Abstract
Background: Multiple external root resorption (MERR) has been reported in systemic sclerosis (SSc) patients in Japan and Spain. To establish whether MERR is a new manifestation, we investigated the prevalence of MERR and systemic and oral manifestations to be associated with MERR in patients with SSc. Methods: Root resorption was detected by dental X-rays, panoramagraphy or cone beam computed tomography (CBCT). The prevalence of systemic and oral manifestations was examined by rheumatologists and dentists, respectively. Autoantibodies were investigated using laboratory tests. Results: MERR was detected in four out of the 41 patients (9.8%) who participated in the present study. The prevalence of digital ulcers was significantly higher in patients with MERR (MERR vs. non-MERR, 75% vs. 16.2%, p < 0.05), whereas that of other systemic manifestations was not. The prevalence of face skin sclerosis (100% vs. 10.8%, p < 0.01), calcinosis at the facial region (75% vs. 0%, p < 0.01), limited mouth opening (75% vs. 18.9% p < 0.05), temporomandibular disorder symptoms (50% vs. 2.7%, p < 0.05), and tongue rigidity (75% vs. 2.7%, p < 0.05) was significantly higher in patients with MERR. Conclusion: SSc patients with MERR had highly homogenous maxillofacial manifestations. Further clinical and basic studies are needed to elucidate the mechanisms underlying MERR in SSc patients.
1. Introduction
Systemic sclerosis (SSc) is a multisystem connective tissue disease that is characterized by three basic features: Immunological abnormalities, vasculopathy and fibrosis of the skin and certain internal organs [1]. It is a rare disease with a reported prevalence of 276 cases per 1,000,000 individuals in the U.S. [2]. The oral manifestations of SSc are oral mucosa atrophy, periodontal ligament (PDL) space widening, apical root resorption, dry mouth associated with Sjögren’s syndrome and calcification in the PDL space [3,4,5,6].
External root (cervical) resorption occurs when the body’s own immune system dissolves the tooth root structure from outside of the tooth [7]. Since methods to assess the condition of the root, including CBCT, have recently been developed, root resorption is now more easily detected.
The causes of single root resorption include inflammation, trauma and orthodontic treatments [8,9,10]. However, the mechanisms underlying “multiple” root resorption remain unclear. The management of external root resorption is surgical debridement when invasion is still in a shallow site, such as dentine [11]. However, in the case of a deeper invasion reaching pulpal tissue, the most appropriate strategy is to monitor the tooth in follow-up visits, and when it is impossible to stop progression, tooth loss occurs. Therefore, the early detection of root resorption is necessary to protect against tooth loss.
We and Arroyo-Bote and colleagues recently this reported multiple external root resorption (MERR) in SSc patients in Japan and Spain [12,13], respectively. These findings prompted us to examine MERR as a new manifestation of SSc. In order to elucidate disease mechanisms, all manifestations need to be investigated. Therefore, to clarify the mechanisms contributing to the pathogenesis of SSc, it is necessary to know whether MERR is a new manifestation of SSc.
In the present study, we investigated the prevalence of MERR in SSc patients. Furthermore, we evaluated systemic and oral manifestations in all subjects to identify the factors associated with MERR.
2. Patients and Methods
2.1. Patients
This was an observational, cross-sectional study. Patients with systemic sclerosis (SSc) who routinely visit the Department of Clinical Immunology and Rheumatology in Hiroshima University Hospital, and were referred to our department between July 2015 and May 2019, were consecutively registered. Patients with an edentulous jaw were excluded. All patients fulfilled the 1980 American College of Rheumatology (ACR) classification criteria for SSc and/or the 2013 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for SSc [14,15].
2.2. Variable Assessment
The classification of limited cutaneous (lc) or diffused cutaneous (dc)-SSc and the prevalence of Raynaud’s phenomenon, microvascular disorder, skin sclerosis, ectopic calcinosis, digital ulcers, myalgia, arthralgia, gastroesophageal reflux disease (GERD), dysphagia, Sjögren’s syndrome, pulmonary fibrosis, pulmonary hypertension, cardiovascular involvements and scleroderma renal crisis (SRC), were evaluated by physician rheumatologists at the Department of Clinical Immunology and Rheumatology. Serum immunological profiles, which included anti-nuclear antibodies (ANA) and anti-centromere antibodies, anti-Scl-70 antibodies, anti-RNA polymerase III antibodies, anti-SS-A antibodies, anti-SS-B antibodies, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibodies, were assessed at the laboratory unit in Hiroshima University Hospital.
