink et al. [7] performed a self-controlled case series study to demonstrate a 1.5 times higher risk of stroke 3 months after an HZO episode (IRR, 1.59; 95% CI, 1.10–2.32). In this study, the risk of ischemic stroke increased (IRR, 1.57; 95% CI, 1.05–2.35) among HZO patients, but not hemorrhagic stroke (IRR, 1.82; 95% CI, 0.62–5.37) [7] A similar finding was presented by Minassian et al. [10] of a higher ischemic stroke risk the first week (IRR, 2.73; 95% CI, 2.22–3.35) and three months (IRR, 1.29; 95% CI, 1.15–1.44) after an HZO episode. However, Yang et al. [19] included three studies [2,3,16] in a meta-analysis to demonstrate increased stroke risk related to HZ, and the pooled RR was 2.62 (95% CI, 0.85–8.06), but the heterogeneity was high (Table 1).
5. Post-Herpes Zoster Length of Follow-Up and Age Stratification with Associated Risk of CVD
The associations between post-HZ length of follow-up and CVD or cerebrovascular events were noted in several epidemiology studies designed as self-controlled case series and meta-analysis reports. The temporal pattern of the risk of stroke was similar in Langan et al. [6], Minassian et al. [10], and Schink et al. [7]. All three studies were based on a self-controlled case series study design. They found that the risk of stroke increased in the first week after an HZ episode then decreased after 6 to 12 months. The peak of the risk of stroke observed by Minassian et al. [10] was within the first weeks (IRR, 2.37; 95% CI, 2.17–2.59) after HZ onset, followed by a gradual decline in the risk (IRR, 1.55; 95% CI, 1.46–1.66 at 2–4 weeks; IRR, 1.17; 95% CI, 1.11–1.22 at 5–12 weeks). Sreenivasan et al. [13] enrolled 4.6 million people in Denmark and revealed a 127% (IRR, 2.27; 95% CI, 1.83–2.82) increase in the risk of stroke within two weeks after an HZ episode, and a 17% (IRR, 1.17; 95% CI, 1.09–1.24) increased risk from 2 weeks to 1 year after an HZ episode. Langan et al. [6] also found that the risk varied according to the time period after HZ with an incidence of 1.63 at 1–4 weeks, 1.42 at 5–12 weeks, and 1.23 at 13–26 weeks after an HZ episode. Regarding the antiviral treatment effect, 55% of HZ patients with antiviral treatment had a reduced stroke risk. However, a direct comparison cannot be made because of the diversity of HZ onset definition in the different studies. For example, Sreenivasan et al. [13] defined HZ onset as the start of antiviral therapy. Minassian et al. [10] used the start of antiviral therapy within 7 days before or after HZ diagnosis. Schink et al. [7] defined HZ onset as admission to hospital or the start of antiviral therapy. Kang et al. [3], Lin et al. [16], and Langan et al. [6] assessed the HZ diagnosis by International Classification of Diseases (ICD) codes. Therefore, the meta-analysis data demonstrated more consistent patterns and results of CVD and cerebrovascular events stratified by length of follow-up after HZ episodes. Liu et al. [17] used data from eight studies to show a gradient of stroke risk decreasing from 2.36 (95% CI, 2.17–2.56) in the first 2 weeks after an HZ episode to 1.56 (95% CI, 1.46–1.66) at 1 month, 1.17 (95% CI, 1.13–1.22) at 1 year, and 1.09 (95% CI, 1.02–1.16) after 1 year (Figure 1). Lian et al. [18] meta-analyzed eight studies and found the short-term risks increased for ischemic stroke after an HZ episode, whereas it was not found in the long-term follow-up. The pooled relative ratios (RRs) for ischemic stroke after an HZ episode were 1.55 (95% CI, 1.46–1.65) within one month, 1.17 (95% CI, 1.12–1.23) within three months, and 1.03 (95% CI, 0.99–1.07) within six months (Figure 1). Similar results were found by Marra et al. [20], indicating that the RR for stroke was 1.78 (95% CI, 