Abstract
Headache is a common complication after diagnostic lumbar puncture (DLP). We aimed to check whether hydration before puncture influences the incidence of post-lumbar puncture headache (PLPH) and affects cerebral blood flow. Ninety-nine patients enrolled for puncture were assigned to a group with (n = 40) or without hydration (n = 59). In the hydration group, 1000 mL 0.9% NaCl was infused and a minimum of 1500 mL oral fluids was recommended within the 24 h before puncture. A Transcranial Doppler (TCD) was performed before and after DLP. Mean velocity (Vm) and pulsatility index (PI) were measured in the middle cerebral arteries (MCAs). PLPH occurred in 28 patients (28.2%): six (15.4%) from the hydrated and 22 (37.3%) from the non-hydrated group (p < 0.023). Patients with PLPH were younger (p < 0.014) and with headaches in their histories (p < 0.036) compared with the non-headache group. Vm values in both MCAs after puncture were significantly lower than before puncture in all patients. In the PLPH group, Vm in MCAs before puncture were significantly higher and the PI was lower than in the non-headache group. Our findings suggest that hydration of patients within 24 h before puncture prevented PLPH. Twenty-four hours after puncture, significant decreases in Vm were observed in the MCAs of all patients. Low baseline values of PI and high Vm predisposed patients to PLPH.
1. Introduction
Diagnostic lumbar puncture (DLP) is a frequently performed medical procedure, which is important in the diagnosis of nervous system diseases. Headache is the most common complication after DLP, with frequency rates ranging from 3% to 40% of patients [1,2,3,4]. Post-lumbar puncture headache (PLPH) probably results from the leakage of cerebrospinal fluid into the epidural cavity through the post-puncture opening in the dura mater. The loss of cerebrospinal fluid causes a drop in intracranial pressure (ICP), leading to the compensative dilatation of cerebral vessels and hence, the headache [1,2,3,5]. Transcranial Doppler ultrasonography (TCD) makes it possible to assess blood flow in the intracranial arteries non-invasively. TCD parameters are influenced both by changes in cerebral vessel diameters and by fluctuations in ICP [6,7,8]. DLP results in significant changes in cerebral blood flow (CBF) that can be visualized by TCD [9,10,11]. The role of fluid supplementation in the prevention of PLPH remains unclear [12]. The results of a few current studies have unambiguously confirmed that hydration of patients after puncture prevents PLPH [13,14,15]. This is why we decided to check whether hydration before puncture influences the incidence of PLPH and causes changes in CBF which are reflected in TCD.
2. Experimental Section
The prospective parallel study involved patients hospitalized in the Neurology Clinic who required scheduled DLP. The exclusion criteria included emergency lumbar punctures, inadequate temporal windows, stenosis of the middle cerebral arteries, hemodynamically significant stenosis of the internal carotid arteries, atrial fibrillation, general severe medical conditions, heart, kidney, and respiratory failures, unconsciousness, speech disorders, and patient immobilization. Patients enrolled for puncture were randomly assigned following simple randomization procedures (using the basic random number generator in Excel) to one of two groups: one containing patients with hydration and one without hydration. In the hydration group, intravenous 1000 mL 0.9% NaCl was infused within the 24 h before puncture and an increased amount of oral fluids was also recommended (minimum 1500 ml). A TCD was performed just before DLP and 24 h after DLP. The lumbar puncture was performed according to the standard procedure in a supine position, by one of the 11 doctors working in the clinic. On the day of puncture, plasma osmolality was determined in the morning. PLPH was diagnosed according to the International Classification of Headaches (ICH-III beta) [16]. The presence and severity of PLPH was assessed using a numerical scale (numeric rating scale, NRS) every day for five days. All the procedures were approved by the Local Ethics Committee of the Ludwik Rydygier Collegium Medicum in Bydgoszcz (KB/285/2015). The subjects gave their informed consent before the start of any procedure.
Lumbar puncture was performed in the lateral recumbent position in the space between L4 and L5, or between L5 and S1, using a 22-gauge needle (BD Spinal Needle Quincke Type Point). The volume of the cerebral fluid collected ranged from 3 mL to 6 mL. Punctures were performed between 11 a.m. and midday.