All SSc patients were subjected to dental X-rays or panoramagraphy to find root resorption. Cone beam computed tomography (CBCT) was used when dentists needed to diagnose root resorption in three dimensions. Clinical assessments are described below.
Root resorption: Root resorption was evaluated as described previously, and a classification higher than 1Ad was defined as external root resorption [16]. More than two teeth with root resorption was defined as multiple root resorption.
PDL space widening: A widened PDL space was evaluated in dental X-ray images, as described previously [17].
Facial skin sclerosis: A smooth, tight, and expressionless face, often referred to as a “mask-like” appearance, with the simultaneous disappearance of wrinkles and perioral furrows. Nasal alae undergo atrophy, often termed a “mouse-like” face [18,19].
Temporomandibular disorder (TMD) symptoms: Patients’ symptoms, collected as categorical data (the presence or absence of TMDs), were recorded through a questionnaire investigating masticatory muscle pain at rest and chewing, neck and shoulder stiffness, temporomandibular junction arthralgia, the feeling of a locked jaw and migraines and headaches [20].
Reduced opening: Restricted mandibular opening of <40 mm [21].
Tongue rigidity: Lingual fibrotic induration and consequent reductions in mobility.
2.3. Ethical Considerations
All subjects provided informed consent for inclusion before they participated in the present study. This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee for Epidemiological Research at Hiroshima University, Japan (approval no. E-1043; 11 December 2017).
2.4. Statistical Analysis
Fisher’s exact tests were used to compare categorical variables in SSc patients with/without MERR. Odds ratios (OR) and 95% confidence intervals (95%CI) are shown below. The Student’s t-test was used to compare disease duration and the age of patients with or without multiple external root resorption (MERR) (MERR/non-MERR). A p value less than 0.05 was considered to be significant. All tests were performed using the internet-based R software package (version R 3.0.3; http://www.r-project.org).
3. Results
Forty-one patients (female, 85.4%) were included in the present study (Table 1). The mean age of these subjects was 62.8 ± 11.2 years (range, 42–85) with a mean disease duration of 9.6 ± 8.7 years (range, 1–40). Among all subjects, 65.9% had lc-SSc. The prevalence of systemic involvement was similar to that reported previously (musculoskeletal; 5–96% [22], gastroesophageal; 50–70% [23,24], interstitial lung; 80% [25], pulmonary hypertension; 15% [26], cardiovascular; 55% [27], and SRC; 10% [28]).

Table 1.
Demographic data of all systemic sclerosis (SSc) patients. Systemic and maxillofacial involvement and antibodies; number (%). When the total number was less than 41, it was described as number/total number.
In the present study, MERR was detected in four SSc patients, including one described in a case report (pt. 1) [12]. The causes of external resorption, including trauma, periodontal and periapical inflammation, orthodontic treatment, internal bleaching or tumors, were not found in the teeth having external resorption. Dental X-rays and CBCT images showed that MERR was observed in six, six, and four teeth in pts. 2, 3, and 4, respectively (Figure 1a, Figure 2a and Figure 3a). A widening PDL space was detected in all patients. A space between canines and premolars in the upper jaw was found in pts. 3 and 4 (Figure 2a and Figure 3a). Furthermore, the deposition of calcinosis in the nasal spur was noted in pts. 1, [12], 2, and 3, while calcinosis in the palatal plate was observed in pt. 4 (Figure 2c and Figure 3c).
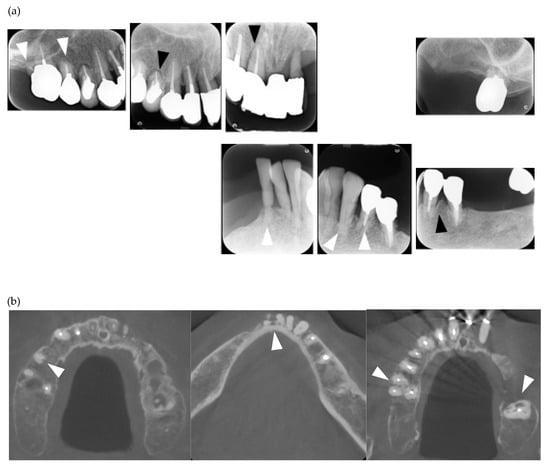
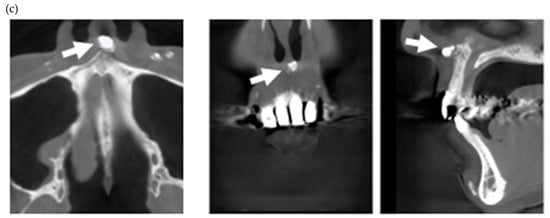
Figure 1.