1.70–1.88) in the first month, 1.43 (95% CI, 1.38–1.47) after three months, 1.20 (95% CI, 1.14–1.26) after 1 year, and no relationship was found after three years (RR, 1.07; 95% CI, 0.99–1.15). Three meta-analysis studies assessed the association between HZ and stroke/TIA or MI at different time periods after an HZ episode (Figure 1). Similar time period stratification (<3 months, <1 year, and >1 year) and random effect meta-analysis results were noted. The pooled RRs for the HZ-associated risk of stroke/TIA presented decreasing gradients of risk from short-term periods of time (<3 months and <1 year) to long-term periods of time (>1 year) [19,21,22]. The RRs for stroke/TIA post-HZ within three months were 1.94 (95% CI, 1.33–2.84) in Yang et al. [19], 1.34 (95% CI, 1.22–1.46) in Erskine et al. [21], and 1.27 (95% CI, 1.05–1.53) in Zhang et al. [22]. The RRs within one year were 1.17 (95% CI, 1.10–1.25) in Yang et al. [19], 1.22 (95% CI, 1.15–1.29) in Erskine et al. [21], and 1.02 (95% CI, 0.99 to 1.04 observed from 3 to 12 months) in Zhang et al. [22] To manage the statistical heterogeneity, Erskine et al. [21] reported the risks of stroke at one year after an HZ episode were significant in the fixed effects model (RR, 1.40; 95% CI, 1.38–1.43), but not in the random effects model (RR, 1.20; 95% CI, 0.82–1.75). Regarding MI, patients with HZ had higher risks of cardiac events at three months using the fixed effects model (RR, 1.31; 95% CI, 1.02–1.70), but not the random effects model (RR, 1.34; 95% CI, 0.98–1.82). The risks of cardiac events at one year and over one year after an HZ episode were higher using the fixed and random effects models (RR, 1.19; 95% CI, 1.01–1.41; RR, 1.12; 95% CI, 1.08–1.16, respectively) (Figure 1). The increased risk of stroke post-HZO at different periods of time was observed in two meta-analysis studies reported by Marra et al. [20] and Erskine et al. [21]. Due to the limited numbers of HZO cases and observation times, the RRs were 1.3–4.4-fold higher risks of stroke/TIA after HZO in short-term periods of time (<1 month, <3 months, and <1 year) (Figure 1).
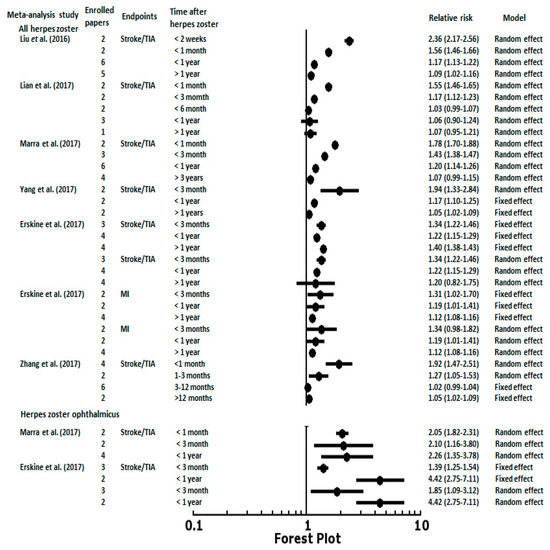
Figure 1.
The results of meta-analyses examining the relative risk (RR) of stroke within different lengths of follow-up after herpes zoster episodes. TIA, transient ischemic attack; MI, myocardial infarction.
Since differences in the choice of controls, periods for between-group comparisons, and controlling for confounders greatly affect outcomes, the results of these studies are hardly comparable. Considering the more reliable method by self-controlled case series analyses, three studies (Langan et al., Minassian et al., and Schink et al.) [6,7,10] confirmed the results of a higher incidence ratio of post herpes zoster associated cardiovascular events in different risk periods after HZ diagnosis. The age-adjusted incidence ratios of ischemic stroke were 1.30–2.37 within 4 weeks, 1.17–1.42 during 5–12 weeks, and no effect was found after 12 weeks [6,7,10]. Similarly, the risk of MI after an HZ episode was increased within 12 weeks and stabilized after 12 weeks [10] (Table 5). The risks of cerebrovascular or cardiovascular complications associated with HZ appear transient in nature, tending to decrease with time, and returning to general population risks by 12 weeks after HZ based on self-controlled case series analyses. These studies have the major benefit of implicitly controlling for fixed between-person confounding effects. However, most prospective cohort studies show a gradient of stroke risk up to 1 year [4,5,13]. More research on the transient increased vascular risks post HZ is needed to guide strategies and policies for risk prevention.