TCD examinations were performed with a Nicolet Sonara transcranial doppler system (producer Viasys Healthcare) and a 2 MHz probe. Patients were examined in the supine position. After finding the temporal window, the middle cerebral artery was identified. The probe was positioned in such a way as to obtain the highest possible velocity of the tested vessel. Mean velocity (Vm) and Gosling’s pulsatility index (PI) were measured at a 54–56 mm depth in the right and left middle cerebral artery (MCA). TCD tests were performed by a physician experienced in the field of neurosonology who was not informed about the presence and severity of PLPH or the hydration of the patient.
The results are presented as mean ± standard deviation (SD). Mean values close to normal distribution were compared for independent samples with Student’s t test. When the distribution was significantly different from the normal distribution, differences between the groups were checked using the non-parametric Mann–Whitney U test. Dependent variables were checked using the Student’s t test for dependent samples. Proportions in the groups were evaluated using the χ2 test. Correlation between the two groups was calculated with Spearman’s rank correlation coefficient. The Shapiro–Wilk test was used to check for normality. Continuous variables were compared between the four groups with an analysis of variance (ANOVA) or the Kruskal-Wallis test, as appropriate. The Dunn test was used for post-hoc comparisons, with p-values adjusted using Holm’s method. Categorical variables were compared with Fisher’s exact test. When categorical variables significantly differed between the four groups, pairwise Fisher’s exact tests were used for post-hoc comparisons, with p-values adjusted using the Holm’s method. Statistical significance was set at p < 0.05, two-tailed. All the calculations were made in R software.
3. Results
Ninety-nine patients were enrolled for this study, including 45 men and 54 women. The mean age was 42.5 ± 14.48 years (range: 18 to 79 years). Patients were diagnosed due to the following: demyelinating disease (21 patients), headaches (14), mononeuropathy (11), abnormal outbreaks in head MR (11), transient ischemic attack (11), polyneuropathy (5), vertigo (5), loss of consciousness (7), and other reasons (epilepsy, transient global amnesia, tremor, syphilis).
Forty patients were hydrated 24 h before puncture, and in 59 patients punctures were performed without prior hydration. Post-puncture headache occurred in 28 patients (28.2%): six (15.4%) in the hydrated group and 22 (37.3%) in the non-hydrated group (p < 0.023). Patients with PLPH were significantly younger and more frequently reported headaches in their medical histories compared with the group without headache. The characteristics of the patients in each group are presented in Table 1 and Table 2.

Table 1.
Clinical characteristics of hydrated and non-hydrated patients; patients who developed post-lumbar puncture headache (PLPH) and PLPH-free individuals.

Table 2.
Characteristics and transcranial Doppler (TCD) parameters before and after puncture in groups of patients who developed post-lumbar puncture headache (PLPH) and in PLPH-free individuals, depending on the hydration.
There were no significant differences in Vm between the right and left MCAs, before and after puncture, while PI values were significantly different between the cerebral hemispheres. PI was higher on the right side before and after the puncture (p < 0.001).
Vm values in both MCAs after puncture were significantly lower than before puncture in all the groups of patients, while PI after puncture increased only in the group of patients with post-puncture headache (Table 2). Percentage changes in Vm (dVm) and PI (dPI) after puncture were highest in the group of patients with PLPH who had not been hydrated (Table 2).
In patients with PLPH, the values of Vm in both MCAs before puncture were significantly higher and PI values were lower than in the group without headache, while no significant differences in Vm and PI values after puncture were observed between the groups (Table 2). The highest Vm values before puncture were found in the hydrated PLPH patients. The Vm values after puncture were higher in the hydrated group than in the non-hydrated group; there were no significant differences in PI between the hydrated and non-hydrated group, before and after the puncture (Table 2).
Moreover, there were no significant differences in the osmolality of the serum before puncture between the hydrated, non-hydrated, PLPH, and non-PLPH group; however, the average osmolality in all the groups was higher than the laboratory standard (Table 2).
The logistic regression model for PLPH (PLPH occurrence as a dependent variable) showed more frequent occurrence of PLPH in patients who were younger (p < 0.014), not hydrated (p < 0.007) and had a history of headaches (p < 0.036).
4. Discussion
Post-lumbar puncture headache is probably caused by leakage of the cerebrospinal fluid through the post-puncture opening, which leads to a decrease in intracranial pressure and dilation of the cerebral vessels [1,2,3,5]. The change of the above parameters may affect the cerebral blood flow assessed by TCD: the dilatation of the cerebral arteries causes a decrease in the flow velocity [6,7,8] (Figure 1). The pulsatility index is an indirect indicator of the ICP changes. The value of the PI illustrates the resistance of a vascular bed (supplied by a given artery) when examined by TCD. There is a linear relationship between changes in PI and ICP. A decrease in PI may result both from lowered ICP and from vascular dilatation [7,8].