Multiple external root resorption and calcinosis at the oral region in pt. 2. A dental X-ray photo and cone beam computed tomography (CBCT) image of teeth (a,b) and the deposition of calcinosis at the nasal spur (c). The white arrowhead indicates root resorption, black arrowhead shows periodontal ligament (PDL) space widening and the white arrow, calcinosis.
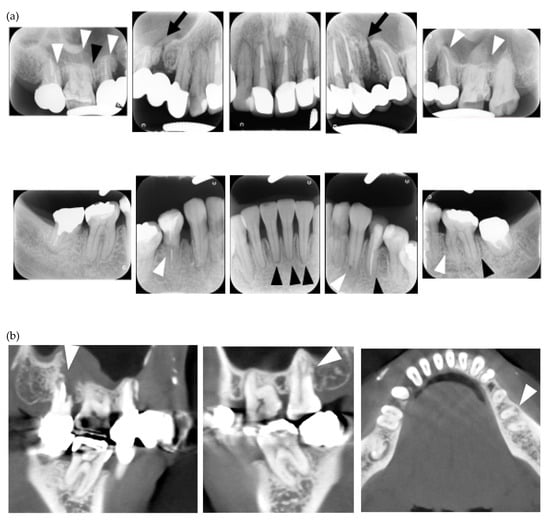
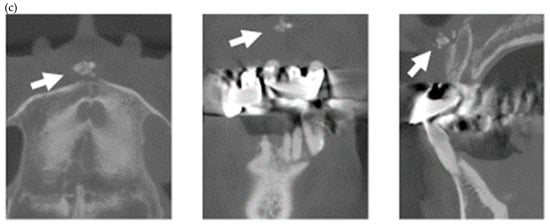
Figure 2.
Multiple external root resorption and calcinosis at the oral region in pt. 3. A dental X-ray photo and CBCT image of teeth (a,b) and the deposition of calcinosis at the nasal spur (c). The white arrowhead points to root resorption, the black arrowhead to PDL space widening, the white arrow at calcinosis and the black arrow indicates the failure to close the space with orthodontic treatment.
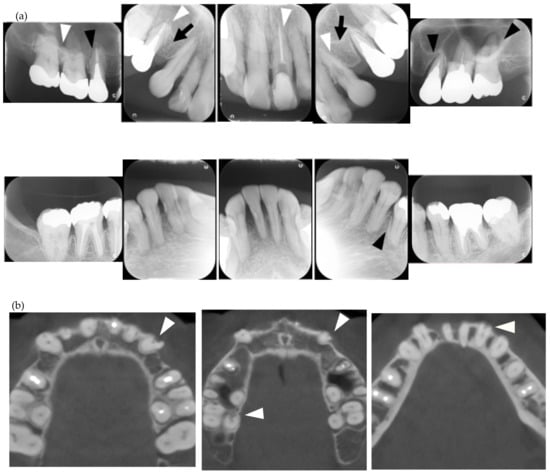
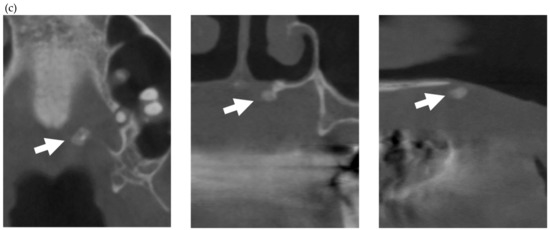
Figure 3.
Multiple external root resorption and calcinosis at the oral region in pt. 4. A dental X-ray photo and CBCT image of teeth and the deposition of calcinosis at the palatal site (a–c). The white arrowhead represents root resorption, the black arrowhead, PDL space widening, and the white arrow, calcinosis, while the black arrow shows a failure to close the space with orthodontic treatment.