Table 5.
Age-adjusted incidence ratios for stroke/TIA or myocardial infarction in length of follow-up time after herpes zoster in three different self-controlled case series studies.
Figure 2 shows the risks of stroke/TIA and MI after an HZ episode in different age groups. As the Zhang et al. [22] and Marra et al. [20] meta-analyses reported, there was a significant increased risk of stroke after an HZ episode in younger subjects. The risk of stroke showed a decreased gradient from younger to elderly patients as in Kwon et al. [12] and Kim et al. [9]. In Breuer et al. [2] and Sundström et al. [4], the risks of CVD or cerebrovascular disease were especially high in patients <40 years, a group with fewer atherosclerosis risk factors. These findings were also compatible with recent studies by Patterson et al. [23,24] that found 5 times (95% CI, 1.37–19.10) higher risks of TIA and 3 times (95% CI, 1.15–7.57) higher risks of composite vascular events (MI, ITA, and stroke) in adults aged <50 years. These studies reported that VZV is a risk factor for stroke, especially in individuals under 50 years of age who develop HZ.
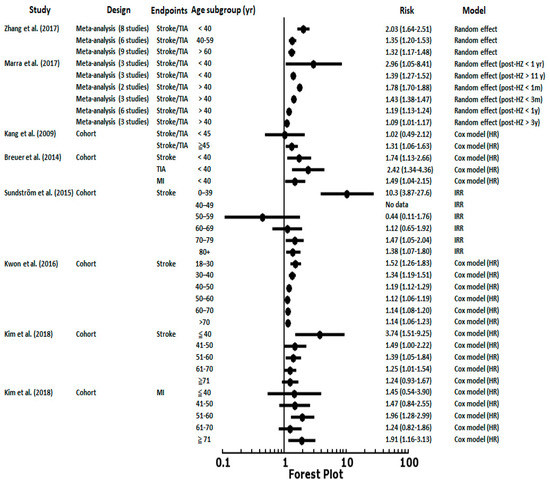
Figure 2.
The results of meta-analyses and enrolled cohorts examining the risk of stroke or myocardial infarction after herpes zoster episodes in different age ranges. TIA, transient ischemic attack; MI, myocardial infarction; HR, hazard ratio; IRR, incident rate ratio.
Figure 3 demonstrates the meta-regression of the post-HZ length of follow up as well as age on stroke/TIA risk. Each circle represents a study subgroup and the size of the circle reflects the influence of that study subgroup on the model (inversely proportionate to the SE of that study). Post-HZ length of follow-up and age influenced the relative risk of stroke/TIA in post-HZ individuals.
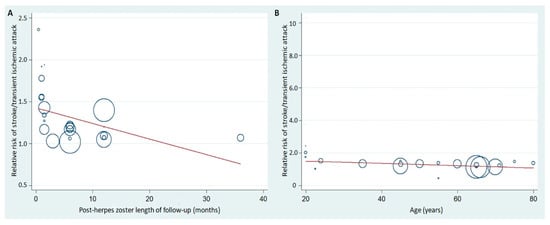
Figure 3.
Meta-regression of the post-HZ length of follow-up (A) or age of the subjects (B) and relative risk of stroke/TIA in post-HZ patients. HZ, herpes zoster.