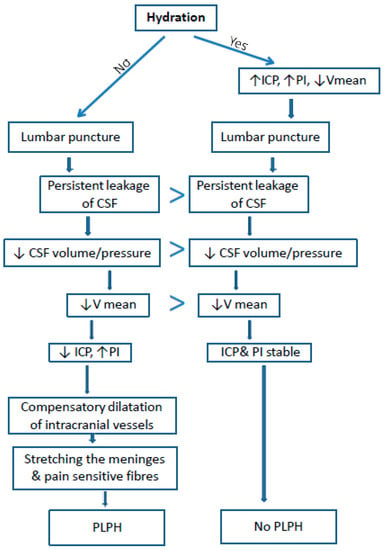
Figure 1.
Proposed mechanism of PLPH pathogenesis and the influence of hydration on the incidence of PLPH and cerebral blood flow (based on our study). ICP–intracranial pressure, PI–Gosling’s pulsatility index, V mean–mean velocity, CSF–cerebro-spinal fluid, PLPH–post lumbar puncture headache.
In our previous study, we showed that patients with a lower baseline PI and higher Vm developed PLPH more often [9]. This was recently confirmed by other authors [10]. From previous studies we know that the PI positively correlates with ICP—a PI change of 2.4% is reflected by a 1 mmHg change in ICP [17]. Therefore, it can be assumed that lower PIs correspond to a lower ICP. This means that patients with lower baseline ICP develop PLPH more frequently. One of the factors that may affect ICP is hydration; therefore, we assumed that mild dehydration of patients before lumbar puncture may influence the incidence of PLPH. Thus, we decided to investigate how the hydration of patients before the puncture affects PI, Vm, and the occurrence of PLPH.
The results of several current studies describe no significant effect of patient hydration on the occurrence of post-lumbar puncture headache [12,13,15]. However, most patients were hydrated after puncture or directly before the procedure. Dietrich hydrated patients for five days after DLP and did not notice a difference in the occurrence of PLPH [13]. The patients involved in the study by Gupta et al. were hydrated directly before and after puncture. They were given 500 mL of saline intravenously 30 min before and after puncture and showed no significant reduction in PLPH compared to the non-hydrated group. Nevertheless, they noticed a significant reduction in pain severity [15]. The effects of intravenous and oral hydration within the 24 h before puncture on the occurrence of PLPH have not been described by any author.
In our study, we showed that in the group of patients hydrated before puncture, PLPH was significantly less frequent than in the group without hydration. In further analyses of the effect of hydration on CBF and PLPH, we additionally used TCD parameters.
In all the patients, puncture was followed by a decrease in Vm in both MCAs, while PI after puncture increased only in the PLPH group. Most likely, the ICP decreased after the puncture which led to the dilatation of the arteries and the decrease in Vm. This decrease was the highest in the group with PLPH. V correlates linearly with PI and ICP (when PI and ICP decreases, there is a Vm increase), a large decrease in Vm in the PLPH group probably caused compensatory vasoconstriction in the microcirculation, which, in turn, caused PI growth. This confirms the theory of PLPH pathophysiology according to which, in patients with PLPH, additional cerebrospinal fluid leakage through the post-puncture opening causes a greater decrease in ICP, and thus, a greater decrease in Vm in the MCA.
In the group of patients with PLPH, baseline Vm values (before puncture) were higher and PI values were lower than in the group without PLPH. It is known that with the increase of ICP, PI increases and Vm decreases. Therefore, it can be concluded that in patients who developed PLPH, the baseline ICP was lower than in the group without PLPH. This could be associated with a greater dehydration before puncture in this group. The baseline low ICP led to PLPH development after puncture, while in the group with higher baseline ICP, despite the reduction of V after puncture, headache did not occur. It is possible that a higher pre-puncture ICP protected patients from excessive vasodilatation and decreased ICP (Figure 1). Nevertheless, that lower PIs and higher Vm in the group of PLPH patients may have resulted from young age can be excluded because Vm decreases with age, while PI increases [18].
It is worth emphasizing that in the group of patients who were hydrated before puncture, post-puncture Vm values were higher than in the group without hydration. This is probably why these patients less frequently noticed the decrease of ICP values and PLPH development.