A summary of the results obtained on systemic or maxillofacial involvement in each patient is provided in Table 2. Two patients also had rheumatic arthritis, while another had autoimmune disease. Calcification in the PDL space was found in one MERR patient and one non-MERR patient. Furthermore, the occlusal force of pt. 1 was 77.4 N in May 2019, and was weaker than that previously reported [12].

Table 2.
A summary of results on systemic and maxillofacial manifestations in all SSc patients.
Systemic and maxillofacial manifestations in MERR and non-MERR patients are shown in Table 3. Disease duration in MERR patients was significantly longer than that in non-MERR patients. The prevalence of digital ulcers was significantly higher in MERR patients (75%) than in non-MERR patients (16.2%, p < 0.05, OR = 17.0, 95%CI = 1.1–1029.3). However, the prevalence of systemic involvement was not significantly different between non-MERR and MERR patients. On the other hand, among maxillofacial manifestations, the prevalence of face skin sclerosis (100% vs. 10.8%, p < 0.01, OR = 127.8 [95%CI = 5.3–3106.8]), calcinosis at the facial region, (100% vs. 0%, p < 0.01, OR = 245.0 [95%CI = 4.1–14,556.6]), limited mouth opening (75% vs. 18.9%, p < 0.05 OR = 11.8, [95%CI = 0.8–693.0]), TMDs (50% vs. 2.7%, p < 0.05, OR = 28.3, [95%CI = 1.1–2130.3]), and tongue rigidity (75% vs. 2.7%, p < 0.05, OR = 75.0 [95%CI = 2.8–2023.9]) was higher in MERR than in non-MERR patients.

Table 3.
Systemic and maxillofacial manifestations in multiple external root resorption (MERR) and non-MERR patients. Systemic and maxillofacial involvement and antibodies; number (%). When the total number was less than 41, it was described as number/total number. OR; Odds ratio, CI; confidence intervals.
4. Discussion
In the present study, MERR was detected in four SSc patients. These patients had higher homogeneity for several maxillofacial manifestations, but not systemic manifestations. This result suggests that there is a new group with distinctive maxillofacial manifestations in SSc.
Although multiple root resorption has been described in case reports of SSc, an epidemiological study was conducted herein for the first time, and the results obtained revealed a relationship between SSc and MERR. Only 30 cases of idiopathic MERR were reported between 1930 and 2015 [29]. Four MERR patients were identified among 41 SSc patients. This result suggests that SSc is strongly associated with the development of MERR. The occlusal force and surface area in pt. 1 was markedly weaker than that reported previously in women of the same age [30], resulting in weakened mastication ability. This may be attributed to the loss of PDL due to root resorption. To prevent this, the early detection and treatment of MERR are needed. Furthermore, pt. 3 and pt. 4 received orthodontic treatment that was not successful. A potential reason for this is the loss of PDL caused by root resorption. A careful examination of the condition of the root in SSc patients is needed prior to orthodontic treatment, and it is not recommended for MERR patients.
No significant differences were observed in the prevalence of systemic involvement between non-MERR and MERR patients, except for digital ulcers, whereas the rate of many maxillofacial manifestations was significantly higher in MERR than in non-MERR patients. Although digital ulcers are associated with systemic involvement or mortality in SSc patients [31,32,33], this is the first study to show a relationship between digital ulcers and the oral manifestation MERR. Further studies, such as a prospective study, will clarify whether digital ulcers are a strong risk factor for MERR. Differences in the prevalence of oral manifestations between SSc patients and healthy subjects have been investigated, and the prevalence of manifestations including TMDs and tongue rigidity was reported to be higher in SSc patients than in healthy subjects [18,20,34]. Furthermore, a previous study revealed a relationship between oral manifestations and systemic involvement or severity in SSc patients [35]. In the present study, MERR was found to be associated with other oral manifestations. MERR may be an important factor for assessing the severity of oral manifestations and systemic involvement. The deposition of calcinosis in the maxillofacial region was found be more frequent among MERR patients than non-MERR patients, whereas no significant differences were observed in the prevalence of subcutaneous calcinosis. Subcutaneous calcinosis in lc-SSc patients has been associated with acroosteolysis, a higher modified Rodnan skin score, and higher serum levels of phosphorus [36]. Taken together with the present results, these findings indicate that abnormalities in bone or tooth metabolism in the maxillofacial region are related to MERR. A higher prevalence of maxillofacial manifestations, such as facial skin sclerosis, mouth opening disorder, and TMDs, was found in MERR than in non-MERR patients. The root condition of SSc patients with these manifestations needs to be carefully assessed, and interdisciplinary treatment between medical doctors and dentists is needed. SSc patients with MERR may have a similar background for cell function disorders in the maxillofacial region, which implies that the mechanisms contributing to the disease may be clarified using disease-specific iPS cells of MERR patients.