6. Varicella Zoster Virus Vasculopathy and Other Potential Mechanisms of Herpes Zoster, Such as CVD Risks
Several studies declared that the vasculopathy caused by VZV was associated with the increased risk of future CV outcomes. A clinical case series found that patients with symptoms of HZ and stroke or TIA presented positive for VZV DNA and anti-VZV antibodies from cerebrospinal fluid samples [25,26]. In a pathology survey, arterial biopsies containing intranuclear Cowdry A inclusions in HZ patients concurrent with stroke were consistent with VZV [27]. Moreover, the brain MRI was abnormal in 97% of VZV vasculopathy cases, frequently revealing lesions at the gray-white matter junctions, deep-seated, and cortical infarctions, and the arteries were involved in 70% of patients as determined by angiography [28].
The potential mechanism of VZV-associated vascular events is shown in Figure 4. VZV vasculopathy caused by the productive virus infection of the cerebral arteries can cause pathological vascular remodeling [25], resulting in clinical ischemic or hemorrhagic stroke. HZ induces vasculopathy via the following mechanisms: (1) Induction of the production of prothrombotic autoimmune antibodies, such as IgM and IgG anticardiolipin antibodies [29]. (2) Autoimmune phenomenon caused by circulating immune complexes [30]. (3) Disruption of the internal elastic lamina, intimal hyperplasia, and decreased smooth muscle cells in the tunica medial layer [25].
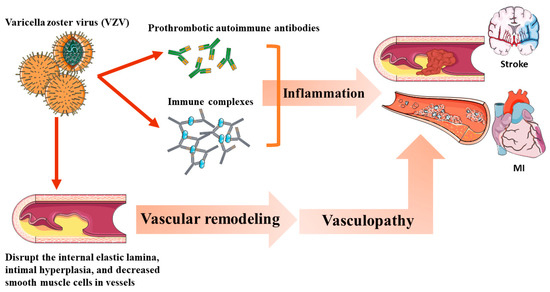
Figure 4.
The potential mechanism of varicella zoster virus (VZV)-associated vascular events.
Virus-induced inflammation accounts for remodeling of the vessel wall that causes an overly thickened intima and inflammation in the vasa vasorum vessels [31,32]. VZV and neutrophils were identified in the arterial adventitia in early vasculopathy. Viral inclusions, DNA, and antigens can be found in the cerebral arteries of VZV vasculopathy in stroke or TIA patients [27,33]. VZV vasculopathy should always be suspected in HZ patients with concurrent TIA, stroke, and/or chronic headache [34]. Most VZV vasculopathies develop within 6 weeks after an HZ episode [35].
The virus sometimes travels from the same trigeminal ganglion along afferent fibers around the carotid artery and its branches [36]. VZV adjacent to small and large intracranial cerebral arteries or extracranial arteries can produce inflammation and pathological vascular remodeling with intima proliferation and media damage, leading to subsequent thrombosis and rupture, culminating in ischemia or hemorrhage [27,36,37,38]. Therefore, VZV can cause cerebrovascular infarctions, aneurysms, and hemorrhages by weakening the cerebral artery walls [27,39]. Moreover, VZV in the cranial nerves has a more direct effect on the cerebral arteries [40]. Stroke patients after an HZ episode showed changes in both brain imaging and angiograms [26,39], which showed vasculitis and lymphocytic infiltration pathology presentation in the infarcted brain [41]. Regarding the pathogenesis of stroke or TIA after an HZ episode located outside of the head and neck, there are several possible biological causes, including (1) an increase in the sympathetic tone, blood pressure, and adverse emotional reactions [42]; (2) altered immunological status caused by VZV reactivation and subsequent vulnerability to cerebrovascular events [42]; and (3) systemic inflammation, autoimmune responses, or hemodynamic changes, leading to cardiovascular events [42].