Can the occurrence of PLPH be predicted based on TCD parameters before the puncture? Movafy showed that pre-puncture Vm was the parameter with the greatest accuracy for predicting PLPH, with a cutoff of Vm > 68.4 cm/s. Our study confirms these observations. In addition, similarly to other authors, we confirmed the correlation between PLPH with age and a history of headaches [1,2].
In our patients, an interhemispheric difference in PI was found before and after puncture, while mean Vm was not significantly different between the right and left MCA. Gobel et al. noticed that patients who develop post-lumbar puncture headache were characterized before lumbar puncture by a significant lateralization of Vm of the MCAs—the flow velocity in the right artery predominated significantly. Unfortunately, they did not evaluate PI value, only Vm. It is difficult to explain our results clearly; this requires further observation and more accurate research.
PLPH is only one type of headache; however, PLPH patients often have a medical history of various types of headaches. The level of hydration affects headaches in general. It is known that dehydration promotes the occurrence of migraine and other types of headaches [19,20]. Spigt noticed that increased hydration reduced the number of pain days in patients with migraine and tension headache [19]. Perhaps pre-puncture hydration prevents the occurrence of PLPH not only by a mechanical CBV increase but also by means of another mechanism.
Although, in our study, we showed that pre-puncture hydration reduced the incidence of PLPH, we did not find differences in plasma osmolality between groups. Perhaps small and rapid changes in the hydration in our patients were not detectable by changes in plasma osmolality. This was also confirmed by other authors [21]. Interestingly, in all the groups, plasma osmolality values were higher than the laboratory norm, which may indicate a dehydration problem in all the patients before DLP. Dehydration may be related with hospitalization, stress, or fasting before the planned examinations (e.g., MRI).
The limitation of this study is the fact that TCD measures only the flow of velocity and not the absolute CBF value. The correlation between CBF and flow velocity is variable. Cerebral blood velocity is an adequate surrogate of absolute flow only if the insonated vessel maintains constant vessel diameter in the experimental conditions. Blood flow velocity is further influenced by several factors, including arterial blood pressure, ICP, hematocrit, PaCO2 and the status of autoregulation, thus making a direct comparison of flow velocity and CBF difficult. Moreover, it is worth noting that a correlation between PI and ICP has been found in the context of intracranial hypertension (after trauma, intracranial heamorrhage, cerebral mass lesion and hydrocephalus) and is strongest when ICP is over 20 mm [7,8,17]. There is no study of PI in patients with intracranial hypotention or dehydration.
Another limitation of the study may be the fact that the hydration level of the patients was evaluated only by a single measurement of plasma osmolality before DLP, while urine osmolality and gravity was not assessed, nor did we assess any hydration level parameters after puncture.
5. Conclusions
Our findings suggest that oral and intravenous hydration of patients within the 24 h before puncture prevented PLPH. The changes of TCD parameters partially confirm the theory of PLPH pathogenesis as a consequence of cerebrospinal fluid leakage through the post-puncture opening, with subsequent vasodilatation and ICP decrease. A history of headaches, low baseline values of PI, and high Vm predispose patients to PLPH occurrence.
Author Contributions
Conceptualization, M.N.; methodology, M.N.; software, B.K.-P.; validation, M.N., B.K.-P.; formal analysis, M.N.; investigation, M.N., B.K.-P.; resources, M.N.; data curation, M.N., B.K.-P.; writing—original draft preparation, M.N.; writing—review and editing, M.N., K.P.-O.; supervision K.P.-O., H.K.