The mechanisms underlying external root resorption have been investigated, but remain unclear. Patel et al. published a review on external cervical resorption [37], and suggested three stages of resorption: Initiation, progression, and reparative. The destruction of PDL or cementum in the initiation stage triggers the formation of clastic cells, hypoxic conditions activate osteoclastogenesis in the progression stage, and finally, repaired by bone-like tissue into the resorption.
Since MERR in SSc patients has mainly been detected in the cervical region, cementum abrasion caused by occlusal force may be a cause of MERR. In SSc patients, vasculopathy causes hypoxia in tissue [38], which indicates that hypoxic conditions in periodontal tissue, including PDL and alveolar bone, activate osteoclasts in MERR patients. Calcification in the PDL space was found in MERR and non-MERR patients. This result suggests that bone or tooth metabolism disorders lead to MERR. Furthermore, the fractured tooth in pt. 1 was histologically analyzed, and the findings obtained revealed that the fracture was repaired with bone-like tissue [12]. These findings suggest excessive bone formation in these patients is related to the resorption.
The limitations of the present study are the small sample number examined and its cross-sectional nature. A larger sample number and prospective study are needed to clarify the relationship between SSc and MERR. The root condition of SSc patients without MERR in the present study will be carefully checked for the appearance of MERR.
5. Conclusions
MERR was clearly identified as a manifestation in patients with SSc, and these patients had highly homogenous maxillofacial manifestations. Since MERR is a silent symptom, root conditions need to be carefully checked in patients with SSc who have maxillofacial features. Furthermore, the prevalence of SSc needs to be clarified in patients with idiopathic multiple root resorption or abnormal root conditions. Further clinical and basic studies that focus on maxillofacial involvement will contribute to the mechanisms of SSc being elucidated in more detail.
Author Contributions
Conceptualization, S.M. and H.K.; methodology, T.M. and S.M.; software, S.M.; validation, N.M., K.O., T.F., and H.N.; formal analysis, T.M. and S.M.; investigation, T.M., S.H., Y.Y., T.S., and S.M.; data curation, M.K. and S.M.; writing—original draft preparation, S.M.; writing—review and editing, M.K. and E.S.; visualization, X.X.; supervision, H.K., E.S., and H.K.
Acknowledgments
This manuscript was proofread by Medical English Service.
Conflicts of Interest
S.H. received a research grant, consultancy fee, advisory fee, or speaker’s bureau from AbbVie, Asahi-Kasei Pharma, Asahi-Kasei Medical, Astellas, Ayumi, Bristol-Myers Squibb, Celgene, Chugai, Eisai, Eli Lilly, Janssen, Kissei, Novartis, Pfizer, Sanofi, Takeda, Tanabe-Mitsubishi, and UCB.