Vascular events occurring within days of an HZ episode could be due to the associated inflammatory response [43]. For example, inflammatory cytokines, such as interleukin-6 (IL-6), are significantly increased in VZV infections that are related to arterial thrombosis [44,45]. There are different etiologies in ischemic stroke and hemorrhagic stroke, and inflammation plays an important role in the etiology of ischemic stroke. Inflammatory cells could contribute to vascular remodeling by secreting soluble factors and potentially disrupting pre-existing atherosclerotic plaques [46]. In recent studies, the asymptomatic reactivation of VZV from the cranial nerves could also lead to infection of the cranial arteries, stroke, and TIA, including in the absence of HZ rash or rash in the noncranial dermatomes [40]. This hypothesis is supported by findings from a VZV-infected animal model of the simian varicella virus in macaques [47]. In this study, the asymptomatic reactivation of the simian varicella virus with the potential to cause cranial arteritis from the trigeminal ganglia was detected in macaques [48]. In a human study, it was shown diabetes patients without a history of HZ can also have VZV antigen in the arterial adventitial tissue within skip lesions of cerebral arteries [49]. Conclusively, individuals who are predisposed to HZ have a higher risk of cerebrovascular disease.
7. Limitations of Current Observational Studies
The limitations of the observational studies included a lack of randomization and a risk of bias. The review studies were controlled for important confounders, such as demographics and CVD risk factors. Some studies were flawed by a small number of enrolled subjectof HZ (Hosamirudsari et al.) [8], few HZO identified patients (Langan et al.) [6], small events numbers (Kang et al., Lin et al., and Sundström et al.) [3,4,16], and misclassification of HZ and herpes simplex (Sreenivasan et al.) [13]. Furthermore, several studies implemented a self-controlled case series design to provide stronger confounding control [6,7,10]. However, the possibility of residual confounding, such as stressful life events or mental health, could not be ruled out. Furthermore, few of the studies evaluated stroke risk according to use of antiviral treatment, which may solidify the evidence of risk association and provide clinical recommendations for adults with high CVD risk after HZ. Correspondingly, only one study evaluated the effect of vaccination on stroke risk after an HZ episode with low power [10]. Additional studies of CVD outcomes among patients vaccinated against HZ could provide a better understanding of HZ as a possible risk factor for CVD. Future cohort studies or clinical trials of patients who received the HZ vaccine could better clarify the association between HZ and CVD or cerebrovascular outcomes. The risk of CVD may depend on whether the HZ outbreak occurs in dermatomes that share innervation with the coronary and cerebral arteries. Although the meta-analysis results demonstrated the association between HZ or HZO and stroke, heterogeneity was observed in the study designs, geographic areas, characteristics of populations, and variant confounding factors’ adjustment. Furthermore, positive result bias may reveal an association between HZ and selected CVD events.
8. Conclusions
To summarize the association of HZ and CVD, 15 published epidemiological studies and six meta-analysis reports were reviewed. Individuals exposed to HZ or HZO had 1.3 to 4-fold increased risks of cerebrovascular events. Higher risks were noted among younger patients (age < 40 years) within one year after an HZ episode. The elevated risk of CV events diminished gradually according to age and length of time after an HZ episode. VZV infection of the cerebral arteries with subsequent inflammation leads to vascular remodeling and thickened intima that contributes to vascular occlusion in patients with VZV vasculopathy. Epidemiological studies and the pathology of VZV vasculopathy both indicate that HZ is an important risk factor for stroke within one year after an HZ episode. However, limited reports indicated that antiviral treatment reduces stroke risk. It was also difficult to confirm the relationship between the zoster vaccine and cerebrovascular events because of the small sample size. Emerging studies are needed to determine the effect of optimal antiviral treatment and zoster vaccination on reducing the burden of cerebrovascular and CVD, especially for patients with pre-existing high CVD risk.
Author Contributions
Conceptualization, P.-H.W. and Y.-T.L.; methodology, P.-H.W. and Y.-S.C.; software, P.-H.W.; validation, P.-H.W., Y.-S.C. and Y.-T.L.; formal analysis, P.-H.W., Y.-S.C. and Y.-T.L.; resources, P.-H.W. and Y.-T.L.; data curation, Y.-T.L.; writing—original draft preparation, P.-H.W. and Y.-T.L.; writing—review and editing, P.-H.W. and Y.-T.L.; visualization, P.-H.W. and Y.-S.C.; supervision, Y.-T.L.; project administration, Y.-T.L.; funding acquisition, Y.-T.L.