Acknowledgments
The language assistance was provided by Proper Medical Writing Sp. z o.o., Warsaw, Poland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spielman, F.J. Post-lumbar puncture headache. Headache 1982, 22, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D. Post-lumbar puncture headache. Cephalalgia 1998, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Bezov, D.; Lipton, R.B.; Ashina, S. Post-dural puncture headache: Part I diagnosis, epidemiology, etiology, and pathophysiology. Headache 2010, 50, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Tullhög, K.; Hansson, O.; Minthon, L.; Londos, E.; Blennow, K. Low incidence of post-lumbar puncture headache in 1089 consecutive memory clinic patients. Eur. Neurol. 2010, 63, 326–330. [Google Scholar] [CrossRef]
- Hannerz, J.; Ericson, K.; Bro Skejo, H.P. MR imaging with gadolinum in patients with and without post-lumbar puncture headache. Acta Radiol. 1999, 40, 135–141. [Google Scholar] [CrossRef]
- Ackerstaff, R.G.; Keunen, R.W.; van Pelt, W.; Van Swijndregt, A.D.M.; Stijnen, T. Influence of biological factors on changes in mean cerebral blood flow velocity in normal ageing: A transcranial Doppler study. Neurol. Res. 1990, 12, 187–191. [Google Scholar] [CrossRef]
- Bellner, J.; Romner, B.; Reinstrup, P.; Kristiansson, K.-A.; Ryding, E.; Brandt, L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg. Neurol. 2004, 62, 45–51. [Google Scholar] [CrossRef]
- Voulgaris, S.G.; Partheni, M.; Kaliora, H.; Haftouras, N.; Pessach, I.S.; Polyzoidis, K.S. Early cerebral monitoring using the transcranial Doppler pulsatility index in patients with severe brain trauma. Med. Sci. Monit. 2005, 11, CR49–CR52. [Google Scholar]
- Nowaczewska, M.; Książkiewicz, B. Cerebral blood flow characteristics in patients with post-lumbar puncture headache. J. Neurol. 2012, 259, 665–669. [Google Scholar] [CrossRef]
- Mowafy, S.M.S.; Abd Ellatif, S.E. Transcranial Doppler role in prediction of post-dural puncture headache in parturients undergoing elective cesarean section: Prospective observational study. J. Anesth. 2019, 33, 426–434. [Google Scholar] [CrossRef]
- Göbel, H.; Klostermann, H.; Lindner, V.; Schenkl, S. Changes in cerebral haemodynamics in cases of post-lumbar puncture headache: A prospective transcranial Doppler ultrasound study. Cephalalgia 1990, 10, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Rodriguez, I.; Ciapponi, A.; Roqué i Figuls, M.; Munoz, L.; Cosp, X.B. Posture and fluids for preventing post-dural puncture headache. Cochrane Database Syst. Rev. 2016, 3, CD009199. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Brandt, T. Incidence of post-lumbar puncture headache is independent of daily fluid intake. Eur. Arch. Psychiatry Neurol. Sci. 1988, 237, 194–196. [Google Scholar] [CrossRef]
- Tucker, M.A.; Adams, J.D.; Brown, L.A.; Ridings, C.B.; Burchfield, J.M.; Robinson, F.B.; McDermott, J.L.; Schreiber, B.A.; Moyen, N.E.; Washington, T.A.; et al. No Change in 24-Hour Hydration Status Following a Moderate Increase in Fluid Consumption. J. Am. Coll. Nutr. 2016, 35, 308–316. [Google Scholar] [CrossRef]
- Gupta, N.; Kowalska, A.; Smith, J.; Coyle, P. Does Intravenous Hydration Before and After Lumbar Puncture Decrease the Occurrence of Post-Lumbar Puncture Headache? Neurology 2014, 82, 1–260. [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Homburg, A.; Jakobsen, M.; Enevoldsen, E. Transcranial Doppler recordings in raised intracranial pressure. Acta Neurol. Scand. 1993, 87, 488–493. [Google Scholar] [CrossRef]
- Muller, M.; Schimrigk, K. A comparative assessment of cerebral haemodynamics in the basilar artery and carotid territory by transcranial Doppler sonography in normal subjects. Ultrasound Med. Biol. 1994, 20, 677–687. [Google Scholar] [CrossRef]
- Spigt, M.G.; Kuijper, E.C.; Schayck, C.P.; Troost, J.; Knipschild, P.G.; Linssen, V.M.; Knottnerus, J.A. Increasing the daily water intake for the prophylactic treatment of headache: A pilot trial. Eur. J. Neurol. 2005, 12, 715–718. [Google Scholar] [CrossRef]
- Woldeamanuel, Y.W.; Cowan, R.P. The impact of regular lifestyle behavior in migraine: A prevalence case-referent study. J. Neurol. 2016, 263, 669–676. [Google Scholar] [CrossRef]
- Sommerfield, L.M.; McAnulty, S.R.; McBride, J.M.; Zwetsloot, J.J.; Austin, M.D.; Mehlhorn, J.D.; Calhoun, M.C.; Young, J.O.; Haines, T.L.; Utter, A.C. Validity of Urine Specific Gravity When Compared with Plasma Osmolality as a Measure of Hydration Status in Male and Female NCAA Collegiate Athletes. J. Strength Cond. Res. 2016, 30, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).