References
- Leroy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; A Medsger, T.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Mayes, M.D.; Lacey, J.V.; Beebe-Dimmer, J.; Gillespie, B.W.; Cooper, B.; Laing, T.J.; Schottenfeld, D.; Beebe-Dimmer, J. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003, 48, 2246–2255. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.G.; Hamburger, J.; Potts, A.J. Orofacial radiological manifestations of systemic sclerosis. Dentomaxillofac. Radiol. 1996, 25, 193–196. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, M.A.Z.; De Figueiredo, J.A.P.; Porter, S. Root Resorption Associated with Mandibular Bone Erosion in a Patient with Scleroderma. J. Endod. 2008, 34, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, M.; Macdonald, D.; Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Gravel, S.; Mohit, S.; El Sayegh, T.; Pope, J.; et al. The Canadian Systemic Sclerosis Oral Health Study IV: Oral radiographic manifestations in systemic sclerosis compared with the general population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 104–111. [Google Scholar] [CrossRef]
- Jung, S.; Minoux, M.; Manière, M.-C.; Martin, T.; Schmittbuhl, M. Previously undescribed pulpal and periodontal ligament calcifications in systemic sclerosis: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e47–e51. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, Z.; Nasser, M.; Mahdian, M.; Fedorowicz, Z.; A Marchesan, M. Interventions for the management of external root resorption. Cochrane Database Syst. Rev. 2015, CD008003. [Google Scholar] [CrossRef]
- Barclay, C.W. Root resorption: Aetiology, classification and clinical management. Dent. Updat. 1993, 20, 248–250. [Google Scholar]
- Patel, S.; Kanagasingam, S.; Ford, T.P. External Cervical Resorption: A Review. J. Endod. 2009, 35, 616–625. [Google Scholar] [CrossRef]
- Mavridou, A.M.; Hauben, E.; Wevers, M.; Schepers, E.; Bergmans, L.; Lambrechts, P. Understanding External Cervical Resorption in Vital Teeth. J. Endod. 2016, 42, 1737–1751. [Google Scholar] [CrossRef]
- Smidt, A.; Nuni, E.; Keinan, D. Invasive Cervical Root Resorption: Treatment Rationale with an Interdisciplinary Approach. J. Endod. 2007, 33, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Memida, T.; Mizuno, N.; Ogawa, I.; Ouhara, K.; Kajiya, M.; Fujita, T.; Sugiyama, E.; Kurihara, H. Reparative bone-like tissue formation in the tooth of a systemic sclerosis patient. Int. Endod. J. 2018, 51, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Bote, S.; Bucchi, C.; Manzanares, M.C.; Céspedes, M.C.M. External Cervical Resorption: A New Oral Manifestation of Systemic Sclerosis. J. Endod. 2017, 43, 1740–1743. [Google Scholar] [CrossRef] [PubMed]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980, 23, 581–590. [CrossRef]
- Hoogen, F.V.D.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Patel, S.; Foschi, F.; Mannocci, F.; Patel, K. External cervical resorption: A three-dimensional classification. Int. Endod. J. 2018, 51, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Mahmood, H.; Cao, C.F.; Jin, L.J. Teeth under High Occlusal Force may Reflect Occlusal Trauma-associated Periodontal Conditions in Subjects with Untreated Chronic Periodontitis. Chin. J. Dent. Res. 2017, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Martin, T.; Schmittbuhl, M.; Huck, O. The spectrum of orofacial manifestations in systemic sclerosis: A challenging management. Oral Dis. 2017, 23, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Albilia, J.B.; Lam, D.K.; Blanas, N.; Clokie, C.M.; Sandor, G.K. Small mouths…Big problems? A review of scleroderma and its oral health implications. J. Can. Dent. Assoc. 2007, 73, 831–836. [Google Scholar]
- Crincoli, V.; Fatone, L.; Fanelli, M.; Rotolo, R.P.; Chialà, A.; Favia, G.; Lapadula, G. Orofacial Manifestations and Temporomandibular Disorders of Systemic Scleroderma: An Observational Study. Int. J. Mol. Sci. 2016, 17, 1189. [Google Scholar] [CrossRef] [PubMed]
- Agerberg, G. Maximal mandibular movements in young men and women. Sven. Tandlak. Tidskr. Swed. Dent. J. 1974, 67, 81–100. [Google Scholar]
- Lóránd, V.; Czirják, L.; Minier, T. Musculoskeletal involvement in systemic sclerosis. La Presse Médicale 2014, 43, e315–e328. [Google Scholar] [CrossRef]
- Clements, P.J.; Becvar, R.; A Drosos, A.; Ghattas, L.; Gabrielli, A. Assessment of gastrointestinal involvement. Clin. Exp. Rheumatol. 2003, 21, 15–18. [Google Scholar]