Funding
The funding sources did not play any role in the conduct of the review, interpretation of the results, or approval of the manuscript. The study was funded by grants from Kaohsiung Medical University Hospital, Taiwan (KMUH104-4M05) and Kaohsiung Municipal Hsiao-Kang Hospital, Taiwan (Kmhk-104-023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vrcek, I.; Choudhury, E.; Durairaj, V. Herpes zoster ophthalmicus: A review for the internist. Am. J. Med. 2017, 130, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Breuer, J.; Pacou, M.; Gautier, A.; Brown, M.M. Herpes zoster as a risk factor for stroke and tia: A retrospective cohort study in the uk. Neurology 2014, 83, e27–e33. [Google Scholar] [CrossRef]
- Kang, J.H.; Ho, J.D.; Chen, Y.H.; Lin, H.C. Increased risk of stroke after a herpes zoster attack: A population-based follow-up study. Stroke 2009, 40, 3443–3448. [Google Scholar] [CrossRef]
- Sundstrom, K.; Weibull, C.E.; Soderberg-Lofdal, K.; Bergstrom, T.; Sparen, P.; Arnheim-Dahlstrom, L. Incidence of herpes zoster and associated events including stroke--a population-based cohort study. BMC Infect. Dis. 2015, 15, 488. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.P.; Wollan, P.C.; Nagel, M.A.; Gilden, D. Risk of stroke and myocardial infarction after herpes zoster in older adults in a us community population. Mayo Clin. Proc. 2016, 91, 33–44. [Google Scholar] [CrossRef]
- Langan, S.M.; Minassian, C.; Smeeth, L.; Thomas, S.L. Risk of stroke following herpes zoster: A self-controlled case-series study. Clin. Infect. Dis. 2014, 58, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Schink, T.; Behr, S.; Thone, K.; Bricout, H.; Garbe, E. Risk of stroke after herpes zoster - evidence from a german self-controlled case-series study. PLoS ONE 2016, 11, e0166554. [Google Scholar] [CrossRef] [PubMed]
- Hosamirudsari, H.; Rashed, P.; Afsari, F.; Akbarpour, S.; Bagherzadeh, A. Correlation between herpes zoster and stroke-a case-control study. J. Med. Virol. 2018, 90, 1370–1374. [Google Scholar] [CrossRef]
- Kim, M.C.; Yun, S.C.; Lee, H.B.; Lee, P.H.; Lee, S.W.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H.; Kwon, S.U. Herpes zoster increases the risk of stroke and myocardial infarction. J. Am. Coll. Cardiol. 2017, 70, 295–296. [Google Scholar] [CrossRef]
- Minassian, C.; Thomas, S.L.; Smeeth, L.; Douglas, I.; Brauer, R.; Langan, S.M. Acute cardiovascular events after herpes zoster: A self-controlled case series analysis in vaccinated and unvaccinated older residents of the united states. PLoS Med. 2015, 12, e1001919. [Google Scholar] [CrossRef]
- Seo, H.M.; Cha, M.J.; Han, J.H.; Han, K.; Park, S.H.; Bang, C.H.; Lee, J.H.; Lee, J.Y.; Choi, E.K.; Park, Y.M. Reciprocal relationship between herpes zoster and cardiovascular diseases: A nationwide population-based case-control study in korea. J. Dermatol. 2018, 45, 1312–1318. [Google Scholar] [CrossRef]
- Kwon, S.U.; Yun, S.C.; Kim, M.C.; Kim, B.J.; Lee, S.H.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Risk of stroke and transient ischaemic attack after herpes zoster. Clin. Microbiol. Infect. 2016, 22, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, N.; Basit, S.; Wohlfahrt, J.; Pasternak, B.; Munch, T.N.; Nielsen, L.P.; Melbye, M. The short-and long-term risk of stroke after herpes zoster-a nationwide population-based cohort study. PLoS ONE 2013, 8, e69156. [Google Scholar] [CrossRef]
- Wang, C.C.; Lin, C.L.; Chang, Y.J.; Wang, G.J.; Sung, F.C.; Kao, C.H. Herpes zoster infection associated with acute coronary syndrome: A population-based retrospective cohort study. Br. J. Dermatol. 2014, 170, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Lin, C.L.; Sung, F.C.; Chou, T.C.; Lee, Y.T. Increased risk of cardiovascular events in patients with herpes zoster: A population-based study. J. Med. Virol. 2014, 86, 772–777. [Google Scholar] [CrossRef]
- Lin, H.C.; Chien, C.W.; Ho, J.D. Herpes zoster ophthalmicus and the risk of stroke: A population-based follow-up study. Neurology 2010, 74, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guan, Y.; Hou, L.; Huang, H.; Liu, H.; Li, C.; Zhu, Y.; Tao, X.; Wang, Q. The short- and long-term risk of stroke after herpes zoster: A meta-analysis. PLoS ONE 2016, 11, e0165203. [Google Scholar] [CrossRef]
- Lian, Y.; Zhu, Y.; Tang, F.; Yang, B.; Duan, R. Herpes zoster and the risk of ischemic and hemorrhagic stroke: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0171182. [Google Scholar] [CrossRef]
- Yang, S.Y.; Li, H.X.; Yi, X.H.; Han, G.L.; Zong, Q.; Wang, M.X.; Peng, X.X. Risk of stroke in patients with herpes zoster: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 301–307. [Google Scholar] [CrossRef]
- Marra, F.; Ruckenstein, J.; Richardson, K. A meta-analysis of stroke risk following herpes zoster infection. BMC Infect. Dis. 2017, 17, 198. [Google Scholar] [CrossRef]
- Erskine, N.; Tran, H.; Levin, L.; Ulbricht, C.; Fingeroth, J.; Kiefe, C.; Goldberg, R.J.; Singh, S. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS ONE 2017, 12, e0181565. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, G.; Huang, Y.; Yu, Q.; Wang, L.; Li, K. Risk of stroke/transient ischemic attack or myocardial infarction with herpes zoster: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2017, 26, 1807–1816. [Google Scholar] [CrossRef]
- Patterson, B.; Rausch, D.A.; Irwin, D.E.; Liang, M.; Yan, S.; Yawn, B. Risk of myocardial infarction and composite vascular events in patients with herpes zoster: 2007-2014 united states claims data analysis. J. Am. Coll. Cardiol. 2018, 71, A177. [Google Scholar] [CrossRef]
- Patterson, B.; Rausch, D.; Irwin, D.; Liang, M.; Yan, S.; Yawn, B. Risk of transient ischemic attack and stroke in patients with herpes zoster: 2007–2014 us claims data analysis (s15.008). Neurology 2018, 90, S15.008. [Google Scholar]
- Nagel, M.A.; Traktinskiy, I.; Azarkh, Y.; Kleinschmidt-DeMasters, B.; Hedley-Whyte, T.; Russman, A.; VanEgmond, E.M.; Stenmark, K.; Frid, M.; Mahalingam, R.; et al. Varicella zoster virus vasculopathy: Analysis of virus-infected arteries. Neurology 2011, 77, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Cohrs, R.J.; Mahalingam, R.; Wellish, M.C.; Forghani, B.; Schiller, A.; Safdieh, J.E.; Kamenkovich, E.; Ostrow, L.W.; Levy, M.; et al. The varicella zoster virus vasculopathies: Clinical, csf, imaging, and virologic features. Neurology 2008, 70, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.; Cohrs, R.J.; Mahalingam, R.; Nagel, M.A. Varicella zoster virus vasculopathies: Diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009, 8, 731–740. [Google Scholar] [CrossRef]
- Amlie-Lefond, C.; Gilden, D. Varicella zoster virus: A common cause of stroke in children and adults. J. Stroke Cerebrovasc. Dis. 2016, 25, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Uthman, I.; Taher, A.; Khalil, I. Hughes syndrome associated with varicella infection. Rheumatol. Int. 2001, 20, 167–168. [Google Scholar] [CrossRef] [PubMed]
- Bodensteiner, J.B.; Hille, M.R.; Riggs, J.E. Clinical features of vascular thrombosis following varicella. Am. J. Dis. Child. 1992, 146, 100–102. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. Complications of varicella zoster virus reactivation. Curr. Treat Options Neurol. 2013, 15, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Yeager, M.E.; El Kasmi, K.C.; Nozik-Grayck, E.; Gerasimovskaya, E.V.; Li, M.; Riddle, S.R.; Frid, M.G. The adventitia: Essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 2013, 75, 23–47. [Google Scholar] [CrossRef]