- Lepri, G.; Guiducci, S.; Bellando-Randone, S.; Giani, I.; Bruni, C.; Blagojevic, J.; Carnesecchi, G.; Radicati, A.; Pucciani, F.; Marco, M.C. Evidence for oesophageal and anorectal involvement in very early systemic sclerosis (VEDOSS): report from a single VEDOSS/EUSTAR centre. Ann. Rheum. Dis. 2015, 74, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Nagaraja, V.; Tseng, C.-H.; Abtin, F.; Suh, R.; Kim, G.; Wells, A.; Furst, D.E.; Clements, P.J.; Roth, M.D.; et al. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: Implications for cohort enrichment in systemic sclerosis-associated interstitial lung disease trials. Arthritis Res. 2015, 17, 372. [Google Scholar] [CrossRef] [PubMed]
- Nihtyanova, S.I.; Tang, E.C.; Coghlan, J.G.; Wells, A.U.; Black, C.M.; Denton, C.P. Improved survival in systemic sclerosis is associated with better ascertainment of internal organ disease: A retrospective cohort study. QJM 2010, 103, 109–115. [Google Scholar] [CrossRef]
- Avouac, J.; Meune, C.; Borderie, D.; Lefèvre, G.; Kahan, A.; Allanore, Y.; Chenevier-Gobeaux, C.; Chenevier-Gobeaux, C. Cardiac Biomarkers in Systemic Sclerosis: Contribution of High-Sensitivity Cardiac Troponin in Addition to N-Terminal Pro-Brain Natriuretic Peptide. Arthritis Rheum. 2015, 67, 1022–1030. [Google Scholar] [CrossRef]
- Bose, N.; Chiesa-Vottero, A.; Chatterjee, S. Scleroderma renal crisis. Semin. Arthritis Rheum. 2015, 44, 687–694. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L.Y.; Yang, J.; Chen, X.F.; Ge, J.Y.; Wu, J.R.; Sun, W.B. Multiple idiopathic cervical root resorption: A case report. Int. Endod. J. 2016, 49, 189–202. [Google Scholar] [CrossRef]
- Motegi, E.; Nomura, M.; Tachiki, C.; Miyazaki, H.; Takeuchi, F.; Takaku, S.; Abe, Y.; Miyatani, M.; Ogai, T.; Fuma, A.; et al. Occlusal force in people in their sixties attending college for elderly. Bull. Tokyo Dent. Coll. 2009, 50, 135–140. [Google Scholar] [CrossRef]
- Meunier, P.; Dequidt, L.; Barnetche, T.; Lazaro, E.; Duffau, P.; Richez, C.; Couzi, L.; Truchetet, M.E.; Seneschal, J.; Fhu, A. Increased risk of mortality in systemic sclerosis-associated digital ulcers: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 405–409. [Google Scholar] [CrossRef]
- Friedrich, S.; Lüders, S.; Glimm, A.M.; Werner, S.G.; Schmittat, G.; Burmester, G.R.; Backhaus, M.; Riemekasten, G.; Ohrndorf, S. Association between baseline clinical and imaging findings and the development of digital ulcers in patients with systemic sclerosis. Arthritis Res. 2019, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Mihai, C.; Landewe, R.; van der Heijde, D.; Walker, U.A.; Constantin, P.I.; Gherghe, A.M.; Ionescu, R.; Rednic, S.; Allanore, Y.; Avouac, J.; et al. Digital ulcers predict a worse disease course in patients with systemic sclerosis. Ann. Rheum. Dis. 2016, 75, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Lo, E.; Gravel, S.; Gyger, G.; El Sayegh, T.; Pope, J.; Fontaine, A.; et al. The Canadian systemic sclerosis oral health study: orofacial manifestations and oral health-related quality of life in systemic sclerosis compared with the general population. Rheumatology 2014, 53, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Hudson, M.; Tatibouet, S.; Steele, R.; Lo, E.; Gravel, S.; Gyger, G.; El Sayegh, T.; Pope, J.; Fontaine, A.; et al. Relationship between disease characteristics and orofacial manifestations in systemic sclerosis: Canadian Systemic Sclerosis Oral Health Study III. Arthritis Rheum. 2015, 67, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Barros, M.M.; Branco, L.C.M.C.; Takayama, L.; Filho, M.A.G.P.; Sampaio-Barros, P.D.; Pereira, R.M.R. Acroosteolysis and bone metabolism parameters distinguish female patients with limited systemic sclerosis with and without calcinosis: A case control study. Clin. Rheumatol. 2019, 1–5. [Google Scholar] [CrossRef]
- Patel, S.; Mavridou, A.M.; Lambrechts, P.; Saberi, N. External cervical resorption-part 1: histopathology, distribution and presentation. Int. Endod. J. 2018, 51, 1205–1223. [Google Scholar] [CrossRef] [PubMed]
- Chora, I.; Guiducci, S.; Manetti, M.; Romano, E.; Mazzotta, C.; Bellando-Randone, S.; Ibba-Manneschi, L.; Matucci-Cerinic, M.; Soares, R. Vascular biomarkers and correlation with peripheral vasculopathy in systemic sclerosis. Autoimmun. Rev. 2015, 14, 314–322. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).