- Gilden, D.H.; Lipton, H.L.; Wolf, J.S.; Akenbrandt, W.; Smith, J.E.; Mahalingam, R.; Forghani, B. Two patients with unusual forms of varicella-zoster virus vasculopathy. N. Engl. J. Med. 2002, 347, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; Gilden, D. Neurological complications of varicella zoster virus reactivation. Curr. Opin. Neurol. 2014, 27, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Miravet, E.; Danchaivijitr, N.; Basu, H.; Saunders, D.E.; Ganesan, V. Clinical and radiological features of childhood cerebral infarction following varicella zoster virus infection. Dev. Med. Child. Neurol. 2007, 49, 417–422. [Google Scholar] [CrossRef]
- Grose, C.; Adams, H.P. Reassessing the link between herpes zoster ophthalmicus and stroke. Expert Rev. Anti. Infect. Ther. 2014, 12, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Franco-Paredes, C.; Bellehemeur, T.; Merchant, A.; Sanghi, P.; DiazGranados, C.; Rimland, D. Aseptic meningitis and optic neuritis preceding varicella-zoster progressive outer retinal necrosis in a patient with aids. AIDS 2002, 16, 1045–1049. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. The challenging patient with varicella-zoster virus disease. Neurol. Clin. Pract. 2013, 3, 109–117. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. The relationship between herpes zoster and stroke. Curr. Neurol. Neurosci. Rep. 2015, 15, 16. [Google Scholar] [CrossRef]
- Gilden, D.; Nagel, M.A.; Cohrs, R.J. Persistence of varicella zoster virus DNA in saliva after herpes zoster. J. Infect. Dis. 2012, 205, 1178–1179. [Google Scholar] [CrossRef]
- Hayman, M.; Hendson, G.; Poskitt, K.J.; Connolly, M.B. Postvaricella angiopathy: Report of a case with pathologic correlation. Pediatr. Neurol. 2001, 24, 387–389. [Google Scholar] [CrossRef]
- Josephson, C.; Nuss, R.; Jacobson, L.; Hacker, M.R.; Murphy, J.; Weinberg, A.; Manco-Johnson, M.J. The varicella-autoantibody syndrome. Pediatr. Res. 2001, 50, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of myocardial infarction and stroke after acute infection or vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Emsley, H.C.; Tyrrell, P.J. Inflammation and infection in clinical stroke. J. Cereb. Blood Flow. Metab. 2002, 22, 1399–1419. [Google Scholar] [CrossRef]
- Vila, N.; Reverter, J.C.; Yague, J.; Chamorro, A. Interaction between interleukin-6 and the natural anticoagulant system in acute stroke. J. Interferon Cytokine Res. 2000, 20, 325–329. [Google Scholar] [CrossRef]
- Nagel, M.A.; Traktinskiy, I.; Stenmark, K.R.; Frid, M.G.; Choe, A.; Gilden, D. Varicella-zoster virus vasculopathy: Immune characteristics of virus-infected arteries. Neurology 2013, 80, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R.; Messaoudi, I.; Gilden, D. Simian varicella virus pathogenesis. Curr. Top. Microbiol. Immunol. 2010, 342, 309–321. [Google Scholar] [PubMed]
- Mahalingam, R.; Traina-Dorge, V.; Wellish, M.; Deharo, E.; Singletary, M.L.; Ribka, E.P.; Sanford, R.; Gilden, D. Latent simian varicella virus reactivates in monkeys treated with tacrolimus with or without exposure to irradiation. J. Neurovirol. 2010, 16, 342–354. [Google Scholar] [CrossRef]
- Nagel, M.A.; Traktinskiy, I.; Choe, A.; Rempel, A.; Gilden, D. Varicella-zoster virus expression in the cerebral arteries of diabetic subjects. Arch. Neurol. 2012, 69, 142–144. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).